Abstract
Lubricants must exhibit good tribological behavior at low temperatures to ensure reliable startups in very cold regions. This study investigates the performance of lubricants, with a specific focus on their capacity for high-temperature lubrication and ensuring reliable low-temperature startup in engines. Experiments were conducted to assess the friction and wear characteristics of polydiethylsiloxane in conjunction with a Si3N4 ball and M50 (8Cr4Mo4V) steel across a temperature range of −80 °C to 25 °C. The results indicate that the coefficient of friction, as determined through friction and wear tests at various temperatures, remained below 0.1. As temperatures progressively decreased, the system’s friction coefficient increased, and wear volumes recorded at 25 °C and −60 °C were 9749.513 µm³ and 105.006 µm³, respectively, culminating in lubrication failure at −100 °C. This failure is primarily attributed to the increased viscosity and decreased mobility of polydiethylsiloxane at extremely low temperatures. Additionally, the reduced temperature increases the strength of the quenched steel, leading to hard particles or protrusions on the material’s surface, which collide with the Si3N4 ball during friction, causing adhesion and spalling. Despite this, polydiethylsiloxane forms a stable protective oil film on the surface, enhancing the system’s lubrication performance. However, below −80 °C, this oil film begins to tear, leading to diminished lubrication efficacy. This study provides valuable data supporting the field of cryogenic lubrication.
1. Introduction
The high-end equipment manufacturing sector demands equipment that epitomizes efficiency, reliability, and longevity [1,2,3]. In this context, lubrication oil emerges as a cornerstone for the reliable functioning of essential moving parts [4,5,6]. The implications of lubrication failures are far-reaching, encompassing increased friction and wear on equipment, which can lead to significant economic losses or, in extreme cases, catastrophic equipment failure with potential safety hazards. Specifically, aviation lubricants are subject to exceptionally rigorous conditions, including high temperatures, velocities, and loads, necessitating unparalleled high-temperature performance. These lubricants must also demonstrate a favorable viscosity–temperature relationship and maintain fluidity at low temperatures (below −40 °C) to ensure dependable performance in cold conditions [7,8,9]. Consequently, exploring the low-temperature fluidity alongside the friction and wear characteristics of aerospace lubricants is crucial for enhancing our understanding of aeroengine reliability under such challenging conditions. High-temperature lubricants need a good viscosity coefficient at low temperatures while improving high-temperature performance to ensure low-temperature startup in very cold regions [10,11].
Polysiloxanes, commonly known as silicone fluids, are distinguished by their exceptional temperature–viscosity performance, showcasing a high lubrication capacity over a wide temperature range [12,13,14]. These fluids are characterized by their structural composition, featuring a main chain of repeating silicone–oxygen (Si–O) bonds, with various organic groups attached to the silicon atoms, adhering to the formula [RnSiO4−n/2]m. This structural versatility yields a wide array of silicone fluids, including ethyl, methyl, methyl phenyl, methyl chlorophenyl varieties, and silicic acid esters [15,16,17]. Notably, silicone fluids have demonstrated unparalleled utility in aerospace bearing lubrication and in environments subjected to extreme conditions, such as radiation exposure [18,19]. However, their integration into broader applications is limited by poor miscibility with other lubricants and additives. Polydiethylsiloxane, or ethyl polysiloxanes, constitute a specialized category within this family, comprising silicon and oxygen polymer chains with ethyl groups appended to either terminal or lateral chains [20,21,22]. Their distinct chemical and physical attributes afford these fluids exceptional stability and fluidity, even under severe temperature fluctuations, rendering them indispensable in various sectors including aerospace, electrical and electronics, textiles, and automotive manufacturing. Pioneering research from the Russian Academy of Sciences has revealed that a blend of polydiethylsiloxane and mineral oils significantly reduces the coefficient of friction more effectively than the individual oils [23]. Furthermore, the introduction of silica nanoparticles into ethyl silicone oil not only augments the oil film’s resistance to tearing but also diminishes friction [24]. Similarly, the incorporation of magnetic nanoparticles markedly enhances the boundary lubrication properties of ethyl silicone oil [25].
This study rigorously examines the viscosity–temperature profile of polydiethylsiloxane across a broad low-temperature spectrum (−80 to 25 °C), aiming to decipher the principles underlying its tribological behavior under such conditions. To assess the influence of low temperatures on the flow characteristics of PDES, a specially modified rheometer was employed. Moreover, this study investigates the frictional behavior and mechanisms of PDES in contact with Si3N4 ceramic and M50 disk systems within the same temperature range, utilizing a low-temperature, three-plate ball friction and wear tester. Through this investigation, the research provides both empirical data and a theoretical basis to support the application of PDES in environments characterized by low temperatures.
