Abstract
Electric potential controlled lubrication, also known as triboelectrochemistry or electrotunable tribology, is an emerging field to regulate the friction, wear, and lubrication performance under charge distribution on the solid–liquid interfaces through an applied electric potential, allowing to achieve superlubrication. Electric potential controlled lubrication is of great significance for smart tunable lubrication, micro-electro-mechanical systems (MEMS), and key components in high-end mechanical equipment such as gears and bearings, etc. However, there needs to be a more theoretical understanding of the electric potential controlled lubrication between micro- and macro-scale conditions. For example, the synergistic contribution of the adsorption/desorption process and the electrochemical reaction process has not been well understood, and there exists a significant gap between the theoretical research and applications of electric potential controlled lubrication. Here, we provide an overview of this emerging field, from introducing its theoretical background to the advantages and characteristics of different experimental configurations (including universal mechanical tribometers, atomic force microscopes, and surface force apparatus/balances) for electric potential controlled lubrication. Next, we review the main experimental achievements in the performance and mechanisms of electrotunable lubrication, especially using ionic lubricants, including electrolyte solutions, ionic liquids, and surfactants. This review aims to survey the literature on electric potential controlled lubrication and provide insights into the design of superlubricants and intelligent lubrication systems for various applications.
1. Introduction
Friction and wear are the dominating factors affecting the lifetime of mechanical equipment, leading to about 80% of failures of mechanical parts and increasing the consumption of materials, which hinders the sustainable growth of society and the economy [1,2,3]. Effectively controlling the frictional properties of materials is an important way to achieve efficient use of resources and sustainable economic development worldwide. Over the past century, a number of methods have been applied to solve engineering problems caused by friction and wear, which have greatly enriched the research on lubrication theory simultaneously.
1.1. Lubrication Methods
In order to solve friction and wear problems on the surface of materials and expand the understanding of lubrication mechanisms, the German scholar Stribeck drew the famous Stribeck curve from 1900 to 1902 by conducting friction experiments on rolling and sliding bearings and divided the lubrication regimes into boundary, mixed, and elastohydrodynamic lubrication [3]. Based on the lubrication states, a variety of ways were proposed to control frictional performance.
In boundary and mixed lubrication regimes, the surfaces of the friction pair material can be modified by surface texture [4,5], chemical grafting [6,7], ion sputtering [8,9], and other surface modification technologies [10] in order to improve the anti-friction and anti-wear abilities of the substrates and the adsorption effect of the lubricant. The textured surface of ultra-high-molecular-weight polyethylene (UHMWPE) reduces the actual contact area between friction pairs, thereby improving the tribological properties of the material surface [11]. The chemical grafting of polyhydroxyethyl methacrylate coating (PHEMA) on the surface of silicone rubber with excellent hydrophilic properties reduces the sliding friction coefficient by 98% compared with the non-grafted surface [12]. A nano-multilayer AlCrSiN/VN coating was deposited on the alloy surface by arc ion plating and pulsed direct current magnetron sputtering, and the friction coefficient of the modified alloy surface was reduced to 0.26 [13]. The addition of graphene nanosheets to ethylene glycol lubricant can promote the formation of a boundary lubrication film, which significantly reduces the shear force in the contact area and provides a new idea for the realization of macro-scale superlubricity [14].
In elastohydrodynamic lubrication (EHL), the tribological behavior of the mating surfaces is mainly affected by the properties of the lubricant, like viscosity and chemical stability, where the viscosity of the lubricant determines the friction energy dissipation and the friction force. For Newtonian liquids, the viscosity does not change with the shear rate [15]; however, the viscosity of non-Newtonian lubricants is significantly affected by velocity. The viscosity of nematic liquid crystal (5CB) and a carboxylic acid lubricant decreases with increasing velocity during rolling contact [16]. This inspired us to control lubrication by adjusting the viscosity. In addition, the shear process of the lubricant between surface asperities may induce some triboelectrochemical reactions, resulting in the lubrication regime transition during friction. For example, polyethylene glycol (PEG) aqueous solution is used to lubricate steel/steel surfaces, and the friction coefficient (CoF) decreases to 0.005 after a running-in period [17].
According to the working conditions and application requirements of the equipment, the mechanism and material properties of the system need to be optimized. For example, the brake system of an automobile needs enhanced friction, while the majority of the transmission system needs to operate with minimum friction. Therefore, achieving real-time and effective active control of the friction between interfaces has posed new challenges and opportunities for tribology in potential applications such as electro-switchable devices, triboelectric generators, micro- and nano-electromechanical systems (MEMS and NEMS), smart devices, etc. [18,19].
1.2. Controllable Lubrication Methods
In order to reduce friction or realize active control of lubrication performance, research on electrotunable friction has been carried out by changing the pH [19,20], temperature [21], light [22], and magnetic field [23,24] of the friction environment to adjust the mechanical properties of the friction pairs and the interface interaction between the lubricant and friction pairs. The anti-friction and anti-wear capabilities can be controlled by forming a fluid film or boundary lubrication layer between friction pairs [25,26,27]. These methods have application prospects in controlling the lubrication properties of materials. Electric potential controlled lubrication is an effective way to promote the interaction of desired lubricants and inhibit the interaction of unwanted lubricants with the surface by applying an electric potential, thereby achieving intelligent lubrication or improving the tribological properties of lubricated mechanical components [28].
The interaction between ions and solid surfaces at the solid–liquid interface determines the interfacial structure of adsorbed layers and charged surfaces, resulting in changes in tribological behavior. The electrostatic attraction between the charged interface and the ions draws counterions from the solution to the interface, which forms an electric double-layer [29,30,31]. Helmholtz [32] likened the double layer to a parallel plate capacitor, with one plate pair corresponding to the highly charged interface and the other corresponding to the center of the closest hydrated counterion (the outer Helmholtz plane, OHP). The structure between the two plates is defined as the Helmholtz layer (also known as the Stern layer), at which the potential decreases linearly. However, the presence of specific adsorption and incomplete solvated ions is not taken into account. Gouy and Chapman [33] used the Poisson–Boltzmann equation to describe the equilibrium distribution of ions, suggesting that the potential in the electric double-layer decreases exponentially, called the Gouy–Chapman–Stern model. The electric double-layer is composed of the inner Stern layer and the outer diffuse layer (Figure 1a). A large number of experiments and simulations agree well with the Gouy–Chapman–Stern model.
The aqueous ionic lubricants have good electrical conductivity and can form a physical adsorbed hydration layer on the solid surface, thus exhibiting excellent lubrication ability at both micro- and macro-scale conditions. Experiments have revealed that the external electric field can adjust the surface lubrication ability of these ionic lubricants. When LiPF6-base ionic liquid is used as the lubricant between steel/steel surfaces, the CoF does not fluctuate significantly with the change when electric potential varies from −0.8 V to −0.2 V; however, by further increasing the external electric potential from −0.2 V to 0 V, the CoF decreases with the increase in electric potential, and after that, the CoF increases with the increase in electric potential from 0 V to 0.8 V [34]. In hydrated ion solutions, the electric field could change the orientation of water molecules in the lubricant, regulate the formation and dissociation of the hydrogen bond network, and affect the hydration of the ions [35,36,37].
Using sensitive techniques, such as the surface force apparatus/balance (SFA or SFB) and atomic force microscopy (AFM), the effect of the applied potential on the formation of an electric double-layer of hydrated ions on the surface of Pt was studied. In addition to the potential-dependent physical adsorption of hydrated ions, the potential also affected the adsorption of H+ ions on the Pt surface and the electron transfer state between the electrodes, inducing an electrochemical reaction [38]. Molecular dynamics simulations—together with experiments for electric potential controlled ion lubrication—show that the ion size and the distribution of intramolecular charge can influence the formation of the lubricating film, and the experimentally measured thickness of adsorbed ions and simulation results are highly consistent [24,28]. Therefore, AFM and SFA have revealed the mechanism of electroactive friction, that is, the applied electric potential can regulate the electrostatic adsorption or electrochemical reaction between the ions and the substrate surface, thus affecting the formation and thickness of nano-lubricating films [34].
At macro-scale conditions, the combination of a universal mechanical tribometer (UMT) and electrochemistry setup has also proved that the lubrication ability of CuS [39] and MoS2 [40] lubricating additives is further strengthened under the action of an electric field, and the lubrication performance of surfactants (zinc dithiophosphate (ZDDP) [41] and sodium dodecyl sulfate (SDS) [18]) is also affected by applied electric potential. Liu et al. [42] found that in pure water or pure base oil, the CoF and surface morphology of some metals can be adjusted to a certain extent under external electrical stimulation. By observing the effect of friction on the electrode at a given temperature, they believe that there is a difference in the electrochemical behavior between the contact and non-contact regions of the solid–liquid interface during friction (Figure 1b). The traditional electrochemical processes without the influence of friction generally include the adsorption/desorption process when the applied surface potential is within the thermodynamic potential window, while for the electrode processes with the influence of friction, triboelectrochemical processes including friction-induced reduction, friction-inhibited reduction, friction-induced oxidation, and friction-inhibited oxidation. Beyond the electrochemical window (usually with applied potentials above ±1 V), a triboelectrochemical reaction will occur, which may also control the friction behavior. In addition, even for the insulated Al2O3/ZrO2 pairs, an indirect electric field introduced between the ball holder and the SDS lubricants can also affect the interaction and lubrication performance between the lubricant and substrate surface, resulting in a varying CoF between 0.12 and 0.35 [43].
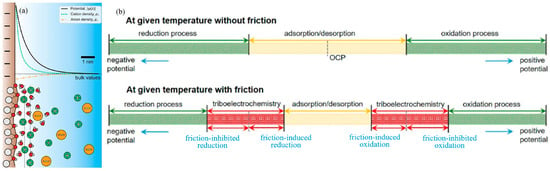
Figure 1.
Electric double-layer and triboelectrochemistry process: (a) Electric double-layer [29]. Large white, small white, and red circles represent atoms of solid surface, hydrogens, and oxygens, respectively. Copyright 2024 Elsevier; (b) schematic diagram of the electrode processes at a given temperature without and with friction [42]. Copyright 2021 the Authors.
So far, most electric potential controlled lubrication experiments were carried out within the electrochemical window (at low applied potential) [42], where mainly physical adsorption/desorption rather than chemical reactions occurred. Although the electrochemical reaction can lead to irreversible changes on the surface of the friction pair or lubricant, and even lead to increased friction in most cases [44], it is still unclear whether the triboelectrochemical reaction can enhance lubrication and reduce friction. At the macro-scale, the friction film formed by the triboelectrochemical interaction between the lubricant and the substrate can reduce the shear strength of the interface. Therefore, the electric potential may promote the electrochemical reaction between the lubricant and surfaces to enhance the lubrication performance but whether this enhancement is reversible still remains unknown. However, due to the difference between micro- and macro-scale experiments, especially under higher loads and speeds at macro-scale conditions, the nano-confined lubricating film may change at macroscopic conditions [45,46]. How to establish the relationship between macro- and micro-scale research already faces a challenge. Further investigation on micro-scale research guiding macro-scale applications is needed.
In addition, most micro-scale electrical potential controlled lubrication studies mainly use materials with good electrical conductivity and strong electrochemical stability as friction surfaces, like gold, platinum, and pyrolytic graphene (HOPG) [47,48,49], which are different from the materials used in actual working conditions (such as steel, nitinol alloy, etc.) [50,51,52], resulting in a significant disconnect between theoretical research and practical applications. Given the extensive research on the electric potential controlled friction of ionic lubricants, as well as the lack of systematic reviews, here we focus on the progress of electric potential controlled lubrication for ionic lubricants. We especially describe the characteristics of experimental configurations and methods, including an electrochemical universal mechanical tribometer (EC-UMT), electrochemical surface force apparatus/balance (EC-SFA/SFB), and an electrochemical atomic force microscope (EC-AFM). In addition, the effects of interactions between different types of water-based lubricants (such as ionic liquids, hydrated ions, and surfactants) and the friction surface effects—under applied electric potential—on friction and wear behavior are summarized, and potential controlled lubrication mechanisms are summarized accordingly. Finally, the key challenges and potential research directions are discussed, and future perspectives for the use of electric potential controlled lubrication in industrial equipment and microsystem equipment (MEMS) are highlighted (Figure 2).
Figure 2.
Electric potential controlled lubrication (including setups and lubricants) with potential applications. Experimental configurations of three common electrochemical setups: EC-AFM [44]—Copyright 2020 American Chemical Society; EC-SFA/SFB [53]—Copyright 2016 American Chemical Society; EC-UMT [54]—Copyright 2021 Springer. Ionic aqueous lubricants: ionic liquids [14]—Copyright 2019 Elsevier; hydrated ions [55]—Copyright 2018 American Chemical Society; surfactants [56]—Copyright 2016 American Chemical Society. Potential applications: electro-switchable devices [57]—Copyright 2022 Elsevier; MEMs and NEMs; triboelectric generators; intelligent lubrication [58]—Copyright 2020 Elsevier; high-end equipment [59]—Copyright 2023 the Authors; biological tissue engineering [60]—Copyright 2011 Elsevier.
