Synergistic Enhancement of Tribological Behavior and Colloidal Stability in CuO Nanolubricants via Ligand Tuning
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Surface-Modified Nanoparticles
2.2. Characterization of Functionalized CuO Nanoparticles
2.3. Evaluation of the Tribological Performance
2.4. Hybrid Optimization by RSM and BO
2.5. Dispersion Stability, Physical, and Rheological Characteristics
3. Results and Discussion
3.1. Surface Chemistry and Thermal Stability of the Modified Nanoparticles
3.2. Optimization Analysis
3.3. Tribological Characteristics
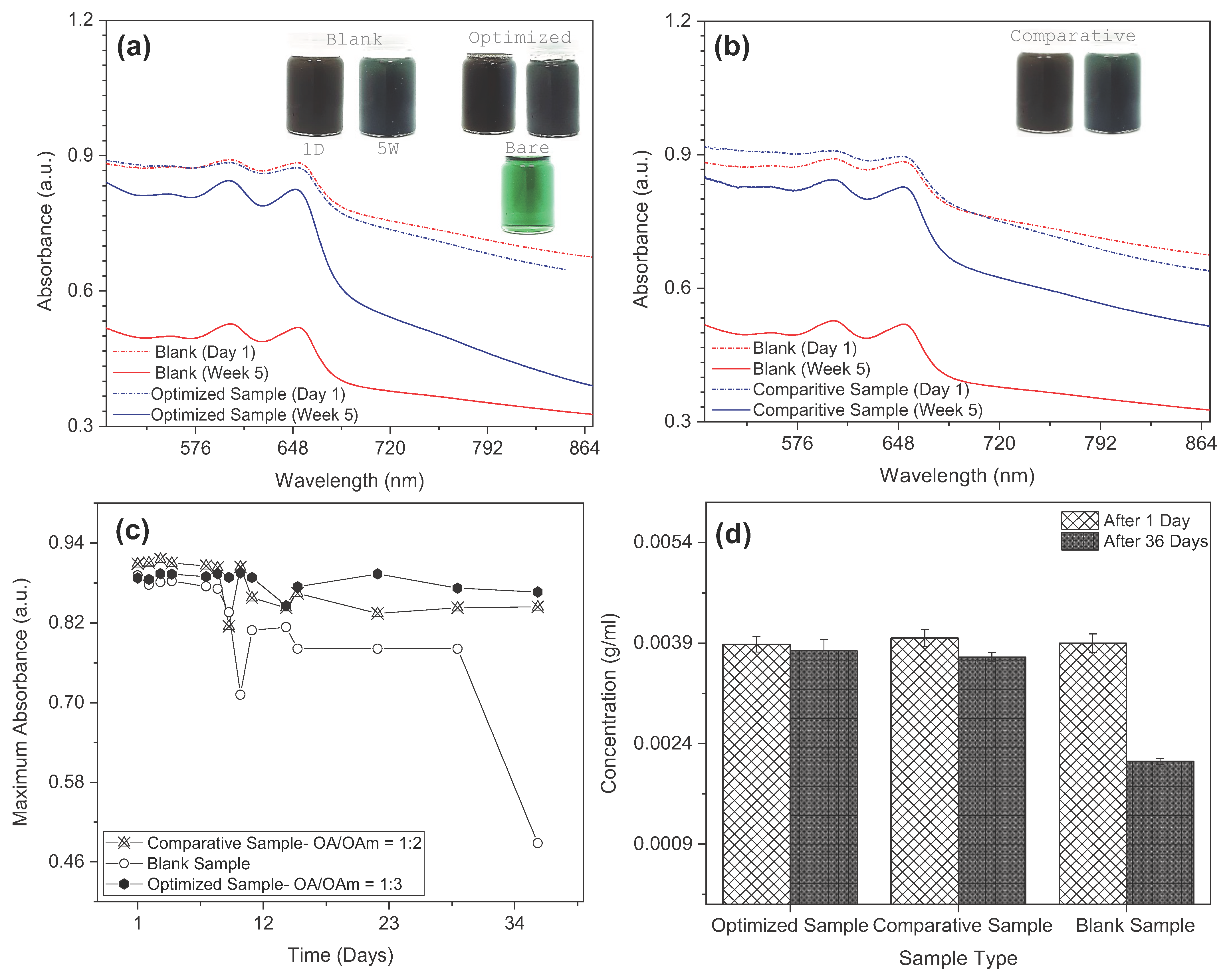
3.4. Colloidal Stability and Rheological Properties
4. Conclusions
- OA/OAm-functionalized CuO nanoparticles significantly enhanced dispersion stability, showing minimal sedimentation over a 5-week period.
- Tribological tests using the ASTM D4172 four-ball method revealed a reduction in the coefficient of friction (COF) by up to 39% compared to unmodified base oil.
- Optimal ligand-functionalized nanolubricants achieved up to 36.5% improvement in wear scar diameter (WSD) reduction.
- Hybrid optimization using RSM and Bayesian Optimization identified an ideal OA–OAm ratio of 1:3 and nanoparticle concentration of 0.04 wt%.