2. Materials and Methods
The experimental materials comprised Si3N4 ceramic balls, procured from Sinoma Hi-Tech Nitride Ceramics Co., Ltd., Beijing, China, and M50 steel (8Cr4Mo4V), sourced from LYC Bearing Corporation, Luoyang, China. The detailed compositions of these materials are presented in Table 1. Solvent materials, including petroleum ether, acetone, and anhydrous ethanol, were obtained from Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China. Polydiethylsiloxane, the primary subject of this study, was acquired from the Synthetic Oil Research Institute, Sinopec Lubricant Co., Ltd., Chongqing, China.

Table 1.
Main composition of M50 steel.
The friction and wear experiments reported herein were performed using an Anton Paar three-plate ball cryogenic test module (Model MCR301, Anton Paar, Graz, Austria). The testing configuration employed Si3N4 ceramic balls with a diameter of 12.7 mm and M50 steel disks measuring 14.9 × 5.9 × 3.1 mm. Figure 1 depicts the operational layout, wherein the three M50 disks are angled at 45° to the rotation axis and affixed in place using screws. Prior to testing, both the ceramic balls and steel disks underwent ultrasonic cleaning in a sequential bath of petroleum ether, acetone, and anhydrous ethanol for a duration of 15 min each to remove any residual contaminants. Subsequent to the cleaning process, the components were dried using nitrogen gas to eliminate moisture and ensure surface purity.
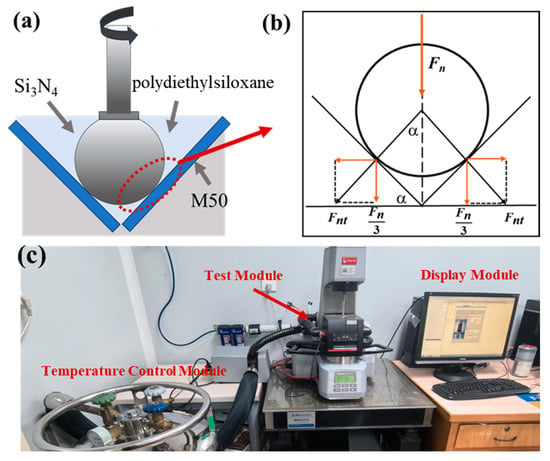
Figure 1.
(a) Working principle of the test instrument, (b) force analysis diagram (Fn: normal force; Fnt: the load on the Si3N4 ball), (c) physical figure.
The experimental setup commenced with the orderly placement of the M50 disks and Si3N4 balls within the three-plate ball test apparatus. The sequence of the procedure is detailed as follows: Initially, a volume of 1.5 mL of polydiethylsiloxane was evenly distributed over the contact area between the ball and plate, after which the system was allowed to reach temperature equilibrium. Subsequently, the ceramic ball was positioned to engage the M50 disk, exerting a normal force of 48 N, corresponding to a contact stress of approximately 1.02 GPa, and was maintained for a duration of one minute. The system’s rotational speed was then methodically increased following a logarithmic progression from 1 rpm to 250 rpm, equivalent to a velocity range of 0.47 to 118 mm/s. Upon completion of the test, the coefficient of friction at each rotational speed was documented, from which the average coefficient of friction was derived.
In this investigation, the tribological performance of the Si3N4 ceramic ball and M50 steel disk pair was assessed under low-temperature conditions, facilitated by the use of liquid nitrogen cooling apparatus. The selected temperature intervals for this study were 25 °C, −20 °C, −40 °C, −60 °C, and −80 °C. This range was chosen to comprehensively understand the tribological behavior’s characteristic laws within a cold environment. To ensure the experimental results’ repeatability and reliability, each test procedure was replicated three times. Following the completion of these tests, both the ceramic balls and steel disks were subjected to a meticulous cleaning process, utilizing petroleum ether, acetone, and anhydrous ethanol in sequence. After cleaning, detailed morphological and compositional analyses of the wear interfaces were conducted to elucidate the effects of low-temperature conditions on the materials’ tribological properties.
Figure 1 illustrates the contact interaction between the Si3N4 ceramic ball and the M50 steel disk, along with the forces generated at their points of contact. A normal force, aligned with the axis of rotation, distributes the contact pressure across three flat plates. The Si3N4 ball rotates at a speed of n, from which the sliding velocity (Vs) is derived. The friction force is determined based on the torque (M), calculated from the radius (r) of the sliding distance and the displacement angle, which is measured using a rheometer. This setup facilitates the precise quantification of tribological parameters by accurately assessing the forces and motions involved in the frictional interaction.