2. Experimental Configurations of Electric Potential Controlled Lubrication
By measuring the normal force and shear force between two surfaces of liquid film lubrication, the film thickness of lubricant, the relative motion of the sliding surface, and the voltage and current applied in situ, the mechanism of electrical potential controlled lubrication and its influencing factors are studied. From the perspective of the contact scale (including the contact area, the film thickness, etc.), we can simply distinguish the experimental configurations into micro-scale and macro-scale measurements. SFA/SFB and AFM are key techniques widely used in these specifications, which also provide molecular-level resolution in the film thickness. UMT is the most commonly used instrument in macro-scale tribological research of electrical potential controlled lubrication, which can conduct experiments at much larger sliding speeds and much higher applied loads compared with SFA/SFB and AFM.
2.1. Electrochemical Surface Force Apparatus/Balance
Based on the ingenious mechanical structure and multiple-beam interferometry, the surface force apparatus/balance (SFA/SFB) technique can be used to measure both normal and lateral forces with a force resolution of <0.1 μN between atomically smooth surfaces in liquids with a distance resolution of ~0.1 nm. Usually, freshly cleaved mica with a smooth surface and a highly reflective silver film on its back is pasted on the cross-placed cylindrical glass disc, which avoids the edge effect compared with the two parallel plates. The distance between the cross-cylindrical surfaces as well as the shape of the surface is determined through the fringes of equal chromatic order (FECO) and multiple-beam interferometry (MBI) [61,62]. Hooke’s law is used to calculate the normal force between two surfaces based on the separation distance. In other words, the normal force is measured by moving the surface at the base of the double-cantilever force spring using the differential micrometer, motor-driven fine micrometer, and piezo tube [61,62]. Experiments show that the normal force versus separation distance curves can be fitted by the Derjaguin–Landau–Vervey–Overbeek (DLVO) theory, which shows the nano-confined interaction between the surfaces clearly [44,45]. The surfaces can also be sheared past each other using a motor-driven micrometer to move the upper surface in the lateral direction, or by using a piezoelectric bimorph slider [61,62]. For SFA2000 designed by J. N. Israelachvili [61], a triangular wave function is applied to shear the surfaces back and forth at a constant velocity usually in a range of 0.1~100 μm/s. The bimorphs are designed with a flexure point in the middle that causes the surfaces to displace linearly as voltages are applied [61].
SFA/SFB has been used to measure the dynamic interactions (mainly including the Van der Waals force, the electric double-layer force, and the hydration force) in aqueous solutions, like hydrated ions, polymer brushes, and amphiphilic surfactants [63]. The fluidity of water molecules affects the confined viscosity of water-based lubricants. In electrolyte solutions, SFA/SFB experiments show that the hydration layer (bound water and free water molecules) has a shear fluidity characteristic as the bulk liquid, and the bound water in the hydration layer is ready to exchange under shear sliding [64,65]. The hydration force between two mica surfaces in monovalent or multivalent electrolyte solutions contributes to excellent superlubrication performance [66,67,68]. Different friction energy dissipation pathways and different hydration layer structures on charged surfaces were found in the nano-confined hydration layer, showing the ion surface coverage dictates the roughness of the hydration layer and its lubricating properties under higher pressures (i.e., lower film thickness), while confined effective viscosity dominates the hydration superlubrication at lower pressures (i.e., larger film thickness) [69,70]. Phospholipids and polymer brushes are also main lubricant components for joint surfaces, and sphingomyelin [71], poly [2-(methylpropenoxy) ethyl phosphocholine] (pMPC) [72], polyethylene oxide (PEO) [73], polystyrene sulfonates [74], and others [75,76,77,78,79] can be grafted on the negatively charged mica surfaces, then, their normal and shear interactions can be studied using SFA/SFB. It was also proposed that the supramolecular synergistic effect of phospholipid and polymers could form a stable hydrated lubrication layer under high pressures, which reduced the shear strength of the contact surface and enabled articular cartilage to exhibit excellent lubrication properties [80,81,82].
As one of the most effective techniques to study nano-tribology, SFA/SFB has promoted the development of the field of intermolecular and surface forces. By optimizing the basic structure of SFA/SFB and combining it with three-electrode components, it has become a powerful means to study electric potential controlled lubrication. Markus Valtiner et al. [83] designed the electrochemical surface forces apparatus (EC-SFA), as shown in Figure 3a. The interactions between the potential controlled gold surface and mica surface, the thickness of anodic oxide film on the gold surface, and the friction force of the metal–ceramic interaction can be measured to show the effect of the applied potential. The typical cyclic voltammetry (CV) curve of the gold surface was measured by this EC-SFA setup (Figure 3b). Jacob Klein’s [53] group studied the interaction between potential controlled gold and mica surfaces in pure water and electrolyte solutions using the EC-SFB setup and proved that the normal interaction between gold and mica was in good agreement with the Poisson–Boltzmann (PB) equation at low electrolyte concentrations. They found different interactions under different electric potentials—pure attraction, non-monotonic interactions from electrostatic repulsion to attraction, and pure repulsion—which provides an effective way to control interface interactions [84]. In addition, the potential controlled lubrication properties of polyelectrolyte solutions are closely related to the ion valence and ion size, and the applied electric potential affects the hydration layer formed by ions [66,67,85]. Therefore, it is feasible to control the physical adsorption/desorption of ions and molecules on the charged surface by adjusting the applied potential, so as to realize the active control of the lubrication response of these nano-confined lubricants [17,86].
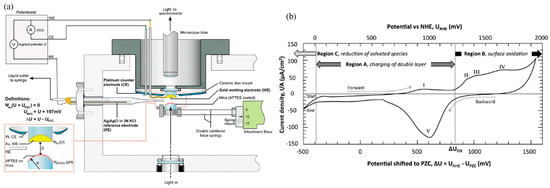
Figure 3.
EC-SFA setup [83]: (a) structure diagram of EC-SFA setup with the electrochemical three-electrode setup; (b) typical cyclic voltammetry (CV) of a gold electrode in 1 mM HNO3 solution at pH = 3 measured using EC-SFA setup. I to V represent current peak at ~950, 1330, 1400, 1650, and 1025 mV measured against the normal hydrogen electrode (NHE) respectively. Copyright 2011 American Chemical Society.
Kazue Kurihara’s group designed the salt bridge structure to apply a potential to the gold surface using an SFA setup, in order to eliminate the influence of Cl− in the electrolyte solution on the experiment, as shown in Figure 4a. The adsorption characteristics of KClO4, K2SO4, and KCl electrolytes on the gold surface were studied under the influence of electric potential [49]. When the applied potential was less than the point of zero charge (pzc), the interaction between the electrode and negatively charged mica surface (σ) was independent of the type of hydrated ions. When the applied potential was greater than pzc, σ became positive in the KClO4 solution but negative in the K2SO4 and KCl solutions (Figure 4b). This proves that different types of hydrated ions have different adsorption performances on the potential controlled gold surface as the electric potential changes [49]. Moreover, the relationship between the surface force and applied potential between Pt/Pt and Pt/mica surfaces in HClO4 solution was measured, which provided the possibility for characterizing the hydrogen evolution process on the Pt electrode [87]. The effect of ion adsorption on the surface potential and charge density of the electrode can be quantitatively evaluated by EC-SFA. It is necessary to further study the effect of electric potential on the electric double-layer and hydration layer structures under shear sliding.
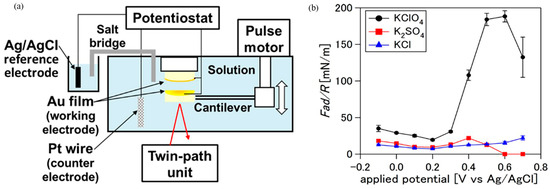
Figure 4.
The twin-path EC-SFA setup with a salt bridge [49]: (a) schematic diagram of this setup, avoiding any contamination of Cl− ions by the salt bridge; (b) the relationship between normal interaction and applied potential between gold and mica surfaces. Copyright 2016 American Chemical Society.
SFA/SFB has a resolution of 0.1 nm and is used to study the mechanism and influencing factors of hydration lubrication at the nanoscale [70]; moreover, the EC-SFA/SFB device is designed by combining SFA/SFB with an electric field, which can study the electrically controlled lubrication phenomenon at atomic level resolution, in situ characterizing the nanometer-scale friction forces and the number of ion layers adsorbed on the substrate surface [69]. A large number of electronic lubrication studies with EC-SFA/SFB devices have demonstrated that the electric potential affects the adsorption/desorption ability of ions in the lubricant on the substrate surface and changes the thickness and uniformity of the formation of nano-lubricating film on the surface of the contact zone. However, whether the experimental results obtained from the SFA/SFB study on the interactions of the contact interface are consistent with the macroscopic phenomena, and whether the electric potential changes the electrolyte viscosity in the nano-confined film need to be further verified [88].
2.2. Electrochemical Atomic Force Microscope
An atomic force microscope (AFM) is a kind of precision inspection instrument with atomic-level high resolution, which can detect the properties and morphology of various materials at the nanoscale in atmospheric and liquid environments [89]. AFM is mainly composed of a force detection system, a displacement system, and a feedback system. The interaction between the tip and the sample surface makes the cantilever beam deform, affecting the deflection of the reflected light; then, it transmits the signal to the feedback system for conversion and generates the characteristic information of the material surface. AFM can provide three-dimensional surface images of the materials without any special treatment [90,91], so it can be used to study the interactions between different materials, such as Si3N4, Al2O3, and diamond [92]. Moreover, the normal and shear interactions in solutions can also be studied exactly as SFA/SFB [93], such as the Debye length of ions with different solubility [94], different valences, and the ion adsorption performance on different charged surfaces [95]. Compared with the complicated process of preparing mica surfaces for SFA/SFB, it is much easier to prepare AFM tips and samples, which may be the reason for more AFM studies than SFA/SFB studies.
By using AFM to study the cell wall structure of Gram-positive bacteria, it was found that the surface of living cells was characterized by disordered peptidoglycan gels formed by large and deep pores, which provided a theoretical basis for studying the mechanical properties of cell molecular structures [96]. Direct force measurements between charged surfaces in solutions of multivalent electrolytes were carried out using AFM, showing that the measured forces are in good agreement with the predictions of the Derjaguin–Landau–Verwey–Overbeek theory of colloidal interactions [94]. Natural articular cartilage has excellent lubrication properties due to hydration contribution. Many studies have found that hydrated ions interact with water molecules to form a hydration layer with low shear resistance, so it is necessary to detect the structure of the hydration layer and its influencing factors at the atomic level, including the solid materials, the type of hydrated ions, and the solubility [97,98]. The tribological behavior of different types of hydrated ions is studied by AFM combined with friction microscopy, showing that the higher the ion concentration, the larger the hydration force. The CoF of monovalent hydrated ions at the same concentration is as follows, K+ < Na+ < Li+ < Cs+, confirming that hydration lubrication is not only affected by the hydration strength of ions but also has a significant relationship with its surface distribution and hydration ability [99]. AFM was also used to study the surface wettability and surface tribological properties of TiO2 and silicon wafers after polishing under heating, ultraviolet (UV), and plasma treatments. It was found that with the contact angle increasing, the CoF of the TiO2 surface decreased, while the CoF of the silicon wafer increased, which was attributed to the formation of a uniform interfacial water structure on solid surfaces [100].
Friction at interfaces will change the distribution of surface charge. AFM and Kelvin probe force microscopy are used to study the influence of different thermal conditions on charge transfer at the micro-scale level. A thermionic emission band-structure model is proposed to describe the phenomenon of contact electrification caused by electron transfer, in turn, caused by temperature difference [101]. Furthermore, AFM can also be used to study the effect of applied electric field on the charge transfer of friction pairs and electrolyte lubricants, which has great guiding significance for controlling the friction and wear behavior of materials. The surface charge can be controlled through applied electric potential, which can further control the lubrication performance. The structure of an electric double-layer (EDL) adsorbed by ionic liquid on the HOPG surface was studied by combining AFM and electric field (i.e., EC-AFM, Figure 5a) [44]. It was found that EDL exhibited obvious 3D reconfiguration under different electric potentials. In addition, polymeric ionic liquids (PILs) adsorbed on a charged Au (111) surface can form a cation-enriched boundary layer that lubricates the interface, and the adhesion characteristics are closely related to the applied electric potential [101]. Figure 5b shows the normal force versus separation distance curves between the SiO2 probe and gold surface in NaCl solution under different applied potentials using EC-AFM. The solid curves correspond to the fitting data obtained at −0.6 and +0.6 V by assuming a classical hydrodynamic model, indicating that the microsphere is subject to strong repulsive forces at all surfaces, which can be attributed to the spatial effect of confining water molecules on the surface, that is, the hydration force [31]. At negative potential, the effective viscosity is relatively low due to the fluidity of surface water molecules. As the applied potential increases, the surface water molecules line up at the interface to form an ice-like surface layer, increasing the confined viscosity of the water layer and thus increasing the repulsive force [36].
