Rheological Study and FTIR Analysis of Thermally Degraded Mineral and Biodegradable Hydraulic Fluids
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thermal Degradation
- For the mineral hydraulic fluid, 130 °C and 150 °C;
- For the two biodegradable hydraulic fluids, 150 °C, 200 °C, and 220 °C.
2.3. Rheological Study
- —shear stress [Pa];
- —viscosity [Pa·s];
- —shear rate [s−1].
- and are material constants— is the consistency index [Pa·sn] and is the flow index (dimensionless).
2.4. FTIR Spectroscopy
3. Results and Discussion
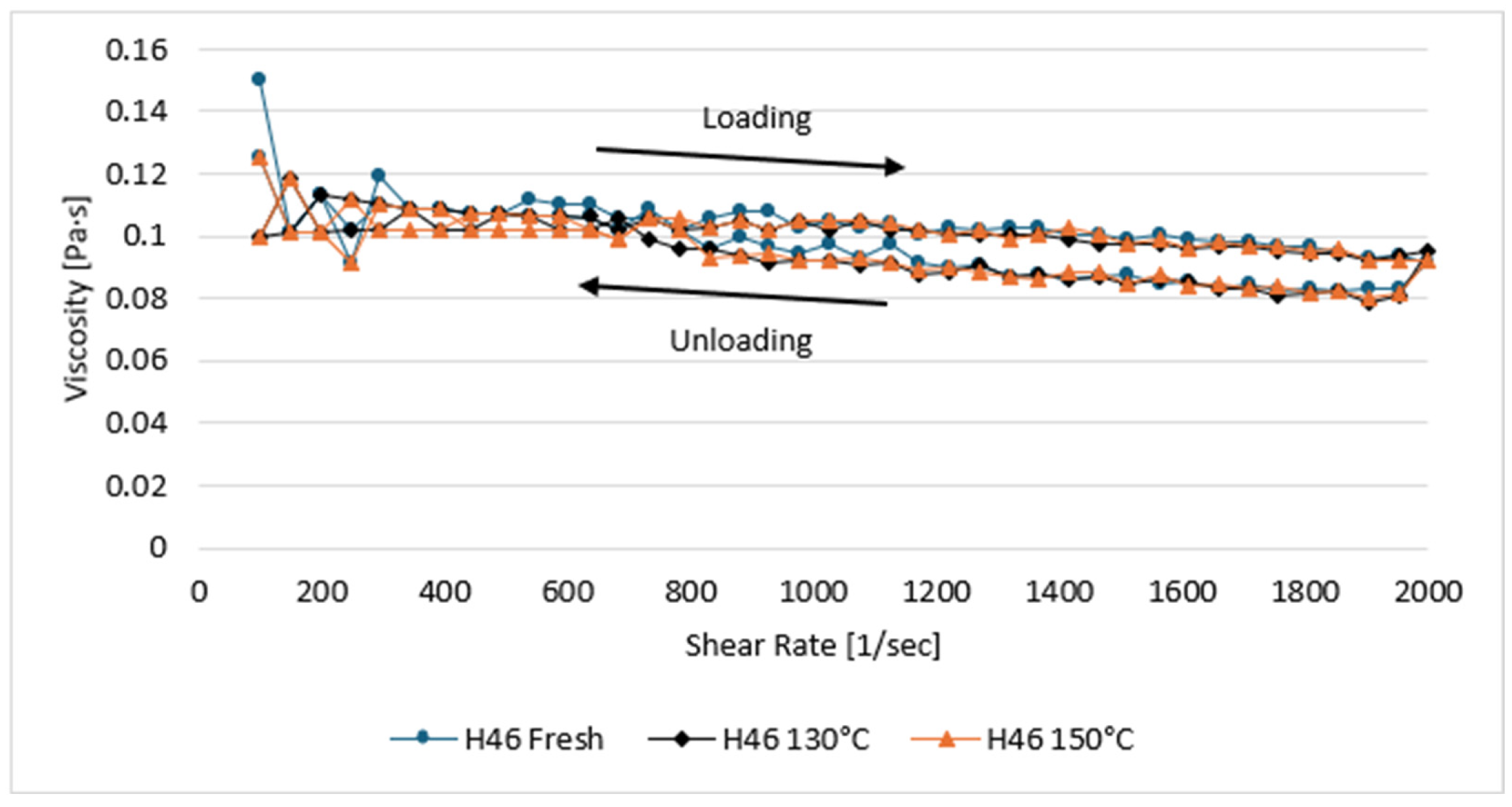
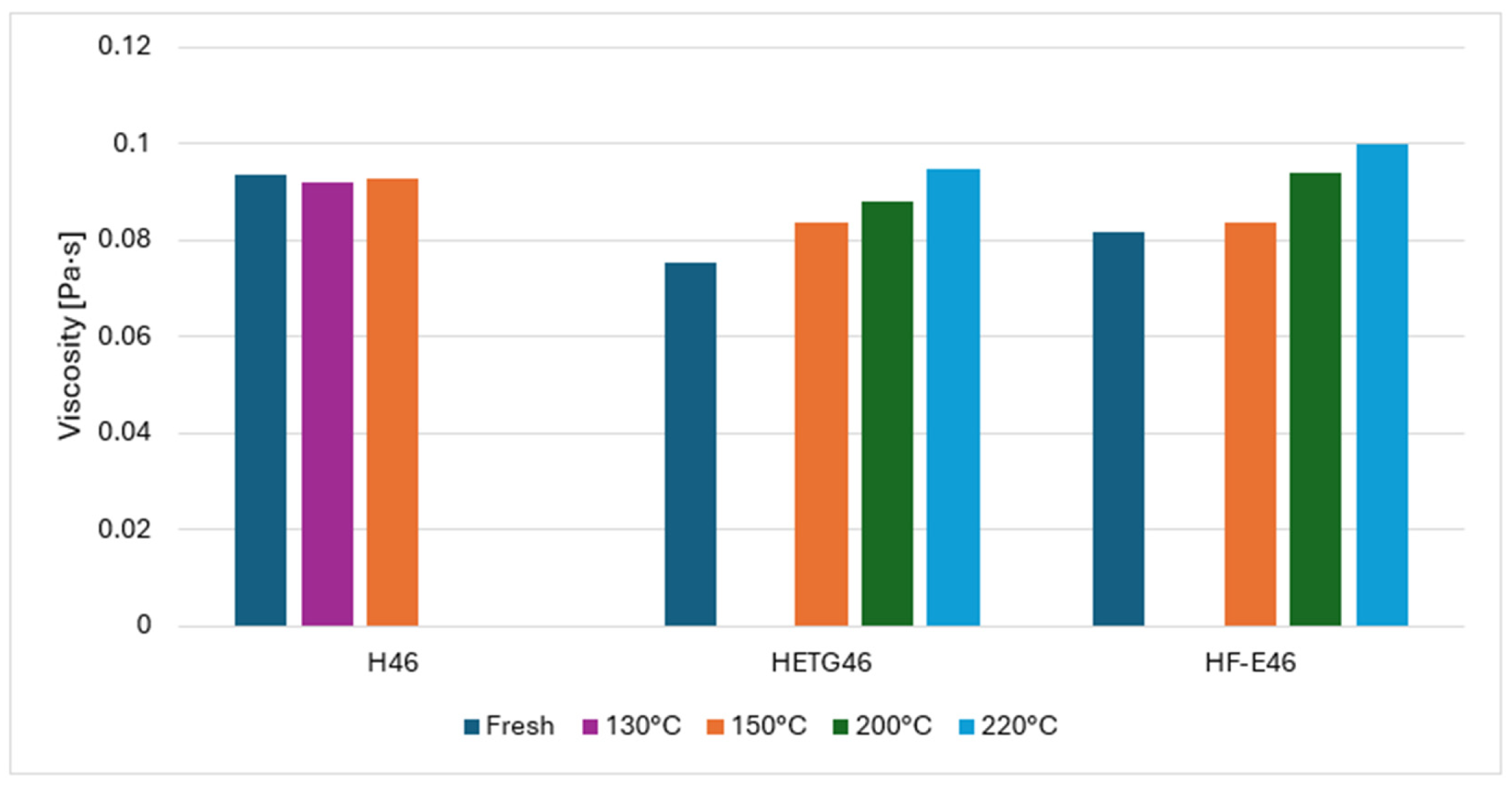
3.1. Rheological Study Results
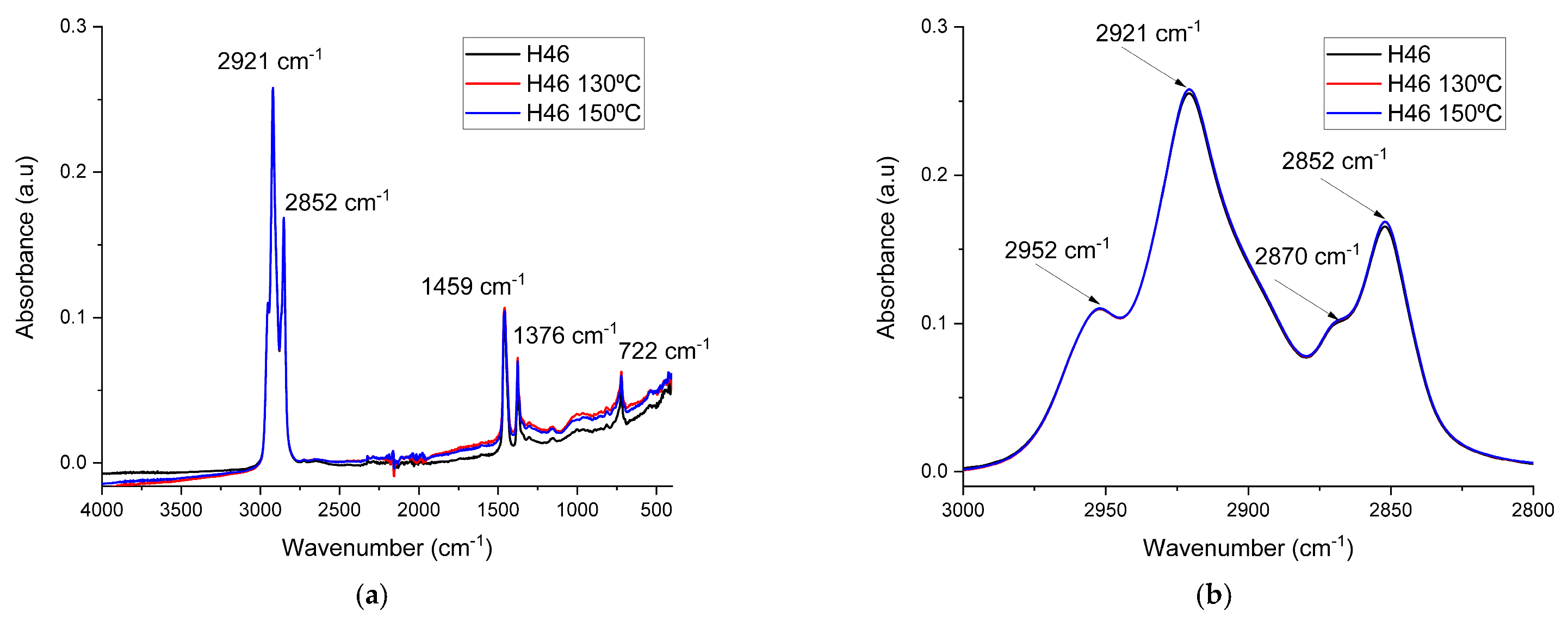
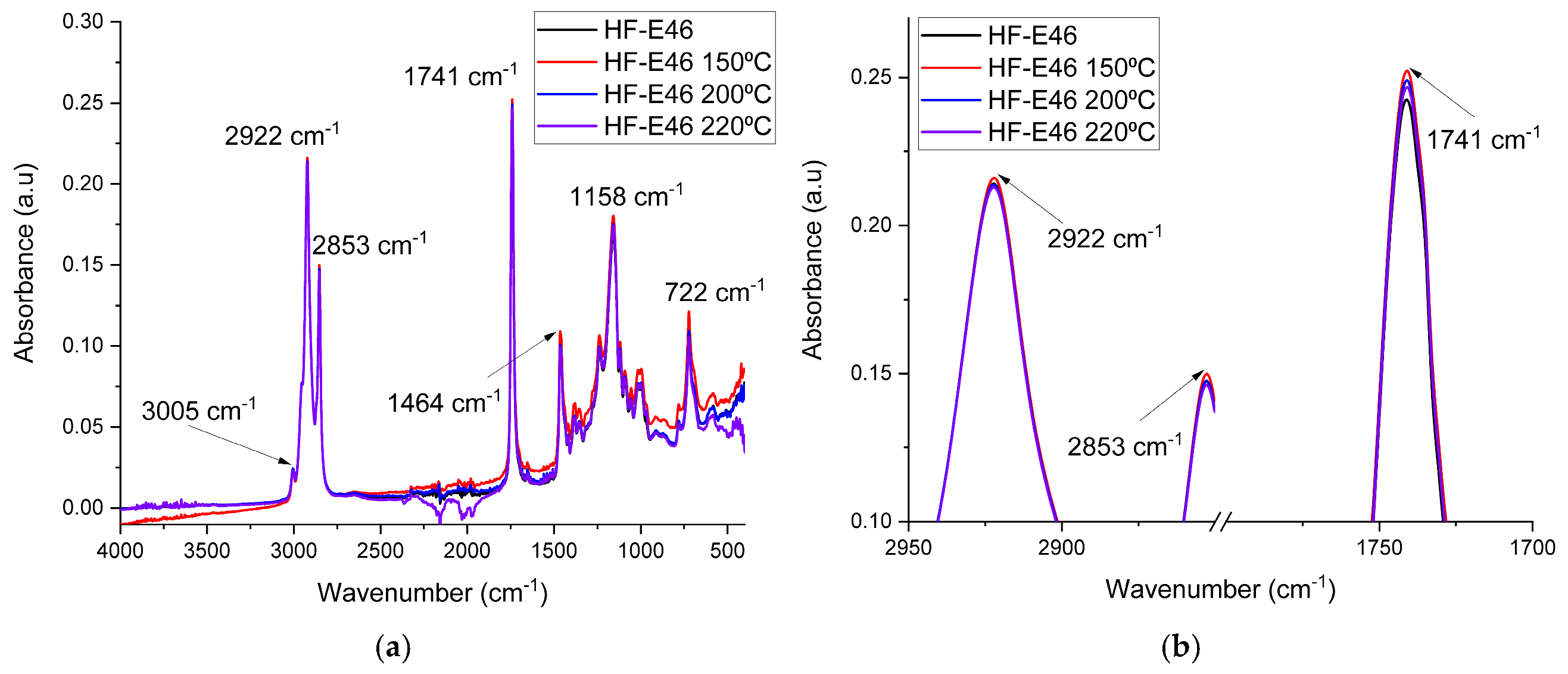
3.2. FTIR Spectroscopy Results
4. Conclusions
- The tested samples are designed to be resistant to high temperatures;
- The time in which thermal degradation was achieved was not long enough for structural changes to occur;
- The chemical stability of the hydraulic oil at normal temperature is ensured by the presence of additives (antioxidants, anticorrosive, antifoaming agents, etc.), which reduce the speed of chemical degradation reactions. At very high temperatures (during the test, up to 200–220 °C), biodegradable oils show an increase in viscosity, a phenomenon attributed to the formation of compounds identified by the absorption bands (peaks) highlighted in the FTIR spectra of the oils tested at different temperatures.