The viscosity coefficient of polydiethylsiloxane at varying temperatures was measured using a rotational rheometer (Model MCR301, Anton Paar, Graz, Austria), as illustrated in Figure 1c. Following the friction and wear experiments, the surface conditions of the M50 disks were evaluated with an optical microscope (Model VHX6000, Keyence, Osaka, Japan). The wear tracks’ three-dimensional morphology, roughness, and volume were precisely investigated by utilizing a white light interferometric profilometer (Model New View 8300, Zygo, Middlefield, CT, USA). Additionally, the wear interface’s morphological features and elemental composition were investigated using a scanning electron microscope (SEM, Model Quanta 200, FEI, Billings, MT, USA) equipped with an energy dispersive spectrometer (EDS, Model Genesis xm-2, EDAX, Pleasanton, CA, USA).
3. Results and Discussion
Polydiethylsiloxane is characterized by its polymer chains, which are composed of alternating silicon and oxygen atoms, with ethyl (–CH2CH3) groups attached to either the ends or sides of these chains. The temperature-dependent viscosity of polydiethylsiloxane was evaluated utilizing an MCR-301 rotational rheometer, which is outfitted with a liquid nitrogen cooling accessory for temperature regulation. Figure 2 displays the variation in viscosity of polydiethylsiloxane across a range of temperatures, showcasing a notable increase in viscosity with decreasing temperature. Specifically, viscosity escalates from 0.0342 Pa·s at 25 °C to 0.782 Pa·s at −60 °C. This trend of rising viscosity is markedly accentuated beyond −80 °C, where the viscosity surges to 36.4 Pa·s at −100 °C. Such findings underscore that polydiethylsiloxane maintains advantageous viscosity–temperature characteristics within a temperature window of 25 to −65 °C, where its viscosity remains ≤1 Pa·s, indicating exceptional fluidity that is beneficial for its performance as a lubricant in low-temperature environments. As temperature increases, the average kinetic energy of a fluid’s molecules correspondingly rises, leading to a reduction in intermolecular forces. This reduction facilitates an increase in the average distance between molecules, effectively diminishing frictional interactions among them. Viscosity, which quantifies the degree of intermolecular friction within a fluid, consequently decreases at elevated temperatures. This decrease is attributable to the enhanced ease with which molecules slide past and flow around each other, reducing mutual impedance. A decrease in viscosity results in a thinner lubricant film, which may adversely affect the effectiveness of lubrication. On the contrary, a higher viscosity indicates that greater energy is required for mechanical operations, potentially impairing mechanical efficiency. PDES has a lower temperature–viscosity coefficient, which is mainly caused by the structure of PDES. The viscosity of silicone oil is determined by the interaction between silicone oil molecules. PDES has a longer carbon chain and therefore has a lower viscosity. In addition, PDES has a smaller low-temperature viscosity change trend and exhibits better viscosity–temperature characteristics than other silicone oils in low-temperature environments.
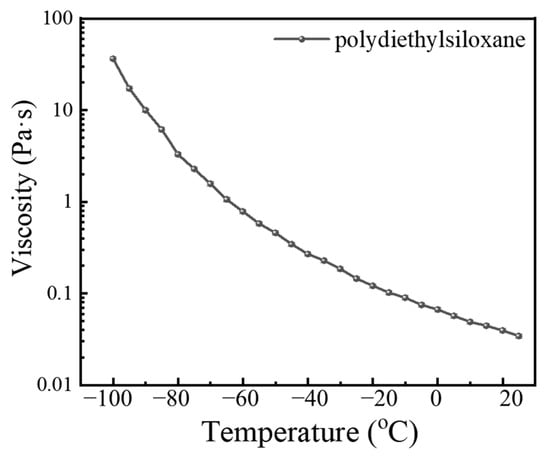
Figure 2.
Viscosity of polydiethylsiloxane.
PDES is a derivative of polysiloxane characterized by a low glass transition temperature (Tg), contributing to its unique physical properties. Notably, PDES exhibits significant flexibility at low temperatures and stability at high temperatures. The Tg of PDES typically ranges from −144 °C to −139 °C [26,27,28,29]. This exceptionally low Tg can be attributed to the flexibility of the silicon–oxygen backbone and the presence of larger ethyl side groups, which increase the free volume and consequently diminish intermolecular forces. Below the Tg, PDES behaves as a hard, brittle solid with very high viscosity, rendering it nearly non-flowing. Above the Tg, however, PDES transitions to a rubbery state, with a marked decrease in viscosity. This decrease is facilitated by increased thermal motion, which enhances the mobility of the molecular chains, thereby reducing internal friction and significantly lowering viscosity with rising temperature.