- The developed nanolubricants maintained Newtonian flow behavior and showed suitable high-temperature, high-shear viscosity performance.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Diana Berman, A.E. Vanishing Friction: Progress toward Mechanistic Understanding and Potential Engineering Applications. Tribol. Online 2024, 19, 237–246. [Google Scholar] [CrossRef]
- Saidi, M.Z.; El Moujahid, C.; Pasc, A.; Canilho, N.; Delgado-Sanchez, C.; Celzard, A.; Fierro, V.; Kouitat-Njiwa, R.; Chafik, T. SHH.1 LL901.Enhanced Tribological Properties of Wind Turbine Engine Oil Formulated with Flower-Shaped MoS2 Nano-Additives. Colloids Surf. A Physicochem. Eng. Asp. 2021, 620, 126509. [Google Scholar] [CrossRef]
- Deshpande, P.; Yelkarasi, C.; Lee, S.; Farfan-Cabrera, L.I.; Erdemir, A. Electrotribochemical Formation of Abrasive Nano-Carbon Particles under Electrified Conditions on Lubricated Sliding Contacts. Carbon 2024, 228, 119425. [Google Scholar] [CrossRef]
- Srivyas, P.D.; Charoo, M.S.; Wani, M.F.; Sehgal, R.; Raina, A.; Haq, M.I.U.; Shekhar, C.; Medhi, T.; Arumugam, S. Impact of Surface Texturing on the Tribological Behaviour of Aluminium-Silicon (Al-Si/Al2O3) Advanced Composite under Dry and Lubricating Conditions. Surf. Topogr. Metrol. Prop. 2022, 10, 035043. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, Y.; Liu, B.; Yu, L.; Tong, Y.; Yan, M.; Yang, Z.; Hao, Y.; Shangguan, L.; Zhang, S.; et al. Research Progresses of Nanomaterials as Lubricant Additives. Friction 2024, 12, 1347–1391. [Google Scholar] [CrossRef]
- Garcia Tobar, M.; Contreras Urgiles, R.W.; Jimenez Cordero, B.; Guillen Matute, J. Nanotechnology in Lubricants: A Systematic Review of the Use of Nanoparticles to Reduce the Friction Coefficient. Lubricants 2024, 12, 166. [Google Scholar] [CrossRef]
- Abdel-Rehim, A.A.; Akl, S.; Elsoudy, S. Investigation of the Tribological Behavior of Mineral Lubricant Using Copper Oxide Nano Additives. Lubricants 2021, 9, 16. [Google Scholar] [CrossRef]
- Chen, Y.; Renner, P.; Liang, H. Dispersion of Nanoparticles in Lubricating Oil: A Critical Review. Lubricants 2019, 7, 7. [Google Scholar] [CrossRef]
- Ali, M.K.A.; Xianjun, H. Colloidal Stability Mechanism of Copper Nanomaterials Modified by Ionic Liquid Dispersed in Polyalphaolefin Oil as Green Nanolubricants. J. Colloid Interface Sci. 2020, 578, 24–36. [Google Scholar] [CrossRef]
- Berti, D.; Palazzo, G. Colloidal Foundations of Nanoscience; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Menelaou, M.; Fiuza-Maneiro, N.; Zheng, G.; Wei, S.; Pérez-Juste, J.; Polavarapu, L.; Sofer, Z. Oleic Acid/Oleylamine Ligand Pair: A Versatile Combination in the Synthesis of Colloidal Nanoparticles. Nanoscale Horiz. 2022, 7, 941–1015. [Google Scholar] [CrossRef]