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATR | Attenuated Total Reflectance |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| FTIR | Fourier Transform Infrared Spectroscopy |
| ICP | Inductively Coupled Plasma |
| IR | Infrared |
| MRI | Magnetic Resonance Imaging |
| PAO | Poly-alpha-olefin |
References
- Livingstone, G.; Cavanaugh, G. The Real Reasons Why Hydraulic Fluids Fail and Strategies to Stop Problems Before They Start. Tribol. Lubr. Technol. 2015, 71, 44–51. [Google Scholar]
- Schick, T.; Hörer, J.; Ellenrieder, K.; Blum, K.-H.; Schmitz, K. Sensitivity Analysis of Operating Parameters in Hydraulic Systems with Respect to the Aging of Hydraulic Fluids. T+S 2022, 69, 25–29. [Google Scholar] [CrossRef]
- Kučera, M.; Aleš, Z. Morphology Analysis of Friction Particles Generated in Tractor Transmission Oils. Acta Technol. Agric. 2017, 20, 57–62. [Google Scholar] [CrossRef]
- Şuvar, N.-S.; Prodan, M.; Nălboc, I.V.; Szollosi-Moţa, A. Analysis of Thermal Degradation Behavior for Some Hydraulic Oils, Using FTIR-TGA Coupling. MATEC Web Conf. 2020, 305, 00006. [Google Scholar] [CrossRef]
- Gatto, V.; Moehle, W.; Cobb, T.; Schneller, E. Oxidation Fundamentals and Its Application to Turbine Oil Testing. J. ASTM Int. 2006, 3, 1–20. [Google Scholar] [CrossRef]