Figure 3 illustrates the friction coefficients and the corresponding average values for the tribopair composed of Si3N4 balls and M50 steel disks, lubricated with polydiethylsiloxane, across a diverse temperature spectrum, specifically at 25, −20, −40, −60, and −80 °C. The data reveal that polydiethylsiloxane exhibits superior lubrication efficacy within the 25 to −80 °C range, consistently maintaining friction coefficients below 0.1. This performance is notably stable when contrasted with its behavior at elevated temperatures (above 200 °C), underscoring polydiethylsiloxane’s remarkable fluidity and lubricating properties even under reduced temperature conditions. Intriguingly, an upward trend in the average friction coefficient is observed as the temperature increases, with the coefficients at 25, −20, −40, −60, and −80 °C documented as 0.0425, 0.0609, 0.0731, 0.0862, and 0.0924, respectively. This trend highlights the nuanced impact of temperature on lubrication dynamics, demonstrating that polydiethylsiloxane maintains its effective lubrication performance across a broad range of lower temperatures. The observed incremental rise in friction coefficients can be largely attributed to the increased viscosity of ethyl silicone oil at lower temperatures, which intensifies the shear forces exerted within the Si3N4 and M50 tribopair against the lubricant layer. Initially, a break-in period for the friction coefficients is noted, after which a phase of stabilization ensues, demonstrating consistent stability across subsequent measurements. This stabilization phase suggests the successful formation of a uniform and effective lubrication film by polydiethylsiloxane under low temperature conditions, confirming its capacity to sustain commendable lubrication properties within the temperature spectrum of −80 to 25 °C for the Si3N4 and M50 tribopair. Nonetheless, at the threshold of −100 °C, the lubrication system exhibits failure, marking the operational temperature limit for polydiethylsiloxane as a lubricant under these test conditions. Compared to Tuyana’s findings, this study demonstrates that due to the favorable viscosity–temperature characteristics of PDES, it exhibits excellent lubrication performance in Si3N4 and M50 systems, with the friction coefficient significantly lower than that observed with PES-2. Additionally, PDES maintains a low friction coefficient (COF < 0.3) even under conditions of low temperature and low speed. These results suggest that PDES has potential as a base oil for low-temperature startups.
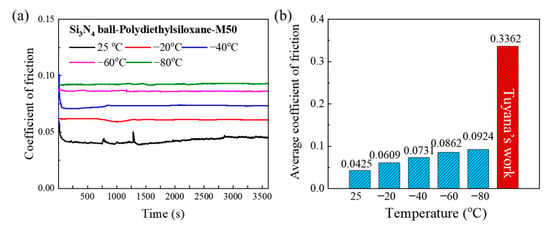
Figure 3.
(a) Coefficient of friction and (b) average coefficient of friction at different temperatures [24].
Subsequent to the execution of the friction wear tests, the wear regions on the M50 steel disks were scrutinized using a VHX-6000 (Keyence, Osaka, Japan) optical microscope, as depicted in Figure 4a–e. Analysis of the light microscope imagery reveals that the wear spots across all tested samples exhibit relatively shallow depths, affirming the proficient lubrication capability of polydiethylsiloxane within the tribosystem composed of Si3N4 ceramic balls and M50 steel disks. Intriguingly, the diameter of the wear spots follows a trend of initially decreasing before subsequently increasing as temperatures decline. This pattern culminates at −80 °C, where an enlargement in the wear scar diameter (WSD) is observed, indicative of the lubrication system’s failure under these extreme conditions. Specifically, the wear scar diameter exhibits an expansion, inaccurately recorded as increasing from 227.7 µm to 254.9 µm. The thickness of the lubricant film at the interface of the friction pair increases as the temperature decreases, which effectively reduces the direct contact area between the friction pair components and thus reduces the wear mark diameter. At low temperatures, the viscosity of the lubricant increases dramatically and the working resistance increases. The thickness of the lubricant film at the interface of the friction pair increases as the temperature decreases, which effectively reduces the direct contact area between the friction pair components and thus reduces the wear mark diameter. In addition, with decreasing temperature, the mechanical properties of the steel material change, which further increases the WSD. The empirical data suggest that the thickness of the lubricant film at the interface of the friction pair escalates with decreasing temperature, which effectively diminishes the direct contact area between the tribopair components, thereby reducing the wear scar diameter. However, at the critical temperature of −80 °C, the viscosity of polydiethylsiloxane experiences a substantial increase, leading to the rupture of the oil film. This rupture undermines the lubricant’s efficacy, culminating in the observed enlargement of the wear spot, a clear manifestation of lubrication failure under such adverse thermal conditions.
