Effect of Substrate Bias on the Structure and Tribological Performance of (AlTiVCrNb)CxNy Coatings Deposited via Graphite Co-Sputtering
Abstract
1. Introduction
2. Experiments and Methods
3. Results and Analysis
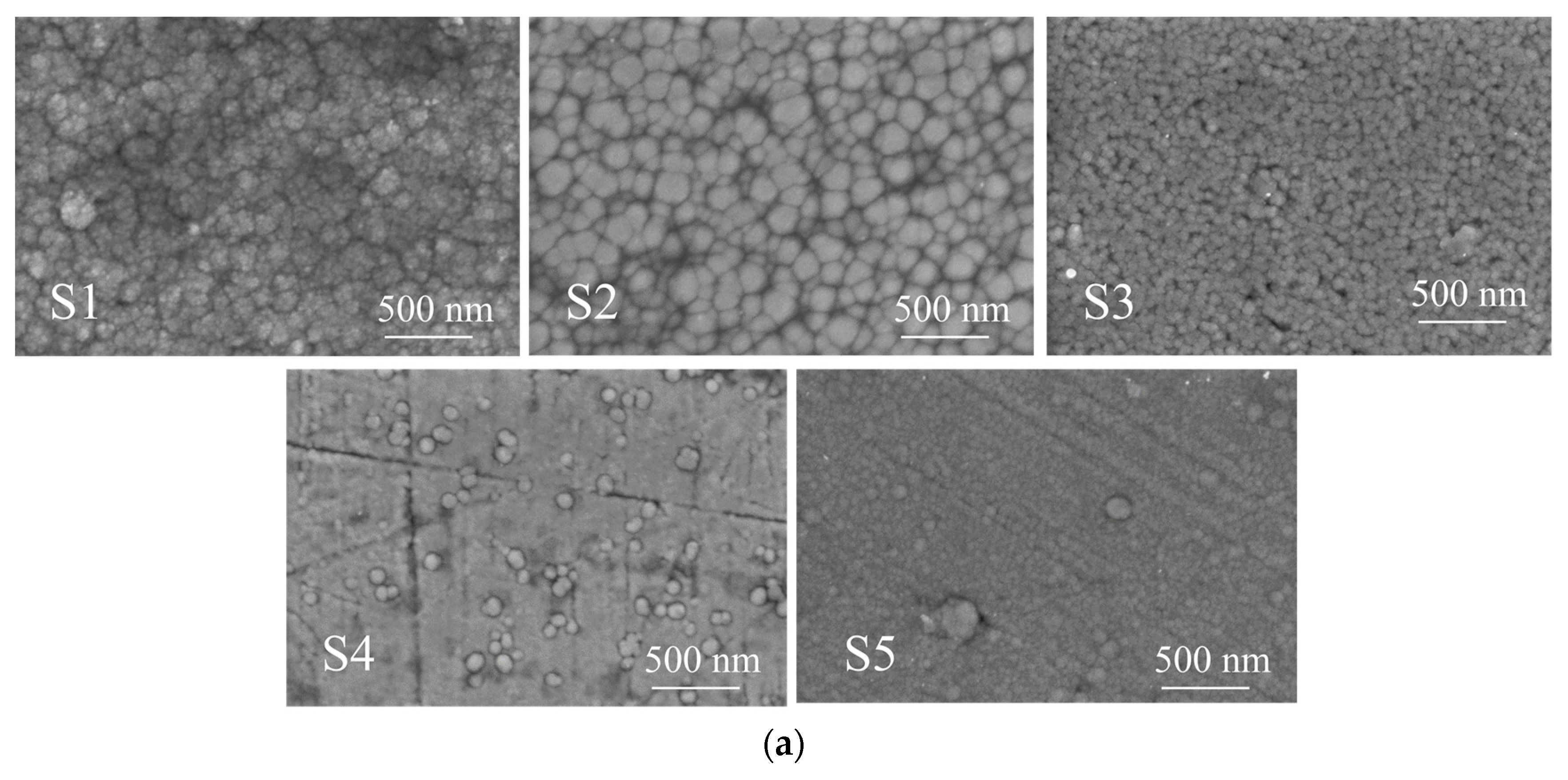
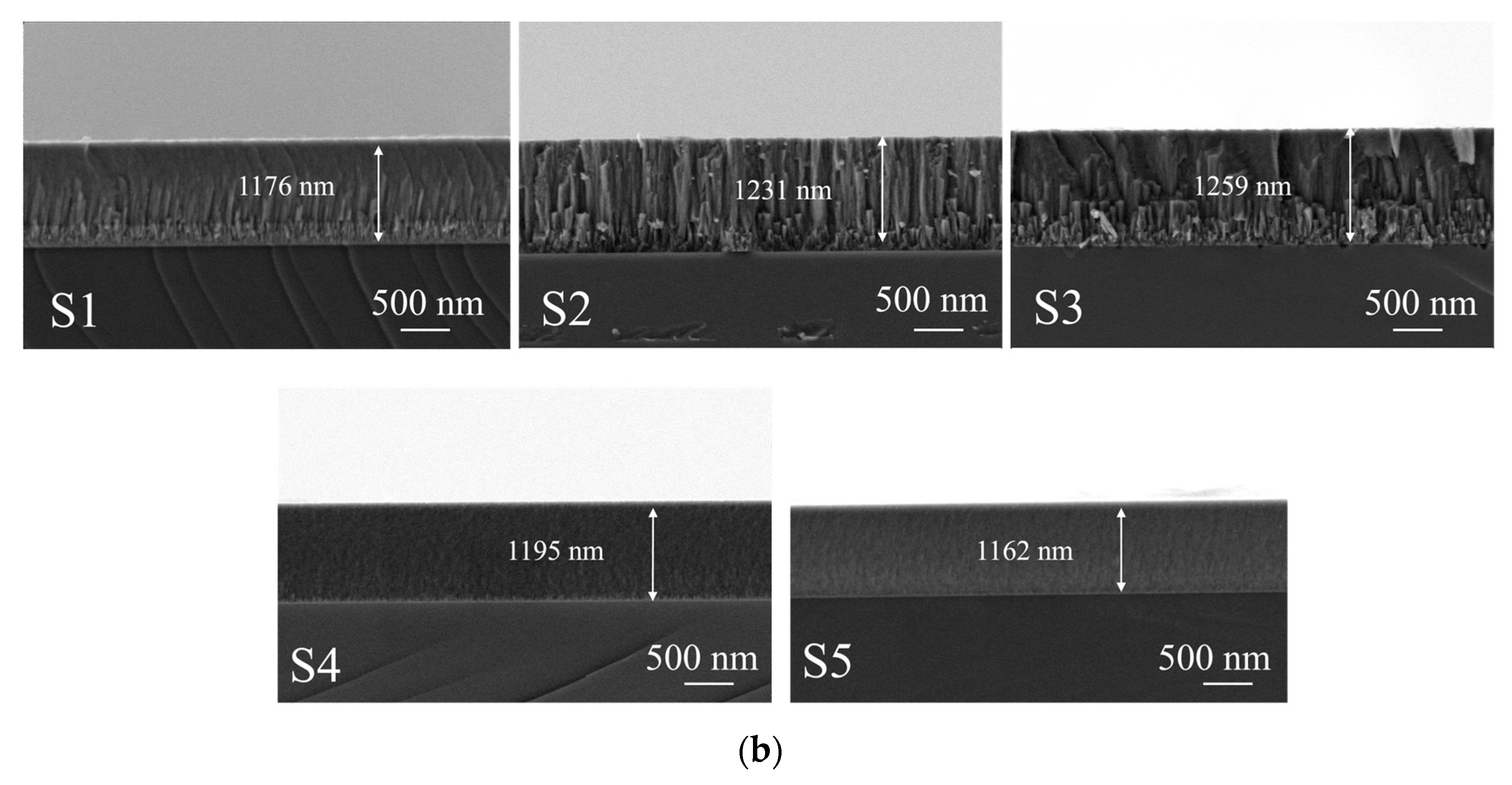
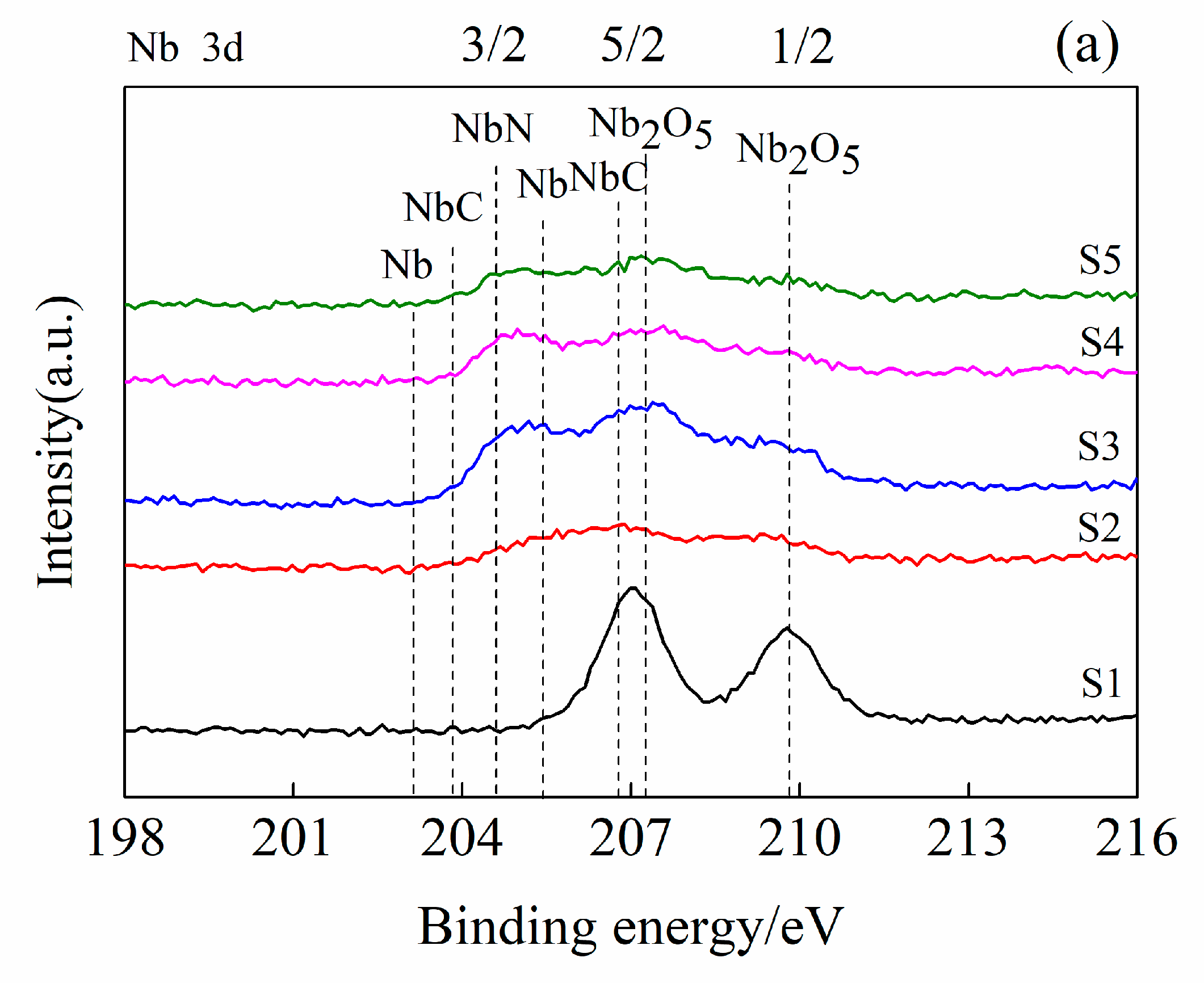
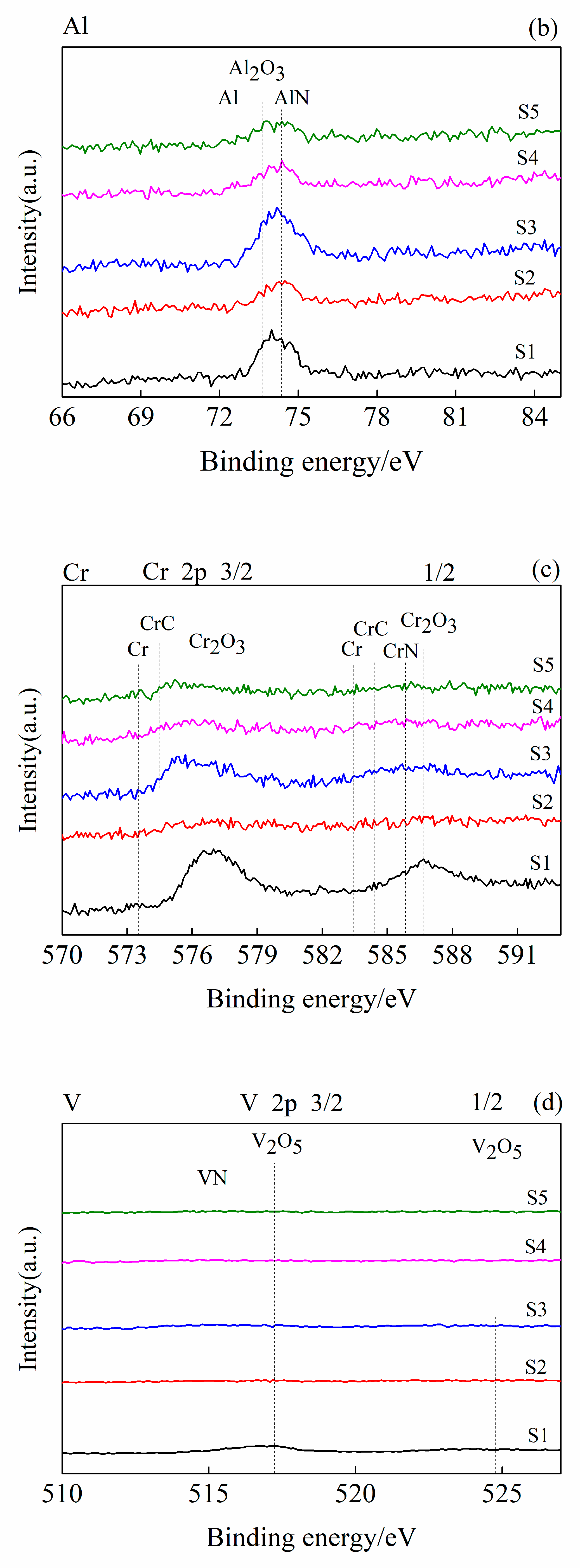
3.1. Coating Structure Characterization
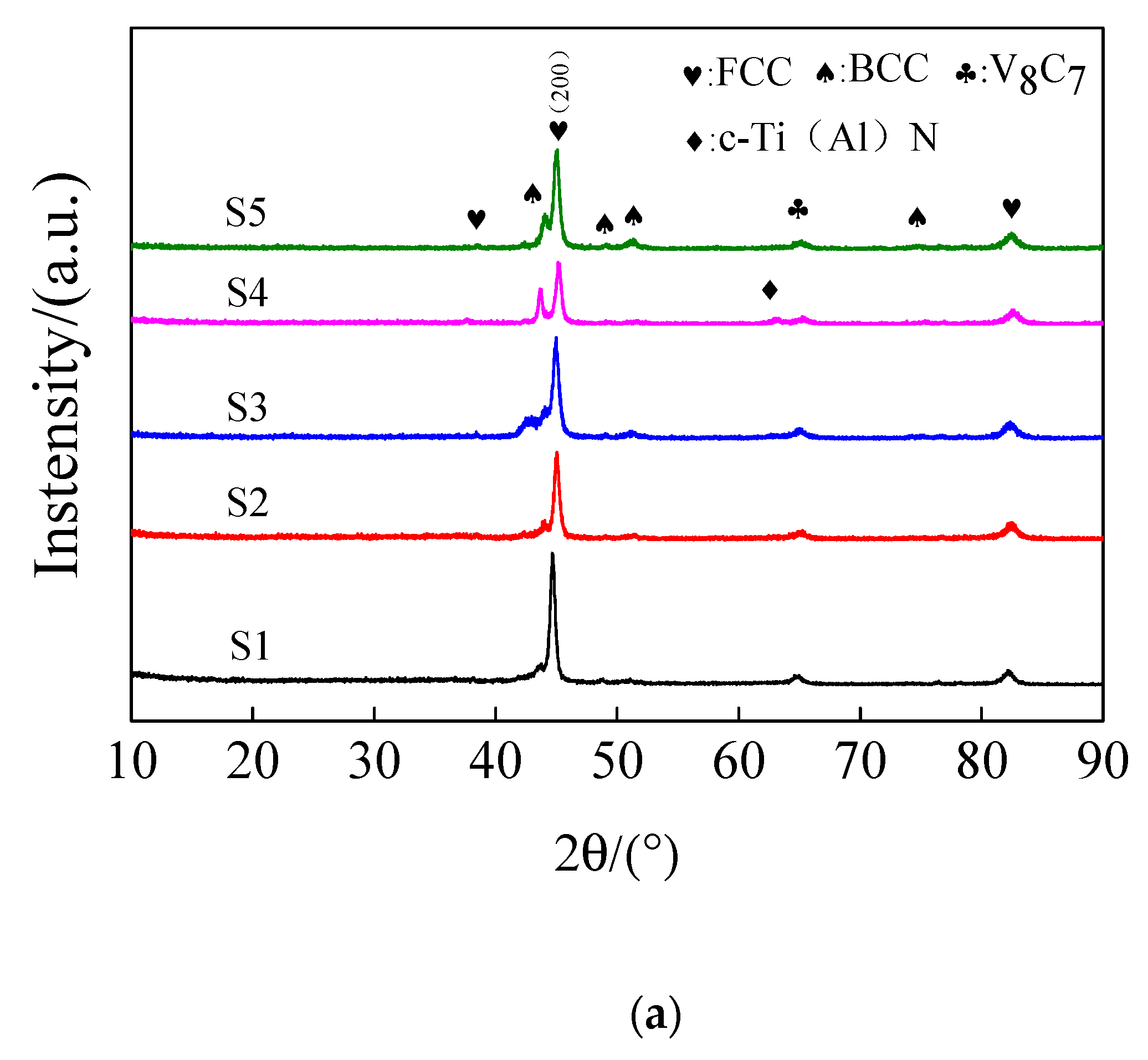
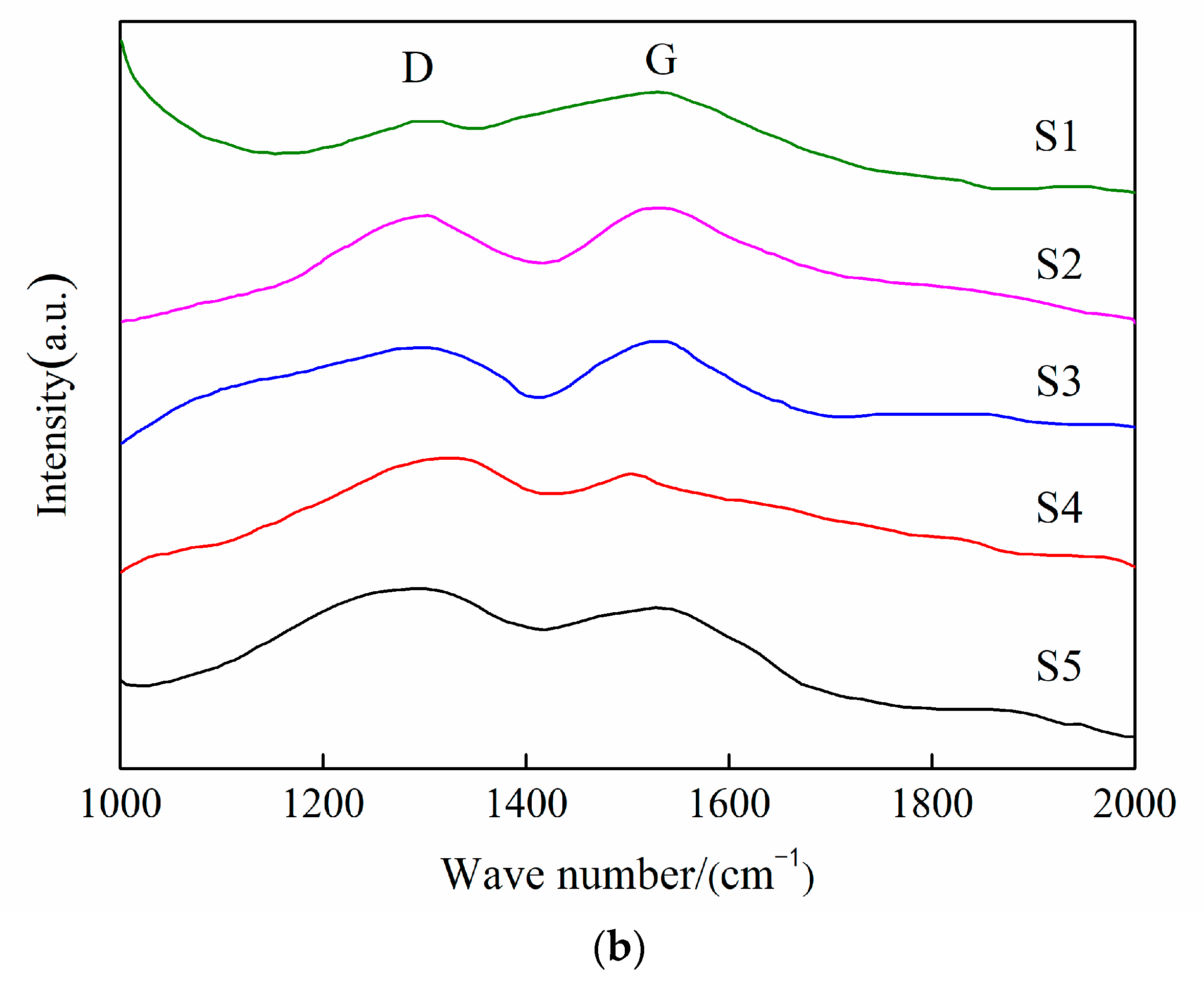
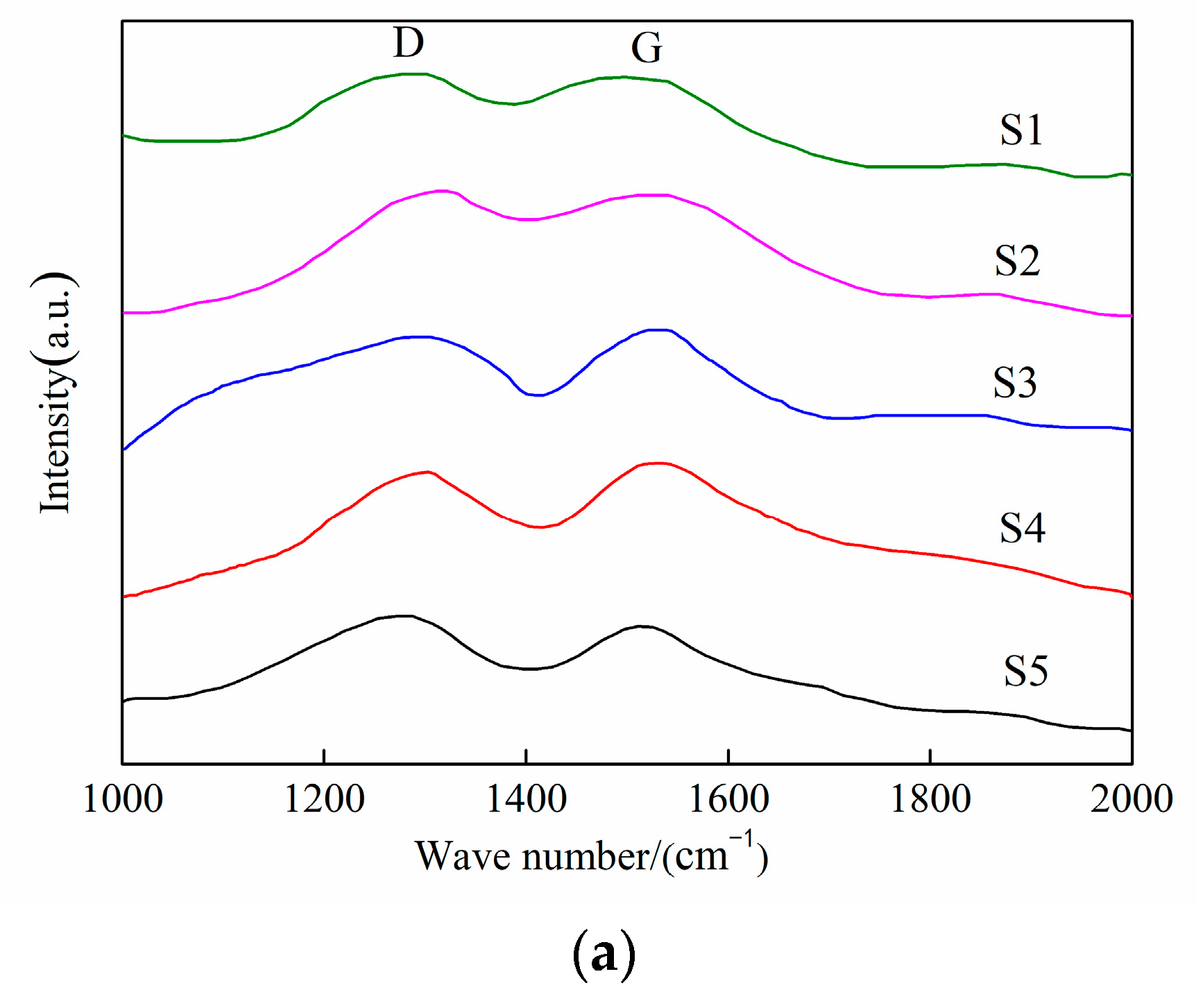
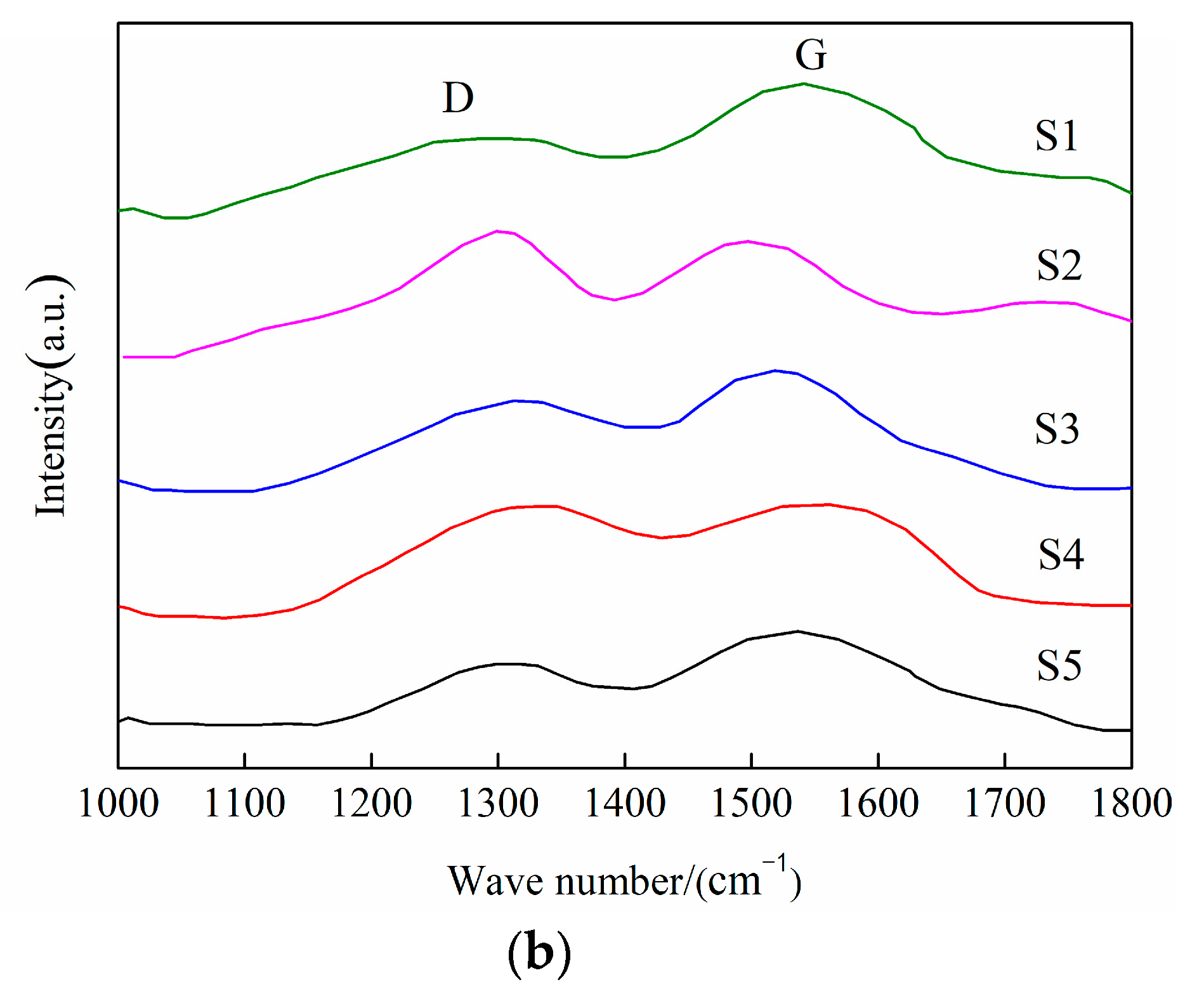
3.2. Coating Structure Analysis
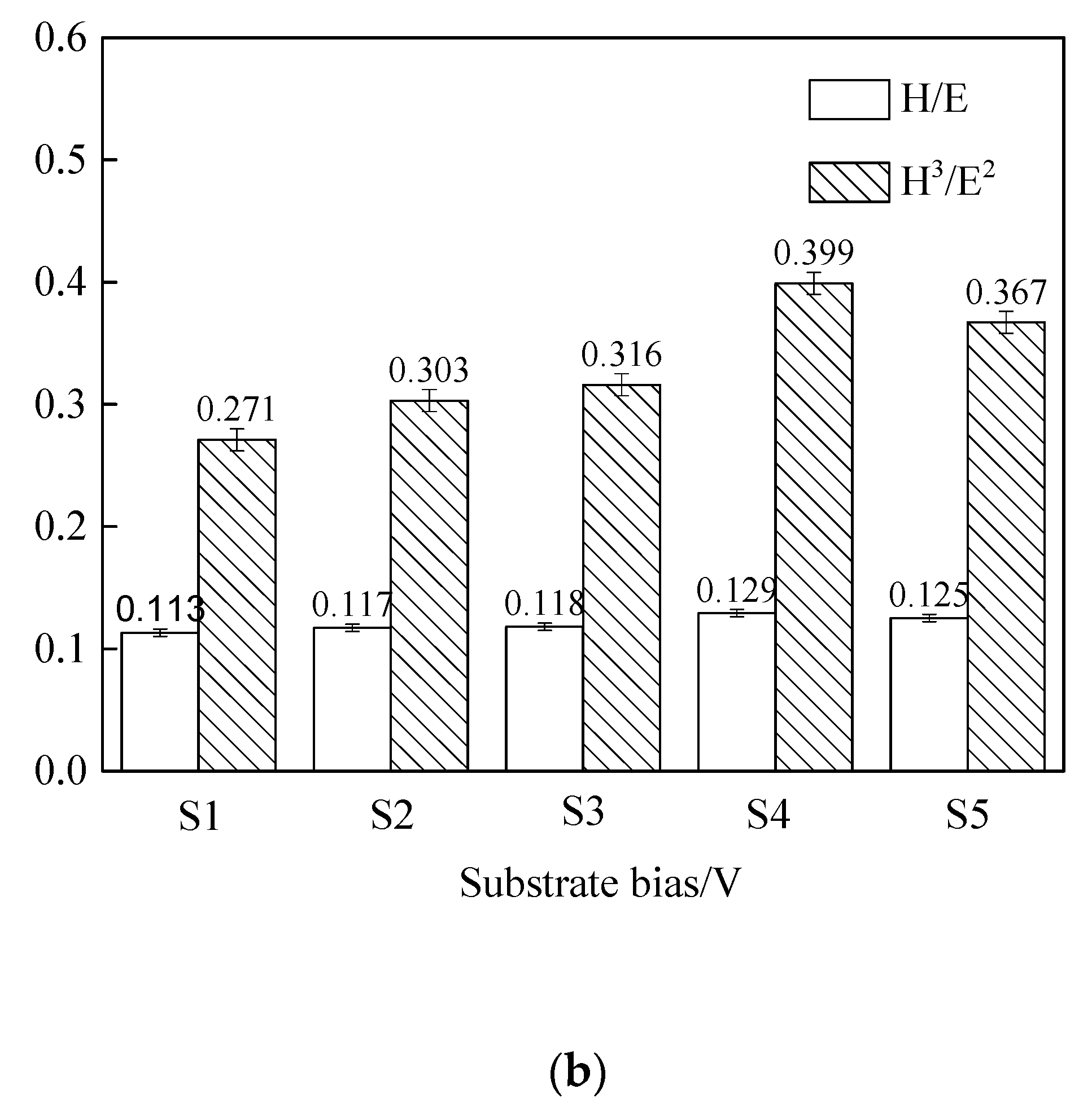
3.3. Hardness and Elastic Modulus Analysis
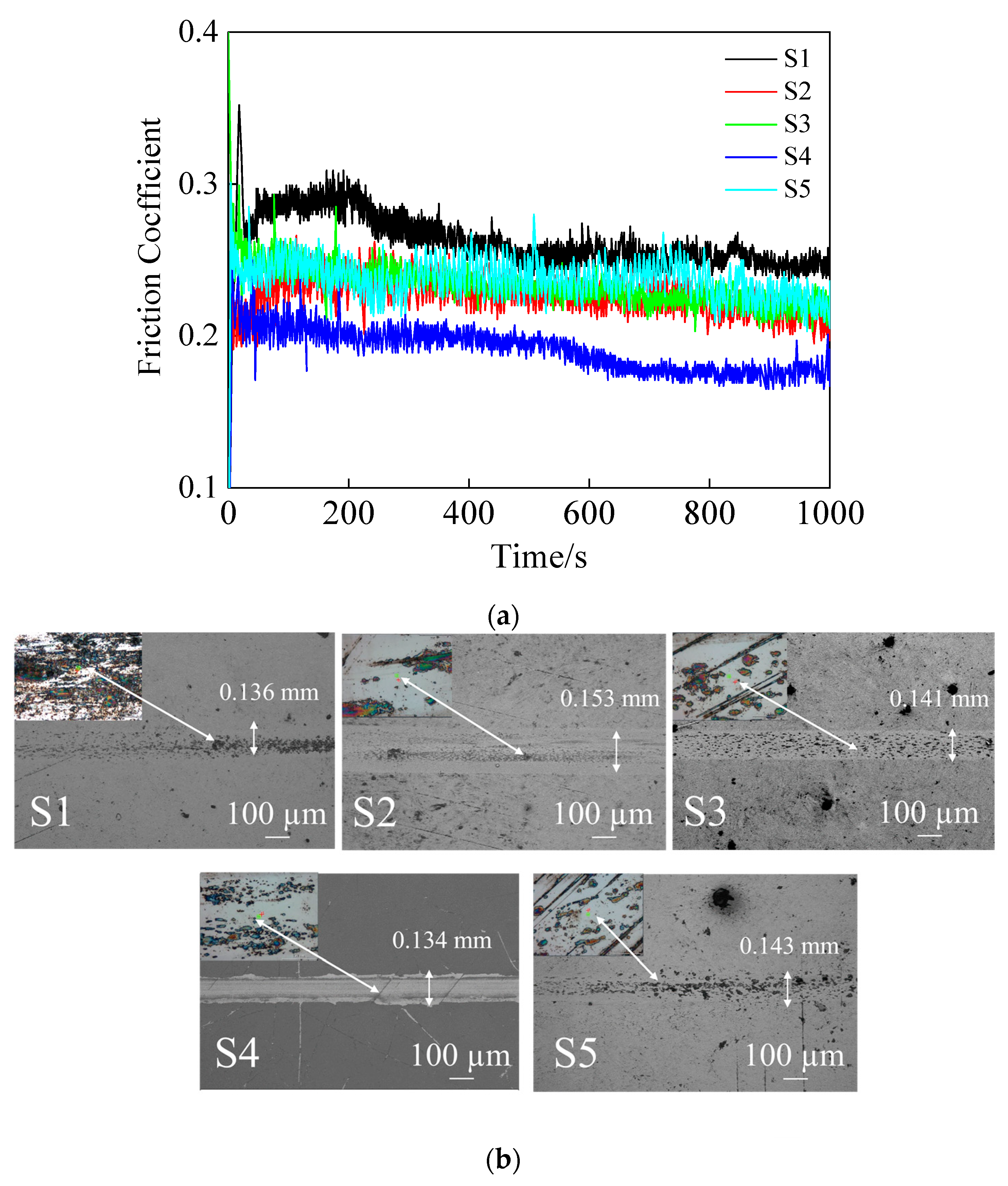
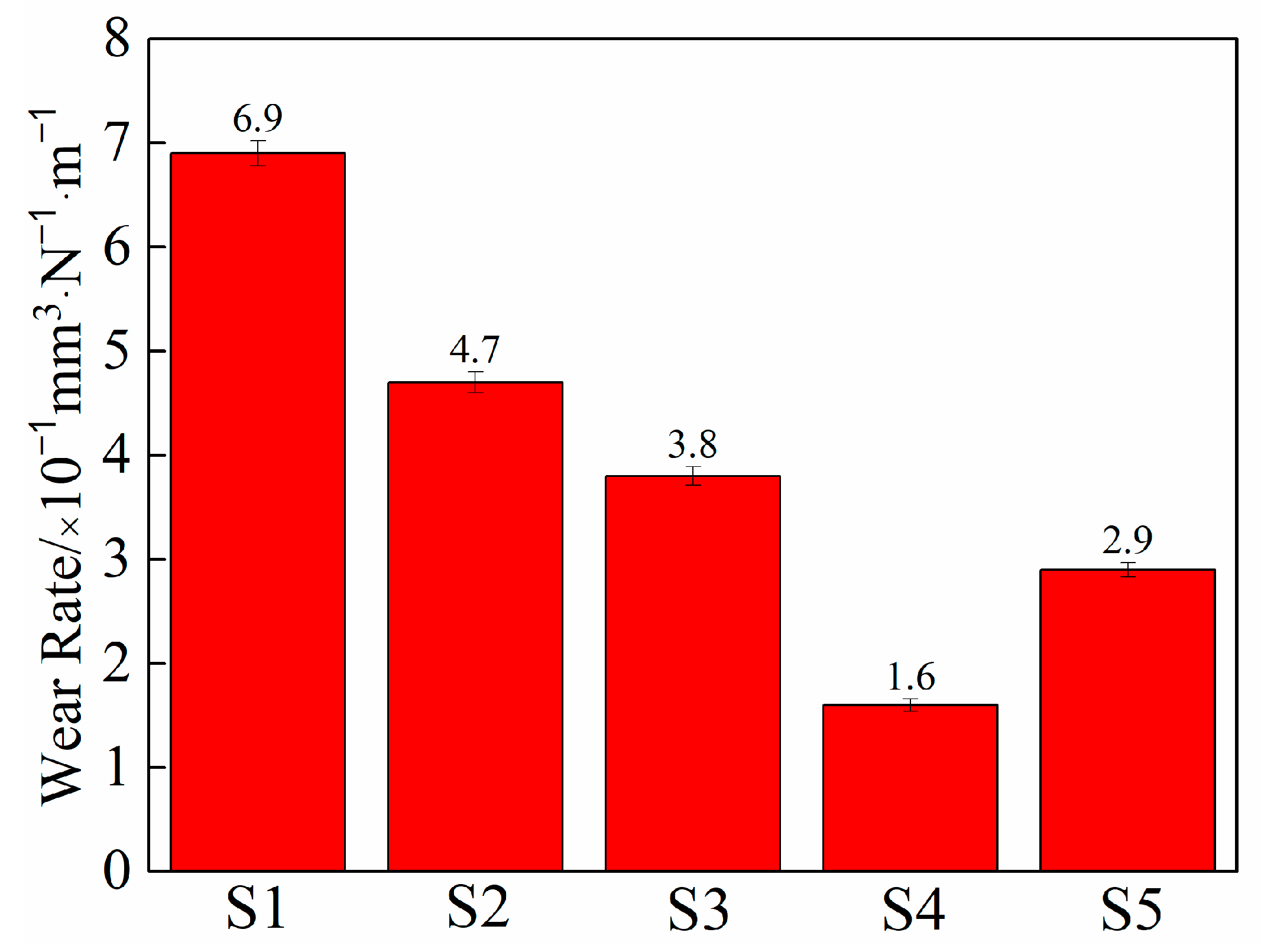
3.4. Tribological Performance Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nie, H.; Zeng, S.; Nie, J.; Lai, C. Effect of VC Addition on microstructure and properties of Laser cladding Fe50Mn30Cr10Co10 High Entropy alloy coating. Mater. Mech. Eng. 2019, 47, 7–11. [Google Scholar]
- Yang, Y.; Zhang, Y.; Li, J.; Zhu, H.; Zhang, J. Research status of high entropy alloying. Metall. Eng. 2021, 8, 10. [Google Scholar]