- Hu, C.; Bai, M.; Lv, J.; Wang, P.; Li, X. Molecular Dynamics Simulation on the Friction Properties of Nanofluids Confined by Idealized Surfaces. Tribol. Int. 2014, 78, 152–159. [Google Scholar] [CrossRef]
- Harris, R.A.; Shumbula, P.M.; van der Walt, H. Analysis of the Interaction of Surfactants Oleic Acid and Oleylamine with Iron Oxide Nanoparticles through Molecular Mechanics Modeling. Langmuir 2015, 31, 3934–3943. [Google Scholar] [CrossRef]
- Akl, S.; Elsoudy, S.; Abdel-Rehim, A.A.; Salem, S.; Ellis, M. Recent Advances in Preparation and Testing Methods of Engine-Based Nanolubricants: A State-of-the-Art Review. Lubricants 2021, 9, 85. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Zhang, S.; Yu, L.; Zhang, P.; Zhang, Z. Preparation of Nickel-Based Nanolubricants via a Facile In Situ One-Step Route and Investigation of Their Tribological Properties. Tribol. Lett. 2013, 51, 73–83. [Google Scholar] [CrossRef]
- Jiang, H.; Hou, X.; Yu, Q.; Ali, M.K.A. Elucidation of the Synergistic Mechanism of Nano Silicon Carbide and Titanium Nitride as Highly Efficient Additives of Polyalphaolefin on Strengthening the Lubrication Performance of Movable Contact Interface. Wear 2023, 532–533, 205076. [Google Scholar] [CrossRef]
- Jiang, H.; Hou, X.; Qian, Y.; Liu, H.; Ali, M.K.A.; Dearn, K.D. A Tribological Behavior Assessment of Steel Contacting Interface Lubricated by Engine Oil Introducing Layered Structural Nanomaterials Functionalized by Oleic Acid. Wear 2023, 524–525, 204675. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, T.; Shukla, A. Investigation of Thermal and Tribological Behaviour of Oil Dispersed Oleic Acid-Functionalized CuO Nano-Particles in Gear Oil Nano-Lubricants. J. Nanofluids 2023, 12, 372–387. [Google Scholar] [CrossRef]
- Aravindakshan, R.; Afnas, V.M.; Reghuraj, A.R.; Christopher, K. Characterization of Engine Oil-Based Nano Lubricant Prepared Using CuO and ZnO Nanoparticles. AIP Conf. Proc. 2024, 3134, 130020. [Google Scholar] [CrossRef]
- Chavan, A.G.; Reddy, Y.P. Formulation and Viscosity Evaluation of Copper Oxide Based Nanolubricants. In Techno-Societal 2022; Springer: Cham, Switzerland, 2024; pp. 763–769. [Google Scholar] [CrossRef]
- Azam, S.; Park, S.-S. Experimental and Predictive Modeling of Dynamic Viscosity in Novel Hybrid Nanolubricants Using Correlation and ANN Approaches. Arab. J. Sci. Eng. 2025, 1–23. [Google Scholar] [CrossRef]
- Elsoudy, S.; Akl, S.; Abdel-Rehim, A.A.; Munyebvu, N.; Howes, P.D. Design of Experiments Coupled with Bayesian Optimisation for Nanolubricant Formulation. Colloids Surf. A Physicochem. Eng. Asp. 2024, 693, 134026. [Google Scholar] [CrossRef]