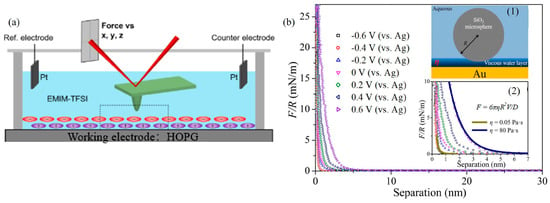
Figure 5.
EC-AFM experiments: (a) EC-AFM setup for studying ionic liquids [44]. Copyright 2020 American Chemical Society; (b) normal force-displacement curve between SiO2 microsphere and gold surface in 0.1 M NaCl solution under different electric potentials [36]. (1) the surface contact geometry, (2) A magnification of the force curves at separations ranging from 0 to 7 nm. Copyright 2020 the Authors.
Graphene has excellent lubrication performance and electrical conductivity and can be used in a wide range of applications in the field of solid lubrication and nano-lubricating additives. EC-AFM was used to study the graphene’s lubrication performance at different electric potentials (Figure 6a) [102]. A layer of graphene was deposited on the surface of Au/SiO2/Si substrate (Figure 6b). Figure 6c,d shows the friction images under different applied voltages and the topography acquired simultaneously during the friction, respectively. The friction force increased with the increase in applied voltage. At different reciprocating speeds under low applied potential (0–3 V), friction increases with the speed increasing, while under high applied potential (6 V), the variation in friction and speed shows the reverse trend. The Prandtl–Tomlinson model suggests that the high electric potential causes the contact surface to generate higher Joule heat, which weakens its mechanical properties, while the increase in speed promotes heat transfer, thus reducing the friction and wear of the contact surface. Therefore, the tribological behavior of hydrated ions, ionic liquids, solid lubricants, etc., can be analyzed under applied electric potential through the output of the surface force-displacement curve and the topography of the contact interface by EC-AFM.
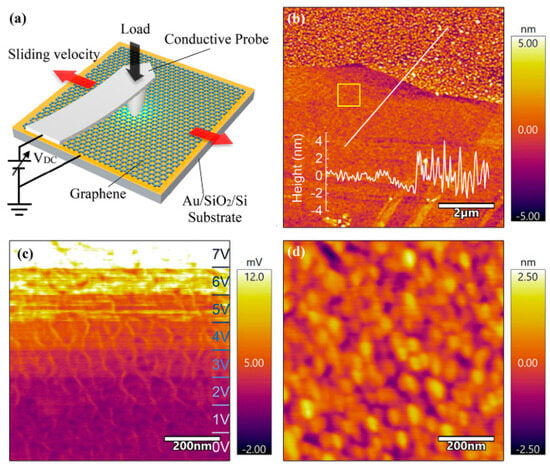
Figure 6.
EC-AFM studies on the relationship between the frictional behavior of the graphene surface and the electric potential [102]: (a) EC-AFM setup, the red arrow indicates the direction of motion; (b) morphology of graphene deposited on Si surface, the 800 nm × 800 nm yellow square represents the frictional contact area under different voltages at the same scanning speed, and the white line represents the height profile; (c) friction image of graphene on Si surface under different electric potentials; (d) topography acquired simultaneously during the friction. Copyright 2020 American Chemical Society.
EC-AFM can be used to study the effect of applied electric potential on the charge transfer of friction pairs and the lubrication performance of conductive lubricants at the microscopic scale, which has great guiding significance for controlling the friction and wear behavior of materials. Moreover, 3D-AFM has shown great potential to accurately characterize the hydration layer structure at the molecular level, and combining EC-AFM with 3D-AFM would be a promising instrument upgrade; however, the use of EC-AFM also needs to avoid the electrochemical reactions because chemical reactions may bring gas bubbles or surface corrosion, seriously restricting the measurement accuracy of the instrument. In addition, the nano-confined hydration layer is crucial for hydration lubrication but it is difficult to accurately obtain the surface spacing at the level of 1 nm or below, resulting in the failure to accurately analyze the hydration layer structure, which is one of the most significant disadvantages compared with EC-SFA.
2.3. Electrochemical Universal Mechanical Tribometer
As the most common macroscopic tribological testing equipment, a universal mechanical tribometer (UMT) simulates the working condition of the specimen changing the motion state under applied load and evaluates the friction and wear performance of the material surface, which plays an important role in improving the performance and reliability of the mechanical components. UMT setup is mainly composed of the drive system, data acquisition system, and microcomputer control system with good compatibility and multi-modular design, which can carry out a variety of performance characterization of friction pairs. With the development of technology and the upgrading and optimization of equipment, UMT can provide simultaneous movement of upper and lower samples to ensure adaptability to multiple friction pairs and can quickly change modules to conduct experiments with different forms of test conditions.
In the past two decades, superlubricity research using UMT has developed fast, providing an effective way to reduce friction energy consumption and material wear, which accelerates the rapid development of lubrication theories. As an example, titanium dioxide (TiO2) can absorb ultraviolet light and exhibit abnormal performance after exposure to light [101,103]. UMT was used to evaluate the superlubricity phenomenon of TiO2 under ultraviolet light between the Si3N4 ball and TiO2 disk pairs. By irradiating the TiO2 surface during the running-in period with ultraviolet light, superlubricity with CoF < 0.01 was achieved, indicating running-in by low-pH solutions contributes to the photoinduced superlubricity. This is due to the formation of the SiO2 layer on the Si3N4 surface and better attraction to lubricant molecules through hydroxyl radicals on the TiO2 surface after radiation [104]. Although superlubricity can be achieved by adjusting the light for the TiO2 surface, there are still some challenges to controlling lubrication in engineering applications for commonly used materials such as steel, brass, and titanium alloy.
Based on the mechanism of electric potential controlled lubrication, the DC/AC power supply is combined with the UMT setup to explore the effects of applied electric potential on the lubrication performance of a macro-scale system. A kind of electrochemical universal mechanical tribometer (EC-UMT) was re-engineered to explore the influence of applied electric potential on the lubrication performance of SDS surfactant between bearing steel ball and 316L steel plate surfaces [18]. Real-time contact resistance was obtained from the ohmmeter by a computer to analyze the performance of the friction film in the contact region and realize online control of the lubrication performance. Figure 7a shows the change in friction coefficient and contact resistance between friction pairs. The contact resistance is large but the friction coefficient is small. According to the experimental phenomena and results, an online feedback control mechanism for the friction behavior of metal friction pairs was established, as shown in Figure 7b [18].
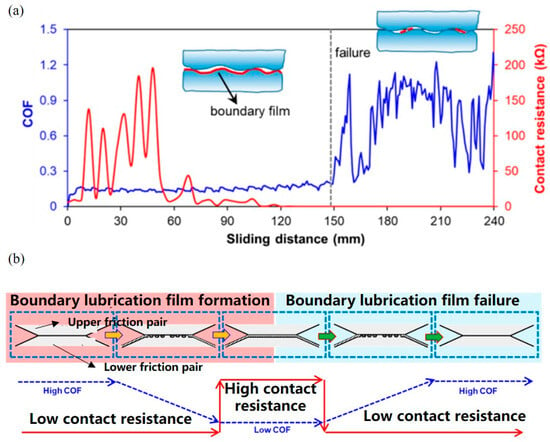
Figure 7.
Real-time tests by EC-UMT setup [18]: (a) the change in friction coefficient and contact resistance of steel/steel interface with lubrication of SDS solution; (b) the transition of lubrication film based on the contact resistance. Copyright 2022 the Authors.
As a prerequisite, friction pairs and lubricants must have good electrical conductivity to achieve electric potential controlled lubrication. Ceramic surfaces have poor electrical conductivity, bringing experimental challenges under applied electric potential. In order to control its tribological performance by EC-UMT, the working electrode (WE) was connected to a conductive steel ball holder, the reference electrode (RE) was connected to liquid, and the counter electrode (CE) was connected to a stainless-steel plate immersed in SDS solution (Figure 8a). EC-UMT experiments showed that CoF changed reversibly in the range of 0.12~0.35 under the influence of electric potential (Figure 8b) [54]. In addition, zinc dialkyl dithiophosphate (ZDDP) is currently one of the most successful lubricant additives with excellent oxidation, wear, and corrosion resistance. Tribological experiments by EC-UMT found that when ZDDP was added to a propylene carbonate (PC)/diethyl succinate (DES) blend system, the lubrication performance was enhanced with the increase in electric potential and the surface wear was also significantly reduced. This is mainly due to the difference in activation energy caused by the adsorption of ZDDP on the stainless steel surface and the triboelectrichemical reactions [105]. In addition, the dispersion and adhesion states of conductive nanoparticles in the lubricants were also changed under applied electric potential. The influence of electric potential on the lubrication performance of CuS nanoparticles modified by octadecylamine added in DES was evaluated [106]. Due to the random conformation of the polymer chain in octadecylamine and the positive charge of the head group, the modified CuS nanoparticles showed good lubrication effect. Due to the disordered structure of the carbon chain, the electron transfer was hindered, and the lubrication response to the applied electric field was weakened. Thus, the entire friction system was less affected by the electric potential [40].
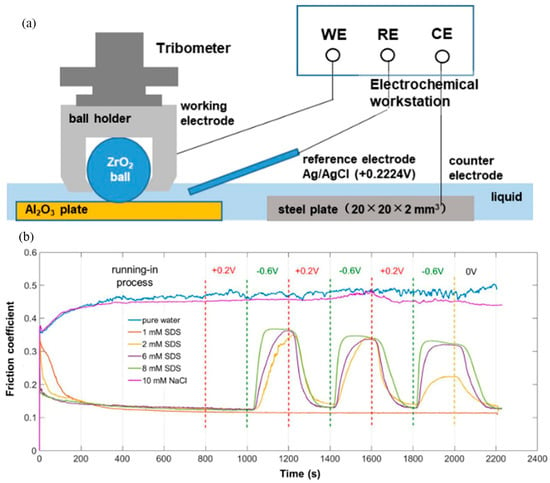
Figure 8.
EC-UMT combined with three electrodes applied an indirect electric field to the friction interface [54]: (a) structure diagram of EC-UMT setup; (b) CoF between Al2O3 plate and ZrO2 ball in pure water, SDS solution, and NaCl solution affected by the applied electric potential, the dashed lines represent the time for applying constant voltage. Copyright 2022 Springer.
The combination of UMT and electrodes can be used to study electric potential controlled lubrication under high load and high speed. It is applicable to a wide range of materials, and the experimental behavior is closely related to practical engineering applications. EC-UMT has been used to study the interactions between friction pairs and lubricants at a macro-scale level, explore the factors affecting lubrication properties, and then propose effective enhancement methods; however, EC-UMT experiments cannot reflect the interface interaction at the micro-scale so it is necessary to explore the physical adsorption and electrochemical interaction between the electrolyte lubricant and the substrate caused by the electric potential in the tribological process with other characterization equipment. Both EC-SFA/SFB and EC-AFM can reflect the surface and interface interactions at nano-confined conditions by analyzing the relationship between force and separation distance as well as the adsorption characteristics at the interface. Therefore, it is necessary to combine EC-UMT from macro-scale aspects and EC-instruments with microscopic characterization to characterize the electric potential controlled lubrication performance in the following studies, although it is always difficult to establish a connection between macro-scale performance and micro-scale mechanism.
Table 1 shows the comparison among EC-SFA/SFB, EC-AFM, and EC-UMT. The measurement accuracy of SFA/SFB and AFM is greatly affected by the test environment (like temperature and humidity). EC-SFA/SFB and EC-AFM have a nanoscale resolution for separation distance and force measurements, while EC-UMT has the capacity to measure high load and high-speed friction. In addition, EC-UMT can be used to study the phenomenon of rotary and linear reciprocating motion at macro-scale conditions, while EC-SFA/SFB and EC-AFM can only be used to study linear reciprocating motion. The continuous improvement and optimization of testing equipment provide an effective way to explore the friction and wear behavior under different working conditions and also lay a foundation for the establishment of electric potential controlled lubrication theory.

Table 1.
Comparison of EC-SFA/SFB, EC-AFM, and EC-UMT setups.