- Deuster, S.; Schmitz, K. Bio-Based Hydraulic Fluids and the Influence of Hydraulic Oil Viscosity on the Efficiency of Mobile Machinery. Sustainability 2021, 13, 7570. [Google Scholar] [CrossRef]
- Fioravanti, A.; Marani, P.; Massarotti, G.P.; Lettieri, S.; Morandi, S.; Carotta, M.C. (Ti, Sn) Solid Solution Based Gas Sensors for New Monitoring of Hydraulic Oil Degradation. Materials 2021, 14, 605. [Google Scholar] [CrossRef]
- Ptak, A.; Taciak, P.; Wieleba, W. Effect of Temperature on the Tribological Properties of Selected Thermoplastic Materials Cooperating with Aluminium Alloy. Materials 2021, 14, 7318. [Google Scholar] [CrossRef] [PubMed]
- Szpunar, M.; Trzepieciński, T.; Żaba, K.; Ostrowski, R.; Zwolak, M. Effect of Lubricant Type on the Friction Behaviours and Surface Topography in Metal Forming of Ti-6Al-4V Titanium Alloy Sheets. Materials 2021, 14, 3721. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.-G.; Kim, J.-K.; Na, S.-J.; Kim, M.-S.; Hong, S.-H. Application of Condition Monitoring for Hydraulic Oil Using Tuning Fork Sensor: A Study Case on Hydraulic System of Earth Moving Machinery. Materials 2022, 15, 7657. [Google Scholar] [CrossRef] [PubMed]
- Pochi, D.; Fanigliulo, R.; Bisaglia, C.; Cutini, M.; Grilli, R.; Fornaciari, L.; Betto, M.; Pari, L.; Gallucci, F.; Capuzzi, L.; et al. Test Rig and Method for Comparative Evaluation of Conventional and Bio-Based Hydraulic Fluids and Lubricants for Agricultural Transmissions. Sustainability 2020, 12, 8564. [Google Scholar] [CrossRef]