- Hisham, S.; Kadirgama, K.; Mohammed, H.A.; Kumar, A.; Ramasamy, D.; Samykano, M.; Rahman, S. Colloidal:Hybrid Nanocellulose-Copper (II) Oxide as Engine Oil Additives for Tribological Behavior Improvement. Molecules 2020, 25, 2975. [Google Scholar] [CrossRef]
- Arboretti, R.; Ceccato, R.; Pegoraro, L.; Salmaso, L. Design of Experiments and Machine Learning for Product Innovation: A Systematic Literature Review. Qual. Reliab. Eng. Int. 2022, 38, 1131–1156. [Google Scholar] [CrossRef]
- Greenhill, S.; Rana, S.; Gupta, S.; Vellanki, P.; Venkatesh, S. Bayesian Optimization for Adaptive Experimental Design: A Review. IEEE Access 2020, 8, 13937–13948. [Google Scholar] [CrossRef]
- Freiesleben, J.; Jan Keim, M.G. Machine Learning and Design of Experiments: Alternative Approaches or Complementary Methodologies for Quality Improvement? Qual. Reliab. Eng. Int. 2020, 36, 1837–1848. [Google Scholar] [CrossRef]
- Jiang, H.; Hou, X.; Su, D.; Liu, H.; Ali, M.K.A. Elucidation of the Thermophysical Mechanism of Hexagonal Boron Nitride as Nanofluids Additives. Adv. Powder Technol. 2021, 32, 2816–2827. [Google Scholar] [CrossRef]
- Navada, M.K.; Rai, R.; Ganesha, A.; Patil, S. Colloidal:Synthesis and Characterization of Size Controlled Nano Copper Oxide Structures for Antioxidant Study and as Eco-Friendly Lubricant Additive for Bio-Oils. Ceram. Int. 2023, 49, 10402–10410. [Google Scholar] [CrossRef]
- Ick, D.; Ho, C.; Whan, T. Applied Surface Science Structural, Optical, and Electronic Properties of Colloidal CuO Nanoparticles Formed by Using a Colloid-Thermal Synthesis Process. Appl. Surf. Sci. 2009, 255, 8794–8797. [Google Scholar] [CrossRef]
- Bouachma, S.; Ayouz-Chebout, K.; Kechouane, M.; Manseri, A.; Yaddadene, C.; Menari, H.; Gabouze, N. Synthesis of PSi-n/CuO-p/Cu2O-n Heterostructure for CO2 Gas Sensing at Room Temperature. Appl. Phys. A 2022, 128, 69. [Google Scholar] [CrossRef]
- Goh, E.G.; Xu, X.; McCormick, P.G. Effect of Particle Size on the UV Absorbance of Zinc Oxide Nanoparticles. Scr. Mater. 2014, 78–79, 49–52. [Google Scholar] [CrossRef]
- Lin, H.; Wang, C.; Shih, H.C.; Chen, J.; Hsieh, C.; Lin, H.; Wang, C.; Shih, H.C. Characterizing Well-Ordered CuO Nanofibrils Synthesized through Gas-Solid Reactions Characterizing Well-Ordered CuO Nanofibrils Synthesized through Gas-Solid Reactions. J. Appl. Phys. 2004, 95, 5889–5895. [Google Scholar] [CrossRef]
- Brunelli, A.; Pojana, G.; Callegaro, S.; Marcomini, A. Agglomeration and Sedimentation of Titanium Dioxide Nanoparticles (n-TiO2) in Synthetic and Real Waters. J. Nanoparticle Res. 2013, 15, 1684. [Google Scholar] [CrossRef]