3. Aqueous Ionic Lubricants
Ionic aqueous solutions as excellent environment-friendly lubricants, have a low viscosity, low viscosity–pressure coefficient, and excellent lubrication performance even under high contact pressures larger than 500 MPa [107,108,109]. The ions in water-based lubricants can form an adsorption layer through the electrostatic interaction, which can bear a large applied load and reduce friction at the interface. Ionic aqueous lubricants even provide favorable conditions for the realization of superlubricity under all lubrication regimes, including boundary lubrication (BL) [69,82,110,111,112,113,114], mixed lubrication (ML) [14,45,55,107,115,116], and elastohydrodynamic lubrication (EHL) [46,117,118,119,120]. In a BL regime, the repulsive force (like hydration repulsion) generated by the adsorption of ions on the friction surfaces is conducive to bearing the applied load and reducing the friction. In an EHL regime, the lubrication film formed by the hydrodynamic effect plays a key role in achieving superlubricity. In addition, in a ML regime, both the separating effect (like hydration effect and tribo-induced boundary films) and hydrodynamic effect contribute to the superlubricity [3]. Here, ionic liquids, hydrated ions, and surfactants are discussed as the ionic aqueous lubricants with both good electrical conductivity and lubrication performance. Their adsorption on the substrate surface is affected by the applied electric potential, which is conducive to the active control of lubrication.
3.1. Ionic Liquids
Ionic liquids (ILs) are room-temperature molten salts that consist of cations and anions, and cations are generally organic, also known as meltable salt with an organic structure at room temperature [121,122]. ILs have good electrical conductivity, low volatility, sufficient chemical stability, high surface tension, and large viscosity, and can be used as lubricant additives, solvents, and catalysts over a wide range of applications in chemical separation, electrochemistry, and nanomaterials preparation [123,124]. ILs can form a lubrication film on the surface of the friction pairs, reducing the direct contact of the asperities, thereby reducing the friction and wear of friction surfaces [14]. Polytetrafluoroethylene (PTFE) has good corrosion resistance, and it is prone to adhesion and abrasive wear, which affects the service life of moving parts. The ionic liquid alkyl amine and carboxylic acids ([DMA][OA] and [DMA][DA]) can significantly improve the wear resistance of PTFE at high contact pressures due to the thick and dense Stern layer as well as the formation of a triboelectrochemical film [125]. Excellent lubricant materials exhibit appropriate shear viscosity during friction and have excellent bearing capacity to resist deformation, thus achieving excellent anti-wear and anti-friction ability under high load conditions. It is believed that to enhance the bearing capacity of liquid lubricants, the interaction between the lubricant and friction pairs should be enhanced. A choline chloride (ChCl)-based ionic liquid analog (ILA) was synthesized using ChCl and MgCl2·6H2O [118]. At a pressure of 160 MPa, the superlubricity of ILA can be realized between various friction pairs (Si3N4 vs. SiO2/Al2O3, steel vs. steel/SiO2). The ion adsorption on negatively charged surfaces, hydrogen-bond interaction-induced bound water, and free water form a low-shear-resistance layer, showing excellent load-bearing and lubrication properties [118].
ILs have excellent electrical conductivity and stable electrochemical performance compared with normal liquid lubricants. Various experiments have proved that the active control of friction can be effectively achieved by controlling the structure of the adsorption layer and the interaction between IL and friction surfaces by applying an electric potential. Rob Atkin group [123,124,125,126,127,128] focuses on the interactions in different ionic liquids on Au (111) or pyrolytic graphene (HOPG) surfaces. It has been found that ionic liquids rearrange on the surface of the substrate under applied potential. In ionic liquids with FAP− ions, the cations at negative potential are arranged more neatly than the anions at positive potential, thus forming a template structure in subsequent layers more efficiently. [EMIM]FAP has a strong interfacial structure because the imidazole ring of [EMIM]+ ions is parallel with the Au (111) surface in favor of the template structure [126]. [HMIM]FAP has the highest repulsive force on the HOPG surface due to the strong cohesive interaction between cations caused by long alkyl chains, forming an ordered structural layer resistant to the shear of SiO2 tip [127]. The electric potential changes the composition of the boundary layer ions of [HMIM]FAP, which realizes the on–off regulation of the superlubricity at the silicon/graphite interface [128].
Different solid surfaces and ionic liquids may lead to different structures of the adsorbed layer [129]. Cations and anions may form H-bonds in ionic liquids, which has been detected in the bulk phase of ILs with hydroxy-functionalized imidazolium and pyridinium cations, leading to the formation of H-bonded cation (C-C) clusters [130]. Figure 9a shows the mechanism of an AFM tip sliding on the surface of a (C-C) H bonding structure. The top of the cation bilayer is ruptured and the tip interacts with the single cation with the increase in load, which causes no (C-C) H bonding structure at the solid–liquid interface [130]. The shear behavior of ionic liquids on the HOPG surface changes with the electric potential as shown in Figure 9b, which indicates that the ionic liquid has formed a low-shear-strength boundary layer rich in cations and anions at the electric potential of both positive (+1 V) and negative potential (−1 V), respectively [131]. In addition, the adsorption state of PIL/[BMIM] [TFSI] ionic liquid on the surface of Au (111) can also be affected by the electric potential (Figure 9c). The adsorption of cations and anions on the substrate surface is competitive under open circuit potential (OCP). The cations have a repulsive effect on the matrix under +1 V, resulting in the interface adsorption layer being easily removed under shear [102]. Therefore, the electric potential controlled lubrication performance can be adjusted by controlling the surface potential and the type of ionic liquids.
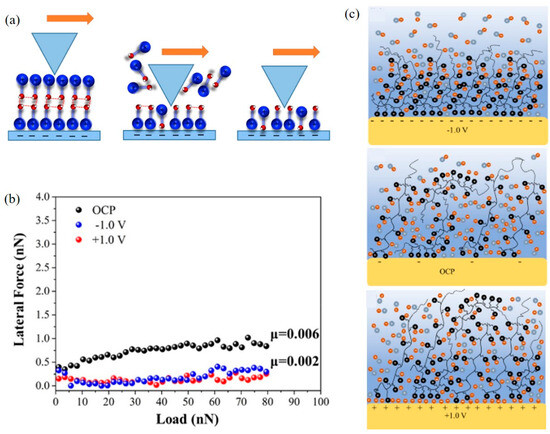
Figure 9.
The ionic liquid interacts with the surface under the control of electric potential: (a) Interaction between AFM tip and (C−C) H-bonding (H−bonds between the hydroxy-functionalized cations) cationic bilayer on negatively charged mica surface—the tip sliding on a single cationic layer when the top of the cationic double-layer breaks, the tip sliding on a single cationic layer without (C−C) H-bonding [130]. Copyright 2020 American Chemical Society; (b) the relationship between the shear force and applied load in [P6,6,6,14][AOT] under different electric potentials [131]. Copyright 2022 Elsevier; (c) schematic diagram of the adsorption layer of 5 wt% PIL/[BMIM][TFSI] on the surface of Au (111) under different electric potentials [102]. The positively charged pendent group of the PIL is dark blue, the BMIM cation is light blue and the TFSI anion is orange. Copyright 2023 American Chemical Society.
There are rich types of ionic liquid lubricants with green and environmental protection characteristics that meet the development of future requirements. Micro-scale studies have found that the lubrication performance of ionic liquids can be controlled by applying electric potentials to adjust the interaction between ionic liquids and friction surfaces. The ionic liquid seems to have limited bearing capacity, and the load applied by AFM is generally less than 100 nN, which is far below the demand of engineering applications. How to further enhance its load bearing capacity still meets great challenges. In addition, the lubrication behavior of ionic liquid strongly depends on the structure of the adsorption layer formed on the charged surface, so it is effective in controlling friction by electric field for smooth surfaces such as HOPG and gold. However, how to accurately regulate the ion adsorption structure for surfaces with a roughness above 1~10 nm still remains unknown. The coupling relationship between surface roughness and ion adsorption structure on lubrication performance is also still unclear. Moreover, whether the lubricating film formed by ionic liquids on the charged surface is a simple physical adsorption layer due to electrostatic interaction or a complex triboelectrochemical film formed by triboelectrochemical reactions still remains unknown at the macro-scale.
3.2. Hydrated Ions
The presence of water molecules tightly bound to ions in aqueous electrolytes leads to the formation of hydrated ions, which have excellent electrical conductivity, solubility, and thermal conductivity in a liquid. Common electrolytes, including NaCl, CuSO4, etc., play an important role in industrial and agricultural production. These ions, like Na+ and K+, can also regulate the osmotic pressure inside and outside the cell. Human synovial joints and other friction tissues have excellent lubrication performance, and it is believed that there is a role of hydration lubrication, i.e., hydration shells formed around the charge lubricating the contact area, which can bear a large load and resemble easy-shear fluid under nanoconfinment, making its lubrication performance excellent; however, the mixing of different energy dissipation mechanisms at the microscopic level has yet to prove the lubrication mechanism of the hydration shell. Klein used SFB to capture hydrated ions on a smooth mica surface at the molecular level and found that the change in friction with sliding speed depends on the surface separation, which is affected by the applied load. Under low load, the energy dissipation of the hydration shell is mainly affected by the viscosity of hydrated ions [70]. At macro-scale conditions, it is challenging to achieve superlubricity of hydrated ions, especially under high applied pressure. Han used UMT to study hydrated alkali metal ions (Li+, Na+, and K+) as lubricants for Si3N4 spheres and sapphire disks and reported that the friction coefficient was around 0.005 under a high pressure of 0.25 GPa [55]. It was also found that the lubrication properties of the hydrated alkali metal ions of the same anion were related to their ability to bind water molecules, and the lubrication properties of the hydrated ions with the same cation were not significantly affected by anions.
Hydration shells, strongly held by the charges they surround, can sustain large pressures without being squeezed out, while by rapidly relaxing they behave like a fluid during shear, which can further modify surface interactions [132]. Kuznetsov et al. [133] studied the ion adsorption on modified electrodes using a colloidal probe to measure the force directly. The electrode surface was modified with a self-assembled monolayer (SAM), and the interactions between SAM and SiO2 tip in HCl, KOH, and KCl solutions under different electric potentials were measured using EC-AFM. When the constant potential was applied, it was found that the SAM surface tightly adsorbed a layer of ions, and the applied electric potential affected the ion distribution in the diffusion layer, indicating that the adsorption of ions on the substrate surface is a charging process. In addition, ion adsorption on a SAM surface is much more pronounced on hydrophobic substrates than on hydrophilic ones because the ion adsorption is dominated by hydroxyl ions rather than hydronium ions [133]. The effect of electric potential on the interaction between hydrated ions and the Au (111) surface was quantitatively studied by EC-AFM (Figure 10a). Under positive potential, the interface will form an ice-like water layer with strong adhesion force, which increases the nonlinear high friction force; while under negative potential, there is a free water layer with minimal adhesion on the gold surface, resulting in a linear ultra-low friction force at the interface. This provides the possibility for a gold/SiO2 interface to achieve the reversible conversion of ultra-low and high friction [36]. The friction force between SiO2 and a graphite surface has an obvious upward trend with the increase in electric potential (from −0.8 V to 0.8 V), which is because the electric potential affects the distribution of hydrated ions on the substrate surface in PBS solution (Figure 10b) [37].
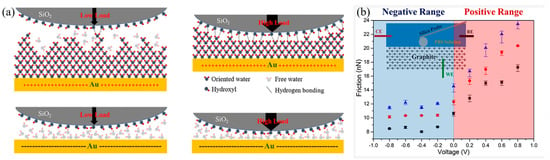
Figure 10.
Electric potential controlled adsorption and lubrication of hydrated ions: (a) The effect of potential and load on hydrogen bonding of hydrated ions at the contact interface [36]. Copyright 2020, the Authors; (b) the relationship between the friction force and the electric potential between SiO2 and HOPG in PBS solution [37]. Squares, circles, and triangles represent the variation of friction with voltage under the load of 1891, 2522, and 3152 nN respectively. Copyright 2020 American Chemical Society.
Hydrated ions adsorbed on charged surfaces can form a hydration layer as the lubrication film, leading to superlubricity even under high pressures above 200 MPa, as measured by UMT experiments [3,50,107]. Moreover, SFA and AFM studies show that the ion surface coverage dictates the roughness of the hydration layer and its lubricating properties, especially under subnanometer confinement [69]. Based on these findings, can we regulate the surface charge density of solid surfaces by applying an electric field, and then potentially regulate the ion adsorption density on charged surfaces? If possible, we may propose an effective method to adjust the interfacial roughness of the hydrated layer so as to control the hydration lubrication behavior. In addition, for metal and semiconductor materials with relatively low surface charge density, it is difficult to adsorb enough hydrated ions on their surfaces to achieve hydration lubrication. It should be noted that this relates to the electric double-layer model used and the inner Helmholtz layer capacitance needs to be further studied. To increase the surface charge density and then enhance the ion adsorption density by applying an electric field to the friction surfaces, is it possible to achieve superlubricity for surfaces that are currently unable to achieve hydration superlubricity? In addition, we also found that most research on electric potential controlled lubrication is based on EC-AFM; therefore, how to achieve electric potential controlled lubrication at macro-scale conditions is worth further exploring.