- Tulík, J.; Hujo, Ľ.; Jablonický, J.; Nosian, J.; Kaszkowiak, J. Properties Evaluation of New Biodegradable Fluid During Accelerated Durability Test. In Proceedings of the International Conference “New Technologies, Development and Applications”, Sarajevo, Bosnia, and Herzegovina, 24–26 June 2021; Springer: Cham, Switzerland, 2021; pp. 205–214. [Google Scholar]
- Totten, G.E.; de Negri, V.J. Handbook of Hydraulic Fluid Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012; ISBN 978-1-4200-8527-3. [Google Scholar]
- Sivriu, A.-M.; Koncsag, C.; Jinescu, G.; Mares, A.-M. Thermal Cracking of Waste Vegetable Oil–a Preliminary Research. UPB Sci. Bull. 2017, 79, 67–74. [Google Scholar]
- Dragne, M.; Pop, E.; Pîşă, I. Technology for Purifying Waste Oils with Nanostructured Materials for Energy Purposes. UPB Sci. Bull. Ser. D 2021, 83, 1223–7027. [Google Scholar]
- Zahid, R.; Bhutta, M.U.; Mufti, R.A.; Abdullah, M.U.; Masjuki, H.H.; Varman, M.; Kalam, M.A.; Ali, M.A.; Aslam, J.; Akhtar, K. Friction and Wear Performance Evaluation of Bio-Lubricants and DLC Coatings on Cam/Tappet Interface of Internal Combustion Engines. Materials 2021, 14, 7206. [Google Scholar] [CrossRef]
- Kamyab, B.; Beims, R.; Chambers, D.W.; Bassi, A.S.; Xu, C. Sustainable Production of High-Performance Bio-Based Hydraulic Fluids from Vegetable Oils: Recent Advances, Current Challenges, and Future Perspectives. Biomass Bioenergy 2024, 183, 107160. [Google Scholar] [CrossRef]