- Quik, J.T.K.; Stuart, M.C.; Wouterse, M.; Peijnenburg, W.; Hendriks, A.J.; van de Meent, D. Natural Colloids Are the Dominant Factor in the Sedimentation of Nanoparticles. Environ. Toxicol. Chem. 2012, 31, 1019–1022. [Google Scholar] [CrossRef]
- Zheng, X.; Li, Y.; Chen, D.; Zheng, A.; Que, Q. Study on Analysis and Sedimentation of Alumina Nanoparticles. Int. J. Environ. Res. Public Health 2019, 16, 510. [Google Scholar] [CrossRef]
- ASTM D4172-21; Standard Test Method for Wear Preventive Characteristics of Lubricating Fluid (Four-Ball Method). Annual Book of ASTM Standards. ASTM International: West Conshohocken, PA, USA, 2021.
- Shang, W.; Cai, T.; Zhang, Y.; Liu, D.; Liu, S. Facile One Pot Pyrolysis Synthesis of Carbon Quantum Dots and Graphene Oxide Nanomaterials: All Carbon Hybrids as Eco-Environmental Lubricants for Low Friction and Remarkable Wear-Resistance. Tribol. Int. 2018, 118, 373–380. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, X.; Zhang, L.; Ali, M.K.A.; Jiang, H.; Ma, Y. Development of Nano Biochar as a Lubricating Oil Additive for Tribological Applications. J. Clean. Prod. 2023, 421, 138519. [Google Scholar] [CrossRef]
- Hong, F.T.; Schneider, A.; Sarathy, S.M. Supplementary Material Enhanced Enhanced Lubrication by Core-Shell TiO2 Nanoparticles Modified with Gallic Acid Ester. Tribol. Int. 2020, 146, 106263. [Google Scholar] [CrossRef]
- Medwal, R.; Gautam, S.; Gupta, S.; Chae, K.H.; Asokan, K.; Deen, G.R.; Rawat, R.S.; Katiyar, R.S.; Annapoorni, S. Hard Magnetic Materials Self-Stabilized Carbon- L 1 0 FePt Nanoparticles for Heated Dot Recording Media. IEEE Magn. Lett. 2018, 9, 5504105. [Google Scholar] [CrossRef]
- El-Trass, A.; Elshamy, H.; El-Mehasseb, I.; El-Kemary, M. CuO Nanoparticles: Synthesis, Characterization, Optical Properties and Interaction with Amino Acids. Appl. Surf. Sci. 2012, 258, 2997–3001. [Google Scholar] [CrossRef]
- Online, V.A.; Wu, W.; Hao, R.; Liu, F.; Su, X.; Hou, Y. Single-Crystalline a-Fe2O3 Nanostructures: Controlled Synthesis and High-Index Plane-Enhanced Photodegradation by Visible Light. J. Mater. Chem. A 2013, 1, 6888–6894. [Google Scholar] [CrossRef]
- Dallas, P.; Bourlinos, A.B.; Niarchos, D.; Petridis, D. Synthesis of Tunable Sized Capped Magnetic Iron Oxide Nanoparticles Highly Soluble in Organic Solvents. J. Mater. Sci. 2007, 42, 4996–5002. [Google Scholar] [CrossRef]
- Klokkenburg, M.; Hilhorst, J.; Erné, B.H. Surface Analysis of Magnetite Nanoparticles in Cyclohexane Solutions of Oleic Acid and Oleylamine. Vib. Spectrosc. 2007, 43, 243–248. [Google Scholar] [CrossRef]
- Zhang, L.; He, R.; Gu, H.C. Oleic Acid Coating on the Monodisperse Magnetite Nanoparticles. Appl. Surf. Sci. 2006, 253, 2611–2617. [Google Scholar] [CrossRef]