3.3. Surfactants
Surfactants with both lipophilic and hydrophilic groups can reduce the surface tension of the solution and can be oriented on the interface of the solution. Usually, the surfactant has two different groups with different properties: one end is a long-chain non-polar group that can dissolve in oil but not water and the other end is a hydrophilic group so that the entire surfactant can dissolve in water. Generally, hydrophilic groups are classified according to their types, which are mainly ionic and non-ionic. Surfactants have been widely applied in the fields of solubilizers, emulsifiers, lubricants, and detergents due to their hydrophilic and lipophilic properties. The critical micelle concentration (CMC) is the lowest concentration of micelles formed by surfactants in water. The hydrophilic and oleophilic groups of surfactant micelles can be stably dispersed in water, which makes them widely applied in the field of nanoscale lubrication [134]. The lubrication performance of surfactants is related to their structure. Fluorinated surfactants increase the stiffness of micelles due to the large hydrophobicity of their carbon chains, which can bear a larger load than hydrogen-containing surfactants [135].
Sodium dodecyl sulfate (SDS) and cetyltrimethyl ammonium bromide (C16TAB) are two common ionic surfactants, which can be adsorbed on solid surfaces (like steel and gold) to form a self-assembled layer with low shear strength. Owing to their excellent chemical stability and electrical conductivity, they have attracted much attention in the anti-friction and anti-wear fields [136,137]. The adsorption characteristics of SDS on a stainless steel surface can be affected by the electric potential and the concentration of NaClO4 solution. It is found that the open-circuit potential (OCP) on the stainless steel surface gradually shifts to the negative direction with the increase in the concentration of SDS (Figure 11a) [138]. An electrochemical workstation, quartz crystal balance (QCM), AFM, or lateral force microscope (LFM) were used to analyze the adsorption behavior of SDS aqueous solution on the stainless steel surface under different electric potentials. It was found that the adsorption mass of SDS on the stainless steel surface decreased with the decrease in electric potential and decreased the fastest in the range of −0.2~−0.4 V but the CoF increased with the potential decreasing [136]; therefore, the electric potential changes the thickness of the boundary film by affecting the adsorption of SDS on the stainless steel surface, thereby controlling the CoF [139]. SDS can be dissolved in both water-based solutions and non-aqueous solutions (like propylene carbonate). The CoF of SDS in a water-based solution is less than that in a non-aqueous solution at positive electric potential, while quite the reverse at negative potential [140]. For the C16TAB solution lubricating the silica surface, there exists a critical load, which makes the lubrication system reversible between low and high friction [56]. Gao et al. connected the C16TAB solution to the reference electrode (RE) and counter electrode (CE), and the gold surface was connected to the working electrode (WE) [138]; lower friction was found on the gold surface under negative potential, which is due to the potential-induced adsorption of C16TAB molecules on the gold surface forming a stable electric double-layer structure with very low shear strength and enhancing the load bearing capacity (Figure 11b) [141].
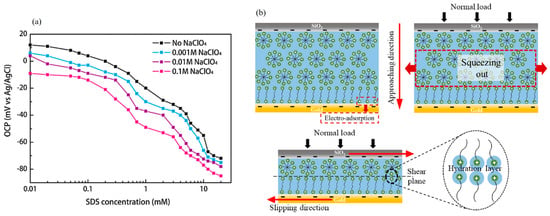
Figure 11.
Electric potential controlled adsorption of surfactants: (a) The relationship between the open circuit potential (OCP) and the solubility of SDS in NaClO4 solutions with different solubility [138]. Copyright 2015 Elsevier; (b) under the action of negative potential, C16TAB solution formed a stable double-layer structure with very low shear strength on the gold surface [141]. Copyright 2023 the Authors.
Derjaguin, Landau, Verwey, and Overbeek proposed the classical DLVO theory by referring to the superposition of the attractive Van der Waals force and the repulsive double-layer force, which quantitatively explained the interactions between the substrate surfaces in a liquid medium. Different experiments have proved that the theory was also reliable in the case of polyvalent ions. However, in electrolyte solutions with large concentrations, appropriate surface charge density, charge regulation characteristics, and ion pairing in solution must be considered to reduce the error of DLVO theory [66]. Ionic liquids, hydrated ions, and surfactants can all be used to realize active control of friction behavior in the field of electric potential controlled lubrication, and a comparison of their lubrication performance is shown in Table 2. A large number of studies have proven the effectiveness of the electric potential controlled lubrication method. Analyzing the electric potential control behavior of ionic lubricants and the influencing factors of lubrication is an effective way to establish the lubrication mechanism. EC-SFA/SFB, EC-AFM, and EC-UMT are the main research methods to study the electric potential controlled lubrication for various solid materials. Ionic liquid has lower conductivity than hydrated ions, and the lubrication ability of the alkyl chain is affected by the stretching motion state under the influence of electric potential; however, it has green environmental protection and stable electrochemical properties and can form a stable adsorption layer to provide lubrication under the action of electric potential.

Table 2.
Electric potential controlled lubrication for three ionic lubricants.
4. Conclusions and Perspectives
4.1. Conclusions
Electric potential controlled lubrication is a robust and readily implementable approach to the active control of friction and lubrication by applying an electric potential to control the lubricating properties of lubrication films. This approach has been demonstrated in SFA/SFB, AFM, and UMT experiments. EC-SFA/SFB and EC-AFM can be used to study the lubricating properties of nanofilms on molecularly smooth surfaces, such as mica, graphite, and Au/Pt coating on mica surfaces. The electric field changes the degree of electric polarization or surface charge density of the matrix material, controlling the adsorption behavior of ions in ionic lubricants on charged surfaces. The influence of an applied electric field on lubrication can be studied at higher velocity and larger contact pressure using EC-UMT for conducting or insulating materials like stainless steel, titanium alloy, etc. With the help of these setups, research methods of electric potential controlled lubrication have been established in micro- and macro-scale conditions, which has laid a foundation for the research of lubrication performance of ionic lubricants under applied electric potential.
The electric potential can change the adsorption/desorption behavior of ionic aqueous lubricants on the solid–liquid surface and different interfacial structures can be formed through the interaction between different types of solid surfaces and ionic aqueous lubricants. Micro- and macro-scale experiments have proved that an applied electric field can control the lubrication performance of ionic lubricants, which is mainly attributed to the physical adsorption of ions in solutions on the charged surface. The formation of a lubrication film with low shear resistance can reduce the sliding resistance in the contact area. In addition, different types of ionic lubricants are greatly affected by applied potential, which can not only physically adsorb on the matrix surface but also show chemical reactions with the matrix, where the reaction products may also significantly affect the friction behavior.
The friction energy dissipation of the system can be effectively controlled through electric potential controlled lubrication. Presently, the following problems have not been resolved and are worth further research, although good progress has been made during the past decade. For conductor materials, an applied electric potential can increase the surface charge density but what is the upper limit of this surface charge density? Then, what is the corresponding upper limit of the counterion adsorption density on the charged surface? How does the applied electric potential affect the electric double-layer, especially the ion distribution characteristics in the Stern layer and the diffuse layer (Figure 1a)? These fundamental questions attract experts in the fields of tribology, physical chemistry, mechanics, and electrical engineering to persistently pursue exciting research goals of electric potential controlled lubrication.
4.2. Perspectives
According to the present research, we propose feasible thoughts to solve existing problems. First, the vast majority of construction machinery contains rotating components, including steam turbines, gas turbines, centrifugal compressors, generators, pumps, water turbines, fans, motors, etc. The rotating speed of these components ranges from 100 to 100,000 rpm, and the working process is accompanied by non-negligible friction and wear, which strongly affects the performance and service life of the equipment. However, most electric potential controlled lubrication experiments were carried out in the form of reciprocating motion. It needs further verification whether the reciprocating experimental findings can be applied to rotary mechanical equipment or not. Therefore, exploring how to simulate the electric potential controlled lubrication experiment with rotating motion at different speeds is the key to improving the performance of rotating machinery. The conductive slip ring may be used to simulate the rotating motion through the relative movement of the inner ring and the outer ring, with the conductive brush in the middle; however, the conductive slip ring may be only applicable to EC-UMT and still has great limitations for EC-SFA and EC-AFM, while in a large number of studies of electrical potential controlled lubrication, SFA/SFB and AFM are used to investigate the lubrication performance of nanolubricating film under the electric potential. The friction phenomenon of rotating machinery in actual production is relatively macroscopic, so improving the EC-UMT setup is an effective way to promote the research of electric potential controlled lubrication with rotating motion.
Second, the electric potential controlled lubrication process during sliding includes two parts, the physical adsorption/desorption process and the chemical reaction, which will change the shear plane and affect the sliding friction. Based on the effective collision model of chemical reactions, Liu et al. proposed a chemical potential equation (including both electrical and mechanical contributions) to describe the triboelectrochemical phenomena in experiments [38]. This triboelectrochemistry model emphasizes the difference between the chemical reactions in the frictional contact zone and the non-contact zone, and can intuitively explain the products of the triboelectrochemical reactions [38,105]. Quite a few studies have confirmed that lubrication can be regulated by changing the voltage within the electrochemical window, and the regulation of the lubrication performance can be reversed by the electric field [38,49,82,103]. If a chemical reaction occurs between the friction surfaces and ionic lubricants, can the reversible regulation of lubrication still be achieved? How to effectively use the electrochemical reaction during sliding to control lubrication is worthy of further consideration. The difficulty lies in optimizing the electric potential controlled setups, designing experimental conditions, and distinguishing the effects of physical adsorption and chemical reaction during electric potential controlled lubrication.
Third, conductive solid surfaces are necessary to apply electric potential and thus adsorb counterions and other molecules with opposite charges. Compared with ionic lubricants, hydrogels have high water content and also show good lubrication performance. By introducing nanoparticles with high conductivity into hydrogels, conductive hydrogels with high conductivity and excellent mechanical properties can be synthesized [142]. By connecting them to an electric field, the micro-network structure of hydrogels together with internal conductive nanoparticles can be controlled, and the macro-lubrication ability of hydrogels can be adjusted. PAAm@κ-Carrageenan hydrogel was prepared using polyacrylamide (PAAm) and carrageenan through the ion migration generated by an electrochemical reaction. Compared with PAAm hydrogel, PAAM@κ-Carrageenan hydrogel shows enhanced mechanical properties by seven times, better self-healing ability, and higher adhesion performance [143]. By using NaAMPS to negatively modify polymer chains in PAAm hydrogels, negative hydrogels with a high evaporation rate and salt tolerance can be synthesized, which provides a new idea for seawater desalination, new energy generation, and potential controlled lubrication [108]. Oil-based lubricants have poor electrical conductivity, which limits their application in the field of electric potential controlled lubrication. By adding 2,5-Dimercapto-1,3,4-thiadiazole derivative (DMTD) and zinc dithiophosphate (ZDDP) to base oil (PAO), applied electric voltage can promote the formation of MoOx friction film on the steel surface to reduce friction and wear [41].
Fourth, the experimental phenomena of electric potential controlled lubrication need to be promoted in engineering applications. For example, metal friction pairs are prone to corrosion and corrosive wear under water lubrication conditions, and it is more difficult to achieve superlubricity for metals. By controlling the oxidation reaction of metal materials with an applied electric field, the corrosion and wear phenomenon can be reduced or eliminated, which also provides a methodology for realizing the superlubricity of metal friction pairs. In the fields of MEMs and NEMs, electro-switchable devices, and triboelectric generators [64], electrical potential controlled lubrication methods can realize real-time control of the friction coefficient [71] and achieve intelligent lubrication. Moreover, ultra-low friction can also be expected by applying a negative electric potential to sliding surfaces, which provides a new idea for achieving superlubricity [144,145]. Combining potential controlled lubrication with superlubricity and two-dimensional materials is a promising research field [146,147,148,149,150,151].
Last but not least, as a powerful tool for studying the surface and interface interactions, molecular dynamics simulation can provide new ideas for the design of lubrication control and the analysis of ion distribution in the hydration layer; however, how to simulate the simultaneous process of physical adsorption and triboelectrochemical reaction remains a big challenge.