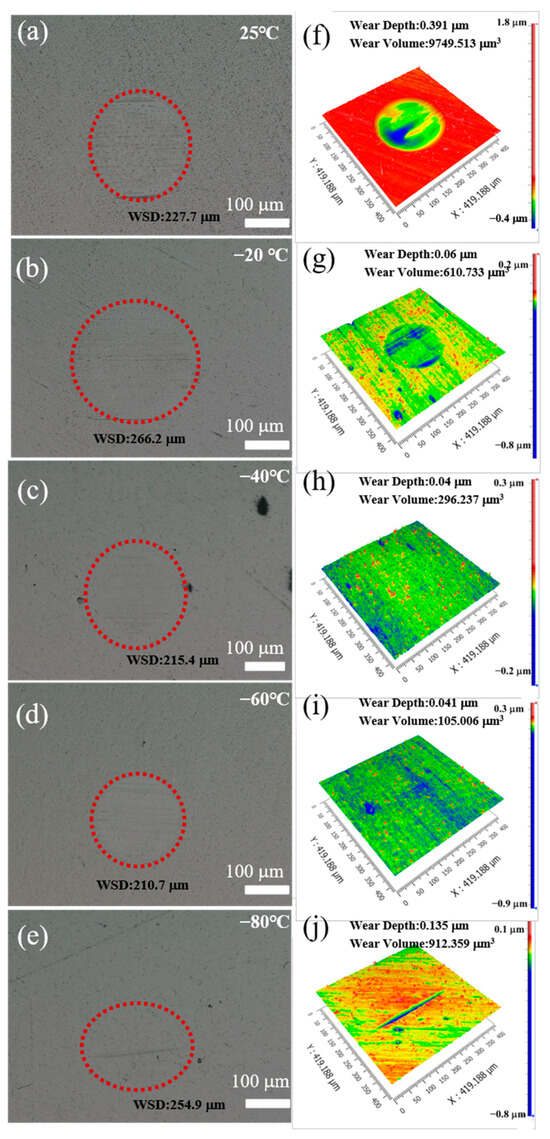
Figure 4.
(a–e) Optical microscope images of M50 wear scars after friction test; (f–j) three-dimensional white light interferometry images after friction test.
The evaluation of the wear rate for the Si3N4 ceramic balls and M50 steel disks was facilitated through the utilization of 3D white light interferometry, which provided detailed images to measure the wear depth and volume on the surface of the M50 steel disks at varying temperatures, as illustrated in Figure 4f–j. The results reveal a distinct pattern in which both wear volume and depth initially decrease as the temperature drops, followed by a subsequent increase. Notably, the wear volumes recorded at 25 °C and −60 °C were 9749.513 μm³ and 105.006 μm³, respectively, indicating a significant reduction of nearly tenfold. This observation underscores the effectiveness of the low-temperature friction interface in substantially reducing material wear. Nevertheless, a notable increase in wear volume was observed at −80 °C, a phenomenon primarily ascribed to the disintegration of the polydiethylsiloxane lubricant film at such low temperatures, which in turn led to an increased wear volume. In a similar trend, the depth of the wear marks decreased with falling temperatures, from 0.391 mm at 25 °C to 0.04 mm at −60 °C, only to rise again to 0.135 mm at −80 °C.
Scanning electron microscopy (SEM) was employed to investigate the morphological changes and elemental composition at the wear interfaces of M50 steel disks subjected to different temperatures, with the findings presented in Figure 5. The SEM images disclose distinct morphological alterations correlating with temperature changes. At ambient temperature, the wear interface is characterized by minor furrows aligned in the friction direction, devoid of significant debris or abrasive particles. This observation suggests a dominance of the two-body abrasive wear mechanism under these conditions. However, as the temperature decreases, there is a notable reduction in the depth and clarity of these furrows, a phenomenon attributed to the increased viscosity of polydiethylsiloxane, which results in a thicker lubricant film at the friction interface. This change facilitates a transition towards hydrodynamic lubrication, marked by elevated internal friction among lubricant molecules, thereby increasing the friction coefficient. Moreover, a distinct white substance was noted, predominantly associated with the accumulation of carbides from the high-temperature surface treatment of the steel. At the reduced temperature of −80 °C, the wear interface exhibits a marked deviation from higher temperature conditions, displaying significant pitting and material spalling. This alteration indicates a shift to primarily adhesive wear, as evidenced by the nearly obliterated plowing furrows. These results suggest that with a decreasing temperature, the wear mechanism evolves from two-body abrasive to adhesive wear, culminating in material failure at the critical temperature of −100 °C. This transition can be further elucidated by the increased hardness and reduced plasticity of the quenched steel at lower temperatures, which diminishes the capacity for plastic deformation. Consequently, hard particles or surface protrusions come into rigid contact with the Si3N4 balls during friction, leading to adhesion and subsequent spalling of the surface material, thereby indicating a significant modification in the wear mechanism.
