Predicting the Viscosity of Ester Biolubricants by the Functional Groups of Their Compounds Using a Sensitivity Parameter Model
Abstract
1. Introduction
2. Experimental Section and Methods
3. Results and Discussion
3.1. TMP-Ester Biolubricant Mixtures Analyzed
3.2. Development of a Model Based on Sensitivity Parameters for Kinematic Viscosity and Viscosity Index in TMP-Ester Biolubricants
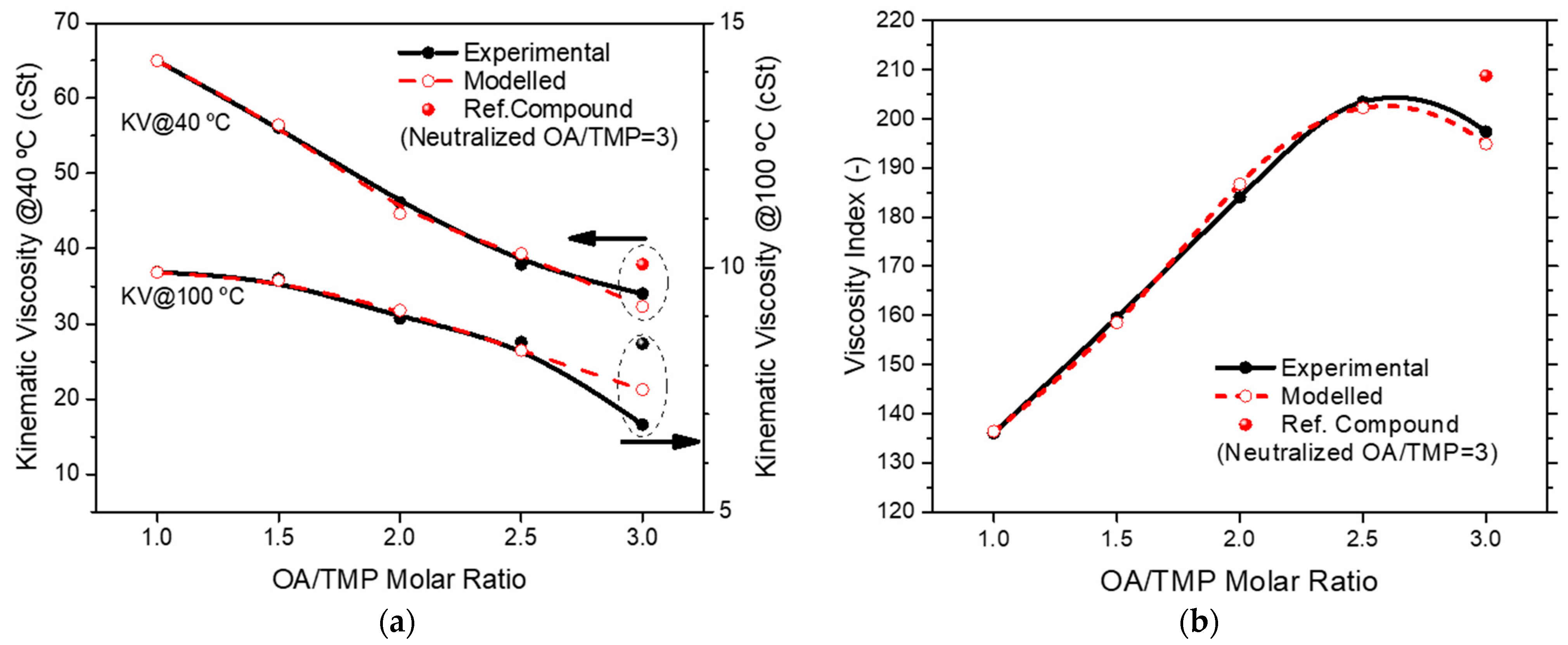
3.3. Influence of the Composition of a Biolubricant Made with Neutralized TMP-Ester Biolubricants on the Viscosity Index
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TMP | trimethylolpropane |
| OA | oleic acid |
| TMPMO | trimethylolpropane monooleate |
| TMPDO | trimethylolpropane dioleate |
| TMPTO | trimethylolpropane trioleate |
| VI | viscosity index |
References
- Gräfen, M.; Hesse, K.; Pollkläsner, D.; Baumann, W. Generic Scenario for the Impact of Cooling Lubricants into the Hydrosphere. Environ. Sci. Pollut. Res. 2003, 10, 301–307. [Google Scholar] [CrossRef]
- Salimon, J.; Salih, N.; Yousif, E. Biolubricants: Raw Materials, Chemical Modifications and Environmental Benefits. Eur. J. Lipid Sci. Technol. 2010, 112, 519–530. [Google Scholar] [CrossRef]
- Fernando, S.; Hanna, M.; Adhikari, S. Lubricity Characteristics of Selected Vegetable Oils, Animal Fats, and Their Derivatives. Appl. Eng. Agric. 2007, 23, 5–11. [Google Scholar] [CrossRef]
- Sathwik Chatra, K.R.; Jayadas, N.H.; Kailas, S.V. Natural Oil-Based Lubricants. In Green Energy and Technology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 287–328. ISBN 9783642236808. [Google Scholar]
- Vaskova, H.; Buckova, M. Thermal Degradation of Vegetable Oils: Spectroscopic Measurement and Analysis. Procedia Eng. 2015, 100, 630–635. [Google Scholar] [CrossRef]
- Maleque, M.A.; Masjuki, H.H.; Sapuan, S.M. Vegetable-Based Biodegradable Lubricating Oil Additives. Ind. Lubr. Tribol. 2003, 55, 137–143. [Google Scholar] [CrossRef]
- Sharma, B.K.; Adhvaryu, A.; Liu, Z.; Erhan, S.Z. Chemical Modification of Vegetable Oils for Lubricant Applications. JAOCS J. Am. Oil Chem. Soc. 2006, 83, 129–136. [Google Scholar] [CrossRef]
- Wagner, H.; Luther, R.; Mang, T. Lubricant Base Fluids Based on Renewable Raw Materials: Their Catalytic Manufacture and Modification. Appl. Catal. A Gen. 2001, 221, 429–442. [Google Scholar] [CrossRef]