Author Contributions
All authors contributed to writing, reviewing, and editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work is financially supported by the National Natural Science Foundation of China (51925506 and 52305198), the XPLORER PRIZE, and the China Postdoctoral Science Foundation (2023M731936).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Holmberg, K.; Erdemir, A. Influence of tribology on global energy consumption, costs and emissions. Friction 2017, 5, 263–284. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, X. Superlubricitive engineering—Future industry nearly getting rid of wear and frictional energy consumption. Friction 2020, 8, 643–665. [Google Scholar] [CrossRef]
- Han, T.; Zhang, S.; Zhang, C. Unlocking the secrets behind liquid superlubricity: A state-of-the-art review on phenomena and mechanisms. Friction 2022, 10, 1137–1165. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Z.; Chen, D.; Xie, Z.; Song, J.; Ruffino, F. Influence of Different Surface Texture Parameters on the Contact Performance of Piston Ring-Sleeve Friction Pair of Hydraulic Cylinders. Adv. Mater. Sci. Eng. 2021, 2021, 5495995. [Google Scholar] [CrossRef]
- Yang, J.; Fu, H.; He, Y.; Gu, Z.; Fu, Y.; Ji, J.; Zhang, Y.; Zhou, Y. Investigation on friction and wear performance of volcano-shaped textured PVD coating. Surf. Coat. Technol. 2022, 431, 128044. [Google Scholar] [CrossRef]
- Feng, S.; Liu, Y.; Li, J.; Wen, S. Superlubricity Achieved with Zwitterionic Brushes in Diverse Conditions Induced by Shear Actions. Macromolecules 2021, 54, 5719–5727. [Google Scholar] [CrossRef]
- Cai, T.; Liu, D.; Liu, S. Fluid-like graphene oxide organic hybrid materials as efficient anti-wear and friction-reducing additive of polyethylene glycol. Tribol. Int. 2021, 159, 106880. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Bai, P.; Jia, W.; Xu, Q.; Meng, Y.; Ma, L.; Tian, Y. Surface wettability effect on aqueous lubrication: Van der Waals and hydration force competition induced adhesive friction. J. Colloid Interface Sci. 2021, 599, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Barril, S.; Mischler, S.; Landolt, D. Triboelectrochemical investigation of the friction and wear behaviour of TiN coatings in a neutral solution. Tribol. Int. 2001, 34, 599–608. [Google Scholar] [CrossRef]
- Ren, X.; Zou, H.; Diao, Q.; Wang, C.; Wang, Y.; Li, H.; Sui, T.; Lin, B.; Yan, S. Surface modification technologies for enhancing the tribological properties of cemented carbides: A review. Tribol. Int. 2023, 180, 108257. [Google Scholar] [CrossRef]
- López-Cervantes, A.; Domínguez-López, I.; Barceinas-Sánchez, J.D.O.; García-García, A.L. Effects of surface texturing on the performance of biocompatible UHMWPE as a bearing material during in vitro lubricated sliding/rolling motion. J. Mech. Behav. Biomed. Mater. 2013, 20, 45–53. [Google Scholar] [CrossRef]
- Zhang, T.; Lu, Q.; Zhang, X.; Liu, S.; Ye, Q.; Zhou, F. Fabrication of polyelectrolyte brush-functionalized two-dimensional covalent organic frameworks as additives for aqueous lubricants. Tribol. Int. 2022, 174, 107737. [Google Scholar] [CrossRef]
- Wang, R.; Li, H.Q.; Li, R.S.; Mei, H.J.; Zou, C.W.; Zhang, T.F.; Wang, Q.M.; Kim, K.H. Thermostability, oxidation, and high-temperature tribological properties of nano-multilayered AlCrSiN/VN coatings. Ceram. Int. 2022, 48, 11915–11923. [Google Scholar] [CrossRef]
- Ge, X.; Li, J.; Wang, H.; Zhang, C.; Liu, Y.; Luo, J. Macroscale superlubricity under extreme pressure enabled by the combination of graphene-oxide nanosheets with ionic liquid. Carbon 2019, 151, 76–83. [Google Scholar] [CrossRef]
- Ma, L.; Luo, J.; Zhang, C.; Ma, Z.; Wang, Y.; Dai, Y. Film formation of yogurt under confined condition. Surf. Interface Anal. 2012, 44, 258–262. [Google Scholar] [CrossRef]
- Tadokoro, C.; Nihira, T.; Nakano, K. Minimization of friction at various speeds using autonomous viscosity control of nematic liquid crystal. Tribol. Lett. 2014, 56, 239–247. [Google Scholar] [CrossRef]
- Bresme, F.; Kornyshev, A.A.; Perkin, S.; Urbakh, M. Electrotunable friction with ionic liquid lubricants. Nat. Mater. 2022, 21, 848–858. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Li, X.; Li, W.; Tian, Y.; Meng, Y. On-Line Feedback Control of Sliding Friction of Metals Lubricated by Adsorbed Boundary SDS Films. Lubricants 2022, 10, 148. [Google Scholar] [CrossRef]
- Sun, K.; Hu, Y.; Dong, Y.; Yao, L.; Song, R.; Xu, Y. Tribological behavior of thermal- and pH-sensitive microgels under steel/CoCrMo alloy contacts. Friction 2022, 11, 602–616. [Google Scholar] [CrossRef]
- Zou, H.; Lin, B.; Zhang, X.; Wang, Z.; Yan, S. Observation of anomalous low friction and wear behavior of polyimide in alkaline environment under high load. Wear 2024, 546–547, 205352. [Google Scholar] [CrossRef]
- Chen, S.; Sun, H.; Liu, J.; Wang, J.; Lu, H.; Hao, J.; Xu, L.; Liu, W. A dual-responsive microemulsion with macroscale superlubricity and largely switchable friction. Mater. Horiz. 2024, 11, 1668–1678. [Google Scholar] [CrossRef]
- Tang, S.; Li, S.; Ma, L.; Tian, Y. Photorheological fluids of azobenzene polymers for lubrication regulation. Friction 2021, 10, 1078–1090. [Google Scholar] [CrossRef]
- Chen, Y.; Li, D.; Zhang, Y.; Li, Z.; Zhou, H. The Influence of the Temperature Rise on the Sealing Performance of the Rotating Magnetic Fluid Seal. IEEE Trans. Magn. 2020, 56, 1–10. [Google Scholar] [CrossRef]
- Chen, Y.; Li, D.; Zhang, Y.; He, C. Numerical Analysis and Experimental Study on Magnetic Fluid Reciprocating Seals. IEEE Trans. Magn. 2019, 55, 1–6. [Google Scholar] [CrossRef]
- Lin, B.; Ding, M.; Sui, T.; Cui, Y.; Yan, S.; Liu, X. Excellent Water Lubrication Additives for Silicon Nitride To Achieve Superlubricity under Extreme Conditions. Langmuir 2019, 35, 14861–14869. [Google Scholar] [CrossRef]
- Yan, S.; Lin, B.; Zhang, X.; Wang, A.; Zhou, X. Investigation of the running-in process of silicon nitride sliding in aqueous solutions of ethylene glycol. Tribol. Int. 2015, 90, 386–392. [Google Scholar] [CrossRef]
- Liu, M.; Liang, H.; Chen, X.; Yin, T.; Bu, Y. Asymmetric Contact Synergy of Unequal-Sized Soft and Hard Clusters in Highly Concentrated ZnCl2 for Heterogeneous Superlubricants. Tribol. Lett. 2024, 72, 41. [Google Scholar] [CrossRef]
- Spikes, H.A. Triboelectrochemistry: Influence of Applied Electrical Potentials on Friction and Wear of Lubricated Contacts. Tribol. Lett. 2020, 68, 90. [Google Scholar] [CrossRef]
- Trewby, W.; Tavakol, M.; Jaques, Y.M.; Voïtchovsky, K. Towards local tracking of solvated metal ions at solid-liquid interfaces. Mater. Today Phys. 2024, 44, 101441. [Google Scholar] [CrossRef]
- Lyklema, J. Fundamentals of Interface and Colloid Science; Academic Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Gonella, G.; Backus, E.H.G.; Nagata, Y.; Bonthuis, D.J.; Loche, P.; Schlaich, A.; Netz, R.R.; Kühnle, A.; McCrum, I.T.; Koper, M.T.M.; et al. Water at charged interfaces. Nat. Rev. Chem. 2021, 5, 466–485. [Google Scholar] [CrossRef] [PubMed]
- Helmholtz, H. Studien über electrische Grenzschichten. Ann. Phys. 1879, 243, 337–382. [Google Scholar] [CrossRef]
- Hjalmarsson, N.; Atkin, R.; Rutland, M.W. Switchable long-range double layer force observed in a protic ionic liquid. Chem. Commun. 2017, 53, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Wu, X.; Shi, Q.; Song, S.; Liu, Y.; Wang, W. Study on the Superlubricity Behavior of Ions under External Electric Fields at Steel Interfaces. Langmuir 2023, 39, 18757–18767. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Zhang, C.; Chen, X.; Li, J.; Wang, W.; Luo, J. Contribution of a Tribo-Induced Silica Layer to Macroscale Superlubricity of Hydrated Ions. J. Phys. Chem. C 2019, 123, 20270–20277. [Google Scholar] [CrossRef]
- Li, S.; Bai, P.; Li, Y.; Pesika, N.S.; Meng, Y.; Ma, L.; Tian, Y. Quantification/mechanism of interfacial interaction modulated by electric potential in aqueous salt solution. Friction 2020, 9, 513–523. [Google Scholar] [CrossRef]
- Gao, T.; Li, J.; Yi, S.; Luo, J. Potential-Dependent Friction on a Graphitic Surface in Ionic Solution. J. Phys. Chem. C 2020, 124, 23745–23751. [Google Scholar] [CrossRef]
- Fujii, S.; Kasuya, M.; Kurihara, K. Characterization of Platinum Electrode Surfaces by Electrochemical Surface Forces Measurement. J. Phys. Chem. C 2017, 121, 26406–26413. [Google Scholar] [CrossRef]
- Liu, C.; Friedman, O.; Li, Y.; Li, S.; Tian, Y.; Golan, Y.; Meng, Y. Electric Response of CuS Nanoparticle Lubricant Additives: The Effect of Crystalline and Amorphous Octadecylamine Surfactant Capping Layers. Langmuir 2019, 35, 15825–15833. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Meng, Y.; Tian, Y. Potential controlled Boundary Lubrication Using MoS2 Additives in Diethyl Succinate. Tribol. Lett. 2020, 68, 72. [Google Scholar] [CrossRef]
- Liu, C.; Li, W.; Ouyang, C.; Tian, Y.; Meng, Y. Voltage-Assisted Tribofilm Formation of Sulfur- and Phosphorus-Free Organic Molybdenum Additive on Bearing Steel Surfaces in Industrial Base Oils. Tribol. Lett. 2022, 70, 19. [Google Scholar] [CrossRef]
- Liu, C.; Tian, Y.; Meng, Y. A Chemical Potential Equation for Modeling Triboelectrochemical Reactions on Solid–Liquid Interfaces. Front. Chem. 2021, 9, 650880. [Google Scholar] [CrossRef]
- Luo, M.; Koper, M.T.M. A kinetic descriptor for the electrolyte effect on the oxygen reduction kinetics on Pt(111). Nat. Catal. 2022, 5, 615–623. [Google Scholar] [CrossRef]
- Zhou, S.; Panse, K.S.; Motevaselian, M.H.; Aluru, N.R.; Zhang, Y. Three-Dimensional Molecular Mapping of Ionic Liquids at Electrified Interfaces. ACS Nano 2020, 14, 17515–17523. [Google Scholar] [CrossRef]
- Liang, H.; Liu, M.; Yin, T.; Zou, S.; Xia, X.; Hua, X.; Fu, Y.; Zhang, J.; Bu, Y.; Ren, X. Carbon Dots as Ligand Operons to Expand Cluster Size Distribution for High Load-bearing Liquid Superlubricity. Adv. Funct. Mater. 2024, 34, 2311600. [Google Scholar] [CrossRef]
- Du, C.; Yu, T.; Wu, Z.; Zhang, L.; Shen, R.; Li, X.; Feng, M.; Feng, Y.; Wang, D. Achieving macroscale superlubricity with ultra-short running-in period by using polyethylene glycol-tannic acid complex green lubricant. Friction 2023, 11, 748–762. [Google Scholar] [CrossRef]
- Lin, W.; Kampf, N.; Klein, J. Designer Nanoparticles as Robust Superlubrication Vectors. ACS Nano 2020, 14, 7008–7017. [Google Scholar] [CrossRef] [PubMed]
- Rosenhek-Goldian, I.; Kampf, N.; Klein, J. Trapped Aqueous Films Lubricate Highly Hydrophobic Surfaces. ACS Nano 2018, 12, 10075–10083. [Google Scholar] [CrossRef]
- Kasuya, M.; Sogawa, T.; Masuda, T.; Kamijo, T.; Uosaki, K.; Kurihara, K. Anion Adsorption on Gold Electrodes Studied by Electrochemical Surface Forces Measurement. J. Phys. Chem. C 2016, 120, 15986–15992. [Google Scholar] [CrossRef]
