Influence of Electrical Stimulation on the Friction Performance of LiPF6-Based Ionic Liquids
Abstract
1. Introduction
2. Materials and Methods
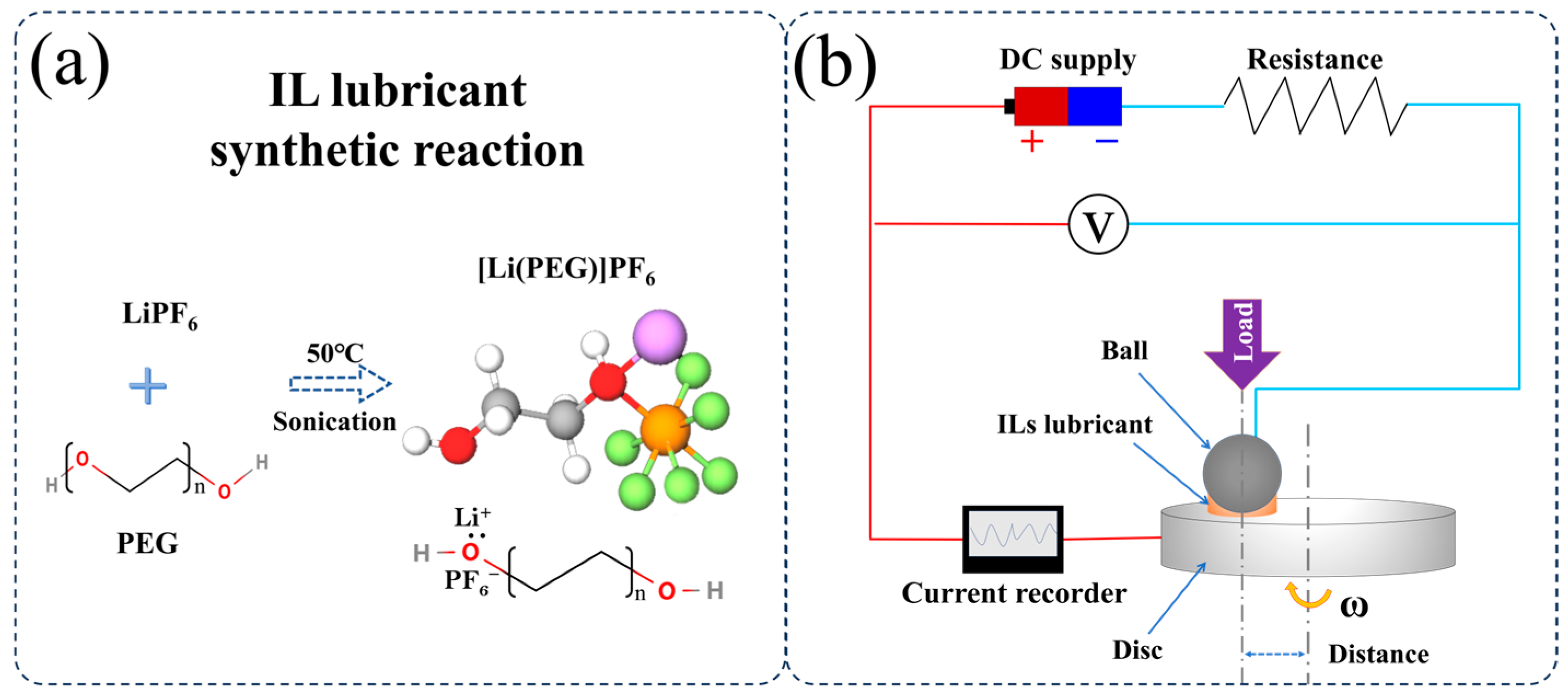
2.1. Materials and Preparation
2.2. Frictional Tests
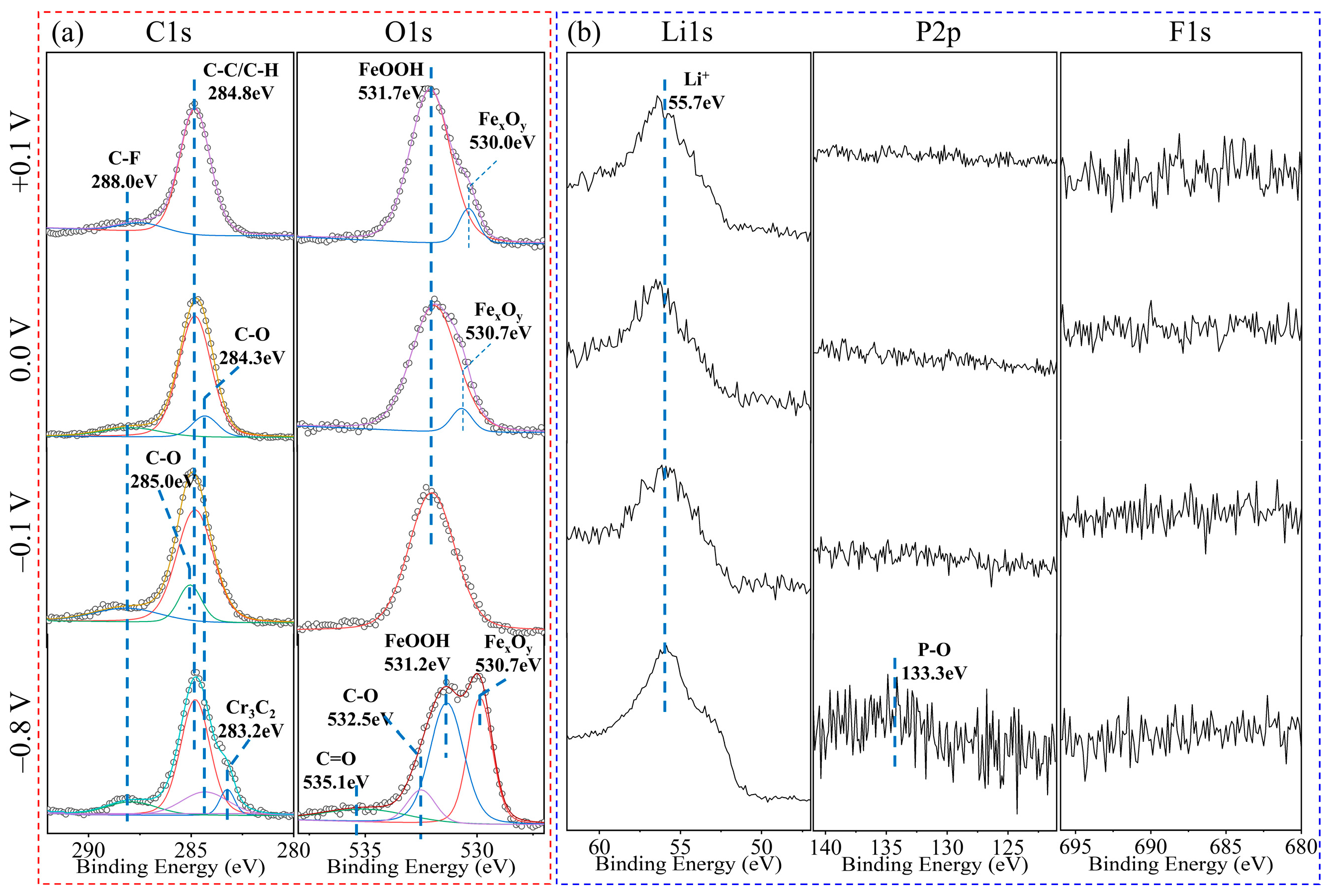
2.3. Surface Analysis
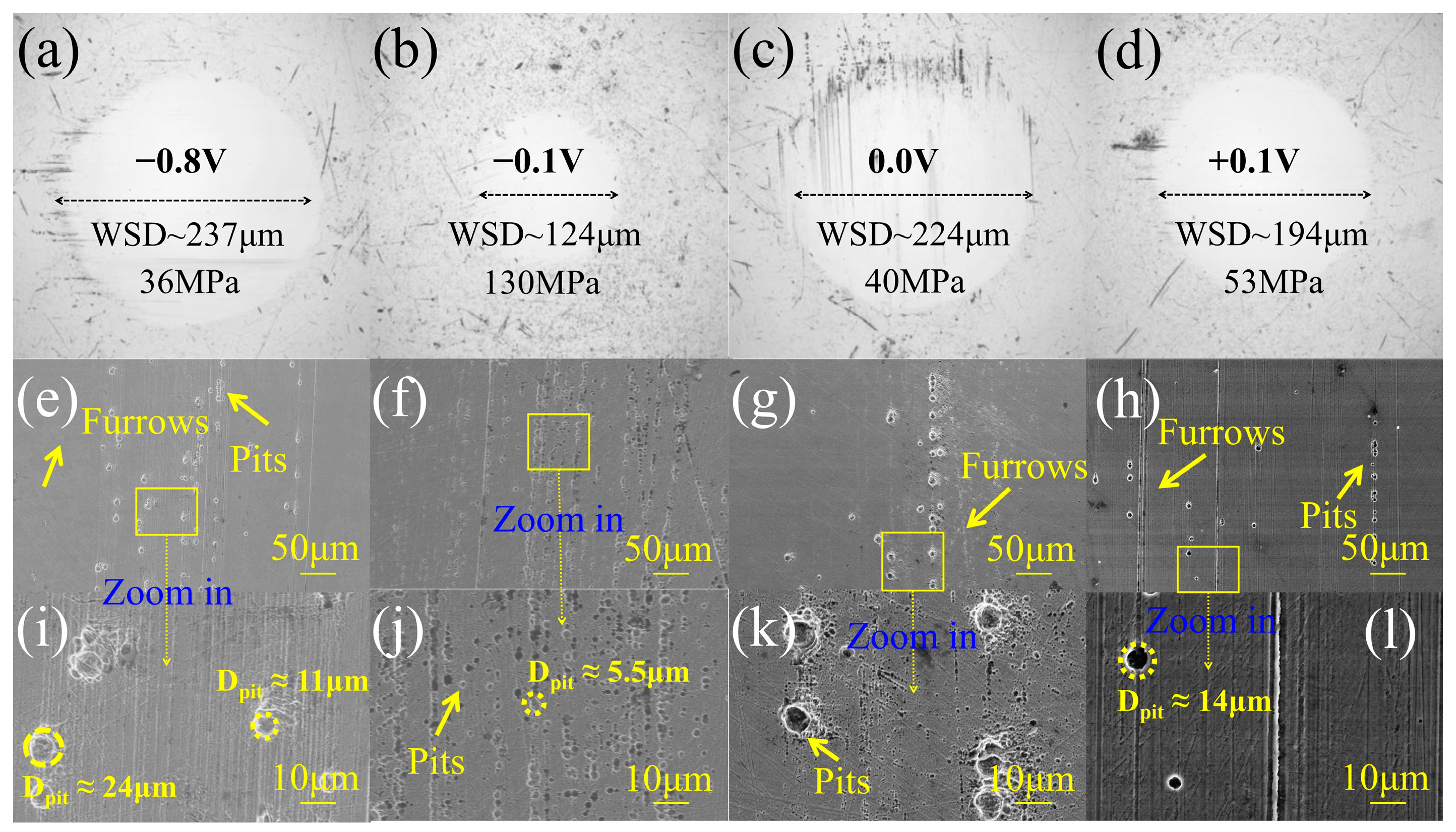
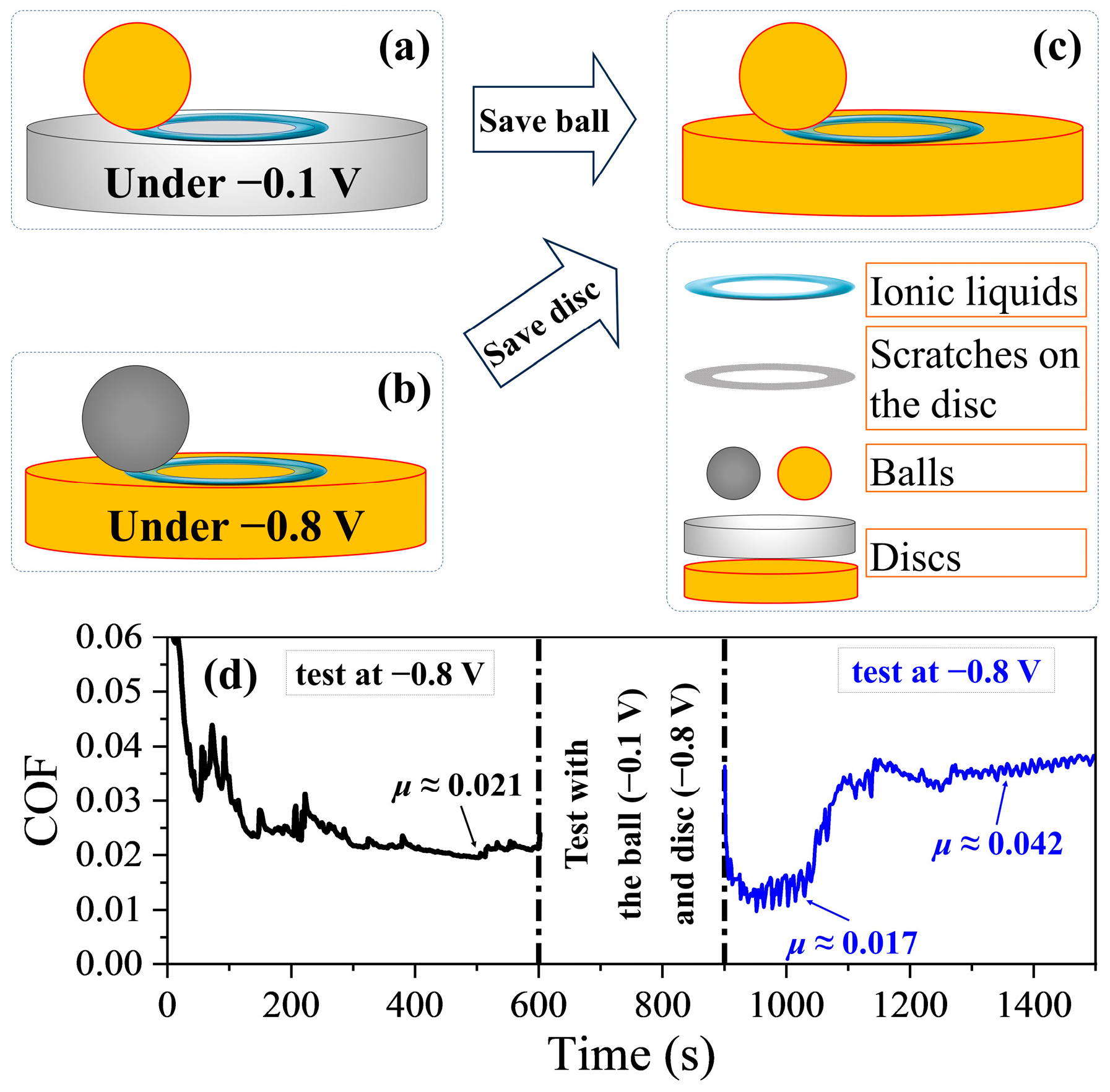
3. Results
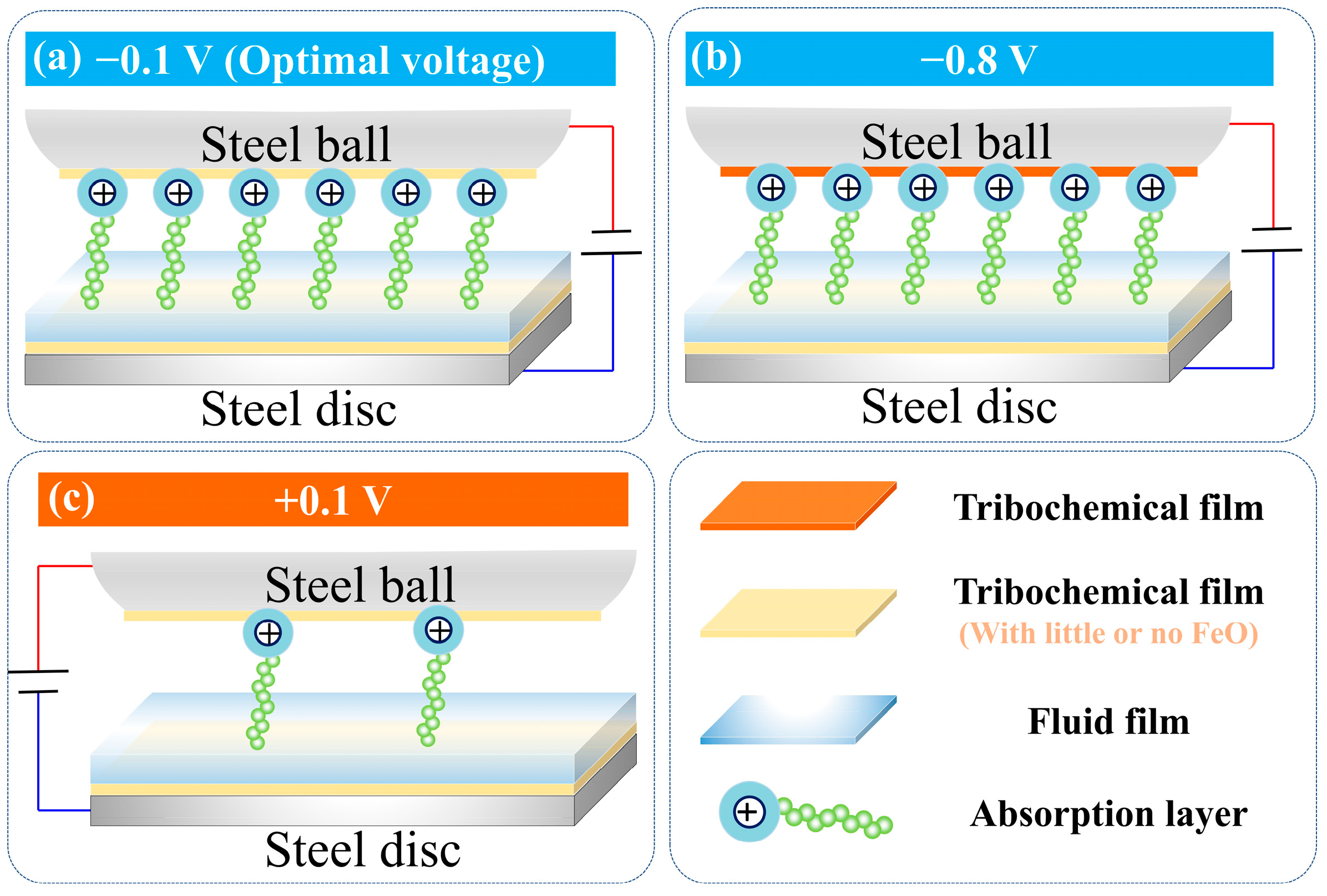
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ILs | Ionic Liquids |
| COF | Coefficient of Friction |
| AFM | Atomic Force Microscope |
| PILs | Polymeric Ionic Liquids |
| ECR | Electrical Contact Resistance |
| WSD | Wear Scar Diameter |
| SEM | Scanning Electron Microscope |
| XPS | X-ray Photoelectron Spectroscope |
| ML | Mixed Lubrication |
References