- Trasarti, A.F.; Segobia, D.J.; Apesteguía, C.R.; Santoro, F.; Zaccheria, F.; Ravasio, N. Selective Hydrogenation of Soybean Oil on Copper Catalysts as a Tool towards Improved Bioproducts. JAOCS J. Am. Oil Chem. Soc. 2012, 89, 2245–2252. [Google Scholar] [CrossRef]
- de Haro, J.C.; Izarra, I.; Rodríguez, J.F.; Pérez, Á.; Carmona, M. Modelling the Epoxidation Reaction of Grape Seed Oil by Peracetic Acid. J. Clean. Prod. 2016, 138, 70–76. [Google Scholar] [CrossRef]
- Isbell, T.A.; Cermak, S.C. Synthesis of Triglyceride Estolides from Lesquerella and Castor Oils. JAOCS J. Am. Oil Chem. Soc. 2002, 79, 1227–1233. [Google Scholar] [CrossRef]
- Graiver, D.; Tran, P.; Patrick, L.; Farminer, K.; Narayan, R. Modifications of Soybean Oil Using Novel Ozone-Based Chemistry. ACS Symp. Ser. 2006, 939, 76–100. [Google Scholar] [CrossRef]
- Kim, H.; Choi, N.; Kim, Y.; Kim, H.R.; Lee, J.; Kim, I.H. Immobilized Lipase-Catalyzed Esterification for Synthesis of Trimethylolpropane Triester as a Biolubricant. Renew. Energy 2019, 130, 489–494. [Google Scholar] [CrossRef]
- Cavalcante, I.M.; de Rocha, N.R.C.; Maier, M.E.; de Lima, A.P.D.; Andrade Neto, D.M.; de Brito, D.H.A.; Petzhold, C.L.; Schanz, M.T.G.F.; Ricardo, N.M.P.S. Synthesis and Characterization of New Esters of Oleic Acid and Glycerol Analogues as Potential Lubricants. Ind. Crops Prod. 2014, 62, 453–459. [Google Scholar] [CrossRef]
- Tao, Y.; Chen, B.; Liu, L.; Tan, T. Synthesis of Trimethylolpropane Esters with Immobilized Lipase from Candida sp. 99–125. J. Mol. Catal. B Enzym. 2012, 74, 151–155. [Google Scholar] [CrossRef]
- Cavalcanti, E.D.C.; Aguieiras, É.C.G.; da Silva, P.R.; Duarte, J.G.; Cipolatti, E.P.; Fernandez-Lafuente, R.; da Silva, J.A.C.; Freire, D.M.G. Improved Production of Biolubricants from Soybean Oil and Different Polyols via Esterification Reaction Catalyzed by Immobilized Lipase from Candida Rugosa. Fuel 2018, 215, 705–713. [Google Scholar] [CrossRef]
- Ramos, F.J.; de Haro, J.C.; Rodríguez, J.F.; Pérez, Á.; Carmona, M. An Environmentally Friendly Production of Ester-Biolubricant from Oleic Acid. Biofuels Bioprod. Biorefining 2022, 16, 1655–1666. [Google Scholar] [CrossRef]
- Rodrigues, J.D.A.; Cardoso, F.D.P.; Lachter, E.R.; Estevão, L.R.M.; Lima, E.; Nascimento, R.S.V. Correlating Chemical Structure and Physical Properties of Vegetable Oil Esters. J. Am. Oil Chem. Soc. 2006, 83, 353–357. [Google Scholar] [CrossRef]