- Long, Y.; Galipaud, J.; Weihnacht, V.; Makowski, S.; Martin, J.M.; De Barros Bouchet, M.-I. Achieving superlubricity using selected tribo-pairs lubricated by castor oil and unsaturated fatty acids. Tribol. Int. 2022, 169, 107462. [Google Scholar] [CrossRef]
- Fu, X.; Cao, L.; Wan, Y.; Li, R. Superlubricity achieved with TiN coatings via the in situ formation of a carbon-based film at the sliding interfaces. Ceram. Int. 2021, 47, 33917–33921. [Google Scholar] [CrossRef]
- Zeng, Q.; Dong, G.; Martin, J.M. Green superlubricity of Nitinol 60 alloy against steel in presence of castor oil. Sci. Rep. 2016, 6, 29992. [Google Scholar] [CrossRef] [PubMed]
- Tivony, R.; Klein, J. Probing the Surface Properties of Gold at Low Electrolyte Concentration. Langmuir 2016, 32, 7346–7355. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fang, J.; Wen, X.; Tian, Y.; Meng, Y. Active Control of Boundary Lubrication of Ceramic Tribo-Pairs in Sodium Dodecyl Sulfate Aqueous Solutions. Tribol. Lett. 2021, 69, 144. [Google Scholar] [CrossRef]
- Han, T.; Zhang, C.; Luo, J. Macroscale Superlubricity Enabled by Hydrated Alkali Metal Ions. Langmuir 2018, 34, 11281–11291. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, C.; Cheng, P.; Chen, X.; Wang, W.; Luo, J. AFM Studies on Liquid Superlubricity between Silica Surfaces Achieved with Surfactant Micelles. Langmuir 2016, 32, 5593–5599. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Shi, F.; Liu, Q.; Pang, Y.; He, D.; Sun, W.; Peng, L.; Yang, J.; Qu, M. Wearable superhydrophobic PPy/MXene pressure sensor based on cotton fabric with superior sensitivity for human detection and information transmission. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128676. [Google Scholar] [CrossRef]
- Kuang, F.; Zhou, X.; Liu, Z.; Huang, J.; Liu, X.; Qian, K.; Gryllias, K. Computer-visionic research on friction vibration and coupling of frictional and torsional vibrations in water-lubricated bearing-shaft system. Tribol. Int. 2020, 150, 106336. [Google Scholar] [CrossRef]
- Zhang, Z.; Ouyang, W.; Liang, X.; Yan, X.; Yuan, C.; Zhou, X.; Guo, Z.; Dong, C.; Liu, Z.; Jin, Y.; et al. Review of the evolution and prevention of friction, wear, and noise for water-lubricated bearings used in ships. Friction 2023, 12, 1–38. [Google Scholar] [CrossRef]
- Mattei, L.; Di Puccio, F.; Piccigallo, B.; Ciulli, E. Lubrication and wear modelling of artificial hip joints: A review. Tribol. Int. 2011, 44, 532–549. [Google Scholar] [CrossRef]
- Israelachvili, J.; Min, Y.; Akbulut, M.; Alig, A.; Carver, G.; Greene, W.; Kristiansen, K.; Meyer, E.; Pesika, N.; Rosenberg, K. Recent advances in the surface forces apparatus (SFA) technique. Rep. Prog. Phys. 2010, 73, 036601. [Google Scholar] [CrossRef]
- Hayler, H.; Groves, T.S.; Guerrini, A.; Southam, A.; Zheng, W.; Perkin, S. The Surface Force Balance: Direct Measurement of Interactions in Fluids and Soft Matter. Rep. Prog. Phys. 2024, 87, 046601. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Tan, Q.; Xiang, L.; Cai, D.; Zeng, H.; Yi, H.; Ni, Z.; Chen, Y. Structure and properties of water film adsorbed on mica surfaces. J. Chem. Phys. 2015, 143, 104705. [Google Scholar] [CrossRef] [PubMed]
- Raviv, U.; Laurat, P.; Klein, J. Fluidity of water confined to subnanometre films. Nature 2001, 413, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Raviv, U.; Klein, J. Fluidity of Bound Hydration Layers. Science 2002, 297, 1540–1543. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Borkovec, M.; Trefalt, G. Forces between solid surfaces in aqueous electrolyte solutions. Adv. Colloid Interface Sci. 2020, 275, 102078. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Chun, B.-W.; Gu, L.; Kuhl, T.L. Effect of Ca2+ ion concentration on adsorption of poly(carboxylate ether)-based (PCE) superplasticizer on mica. J. Colloid Interface Sci. 2018, 527, 195–201. [Google Scholar] [CrossRef]
- Gaisinskayakipnis, A.; Ma, L.; Kampf, N.; Klein, J. Frictional Dissipation Pathways Mediated by Hydrated Alkali Metal Ions. Langmuir 2016, 32, 4755–4764. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Cao, W.; Xu, Z.; Adibnia, V.; Olgiati, M.; Valtiner, M.; Ma, L.; Zhang, C.; Ma, M.; Luo, J.; et al. Hydration layer structure modulates superlubrication by trivalent La3+ electrolytes. Sci. Adv. 2023, 9, eadf3902. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Gaisinskaya-Kipnis, A.; Kampf, N.; Klein, J. Origins of hydration lubrication. Nat. Commun. 2015, 6, 6060. [Google Scholar] [CrossRef]
- Cao, Y.; Kampf, N.; Lin, W.; Klein, J. Normal and shear forces between boundary sphingomyelin layers under aqueous conditions. Soft Matter 2020, 16, 3973–3980. [Google Scholar] [CrossRef]
- Chen, M.; Briscoe, W.H.; Armes, S.P.; Klein, J. Lubrication at physiological pressures by polyzwitterionic brushes. Science 2009, 323, 1698–1701. [Google Scholar] [CrossRef]
- Chai, L.; Klein, J. Shear Behavior of Adsorbed Poly(ethylene Oxide) Layers in Aqueous Media. Macromolecules 2008, 41, 1831–1838. [Google Scholar] [CrossRef]
- Yu, J.; Jackson, N.; Xu, X.; Morgenstern, Y.; Kaufman, Y.; Ruths, M.; de Pablo, J.; Tirrell, M. Multivalent counterions diminish the lubricity of polyelectrolyte brushes. Science 2018, 360, 1434–1438. [Google Scholar] [CrossRef] [PubMed]
- Banquy, X.; Burdyńska, J.; Dong, W.L.; Matyjaszewski, K.; Israelachvili, J. Bioinspired Bottle-Brush Polymer Exhibits Low Friction and Amontons-like Behavior. J. Am. Chem. Soc. 2014, 136, 6199–6202. [Google Scholar] [CrossRef] [PubMed]
- Banquy, X.; Lee, D.W.; Das, S.; Hogan, J.; Israelachvili, J.N. Shear-Induced Aggregation of Mammalian Synovial Fluid Components under Boundary Lubrication Conditions. Adv. Funct. Mater. 2014, 24, 3152–3161. [Google Scholar] [CrossRef]
- Kang, T.; Banquy, X.; Heo, J.; Lim, C.; Lynd, N.A.; Lundberg, P.; Oh, D.X.; Lee, H.-K.; Hong, Y.-K.; Hwang, D.S.; et al. Mussel-Inspired Anchoring of Polymer Loops That Provide Superior Surface Lubrication and Antifouling Properties. ACS Nano 2016, 10, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Faivre, J.; Shrestha, B.R.; Burdynska, J.; Xie, G.; Moldovan, F.; Delair, T.; Benayoun, S.; David, L.; Matyjaszewski, K.; Banquy, X. Wear Protection without Surface Modification Using a Synergistic Mixture of Molecular Brushes and Linear Polymers. ACS Nano 2017, 11, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Adibnia, V.; Olszewski, M.; De Crescenzo, G.; Matyjaszewski, K.; Banquy, X. Superlubricity of Zwitterionic Bottlebrush Polymers in the Presence of Multivalent Ions. J. Am. Chem. Soc. 2020, 142, 14843–14847. [Google Scholar] [CrossRef] [PubMed]
- Reddyhoff, T.; Ewen, J.P.; Deshpande, P.; Frogley, M.D.; Welch, M.D.; Montgomery, W. Macroscale Superlubricity and Polymorphism of Long-Chain n-Alcohols. ACS Appl. Mater. Interfaces 2021, 13, 9239–9251. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Luo, J. Superlubricity behavior with phosphoric acid-water network induced by rubbing. Langmuir 2011, 27, 9413–9417. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, Y.; Shen, J.; Cai, Z.; Zhao, C.; Chen, H.; Luo, X.; Hu, N.; Cui, W.; Huang, W. Injectable hydrogel microspheres with self-renewable hydration layers alleviate osteoarthritis. Sci. Adv. 2022, 8, eabl6449. [Google Scholar] [CrossRef] [PubMed]
- Valtiner, M.; Banquy, X.; Kristiansen, K.; Greene, G.W.; Israelachvili, J.N. The electrochemical surface forces apparatus: The effect of surface roughness, electrostatic surface potentials, and anodic oxide growth on interaction forces, and friction between dissimilar surfaces in aqueous solutions. Langmuir 2012, 28, 13080. [Google Scholar] [CrossRef] [PubMed]
- Tivony, R.; Klein, J. Modifying surface forces through control of surface potentials. Faraday Discuss. 2017, 199, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Lecadre, F.; Kasuya, M.; Harano, A.; Kanno, Y.; Kurihara, K. Low-Temperature Surface Forces Apparatus to Determine the Interactions between Ice and Silica Surfaces. Langmuir 2018, 34, 11311–11315. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, O.Y.; Bresme, F.; Kornyshev, A.A.; Urbakh, M. Electrotunable Friction with Ionic Liquid Lubricants: How Important Is the Molecular Structure of the Ions? J. Phys. Chem. Lett. 2015, 6, 3998–4004. [Google Scholar] [CrossRef]
- Fréchette, J.; Vanderlick, T.K. Electrocapillary at Contact: Potential-Dependent Adhesion between a Gold Electrode and a Mica Surface. Langmuir 2005, 21, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cheng, J.; Delparastan, P.; Wang, H.; Sigg, S.J.; DeFrates, K.G.; Cao, Y.; Messersmith, P.B. Molecular design principles of Lysine-DOPA wet adhesion. Nat. Commun. 2020, 11, 3895. [Google Scholar] [CrossRef] [PubMed]
- Fukuma, T.; Garcia, R. Atomic- and Molecular-Resolution Mapping of Solid–Liquid Interfaces by 3D Atomic Force Microscopy. ACS Nano 2018, 12, 11785–11797. [Google Scholar] [CrossRef] [PubMed]
- Butt, H.J. Electrostatic interaction in atomic force microscopy. Biophys. J. 1991, 60, 777–785. [Google Scholar] [CrossRef]
- Ducker, W.A.; Senden, T.J.; Pashley, R.M. Direct measurement of colloidal forces using an atomic force microscope. Nature 1991, 353, 239–241. [Google Scholar] [CrossRef]
- Butt, H.J. Measuring electrostatic, van der Waals, and hydration forces in electrolyte solutions with an atomic force microscope. Biophys. J. 1991, 60, 1438. [Google Scholar] [CrossRef] [PubMed]
- Ducker, W.A.; Senden, T.J.; Pashley, R.M. Measurement of forces in liquids using a force microscope. Langmuir 1992, 8, 1831–1836. [Google Scholar] [CrossRef]
- Kohonen, M.M.; Karaman, M.E.; Pashley, R.M. Debye Length in Multivalent Electrolyte Solutions. Langmuir 2000, 16, 5749–5753. [Google Scholar] [CrossRef]
- Trulsson, M.; Jönsson, B.; Åkesson, T.; Forsman, J.; Labbez, C. Repulsion between Oppositely Charged Surfaces in Multivalent Electrolytes. Phys. Rev. Lett. 2006, 97, 068302. [Google Scholar] [CrossRef] [PubMed]
- Pasquina-Lemonche, L.; Burns, J.; Turner, R.D.; Kumar, S.; Tank, R.; Mullin, N.; Wilson, J.S.; Chakrabarti, B.; Bullough, P.A.; Foster, S.J.; et al. The architecture of the Gram-positive bacterial cell wall. Nature 2020, 582, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Q.; Li, Q.; Dong, M. The role of hydrated anions in hydration lubrication. Nano Res. 2023, 16, 1096–1100. [Google Scholar] [CrossRef]
- Dishon, M.; Zohar, O.; Sivan, U. Effect of cation size and charge on the interaction between silica surfaces in 1:1, 2:1, and 3:1 aqueous electrolytes. Langmuir 2011, 27, 12977–12984. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Q.; Zhang, D.; Wang, Y.; Zhang, Y.; Li, Q.; Dong, M. Probing the hydration friction of ionic interfaces at the atomic scale. Nanoscale Horiz. 2022, 7, 368–375. [Google Scholar] [CrossRef]