- Holmberg, K.; Andersson, P.; Erdemir, A. Global energy consumption due to friction in passenger cars. Tribol. Int. 2012, 47, 221–234. [Google Scholar] [CrossRef]
- Whittle, M.; Trevelyan, J.; Tavner, P.J. Bearing currents in wind turbine generators. J. Renew. Sustain. Energy 2013, 5, 053128. [Google Scholar] [CrossRef]
- He, F.; Xie, G.; Luo, J. Electrical bearing failures in electric vehicles. Friction 2020, 8, 4–28. [Google Scholar] [CrossRef]
- Xie, G.; Guo, D.; Luo, J. Lubrication under charged conditions. Tribol. Int. 2015, 84, 22–35. [Google Scholar] [CrossRef]
- Ge, X.; Chai, Z.; Shi, Q.; Liu, Y.; Wang, W. Graphene superlubricity: A review. Friction 2023, 11, 1953–1973. [Google Scholar] [CrossRef]
- Matta, C.; Joly-Pottuz, L.; Bouchet, M.I.D.B.; Martin, J.M.; Kano, M.; Zhang, Q.; Goddard, W.A. Superlubricity and tribochemistry of polyhydric alcohols. Phys. Rev. B 2008, 78, 085436. [Google Scholar] [CrossRef]