- DeRieux, W.S.W.; Li, Y.; Lin, P.; Laskin, J.; Laskin, A.; Bertram, A.K.; Nizkorodov, S.A.; Shiraiwa, M. Predicting the Glass Transition Temperature and Viscosity of Secondary Organic Material Using Molecular Composition. Atmos. Chem. Phys. 2018, 18, 6331–6351. [Google Scholar] [CrossRef]
- Rothfuss, N.E.; Petters, M.D. Influence of Functional Groups on the Viscosity of Organic Aerosol. Environ. Sci. Technol. 2017, 51, 271–279. [Google Scholar] [CrossRef]
- Gervasi, N.R.; Topping, D.O.; Zuend, A. A Predictive Group-Contribution Model for the Viscosity of Aqueous Organic Aerosol. Atmos. Chem. Phys. 2020, 20, 2987–3008. [Google Scholar] [CrossRef]
- ASTM D2270-10(2016); Standard Practice for Calculating Viscosity Index from Kinematic Viscosity at 40 °C and 100 °C. ASTM International: West Conshohocken, PA, USA, 2016.
- Zainal, N.A.; Zulkifli, N.W.M.; Gulzar, M.; Masjuki, H.H. A Review on the Chemistry, Production, and Technological Potential of Bio-Based Lubricants. Renew. Sustain. Energy Rev. 2018, 82, 80–102. [Google Scholar] [CrossRef]
- Owuna, F.J.; Dabai, M.U.; Sokoto, M.A.; Dangoggo, S.M.; Bagudo, B.U.; Birnin-Yauri, U.A.; Hassan, L.G.; Sada, I.; Abubakar, A.L.; Jibrin, M.S. Chemical Modification of Vegetable Oils for the Production of Biolubricants Using Trimethylolpropane: A Review. Egypt. J. Pet. 2020, 29, 75–82. [Google Scholar] [CrossRef]
- Kendall, J.; Monroe, K.P. The Viscosity of Liquids.II.The Viscosity-Composition Curve for Ideal Liquid Mixtures. J. Am. Chem. Soc. 1917, 39, 1787–1802. [Google Scholar] [CrossRef]
- Zhai, Y.M.; Wang, Y.; Yang, W.; Xie, B.H.; Yang, M.B. Dynamic Rheological Behavior of Copolymerized Linear Low-Density Polyethylenes: Effect of Molecular Weight and Its Distribution. J. Macromol. Sci. Part B Phys. 2009, 48, 844–855. [Google Scholar] [CrossRef]
- Raju, V.R.; Smith, G.G.; Marin, G.; Knox, J.R.; Graessley, W.W. Properties of Amorphous and Crystallizable Hydrocarbon Polymers. Melt Rheology of Fractions of Linear Polyethylene. J. Polym. Sci. Polym. Phys. Ed. 1979, 17, 1183–1195. [Google Scholar] [CrossRef]
- Colby, R.H.; Fetters, L.J.; Graessley, W.W. Melt Viscosity-Molecular Weight Relationship for Linear Polymers. Macromolecules 1987, 20, 2226–2237. [Google Scholar] [CrossRef]
- Asadauskas, S.; Perez, J.M.; Duda, J.L. Lubrication Properties of Castor Oil—Potential Basestock for Biodegradable Lubricants. Lubr. Eng. 1997, 53, 35–40. [Google Scholar]
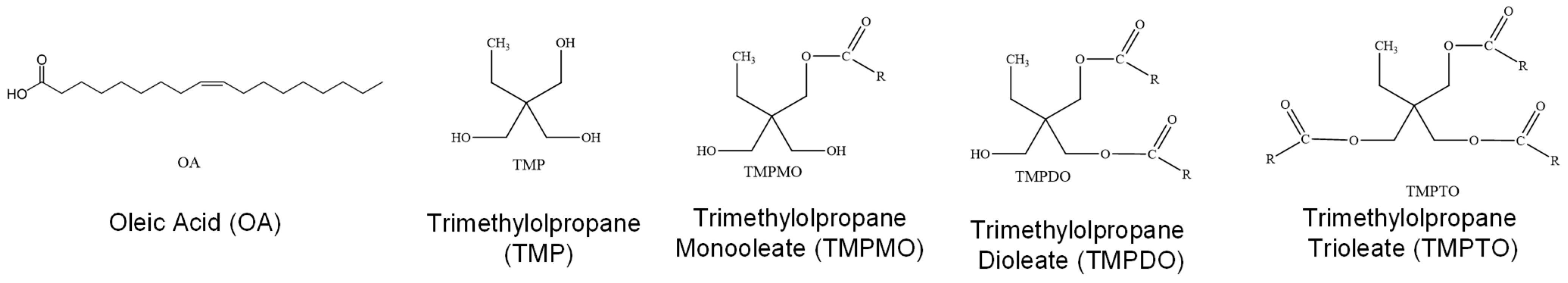