- Ma, P.; Liu, Y.; Sang, X.; Tan, J.; Ye, S.; Ma, L.; Tian, Y. Homogeneous interfacial water structure favors realizing a low-friction coefficient state. J. Colloid Interface Sci. 2022, 626, 324–333. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Jones, S.; Segalman, R.; Warr, G.G.; Atkin, R. Interfacial nanostructure and friction of a polymeric ionic liquid-ionic liquid mixture as a function of potential at Au(111) electrode interface. J. Colloid Interface Sci. 2022, 606, 1170–1178. [Google Scholar] [CrossRef]
- Lang, H.; Peng, Y.; Zou, K.; Huang, Y.; Song, C. Velocity-Dependent Friction of Graphene at Electrical Contact Interfaces. Langmuir 2023, 39, 11363–11370. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, W.; Fan, Y.; Song, Q.; Xiao, S. TiO2 metasurfaces: From visible planar photonics to photochemistry. Sci. Adv. 2019, 5, eaax0939. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Ma, P.; Ma, L.; Tian, Y.; Luo, J. Investigation of the running-in process in photoinduced superlubricity. Front. Mater. 2023, 10, 1109890. [Google Scholar] [CrossRef]
- Cao, H.; Meng, Y. Electrochemical effect on boundary lubrication of ZDDP additive blended in propylene carbonate/diethyl succinate. Tribol. Int. 2018, 126, 229–239. [Google Scholar] [CrossRef]
- Liu, C.; Friedman, O.; Meng, Y.; Tian, Y.; Golan, Y. CuS Nanoparticle Additives for Enhanced Ester Lubricant Performance. ACS Appl. Nano Mater. 2018, 1, 7060–7065. [Google Scholar] [CrossRef]
- Han, T.; Yi, S.; Zhang, C.; Li, J.; Chen, X.; Luo, J.; Banquy, X. Superlubrication Obtained with Mixtures of Hydrated Ions and Polyethylene Glycol Solutions in the Mixed and Hydrodynamic Lubrication Regimes. J. Colloid Interface Sci. 2020, 579, 479–488. [Google Scholar] [CrossRef]
- Wang, K.; Liu, L.; Song, A.; Ma, T.; Wang, H.; Luo, J.; Liu, Y. Macroscale superlubricity under ultrahigh contact pressure in the presence of layered double hydroxide nanosheets. Nano Res. 2022, 15, 4700–4709. [Google Scholar] [CrossRef]
- Ren, X.; Yang, X.; Xie, G.; He, F.; Wang, R.; Zhang, C.; Guo, D.; Luo, J. Superlubricity under ultrahigh contact pressure enabled by partially oxidized black phosphorus nanosheets. npj 2D Mater. Appl. 2021, 5, 44. [Google Scholar] [CrossRef]
- Han, T.; Zhang, C.; Li, J.; Yuan, S.; Chen, X.; Zhang, J.; Luo, J. Origins of Superlubricity Promoted by Hydrated Multivalent Ions. J. Phys. Chem. Lett. 2020, 11, 184–190. [Google Scholar] [CrossRef]
- Yuan, S.; Zhang, C. A sulfonated modification of PEEK for ultralow friction. Friction 2022, 11, 881–893. [Google Scholar] [CrossRef]
- Han, T.; Zhao, M.; Sun, C.; Zhao, R.; Xu, W.; Zhang, S.; Singh, S.; Luo, J.; Zhang, C. Macroscale Superlubricity of Hydrated Anions in the Boundary Lubrication Regime. ACS Appl. Mater. Interfaces 2023, 15, 42094–42103. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Han, T.; Zhang, C.; Luo, J. Superlubricating UHMWPE composites with functionalized carbon nanotubes reinforcement applicable to artificial joints. Tribol. Int. 2024, 191, 109142. [Google Scholar] [CrossRef]
- Wei, Q.; Cai, M.; Zhou, F.; Liu, W. Dramatically tuning friction using responsive polyelectrolyte brushes. Macromolecules 2013, 46, 9368–9379. [Google Scholar] [CrossRef]
- Yi, S.; Li, J.; Liu, Y.; Ge, X.; Zhang, J.; Luo, J. In-situ formation of tribofilm with Ti3C2Tx MXene nanoflakes triggers macroscale superlubricity. Tribol. Int. 2021, 154, 106695. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Ge, X.; Yi, S.; Wang, H.; Liu, Y.; Luo, J. Macroscale Superlubricity Achieved on the Hydrophobic Graphene Coating with Glycerol. ACS Appl. Mater. Interfaces 2020, 12, 18859–18869. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Halmans, T.; Li, J.; Luo, J. Molecular behaviors in thin film lubrication—Part three: Superlubricity attained by polar and nonpolar molecules. Friction 2019, 7, 625–636. [Google Scholar] [CrossRef]
- Liang, H.; Yin, T.; Liu, M.; Fu, C.; Xia, X.; Zou, S.; Hua, X.; Fu, Y.; Bu, Y. Unravelling High-Load Superlubricity of Ionic Liquid Analogues by In Situ Raman: Incomplete Hydration Induced by Competitive Exchange of External Water with Crystalline Water. J. Phys. Chem. Lett. 2023, 14, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, H.; Yan, H.; Huang, C.; Wu, L.; Wang, T. Superlubricity by polyimide-induced alignment. Friction 2023, 11, 1690–1707. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, X.; Huang, G.; Chen, H.; Yu, H.; Feng, D.; Qiao, D. Macroscale superlubricity achieved via hydroxylated hexagonal boron nitride nanosheets with ionic liquid at steel/steel interface. Friction 2022, 10, 1365–1381. [Google Scholar] [CrossRef]
- Zhou, Y.; Qu, J. Ionic Liquids as Lubricant Additives: A Review. ACS Appl. Mater. Interfaces 2017, 9, 3209–3222. [Google Scholar] [CrossRef]
- Zhou, F.; Liang, Y.; Liu, W. Ionic liquid lubricants: Designed chemistry for engineering applications. Chem. Soc. Rev. 2009, 38, 2590–2599. [Google Scholar] [CrossRef]
- Fedorov, M.V.; Kornyshev, A.A. Ionic Liquids at Electrified Interfaces. Chem. Rev. 2014, 114, 2978–3036. [Google Scholar] [CrossRef]
- Kondrat, S.; Feng, G.; Bresme, F.; Urbakh, M.; Kornyshev, A.A. Theory and Simulations of Ionic Liquids in Nanoconfinement. Chem. Rev. 2023, 123, 6668–6715. [Google Scholar] [CrossRef]
- Zheng, Q.; Chhattal, M.; Bai, C.; Zheng, Z.; Qiao, D.; Gong, Z.; Zhang, J. Superlubricity of PTFE triggered by green ionic liquids. Appl. Surf. Sci. 2023, 614, 156241. [Google Scholar] [CrossRef]
- Li, H.; Endres, F.; Atkin, R. Effect of alkyl chain length and anion species on the interfacial nanostructure of ionic liquids at the Au(111)–ionic liquid interface as a function of potential. Phys. Chem. Chem. Phys. 2013, 15, 14624–14633. [Google Scholar] [CrossRef]
- Li, H.; Wood, R.J.; Endres, F.; Atkin, R.J.J. Influence of alkyl chain length and anion species on ionic liquid structure at the graphite interface as a function of applied potential. J. Phys. Condens. Matter 2014, 26, 284115. [Google Scholar] [CrossRef]
- Li, H.; Wood, R.J.; Rutland, M.W.; Atkin, R. An ionic liquid lubricant enables superlubricity to be “switched on” in situ using an electrical potential. Chem. Commun. 2014, 50, 4368–4370. [Google Scholar] [CrossRef]
- Li, H.; Rutland, M.W.; Atkin, R. Ionic liquid lubrication: Influence of ion structure, surface potential and sliding velocity. Phys. Chem. Chem. Phys. 2013, 15, 14616–14623. [Google Scholar] [CrossRef]
- Li, H.; Niemann, T.; Ludwig, R.; Atkin, R. Effect of Hydrogen Bonding between Ions of Like Charge on the Boundary Layer Friction of Hydroxy-Functionalized Ionic Liquids. J. Phys. Chem. Lett. 2020, 11, 3905–3910. [Google Scholar] [CrossRef]
- Zhang, Y.; Marlow, J.B.; Millar, W.; Aman, Z.M.; Silvester, D.S.; Warr, G.G.; Atkin, R.; Li, H. Nanostructure, electrochemistry and potential-dependent lubricity of the catanionic surface-active ionic liquid [P6,6,6,14] [AOT]. J. Colloid Interface Sci. 2022, 608, 2120–2130. [Google Scholar] [CrossRef]
- Lin, W.; Klein, J. Control of surface forces through hydrated boundary layers. Curr. Opin. Colloid Interface Sci. 2019, 44, 94–106. [Google Scholar] [CrossRef]
- Kuznetsov, V.; Papastavrou, G. Ion Adsorption on Modified Electrodes as Determined by Direct Force Measurements under Potentiostatic Control. J. Phys. Chem. C 2014, 118, 2673–2685. [Google Scholar] [CrossRef]
- Li, J.; Luo, J. Normal and Frictional Force Hysteresis between Self-Assembled Fluorosurfactant Micelle Arrays at the Nanoscale. Adv. Mater. Interfaces 2018, 5, 1700802. [Google Scholar] [CrossRef]
- Li, J.; Dou, Z.-P.; Liu, Y.; Luo, J.; Xiao, J.-X. Improvement of Load Bearing Capacity of Nanoscale Superlow Friction by Synthesized Fluorinated Surfactant Micelles. ACS Appl. Nano Mater. 2018, 1, 953–959. [Google Scholar] [CrossRef]
- Li, J.; Luo, J. Superlow Friction of Graphite Induced by Self-Assembly of SDS Molecular Layers. Langmuir 2017, 33, 12596–12601. [Google Scholar] [CrossRef]
- Li, J.; Luo, J. Nonlinear Frictional Energy Dissipation between Silica-Adsorbed Surfactant Micelles. J. Phys. Chem. Lett. 2017, 8, 2258–2262. [Google Scholar] [CrossRef]
- Zhang, J.; Meng, Y.; Tian, Y.; Zhang, X. Effect of concentration and addition of ions on the adsorption of sodium dodecyl sulfate on stainless steel surface in aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2015, 484, 408–415. [Google Scholar] [CrossRef]
- He, S.; Meng, Y.; Tian, Y. Correlation Between Adsorption/Desorption of Surfactant and Change in Friction of Stainless Steel in Aqueous Solutions Under Different Electrode Potentials. Tribol. Lett. 2011, 41, 485–494. [Google Scholar] [CrossRef]
- Yang, X.; Meng, Y.; Tian, Y. Potential controlled Boundary Lubrication of Stainless Steels in Non-aqueous Sodium Dodecyl Sulfate Solutions. Tribol. Lett. 2014, 53, 17–26. [Google Scholar] [CrossRef]
- Gao, T.; Li, J.; Wang, W.; Luo, J. Extremely low friction on gold surface with surfactant molecules induced by surface potential. Friction 2023, 11, 513–523. [Google Scholar] [CrossRef]
- Tang, L.; Wang, L.; Yang, X.; Feng, Y.; Li, Y.; Feng, W. Poly(N-isopropylacrylamide)-based smart hydrogels: Design, properties and applications. Prog. Mater. Sci. 2021, 115, 100702. [Google Scholar] [CrossRef]
- Miao, Y.; Xu, M.; Zhang, L. Electrochemistry-Induced Improvements of Mechanical Strength, Self-Healing, and Interfacial Adhesion of Hydrogels. Adv. Mater. 2021, 33, 2102308. [Google Scholar] [CrossRef]
- Dhanola, A.; Khanna, N.; Gajrani, K.K. A critical review on liquid superlubricitive technology for attaining ultra-low friction. Renew. Sustain. Energy Rev. 2022, 165, 112626. [Google Scholar] [CrossRef]
- Kasuya, M.; Kubota, D.; Fujii, S.; Kurihara, K. Nano-confined electrochemical reaction studied by electrochemical surface forces apparatus. Faraday Discuss. 2022, 233, 206–221. [Google Scholar] [CrossRef]
- Wen, X.; Bai, P.; Li, Y.; Cao, H.; Li, S.; Wang, B.; Fang, J.; Meng, Y.; Ma, L.; Tian, Y. Effects of abrasive particles on liquid superlubricity and mechanisms for their removal. Langmuir 2021, 37, 3628–3636. [Google Scholar] [CrossRef]
- Wen, X.; Bai, P.; Meng, Y.; Ma, L.; Tian, Y. High-temperature superlubricity realized with chlorinated-phenyl and methyl-terminated silicone oil and hydrogen-ion running-in. Langmuir 2022, 38, 10043–10051. [Google Scholar] [CrossRef]
- Wu, L.; Wang, F.; Zhang, C.; Zhang, X.; Gu, L.; Shi, W. Silver/Graphene Nanocomposite as an Additive for Aqueous Lubrication. ACS Appl. Nano Mater. 2023, 6, 1603–1609. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, C.; Gesang, Y.; Shang, H.; Wang, R.; Liang, Y.; Wang, T.; Chen, W.; Shao, T. Fretting wear evolution of γ-TiAl alloy. Tribol. Int. 2021, 154, 106721. [Google Scholar] [CrossRef]
- Yi, M.; Qiu, J.; Xu, W. Tribological performance of ultrathin MoS2 nanosheets in formulated engine oil and possible friction mechanism at elevated temperatures. Tribol. Int. 2022, 167, 107426. [Google Scholar] [CrossRef]
- Yi, M.; Zhang, C. The synthesis of two-dimensional MoS2 nanosheets with enhanced tribological properties as oil additives. RSC Adv. 2018, 8, 9564–9573. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).