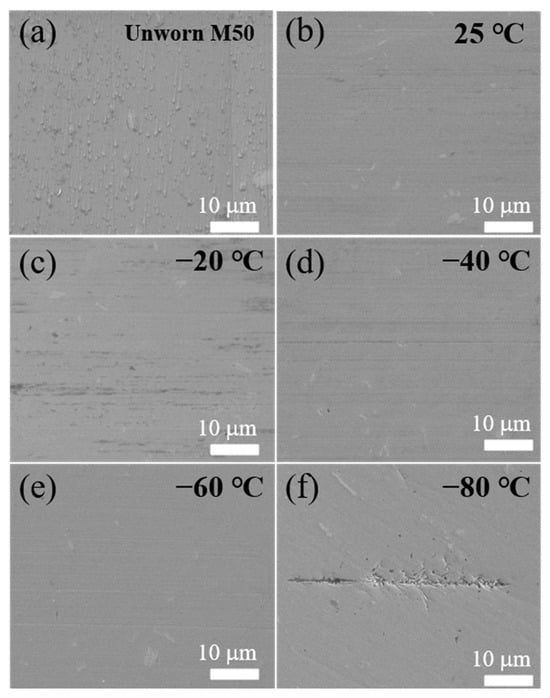
Figure 5.
SEM images of M50 disk after friction tests at different temperatures: (a) unworn M50 disk surface; (b) 25 °C; (c) −20 °C; (d) −40 °C; (e) −60 °C; (f) −80 °C.
A noteworthy observation is the relative increase in Iron (Fe) content at the wear interface compared to the unworn areas of the M50 disks. Furthermore, the analysis shows that the elemental composition within the wear region remains largely consistent across a range of low temperatures. This uniformity highlights the robust physical and chemical properties of polydiethylsiloxane under cold conditions, affirming its capability to safeguard the integrity of the wear interface. The observed stabilization of the friction interface, following the interaction between the ceramic ball and the M50 steel disk, suggests a diminished formation of new wear particles. This stabilization contributes to the maintenance of a consistent friction coefficient over the course of the wear process. The elemental constancy at the wear interface, despite temperature fluctuations, implies the absence of new compound formation during the wear process. Consequently, the wear performance at low temperatures is predominantly dictated by the viscosity and film-forming characteristics of polydiethylsiloxane. Notably, at −80 °C, there is a marked increase in the viscosity of polydiethylsiloxane, leading to the rupture of the lubricant film. This rupture incites higher friction coefficients and increased wear, underscoring the pivotal influence of lubricant viscosity on wear dynamics in low-temperature environments.
Figure 6 elucidates the wear mechanism of polydiethylsiloxane at low temperatures, showcasing its exemplary lubrication performance. This performance is chiefly ascribed to polydiethylsiloxane’s inherent properties and an augmentation in the thickness of the lubricant film. As the Si3N4 ceramic ball undergoes rotation, polydiethylsiloxane generates dynamic pressure at the friction interface, ensuring the maintenance of an optimal oil film thickness. With a reduction in test temperatures, polydiethylsiloxane’s viscosity increases, consequently diminishing its mobility. Nonetheless, this heightened viscosity is instrumental in creating a more substantial dynamic pressure between the Si3N4 ceramic ball and the M50 steel disk, promoting the development of a thicker oil film and thereby elevating the lubrication system’s efficiency. Additionally, the mechanical properties of M50 steel at varying temperatures significantly impact the wear behavior observed during testing. At an ambient temperature, M50 steel exhibits strong mechanical resilience, potentially leading to the accumulation of abrasive particles on the wear surface and subsequent furrow formation. Conversely, a decrease in temperature renders M50 steel more brittle, compromising the protective capacity of the polydiethylsiloxane film and ultimately precipitating lubrication failure. This intricate relationship between the viscoelastic properties of polydiethylsiloxane and the evolving mechanical characteristics of M50 steel underlines the complex dynamics governing the wear mechanism, illustrating the nuanced interaction between lubricant functionality and material behavior in low-temperature contexts.
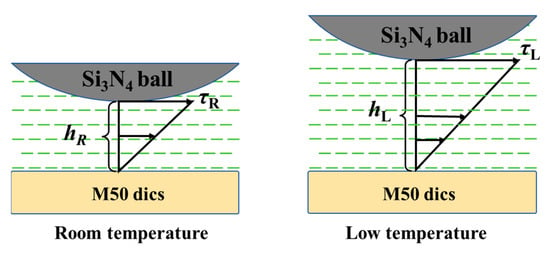
Figure 6.
Diagram of low-temperature wear mechanisms.