| Process | Functional Group Change, i | Change Made | Sensitivity Parameter, @40 °C (-) | Sensitivity Parameter, @100 °C (-) | |
|---|---|---|---|---|---|
| TMPTO → OA | TMP-(R-COO)3 → R-COOH | 3 -COO → pure OA | 3 | −0.0614 | −0.0848 |
| TMPDO → TMPMO | TMP-(R-COO)3 → (R-COO)-TMP-(OH)2 | 3 -COO → 1 -COO + 2 -OH | 2 | 0.2633 | 0.0796 |
| TMPTO → TMPDO | TMP-(R-COO)3 → (R-COO)2-TMP-OH | 3 -COO → 2 -COO + 1 -OH | 1 | 0.0476 | 0.0680 |
| Ref: TMPTO → TMPTO | Ref: TMP-(R-COO)3 → TMP-(R-COO)3 | N/A | 0 | 0 | 0 |
| r2 | 0.9931 | 0.9667 |
| Product | OA/TMP | Molar Fraction | Modeled @40 °C (cSt) | @100 °C (cSt) | Modeled VI (-) | |||
|---|---|---|---|---|---|---|---|---|
| TMPTO | TMPDO | TMPMO | OA | |||||
| Neutralized TMP-ester | 1 | 0.323 | 0.266 | 0.412 | - | 68.0 | 10.3 | 137 |
| 1.5 | 0.350 | 0.380 | 0.270 | - | 57.5 | 9.9 | 160 | |
| 2 | 0.653 | 0.239 | 0.107 | - | 45.5 | 9.1 | 188 | |
| 2.5 | 0.819 | 0.150 | 0.031 | - | 40.4 | 8.7 | 204 | |
| 3 * | 1.000 | 0.000 | 0.000 | - | 37.9 * | 8.4 * | 209 * | |
| Compound | @40 °C (cSt) | @100 °C (cSt) | Modeled VI (-) | Molecular Weight (g·mol−1) |
|---|---|---|---|---|
| TMPMO | 127.5 | 12.2 | 82 | 398 |
| TMPDO | 42.3 | 9.9 | 230 | 662 |
| TMPTO * | 37.9 * | 8.4 * | 209 * | 926 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, F.J.; de Haro, J.C.; Rodríguez, J.F.; Pérez, Á.; Carmona, M. Predicting the Viscosity of Ester Biolubricants by the Functional Groups of Their Compounds Using a Sensitivity Parameter Model. Lubricants 2025, 13, 179. https://doi.org/10.3390/lubricants13040179
Ramos FJ, de Haro JC, Rodríguez JF, Pérez Á, Carmona M. Predicting the Viscosity of Ester Biolubricants by the Functional Groups of Their Compounds Using a Sensitivity Parameter Model. Lubricants. 2025; 13(4):179. https://doi.org/10.3390/lubricants13040179
Chicago/Turabian StyleRamos, F. Javier, Juan Carlos de Haro, Juan Francisco Rodríguez, Ángel Pérez, and Manuel Carmona. 2025. "Predicting the Viscosity of Ester Biolubricants by the Functional Groups of Their Compounds Using a Sensitivity Parameter Model" Lubricants 13, no. 4: 179. https://doi.org/10.3390/lubricants13040179
APA StyleRamos, F. J., de Haro, J. C., Rodríguez, J. F., Pérez, Á., & Carmona, M. (2025). Predicting the Viscosity of Ester Biolubricants by the Functional Groups of Their Compounds Using a Sensitivity Parameter Model. Lubricants, 13(4), 179. https://doi.org/10.3390/lubricants13040179