- Li, X.; Wang, Y.; Zhang, J.; Wu, K.; Liu, G.; Sun, J. Research progress of High-entropy alloy coating. Surf. Technol. 2023, 52, 1–20. [Google Scholar]
- Ragachev, A.; Yarmolenko, M.; Rogachev, A.; Gorbachev, D.; Zhou, B. Chemical composition, morphology and optical properties of zinc sulfide coatings deposited by low-energy electron beam evaporation. Appl. Surf. Sci. 2014, 303, 23–29. [Google Scholar] [CrossRef]
- Wang, H.; Zhuang, D.; Wang, K.; Liu, J.; Fang, X.; Li, Y. Microstructures and wear-resistance behaviors of ferrous sulfide coatings. Acta Metall. Sin.-Chin. Ed. 2003, 39, 1031–1036. [Google Scholar]
- Kang, C.; Jeon, B.; Kumar, R.; Park, S.; Park, H.; Kim, S. Stability of Coatings on Sulfide Minerals in Acidic and Low-Temperature Environments. Mine Water Environ. 2017, 36, 436–442. [Google Scholar] [CrossRef]
- Liang, H.; Qiao, D.; Miao, J.; Cao, Z.; Jiang, H.; Wang, T. Anomalous microstructure and tribological evaluation of AlCrFeNiW0.2Ti0.5 high-entropy alloy coating manufactured by laser cladding in seawater. Mater. Sci. Technol. 2021, 85, 224–234. [Google Scholar] [CrossRef]
- Li, J.; Peng, Z.; Ma, M.; Lu, J.; Wu, Y. Effect of CeO2 on microstructure and hardness of AlCoCuFeMnNi high-entropy alloy coating. Jinshu Rechuli/Heat Treat. Met. 2017, 42, 19–23. [Google Scholar]