4. Conclusions
This study investigated the viscosity–temperature relationships and tribological characteristics of polydiethylsiloxane, a lubricant noted for its excellent low-temperature viscosity performance. The research employed Si3N4 ceramic balls and M50 steel disks as frictional counterparts to establish a comprehensive theoretical and practical understanding of polydiethylsiloxane in cold environments. A key element of this research was measuring the low-temperature viscosity of polydiethylsiloxane using an Anton Paar rheology tester. The results showed that viscosity increased from 0.0342 Pa·s at 25 °C to 0.782 Pa·s at −60 °C, highlighting the outstanding flow properties despite significant temperature reductions. The friction coefficient was evaluated across temperatures of 25, −20, −40, −60, and −80 °C using a specialized low-temperature, three-plate ball friction and wear tester. The lubricant maintained stable friction coefficients at low temperatures, performing better than at higher temperatures (230 °C). Although the friction coefficient rose with decreasing temperatures, it remained below 0.1 until −100 °C, where it sharply increased to 0.656. Morphological and elemental analyses of the wear interface via scanning electron microscopy and white light interferometry indicated that wear spot sizes initially decreased and then increased as temperatures fell. This suggests that polydiethylsiloxane forms a stable lubricant film at lower temperatures, which begins to fail below −80 °C due to viscosity-induced film breakage, thus reducing lubrication effectiveness. These findings highlight the critical role of polydiethylsiloxane’s viscosity and lubrication properties in ensuring the operational reliability of aviation lubricant systems at low temperatures, providing valuable insights for their practical application.
Author Contributions
Data curation, X.M. and H.J.; investigation, Y.T. (Yong Tang), L.Y. and N.L.; supervision, Y.T. (Yu Tian); writing—original draft, J.H.; writing—review and editing, P.B., Y.M. and Y.T. (Yu Tian). All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 52275198) and the Postdoctoral Fellowship Program of CPSF (No. GZC20231287).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors are also thankful for Bingjian Gao’s work.
Conflicts of Interest
Authors Junhao Han, Yong Tang, Xianzhen Ma, Hao Jia, Ningxia Liu were employed by the company AVIC Xinxiang Aviation Industry (Group) Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Du, Y.; Li, C. Implementing energy-saving and environmental-benign paradigm: Machine tool remanufacturing by OEMs in China. J. Clean. Prod. 2014, 66, 272–279. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Shi, L.; Tan, Y.; Qiao, F. Engineering management for high-end equipment intelligent manufacturing. Front. Eng. Manag. 2018, 5, 420–450. [Google Scholar] [CrossRef]
- Pan, X.; Lu, J.; Huo, J.; Gao, J.; Wu, H. A review on self-recovery regulation (SR) technique for unbalance vibration of high-end equipment. Chin. J. Mech. Eng. 2020, 33, 89. [Google Scholar] [CrossRef]
- Liu, H. Research on Freon Compressor Design. Eng. Adv. 2023, 3, 350–354. [Google Scholar] [CrossRef]
- Yao, Q.; Dai, L.; Tang, J.; Wu, H.; Liu, T. High-speed rolling bearing lubrication reliability analysis based on probability box model. Probabilistic Eng. Mech. 2024, 76, 103612. [Google Scholar] [CrossRef]
- Lugt, P.M. A review on grease lubrication in rolling bearings. Tribol. Trans. 2009, 52, 470–480. [Google Scholar] [CrossRef]
- Yao, T.; Zhang, N.; Hu, J.; Liao, X.; Shen, Y.; Gan, Z. Effect of temperature on the chemical composition and physicochemical properties of diester aviation lubrication oil. Int. J. Chem. Eng. 2020, 2020, 8829206. [Google Scholar] [CrossRef]
- Mia, S.; Ohno, N. Relation between low temperature fluidity and sound velocity of lubricating oil. Tribol. Int. 2010, 43, 1043–1047. [Google Scholar] [CrossRef]
- Cyriac, F.; Lugt, P.M.; Bosman, R. Yield stress and low-temperature start-up torque of lubricating greases. Tribol. Lett. 2016, 63, 6. [Google Scholar] [CrossRef]
- Kucinschi, B.R.; Shieh, T.H. Estimation of oil supply time during engine start-up at very low temperatures. SAE Int. J. Fuels Lubr. 2016, 9, 363–369. [Google Scholar] [CrossRef]
- Usman, A.; Park, C.W. Transient lubrication of piston compression ring during cold start-up of SI engine. Int. J. Precis. Eng. Manuf.-Green Technol. 2016, 3, 81–90. [Google Scholar] [CrossRef]