- Seyhan, M.; Kucharczyk, W.; Yarar, U.E.; Rickard, K.; Rende, D.; Baysal, N.; Bucak, S.; Ozisik, R. Interfacial Surfactant Competition and Its Impact on Poly(Ethylene Oxide)/Au and Poly(Ethylene Oxide)/Ag Nanocomposite Properties. Nanotechnol. Sci. Appl. 2017, 10, 69–77. [Google Scholar] [CrossRef]
- Zhulina, E.B.; Borisov, O.V.; Priamitsyn, V.A. Theory of Steric Stabilization of Colloid Dispersions by Grafted Polymers. J. Colloid Interface Sci. 1990, 137, 495–511. [Google Scholar] [CrossRef]
- Rashad, M.; Rüsing, M.; Berth, G.; Lischka, K.; Pawlis, A. CuO and Co3O4 Nanoparticles: Synthesis, Characterizations, and Raman Spectroscopy. J. Nanomater. 2013, 2013, 714853. [Google Scholar] [CrossRef]
- Ganga, B.G.; Santhosh, P.N. Manipulating Aggregation of CuO Nanoparticles: Correlation between Morphology and Optical Properties. J. Alloys Compd. 2014, 612, 456–464. [Google Scholar] [CrossRef]
- El Mendili, Y.; Grasset, F.; Randrianantoandro, N.; Nerambourg, N.; Greneche, J.-M.; Bardeau, J.-F. Improvement of Thermal Stability of Maghemite Nanoparticles Coated With Oleic Acid and Oleylamine Molecules: Investigations Under Laser Irradiation. J. Phys. Chem. C 2015, 119, 10662–10668. [Google Scholar] [CrossRef]
- Strehle, M.A.; Berg, D.; Reitzenstein, S.; Petra, R. Nondestructive Analysis of Single Rapeseeds by Means of Raman Spectroscopy. J. Raman Spectrosc. 2006, 38, 301–308. [Google Scholar] [CrossRef]
- Jakoby, B.; Scherer, M.; Buskies, M.; Eisenschmid, H. An Automotive Engine Oil Viscosity Sensor. IEEE Sens. J. 2003, 3, 562–568. [Google Scholar] [CrossRef]
- Jendrzej, S.; Gökce, B.; Barcikowski, S. Colloidal Stability of Metal Nanoparticles in Engine Oil under Thermal and Mechanical Load. Chem. Eng. Technol. 2017, 40, 1569–1576. [Google Scholar] [CrossRef]
- De La Presa, P.; Multigner, M.; De La Venta, J.; García, M.A.; Ruiz-González, M.L. Structural and Magnetic Characterization of Oleic Acid and Oleylamine-Capped Gold Nanoparticles. J. Appl. Phys. 2006, 100, 123915. [Google Scholar] [CrossRef]
- Choi, C.; Yoo, H.S.; Oh, J.M. Preparation and Heat Transfer Properties of Nanoparticle-in-Transformer Oil Dispersions as Advanced Energy-Efficient Coolants. Curr. Appl. Phys. 2008, 8, 710–712. [Google Scholar] [CrossRef]
- Hou, X.; Guan, W.; Jiang, H.; Wang, Y.; Kamal, M.; Ali, A. Tribology International Improving Physicochemical and Tribological Characteristics of Bio-Lubricants Using Carbon-Based Hybrid Nanomaterials Modified by Tertiary-Butyl-Hydroquinone. Tribol. Int. 2024, 194, 109442. [Google Scholar] [CrossRef]