- Zhang, Y.; Han, T.; Xiao, M.; Shen, Y. Preparation of Diamond Reinforced NiCoCrTi0.5Nb0.5High-Entropy Alloy Coating by Laser Cladding: Microstructure and Wear Behavior. J. Therm. Spray Technol. 2020, 29, 1827–1837. [Google Scholar] [CrossRef]
- Wang, J. Effect of Y on Microstructure and Properties of Al0.8FeCrCoNiCu0.5 High Entropy Alloy Coating on 5083 Aluminum by Laser Cladding. Lubricants 2023, 11, 50. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Q.; Zhou, F. Microstructure and Wear Resistance of High-Melting-Point AlCrFeMoNbxTiW High-Entropy Alloy Coating by Laser Cladding. Chin. J. Rare Met. 2017, 41, 1327. [Google Scholar]
- Zhang, Y.; Han, T.; Xiao, M.; Shen, Y. Tribological behavior of diamond reinforced FeNiCoCrTi0.5 carbonized high-entropy alloy coating. Surf. Coat. Technol. 2020, 401, 126233. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Di, Y.; Lin, J.; Hao, X.; Wang, Y.; Chen, L.; Zhang, X. Effect of Carbon Content on friction, wear and corrosion resistance of Laser cladding CoCrFeMnNiCx high entropy alloy coating. China Surf. Eng. 2019, 33, 118–127. [Google Scholar]