- Zolper, T.J.; Jungk, M.; Marks, T.J.; Chung, Y.-W.; Wang, Q. Modeling polysiloxane volume and viscosity variations with molecular structure and thermodynamic state. J. Tribol. 2014, 136, 011801. [Google Scholar] [CrossRef]
- Kakiuchida, H.; Takahashi, M.; Tokuda, Y.; Masai, H.; Kuniyoshi, M.; Yoko, T. Viscoelastic and structural properties of a phenyl-modified polysiloxane system with a three-dimensional structure. J. Phys. Chem. B 2006, 110, 7321–7327. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Choi, Y.T.; Liao, C.R.; Wereley, N.M. Long term stability of magnetorheological fluids using high viscosity linear polysiloxane carrier fluids. Smart Mater. Struct. 2016, 25, 075006. [Google Scholar] [CrossRef]
- Aziz, T.; Fan, H.; Khan, F.U.; Haroon, M.; Cheng, L. Modified silicone oil types, mechanical properties and applications. Polym. Bull. 2019, 76, 2129–2145. [Google Scholar] [CrossRef]
- Luan, X.; Zhang, E.; Chen, Y.; Ma, R.; Gong, K.; Li, W.; Wang, X. Chemically modified silicone oil with enhanced tribological and anti-foaming properties. Lubricants 2022, 10, 364. [Google Scholar] [CrossRef]
- Wei-Min, L.I.U.; Jun, X.; Da-Peng, F.E.N.G.; Xiao-Bo, W.A.N.G. The research status and prospect of synthetic lubricating oils. Tribology 2013, 33, 91–104. [Google Scholar]
- Schiefer, H.M.; Awe, R.W.; Whipple, C.L. Extending the Utility of Silicone Lubricants through Structural Modifications. J. Chem. Eng. Data 1961, 6, 155–160. [Google Scholar] [CrossRef]
- Brophy, J.E.; Militz, R.O.; Zisman, W.A. Dimethyl-Silicone-Polymer fluids and their performance characteristics in unilaterally loaded journal bearings. Trans. Am. Soc. Mech. Eng. 1946, 68, 355–360. [Google Scholar] [CrossRef]
- Meng, Y.; Yue, L.; Wen, X.; Wei, P.; Zhou, X.; Cheng, J.; Bai, P.; Zhao, Q.; Meng, Y.; Tian, Y. The influence of polydiethylsiloxane (PDES) concentration on the tribolfilm of chlorophenyl silicone oil (CPSO) under high-temperature lubrication. J. Mater. Res. Technol. 2024, 29, 1557–1564. [Google Scholar] [CrossRef]
- Pavelko, G.F.; Bordubanova, E.G.; Zaimovskaya, T.A.; Bondarenko, G.N.; Lyadov, A.S.; Parenago, O.P. Anomalous dependence of wear properties on the mixture composition of hydrocarbon oils with polyorganosiloxanes. J. Frict. Wear 2018, 39, 241–244. [Google Scholar] [CrossRef]
- Zhang, W.; Luan, W.; Dong, H.; Wu, C.; Qu, Z. The density, viscosity, speed of sound, excess properties of binary mixtures of linear polydimethylsiloxane and various alkanes at temperatures in the range (288.15–328.15) K. J. Chem. Thermodyn. 2021, 161, 106537. [Google Scholar] [CrossRef]
- Pavelko, G.F. Extremely high wear of steel surfaces in polyorganosiloxane mixtures containing 10 wt% hydrocarbon oils. J. Frict. Wear 2023, 44, 117–119. [Google Scholar] [CrossRef]
- Dembelova, T.; Badmaev, B.; Makarova, D.; Mashanov, A.; Mishigdorzhiyn, U. Rheological and tribological study of polyethylsiloxane with SiO2 nanoparticles additive. Lubricants 2022, 11, 9. [Google Scholar] [CrossRef]
- Bolotov, A.N.; Novikov, V.V.; Novikova, O.O. Lubricating oils obtained via modifying magnetic nanofluids. Inorg. Mater. Appl. Res. 2020, 11, 865–871. [Google Scholar] [CrossRef]
- Brewer, J.R.; Tsuchihara, K.; Morita, R.; Jones, J.R.; Bloxsidge, J.P.; Kagao, S.; Otsuki, T.; Fujishige, S. Poly (diethylsiloxane-co-diphenylsiloxane) and poly (diethylsiloxane-co-3, 3, 3-trifluoropropylmethylsiloxane): Synthesis, characterization and low-temperature properties. Polymer 1994, 35, 5109–5117. [Google Scholar] [CrossRef]
- Liu, L.; Yang, S.; Zhang, Z.; Wang, Q.; Xie, Z. Synthesis and characterization of poly (diethylsiloxane) and its copolymers with different diorganosiloxane units. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 2722–2730. [Google Scholar] [CrossRef]
- Zlatanic, A.; Radojcic, D.; Wan, X.; Messman, J.M.; Dvornic, P.R. Suppression of crystallization in polydimethylsiloxanes and chain branching in their phenyl-containing copolymers. Macromolecules 2017, 50, 3532–3543. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, J.; Zhao, N.; Li, Z. Fast synthesis of high molecular weights polydiethylsiloxanes and random poly (dimethylsiloxane-co-diethylsiloxane) copolysiloxanes via cyclic trimeric phosphazene base catalyzed ring-opening (co) polymerization. Eur. Polym. J. 2022, 173, 111280. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).