- Shekhar, C.; Wani, M.F.; Sehgal, R.; Saleem, S.S.; Ziyamukhamedova, U.; Tursunov, N. Recent Progress in Particulate Reinforced Copper-Based Composites: Fabrication, Microstructure, Mechanical, and Tribological Properties—A Review. Adv. Eng. Mater. 2025, 27, 2401748. [Google Scholar] [CrossRef]
- Shekhar, C.; Wani, M.F.; Sehgal, R.; Ziyamukhamedova, U.; Tursunov, N. A Novel Ceramic Reinforced Metal Matrix Composite (Cu–Ni/TiC–CaF2): Fabrication, Microstructure, Mechanical and Tribological Characterization. Metall. Mater. Trans. B 2025, 56, 1289–1315. [Google Scholar] [CrossRef]
- Keabadile, O.P.; Aremu, A.O.; Elugoke, S.E.; Fayemi, O.E. Green and Traditional Synthesis of Copper Oxide Nanoparticles—Comparative Study. Nanomaterials 2020, 10, 2502. [Google Scholar] [CrossRef]
- Dubey, M.K.; Chaudhary, R.; Emmandi, R.; Seth, S.; Mahapatra, R.; Harinarain, A.K.; Ramakumar, S.S.V. Results in Engineering Tribological Evaluation of Passenger Car Engine Oil: Effect of Friction Modifiers. Results Eng. 2022, 16, 100727. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, L.; Yu, W.; Xie, H. Intriguingly High Thermal Conductivity Increment for CuO Nanowires Contained Nanofluids with Low Viscosity. Sci. Rep. 2018, 8, 5282. [Google Scholar] [CrossRef]
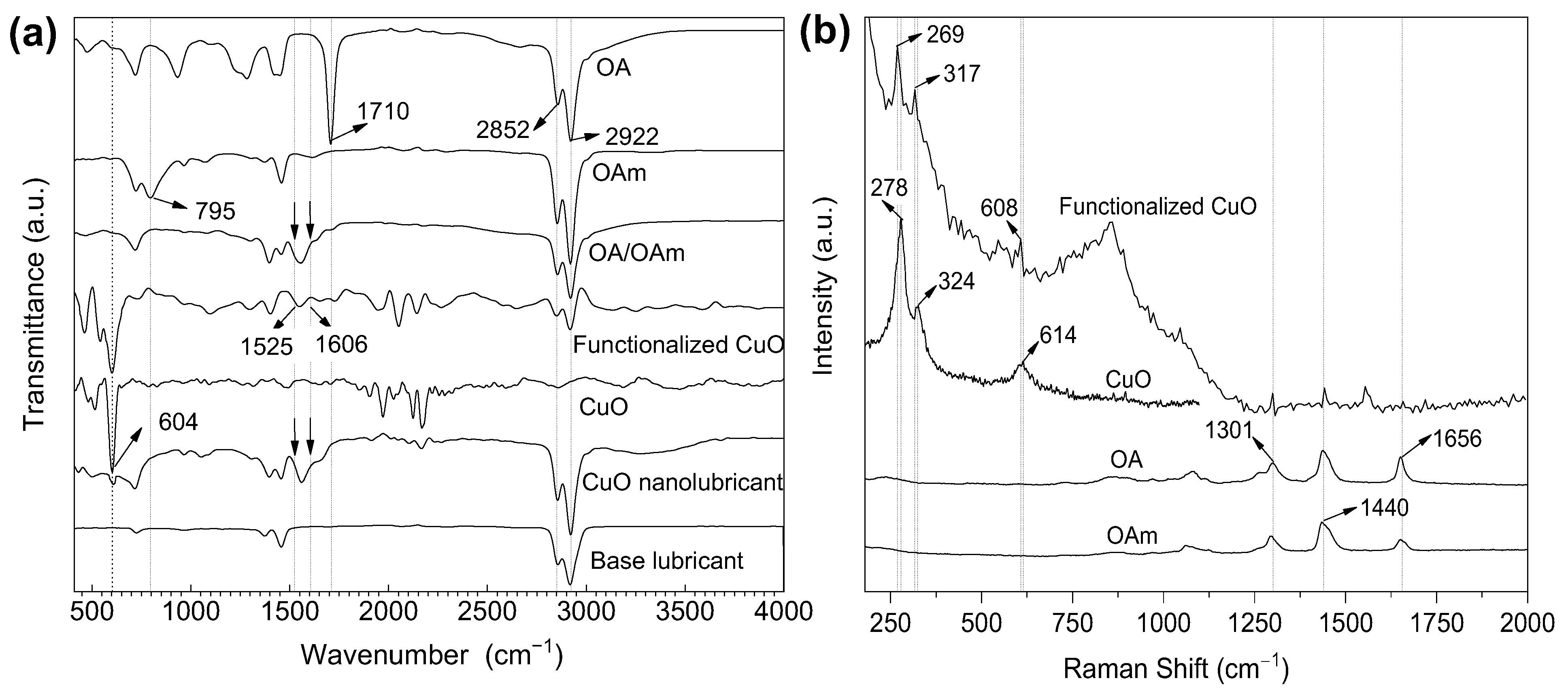

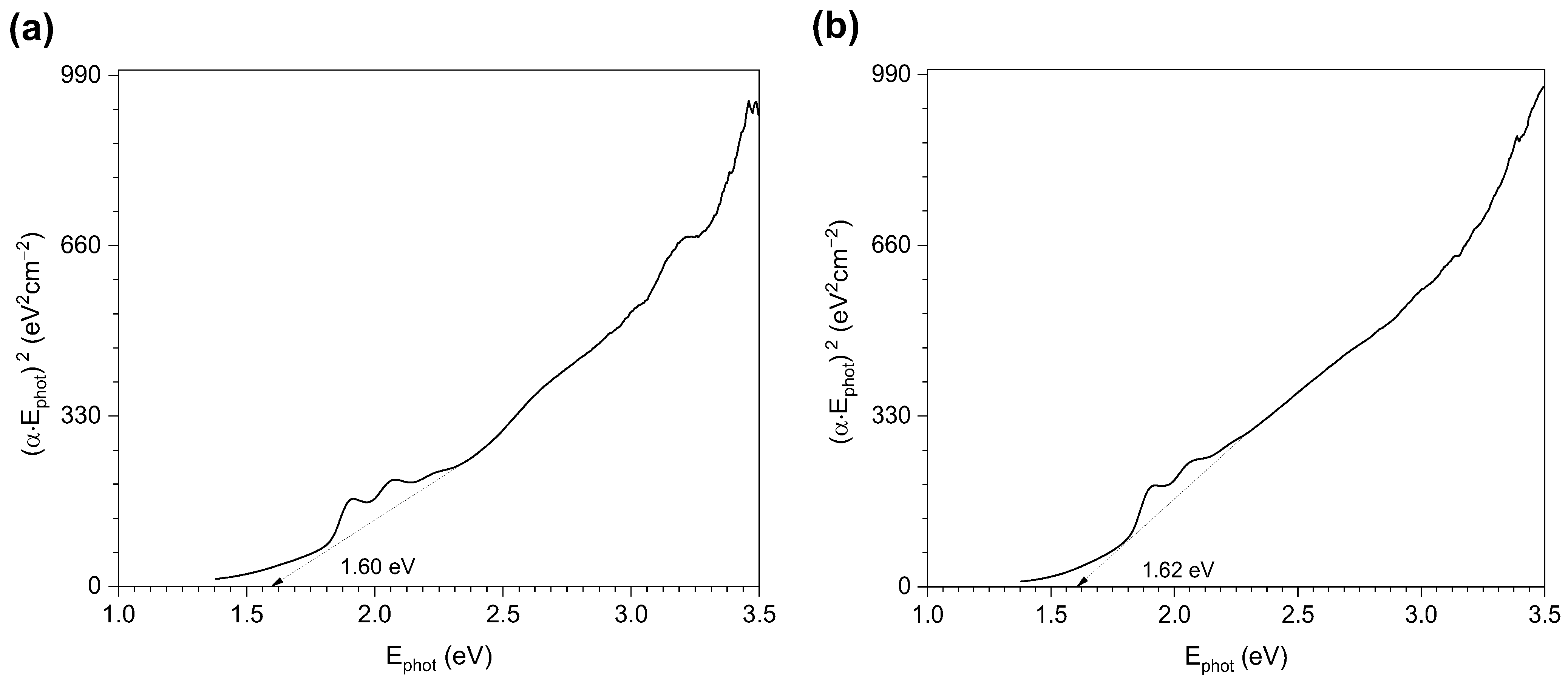
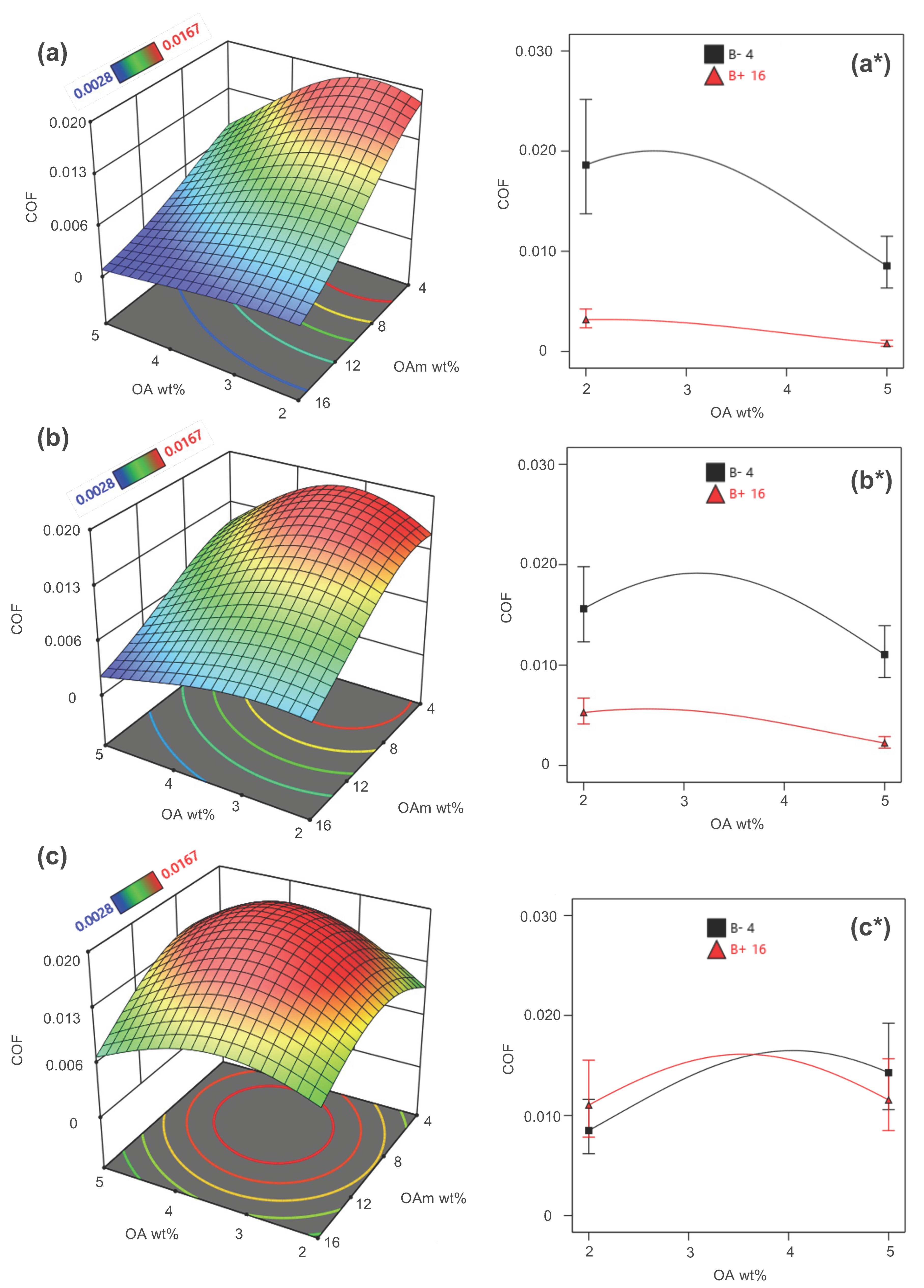
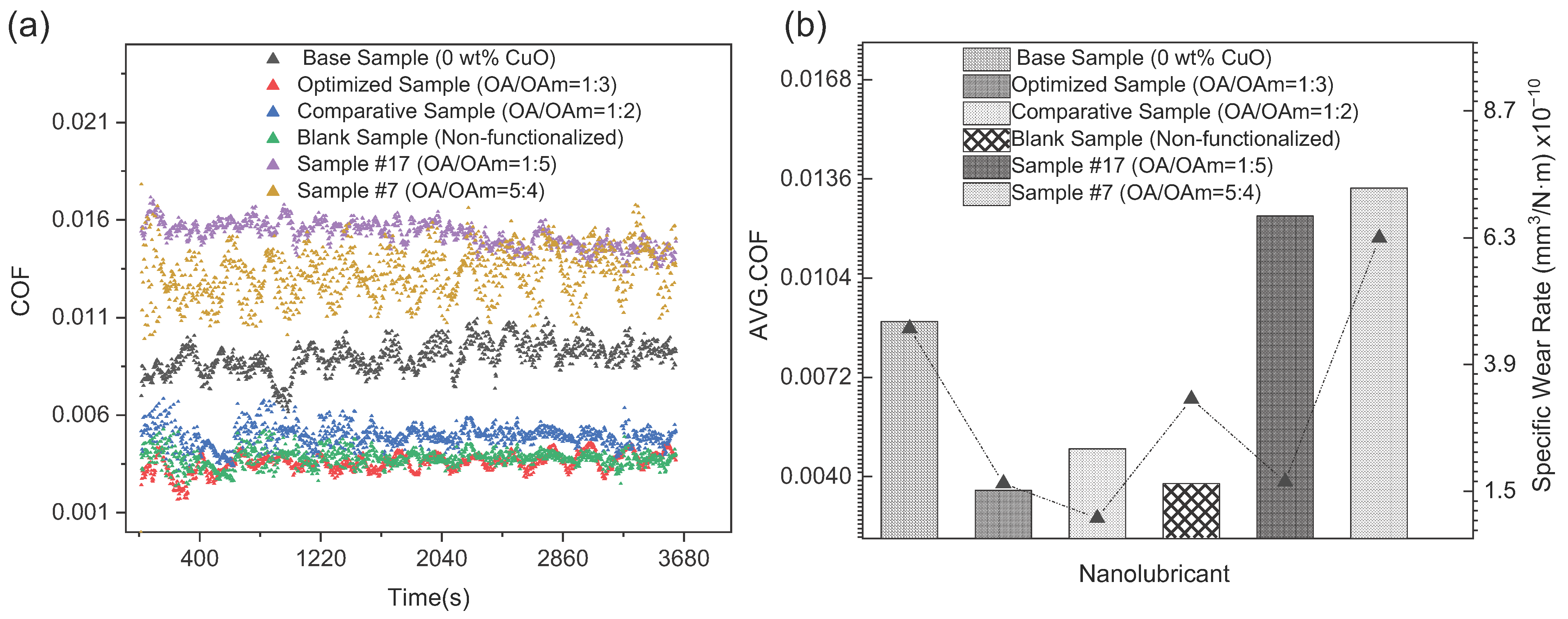

| Substance (Purity) | Appearance | Empirical Formula | Molar Mass (g·mol−1) | Boiling Point (°C) | |
| Copper Oxide (99.99%) | Spherical Dark Gray Particles (35 ± 5 nm) | CuO | 79.55 | - | |
| Oleylamine (98%—primary amine) | Colorless to Yellow | C18H37N | 267.49 | 348–350 | |
| Oleic acid (99.99% GC) | Very Faint Yellow | C18H34O2 | 282.46 | 194–195 | |
| Base Lubricant | Density at 15 °C (kg/m3) | Kinematic Viscosity (cSt) | Viscosity Index | Flashpoint (°C) | Pour Point (°C) |
| SAE 20W50 | 892.1 | 176.4 (40 °C) 19.1 (100 °C) | 123 | 240 | –24 |
| Material | Density (g/cm3) | Diameter (mm) | Hardness (HRC) | Poisson’s Ratio | Elastic Modulus (GPa) |
| GCr15 | 7.8 | 12.7 | 63–65 | 0.3 | 208 |
| Load (Kg, N) | Hertz Pressure (GPa) | Rotating Speeds (rpm) | Linear Speed (m/s) | Temperature (°C) | |
| 40 kg ± 0.2 kg, 392 N ± 2 N | 892.1 | 1200 | 0.461 | 75 °C ± 2 °C | |
| Factor | Code | Units | Coded Variable Levels | ||
|---|---|---|---|---|---|
| –1 | 0 | 1 | |||
| Oleic Acid Concentration (OA) | A | wt% | 2 | 3.5 | 5 |
| Oleylamine Concentration (OAm) | B | wt% | 4 | 10 | 16 |
| Copper Oxide Concentration (CuO) | C | wt% | 0.04 | 0.05 | 0.07 |
| Run | Factors | Ligand Ratio | Response (COF) | ||
|---|---|---|---|---|---|