- Ilyin, S.O. Structural Rheology in the Development and Study of Complex Polymer Materials. Polymers 2024, 16, 2458. [Google Scholar] [CrossRef]
- Stanciu, I. Rheological Properties of Rapeseed Oil and Hydraulic Oil. UPB Sci. Bull. 2011, 73. [Google Scholar]
- Xu, J.; Liu, S.; Gao, M.; Zuo, Y. Classification of Lubricating Oil Types Using Mid-Infrared Spectroscopy Combined with Linear Discriminant Analysis–Support Vector Machine Algorithm. Lubricants 2023, 11, 268. [Google Scholar] [CrossRef]
- Subramanian, A.; Rodriguez-Saona, L. Fourier Transform Infrared (FTIR) Spectroscopy. In Infrared Spectroscopy for Food Quality Analysis and Control; Da-Wen, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 145–178. ISBN 978-0-12-374136-3. [Google Scholar]
- Nicolet, T.; All, C. Introduction to Fourier Transform Infrared Spectrometry. Thermo Nicolet Corp. 2001, 3, 45–50. [Google Scholar]
- Dutta, A. Fourier Transform Infrared Spectroscopy. In Spectroscopic Methods for Nanomaterials Characterization; Sabu, T., Raju, T., Ajesh, K.Z., Raghvendra, K.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 73–93. ISBN 978-0-323-46140-5. [Google Scholar]
- Hnilicová, M.; Kučera, M.; Pavlů, J. Analysis of Hydraulic Oil in Handling Lines Baljer & Zembrod Using the Methods of Tribotechnical Diagnostics. KEM 2015, 669, 451–458. [Google Scholar] [CrossRef]