- Ge, X.; Li, J.; Luo, R.; Zhang, C.; Luo, J. Macroscale superlubricity enabled by the synergy effect of graphene-oxide nanoflakes and ethanediol. ACS Appl. Mater. Interfaces 2018, 10, 40863–40870. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Li, J.; Wang, H.; Zhang, C.; Liu, Y.; Luo, J. Macroscale superlubricity under extreme pressure enabled by the combination of graphene-oxide nanosheets with ionic liquid. Carbon 2019, 151, 76–83. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, L.; Li, G.; Guo, Y.; Qi, H.; Zhang, G. Significantly enhancing tribological performance of epoxy by filling with ionic liquid functionalized graphene oxide. Carbon 2018, 136, 309–319. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Gao, S.; Chen, Q.; Peng, L.; Liu, K.; Wei, X. Superlubricity between MoS2 monolayers. Adv. Mater. 2017, 29, 1701474. [Google Scholar] [CrossRef]
- Gao, K.; Jiao, J.; Wang, Z.; Xie, G.; Luo, J. Superlubricity induced by partially oxidized black phosphorus on engineering steel. Friction 2023, 11, 1592–1605. [Google Scholar] [CrossRef]
- Gao, K.; Lai, Z.; Jia, Q.; Zhang, B.; Wei, X.; Zhang, J. Bilayer a-C:H/MoS2 film to realize superlubricity in open atmosphere. Diam. Relat. Mater. 2020, 108, 107973. [Google Scholar] [CrossRef]
- Yu, G.; Gong, Z.; Jiang, B.; Wang, D.; Bai, C.; Zhang, J. Superlubricity for hydrogenated diamond like carbon induced by thin MoS2 and DLC layer in moist air. Diam. Relat. Mater. 2020, 102, 107668. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, X.; Yu, H.; Chen, H.; Feng, D.; Qiao, D. Insight into macroscale superlubricity of polyol aqueous solution induced by protic ionic liquid. Friction 2022, 10, 2000–2017. [Google Scholar] [CrossRef]