- Xin, B.; Zhang, A.; Han, J.; Meng, J. The tribological properties of carbon doped Al0.2Co1.5CrFeNi1.5Ti0.5 high entropy alloys. Wear 2021, 484, 204045. [Google Scholar] [CrossRef]
- Che, L.; Liu, J.; Peng, F.; Xiong, H.; Zhang, F.; Ma, X.; Gao, N.; Liu, X. Effect of C alloying on microstructure and tensile properties of AlCrFeCoNi2.1 eutectic high entropy alloy. J. Netshape Form. Eng. 2022, 8, 14. [Google Scholar]
- Zhuang, D.; Tao, W.; Ni, H.; Wang, A.; Du, B.; Zhang, S.; Lian, X. TiC morphology and corrosion resistance of CrMnFeCoNi+x(TiC) coatings prepared by laser cladding. Mater. Charact. 2023, 205, 113339. [Google Scholar] [CrossRef]
- Muhammad Nadzri, N.; Halin, D.; Al Bakri Abdullah, M.; Joseph, S.; Mohd Salleh, M.; Vizureanu, P.; Burduhos-Nergis, D.; Sandu, A. High-Entropy Alloy for Thin Film Application: A Review. Coatings 2022, 12, 1842. [Google Scholar] [CrossRef]
- Barber, Z.H. The Structure of Vapor-Deposited Materials. Mater. Sci. Mater. Eng. 2016. [Google Scholar] [CrossRef]
- He, Q.; DePaiva, J.; Kohlscheen, J.; Beake, B.; Veldhuis, S. Study of wear performance and tribological characterization of AlTiN PVD coatings with different Al/Ti ratios during ultra-high speed turning of stainless steel 304. Int. J. Refract. Met. Hard Mater. 2021, 96, 102–110. [Google Scholar] [CrossRef]