- Tulík, J.; Hujo, Ľ.; Kosiba, J.; Jablonický, J.; Jánošová, M. Evaluation of New Biodegradable Fluid on the Basis of Accelerated Durability Test, FTIR and ICP Spectroscopy. Res. Agric. Eng. 2017, 63, 1–9. [Google Scholar] [CrossRef]
- Santos, J.C.O.; Santos, I.M.G.; Souza, A.G. Thermal Degradation Process of Synthetic Lubricating Oils: Part I—Spectroscopic Study. Pet. Sci. Technol. 2015, 33, 1238–1245. [Google Scholar] [CrossRef]
- Mang, T.; Dresel, W. Lubricants and Lubrications, 2nd Completely Revised and Extended ed.; Wiley-VCH: Weinheim, Germany, 2007; ISBN 978-3-527-31497-3. [Google Scholar]
- Technical Topic: Biodegradable Hydraulic Fluids|BIONA JERSÍN—Bio-Oils and Plastic Lubricants. Available online: https://www.biona-oils.com/news/technical-topic-biodegradable-hydraulic-fluids (accessed on 16 July 2025).
- Nuto H 46. Available online: https://www.mobil.eg/en-eg/lubricants/industrial-products/nuto-h-46 (accessed on 25 July 2025).
- Product Information Nr. 33846000 KAJO-BIO-Hydrauliköl HETG 46. Available online: https://www.lubricon.com.au/wp-content/uploads/2021/06/KAJO-BIO-Hydrauliki%CC%82l-HETG-46-EN.pdf (accessed on 13 February 2025).
- Shell Naturelle HF-E 46—Technical Data Sheet. Available online: https://shop.sclubricants.com/pub/media/pds/shell/Shell-Naturelle-Fluid-HF-E-46-datasheet.pdf (accessed on 13 February 2025).