| A: Oleic Acid Concentration (OA) | B: Oleylamine Concentration (OAm) | C: Copper Oxide Concentration (CuO) | OA–OAm | ||
| 1 | 5 | 10 | 0.07 | 1/2 | 0.0167 |
| 2 | 3.5 | 16 | 0.07 | 2/9 | 0.0167 |
| 3 | 3.5 | 10 | 0.055 | 1/3 | 0.0153 |
| 4 | 2 | 4 | 0.055 | 1/2 | 0.0136 |
| 5 | 3.5 | 4 | 0.04 | 7/8 | 0.0159 |
| 6 | 2 | 10 | 0.07 | 1/5 | 0.0103 |
| 7 | 5 | 4 | 0.055 | 5/4 | 0.0133 |
| 8 | 3.5 | 10 | 0.055 | 1/3 | 0.0150 |
| 9 | 5 | 16 | 0.055 | 1/3 | 0.0035 |
| 10 | 3.5 | 16 | 0.04 | 2/9 | 0.0028 |
| 11 | 2 | 16 | 0.055 | 1/8 | 0.0030 |
| 12 | 5 | 10 | 0.04 | 1/2 | 0.0038 |
| 13 | 3.5 | 10 | 0.055 | 1/3 | 0.0152 |
| 14 | 3.5 | 10 | 0.055 | 1/3 | 0.0152 |
| 15 | 3.5 | 10 | 0.055 | 1/3 | 0.0154 |
| 16 | 3.5 | 4 | 0.07 | 7/8 | 0.0151 |
| 17 | 2 | 10 | 0.04 | 1/5 | 0.0124 |
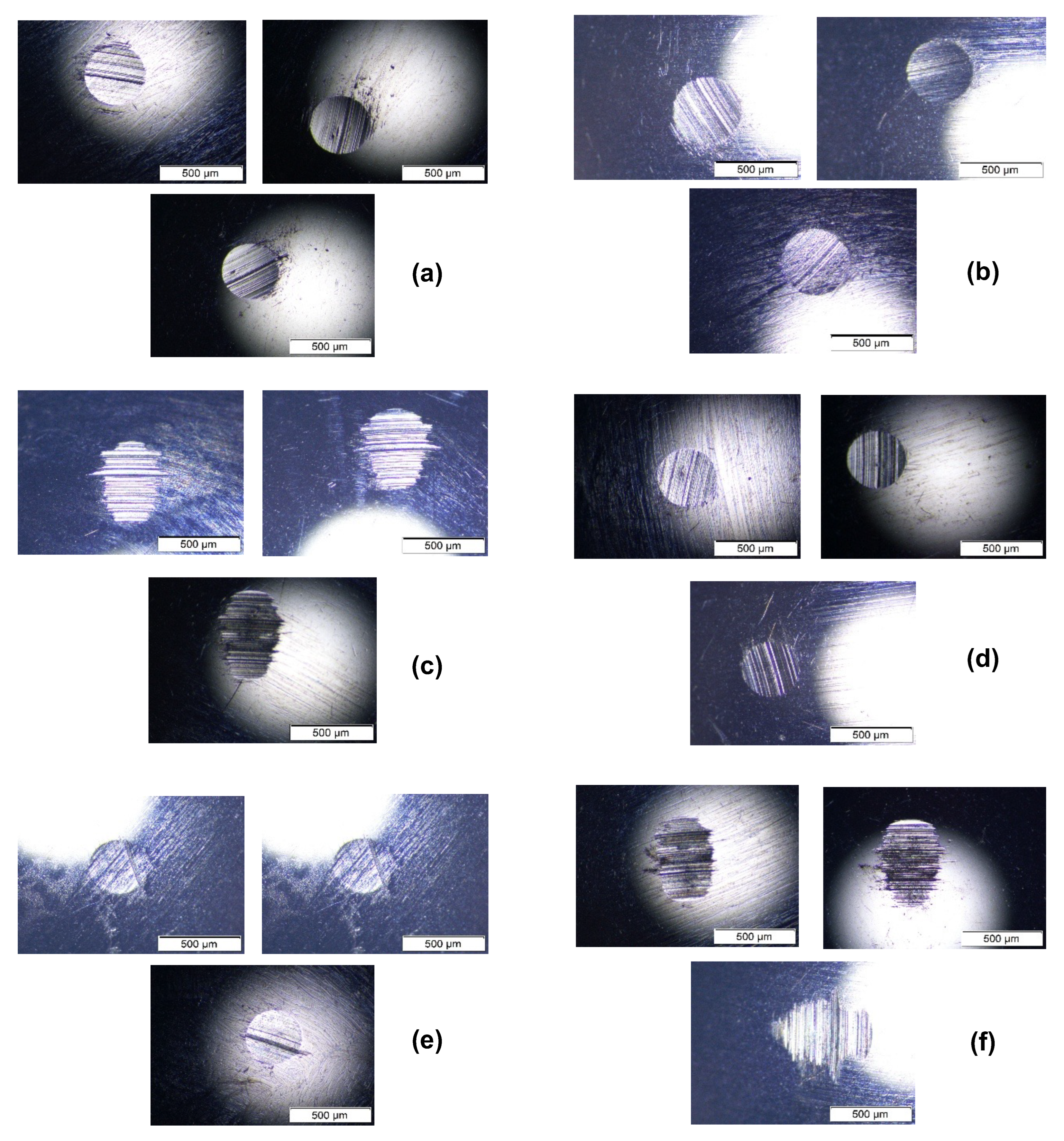
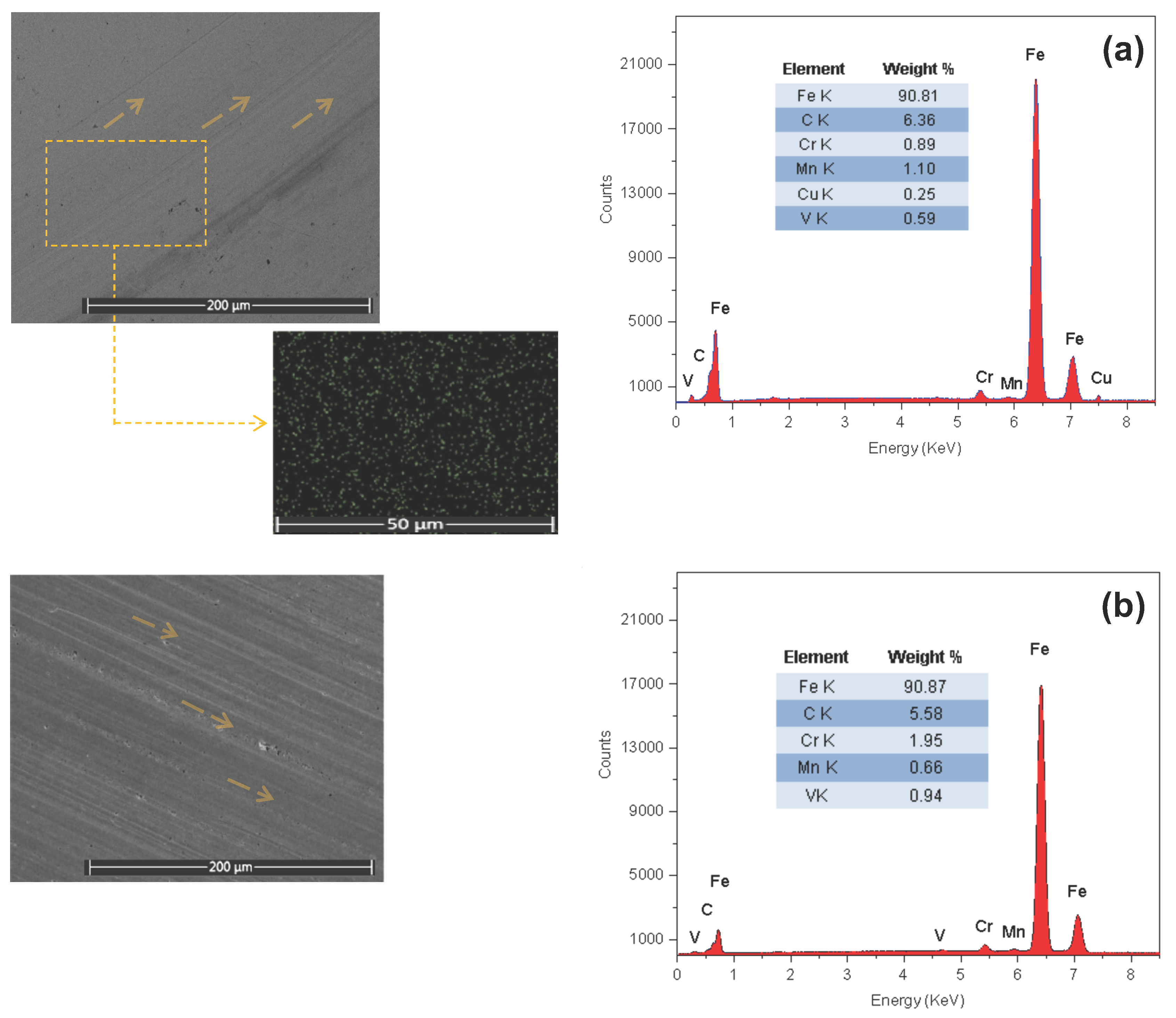
| Lubricant Sample | CuO wt% | OA–OAm Ligand Pair Ratio | Specific Wear Rate—SWR (mm3/N·m) × 10−10 | Percentage Reduction in SWR % vs. Base | Wear Scar Diameter—WSD (um) | AVG COF | Percentage Reduction in COF % vs. Base |
|---|---|---|---|---|---|---|---|
| Base Sample | 0 | 0 | 4.583 | - | 443.121 | 0.0089 | - |
| Optimized Sample | 0.04 | 1:3 | 1.650 | 63.99 | 369.893 | 0.0035 | 60.67 |
| Comparative Sample | 0.04 | 1:2 | 0.987 | 78.47 | 364.320 | 0.0049 | 44.94 |
| Blank Sample | 0.04 | 0 | 3.244 | 29.22 | 406.467 | 0.0037 | 58.43 |
| Sample #17 | 0.04 | 1:5 | 1.680 | 63.34 | 353.760 | 0.0124 | 30.34 |
| Sample #7 | 0.05 | 5:4 | 6.300 | −37.49% (Increase in SWR) | 479.893 | 0.0133 | −49.44 (Increase in COF) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsoudy, S.; Akl, S.; Abdel-Rehim, A.A.; Lane, E.; Hadawey, A.; Howes, P.D. Synergistic Enhancement of Tribological Behavior and Colloidal Stability in CuO Nanolubricants via Ligand Tuning. Lubricants 2025, 13, 358. https://doi.org/10.3390/lubricants13080358
Elsoudy S, Akl S, Abdel-Rehim AA, Lane E, Hadawey A, Howes PD. Synergistic Enhancement of Tribological Behavior and Colloidal Stability in CuO Nanolubricants via Ligand Tuning. Lubricants. 2025; 13(8):358. https://doi.org/10.3390/lubricants13080358
Chicago/Turabian StyleElsoudy, Sherif, Sayed Akl, Ahmed A. Abdel-Rehim, Esme Lane, Abas Hadawey, and Philip D. Howes. 2025. "Synergistic Enhancement of Tribological Behavior and Colloidal Stability in CuO Nanolubricants via Ligand Tuning" Lubricants 13, no. 8: 358. https://doi.org/10.3390/lubricants13080358
APA StyleElsoudy, S., Akl, S., Abdel-Rehim, A. A., Lane, E., Hadawey, A., & Howes, P. D. (2025). Synergistic Enhancement of Tribological Behavior and Colloidal Stability in CuO Nanolubricants via Ligand Tuning. Lubricants, 13(8), 358. https://doi.org/10.3390/lubricants13080358