- Alfonso, J.; Torres, J.; Marco, J. Influence of the substrate bias voltage on the crystallographic structure and surface composition of Ti6A14V thin films deposited by rf magnetron sputtering. Braz. J. Phys. 2006, 36, 994–996. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Y.; Olugbade, E. Microstructure and process optimization of AlCrFeCoNiCu high-entropy alloy by laser deposition. Opt. Precis. Eng. 2019, 42, 184–195. [Google Scholar]
- Cheng, C.; Li, H.; Zhang, C.; Guo, C.; Li, J.; Zhang, H.; Lin, S.; Wang, Q. Effect of substrate bias on structure and properties of (AlTiCrZrNb)N high-entropy alloy nitride coatings through arc ion plating. Surf. Coat. Technol. 2023, 467, 129692. [Google Scholar] [CrossRef]
- Dai, C.; Fu, Y.; Guo, J.; Du, C. Effects of substrate temperature and deposition time on the morphology and corrosion resistance of FeCoCrNiMo0.3 high-entropy alloy coating fabricated by magnetron sputtering. J. Miner. Metall. Mater. 2020, 27, 10. [Google Scholar] [CrossRef]
- Ivanov, Y.; Akhmadeev, Y.; Koval, N.; Shugurov, V.; Petrikova, E.; Krysina, O.; Prokopenko, N.; Tolkachev, O. Nitride Coatings Based on a High-Entropy Alloy Formed by the Ion-Plasma Method. High Energy Chem. 2023, 57 (Suppl. 1), S77–S80. [Google Scholar] [CrossRef]
- Lim, J.; Mimura, K.; Miyake, K.; Yamashita, M.; Isshiki, M. Effect of Substrate Bias Voltage on the Thermal Stability of Cu/Ta/Si Structures Deposited by Ion Beam Deposition. Jpn. J. Appl. Phys. 2003, 42, 2780–2785. [Google Scholar] [CrossRef]
- Hamzah, E.; Ali, M.; Toff, M. Effect of Substrate Bias on Friction Coefficient, Adhesion Strength and Hardness of Tin-Coated Tool Steel. Surf. Rev. Lett. 2006, 13, 763–771. [Google Scholar] [CrossRef]
- Cui, K.; Zhang, Y. High-Entropy Alloy Films. Coatings 2023, 13, 635. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, X.; Ou, Y.; Wei, R. The structure, oxidation resistance, mechanical and tribological properties of CrTiAlN coatings. Surf. Coat. Technol. 2015, 277, 58–66. [Google Scholar] [CrossRef]
- Cai, H.; Xue, Y.; Wang, J.; Ye, J.; He, J. Effect of Sputtering Power on High Temperature Tribolo-gical Behavior of La-Ti/WS2 Composite Films. Rare Met. Mater. Eng. 2023, 52, 1201–1209. [Google Scholar]