- Broboana, D.; Balan, C. Investigations on the Rheology of Water-in-Crude Oil Emulsions. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2007, 69, 35–50. [Google Scholar]
- Motelica, L.; Ficai, D.; Oprea, O.; Ficai, A.; Trusca, R.-D.; Andronescu, E.; Holban, A.M. Biodegradable Alginate Films with ZnO Nanoparticles and Citronella Essential Oil—A Novel Antimicrobial Structure. Pharmaceutics 2021, 13, 1020. [Google Scholar] [CrossRef]
- Motelica, L.; Vasile, B.-S.; Ficai, A.; Surdu, A.-V.; Ficai, D.; Oprea, O.-C.; Andronescu, E.; Mustățea, G.; Ungureanu, E.L.; Dobre, A.A. Antibacterial Activity of Zinc Oxide Nanoparticles Loaded with Essential Oils. Pharmaceutics 2023, 15, 2470. [Google Scholar] [CrossRef]
- Van Der Weerd, J.; Van Loon, A.; Boon, J.J. FTIR Studies of the Effects of Pigments on the Aging of Oil. Stud. Conserv. 2005, 50, 3–22. [Google Scholar] [CrossRef]
- Stelescu, M.D.; Oprea, O.-C.; Sonmez, M.; Ficai, A.; Motelica, L.; Ficai, D.; Georgescu, M.; Gurau, D.F. Structural and Thermal Characterization of Some Thermoplastic Starch Mixtures. Polysaccharides 2024, 5, 504–522. [Google Scholar] [CrossRef]
- Lacatusu, I.; Badea, N.; Murariu, A.; Oprea, O.; Bojin, D.; Meghea, A. Antioxidant Activity of Solid Lipid Nanoparticles Loaded with Umbelliferone. Soft Mater. 2013, 11, 75–84. [Google Scholar] [CrossRef]
- Hadjadj, Y.; Ghunem, R.A.; Alqudsi, A.Y. Monitoring Oxidation in Natural Ester Insulating Liquids Through FTIR Spectroscopy Analysis. In Proceedings of the 2024 IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Auburn, AL, USA, 6–9 October 2024; IEEE: New York, NY, USA, 2024; pp. 1–4. [Google Scholar]
- Balamurugan, V.T.; Haritha, J.; ArunChendhuran, R.; Srivel, S.; AmalaJerrin, J.N.; Kishore, S.; Pachamuthu, M.P. Classification of Groundnut Oil Using Advanced ATR-MIR Spectroscopy and Chemometrics. Food Anal. Methods 2022, 15, 1778–1786. [Google Scholar] [CrossRef]
- Stelescu, M.D.; Oprea, O.-C.; Motelica, L.; Ficai, A.; Trusca, R.-D.; Sonmez, M.; Nituica, M.; Georgescu, M. Obtaining and Characterizing New Types of Materials Based on Low-Density Polyethylene and Thermoplastic Starch. J. Compos. Sci. 2024, 8, 134. [Google Scholar] [CrossRef]
- Ogbu, I.M.; Ajiwe, V.I.E. FTIR Studies of Thermal Stability of the Oils and Methyl Esters from Afzelia Africana and Hura Crepitans Seeds. Renew. Energy 2016, 96, 203–208. [Google Scholar] [CrossRef]



| Properties | H46 | HETG46 | HF-E46 |
|---|---|---|---|
| ISO viscosity class | 46 | 46 | 46 |
| 876 | 918 | 921 | |
| Viscosity index, min | 98 | 210 | 188 |
| 44 | - | 47.2 | |
| 6.6 | 10 | 9.41 | |
| 226 | >270 | 320 | |
| −24 | −30 | −42 | |
| 1A | - | - | |
| - | 0.5 | - | |
| - | 95 | >80 | |
| - | >80 | 76 | |
| Auto-ignition temperature [°C ] | - | - | >400 |
| Oil Type | Oil Condition | Rheological Model | ||||
|---|---|---|---|---|---|---|
| Newtonian | Power Law | |||||
| Correlation Coefficient [%] | Correlation Coefficient [ % ] | |||||
| H46 | Fresh | 0.0936 | 99.29 | 0.199 | 0.897 | 94.20 |
| 130 °C | 0.0922 | 99.32 | 0.167 | 0.919 | 93.90 | |
| 150 °C | 0.0927 | 99.34 | 0.165 | 0.922 | 94.00 | |
| HETG46 | Fresh | 0.0752 | 99.49 | 0.190 | 0.874 | 96.00 |
| 150 °C | 0.0836 | 99.55 | 0.279 | 0.834 | 96.90 | |
| 200 °C | 0.0882 | 99.59 | 0.229 | 0.869 | 97.60 | |
| 220 °C | 0.0947 | 99.62 | 0.280 | 0.851 | 97.60 | |
| HF-E46 | Fresh | 0.0817 | 99.67 | 0.220 | 0.864 | 97.50 |
| 150 °C | 0.0835 | 99.64 | 0.229 | 0.861 | 98.10 | |
| 200 °C | 0.0941 | 99.67 | 0.283 | 0.848 | 98.30 | |
| 220 °C | 0.0998 | 99.79 | 0.195 | 0.908 | 98.30 | |
| Sample | A2952 | A2921 | A2921/A2952 |
|---|---|---|---|
| H46 | 0.438 | 4.371 | 9.979 |
| H46 130 °C | 0.444 | 4.441 | 10.002 |
| H46 150 °C | 0.441 | 4.436 | 10.059 |
| Sample | A2921 | A1743 | A1743/A2921 | A1458 | A1160 | A1160/A1458 |
|---|---|---|---|---|---|---|
| HETG46 | 6.281 | 4.988 | 0.794 | 1.543 | 3.524 | 2.284 |
| HETG46 150 °C | 6.344 | 5.065 | 0.798 | 1.561 | 3.644 | 2.334 |
| HETG46 200 °C | 6.373 | 5.102 | 0.801 | 1.568 | 3.666 | 2.338 |
| HETG46 220 °C | 6.367 | 5.097 | 0.801 | 1.564 | 3.641 | 2.328 |
| Sample | A2922 | A1741 | A1741/A2922 | A1464 | A1158 | A1158/A1464 |
|---|---|---|---|---|---|---|
| HF-E46 | 6.487 | 5.112 | 0.788 | 1.813 | 3.802 | 2.097 |
| HF-E46 150 °C | 6.559 | 5.187 | 0.791 | 1.842 | 3.922 | 2.129 |
| HF-E46 200 °C | 6.454 | 5.239 | 0.812 | 1.804 | 3.856 | 2.137 |
| HF-E46 220 °C | 6.453 | 5.242 | 0.812 | 1.808 | 3.967 | 2.139 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teleașă, A.M.; Stoica, N.-A.; Rădulescu, A.V.; Cănănău, S.; Motelica, L.; Rădoi, R.I. Rheological Study and FTIR Analysis of Thermally Degraded Mineral and Biodegradable Hydraulic Fluids. Lubricants 2025, 13, 462. https://doi.org/10.3390/lubricants13100462
Teleașă AM, Stoica N-A, Rădulescu AV, Cănănău S, Motelica L, Rădoi RI. Rheological Study and FTIR Analysis of Thermally Degraded Mineral and Biodegradable Hydraulic Fluids. Lubricants. 2025; 13(10):462. https://doi.org/10.3390/lubricants13100462
Chicago/Turabian StyleTeleașă, Andreea Mirela, Nicolae-Alexandru Stoica, Alexandru Valentin Rădulescu, Sorin Cănănău, Ludmila Motelica, and Radu Iulian Rădoi. 2025. "Rheological Study and FTIR Analysis of Thermally Degraded Mineral and Biodegradable Hydraulic Fluids" Lubricants 13, no. 10: 462. https://doi.org/10.3390/lubricants13100462
APA StyleTeleașă, A. M., Stoica, N.-A., Rădulescu, A. V., Cănănău, S., Motelica, L., & Rădoi, R. I. (2025). Rheological Study and FTIR Analysis of Thermally Degraded Mineral and Biodegradable Hydraulic Fluids. Lubricants, 13(10), 462. https://doi.org/10.3390/lubricants13100462