- Wang, W.; Pelenovich, V.; Yousaf, M.; Yan, S.; Bin, H.; Wang, Z.; Tolstogouzov, A.; Kumar, P.; Yang, B.; Fu, D. Microstructure, mechanical and tribological properties of WC/a-C:H coatings deposited by cathodic arc ion-plating. Vacuum 2016, 132, 31–39. [Google Scholar] [CrossRef]
- Wu, R.; Zhang, Y.; Zhang, F.; Hou, B.; Liu, J.; Chen, Y.; Yang, S. Effect of Heat Treatment on Microstructure and properties of AlFeCoCrNiTi0.2 High entropy alloy. Nonferr. Met. Eng. 2019, 13, 41–48. [Google Scholar]
- Chang, W.; Cai, H.; Lei, X.; Xue, Y.; Li, H. High-temperature Tribological Properties of (AlCrNbTiVCe)N Coating Deposited by Co-sputtering Rare Earth Ce. Rare Met. Mater. Eng. 2023, 52, 527–534. [Google Scholar]



| Target | Al | Cr | Nb | Ti | V | C |
|---|---|---|---|---|---|---|
| AlTiVCrNb | 19.4 | 19.7 | 20.7 | 20.3 | 19.9 | - |
| C | - | - | - | - | - | 99.9 |
| Samples | Graphite Target Power /(W) | Substrate Bias (Zc)/(V) | AlTiVCrNb Target Power/(W) | Roughness of the Coating RZ/(µm) | Coating Thickness (nm) | Cr Sputtering Target Power/(W) |
|---|---|---|---|---|---|---|
| S1 | 150 | 0 | 150 | 0.085 ± 0.002 | 1176 ± 10 | 100 |
| S2 | 150 | 30 | 150 | 0.083 ± 0.002 | 1231 ± 10 | 100 |
| S3 | 150 | 60 | 150 | 0.047 ± 0.002 | 1259 ± 10 | 100 |
| S4 | 150 | 90 | 150 | 0.029 ± 0.002 | 1195 ± 10 | 100 |
| S5 | 150 | 120 | 150 | 0.016 ± 0.002 | 1162 ± 10 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, H.; Guo, P.; Xue, Y.; Pei, L.; Zhang, Y.; Ye, J. Effect of Substrate Bias on the Structure and Tribological Performance of (AlTiVCrNb)CxNy Coatings Deposited via Graphite Co-Sputtering. Lubricants 2024, 12, 325. https://doi.org/10.3390/lubricants12090325
Cai H, Guo P, Xue Y, Pei L, Zhang Y, Ye J. Effect of Substrate Bias on the Structure and Tribological Performance of (AlTiVCrNb)CxNy Coatings Deposited via Graphite Co-Sputtering. Lubricants. 2024; 12(9):325. https://doi.org/10.3390/lubricants12090325
Chicago/Turabian StyleCai, Haichao, Pengge Guo, Yujun Xue, Lulu Pei, Yinghao Zhang, and Jun Ye. 2024. "Effect of Substrate Bias on the Structure and Tribological Performance of (AlTiVCrNb)CxNy Coatings Deposited via Graphite Co-Sputtering" Lubricants 12, no. 9: 325. https://doi.org/10.3390/lubricants12090325
APA StyleCai, H., Guo, P., Xue, Y., Pei, L., Zhang, Y., & Ye, J. (2024). Effect of Substrate Bias on the Structure and Tribological Performance of (AlTiVCrNb)CxNy Coatings Deposited via Graphite Co-Sputtering. Lubricants, 12(9), 325. https://doi.org/10.3390/lubricants12090325