- Han, T.; Zhang, C.; Chen, X.; Li, J.; Wang, W.; Luo, J. Contribution of a tribo-induced silica layer to macroscale superlubricity of hydrated ions. J. Phys. Chem. C 2019, 123, 20270–20277. [Google Scholar] [CrossRef]
- Han, T.; Yi, S.; Zhang, C.; Li, J.; Chen, X.; Luo, J.; Banquy, X. Superlubrication obtained with mixtures of hydrated ions and polyethylene glycol solutions in the mixed and hydrodynamic lubrication regimes. J. Colloid Interface Sci. 2020, 579, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, H.; Liu, Y.; Zhang, C.; Luo, J. Controllable superlubricity system of polyalkylene glycol aqueous solutions under various applied conditions. Macromol. Mater. Eng. 2020, 305, 2000141. [Google Scholar] [CrossRef]
- Zeng, Q.; Dong, G.; Martin, J.M. Green superlubricity of Nitinol 60 alloy against steel in presence of castor oil. Sci. Rep. 2016, 6, 29992. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, L.; Xiao, C.; Zhang, S.; Zhang, C.; Zhou, N.; Qin, T.; Qian, L.; Zhang, J. How to improve superlubricity performance of diketone at steel interface: Effects of oxygen gas. Friction 2023, 11, 927–937. [Google Scholar] [CrossRef]
- Reddyhoff, T.; Ewen, J.P.; Deshpande, P.; Frogley, M.D.; Welch, M.D.; Montgomery, W. Macroscale superlubricity and polymorphism of long-chain n-alcohols. ACS Appl. Mater. Interfaces 2021, 13, 9239–9251. [Google Scholar] [CrossRef]
- Du, C.; Yu, T.; Wu, Z.; Zhang, L.; Shen, R.; Li, X.; Feng, M.; Feng, Y.; Wang, D. Achieving macroscale superlubricity with ultra-short running-in period by using polyethylene glycol-tannic acid complex green lubricant. Friction 2023, 11, 748–762. [Google Scholar] [CrossRef]
- Arad, S.; Rapoport, L.; Moshkovich, A.; van Moppes, D.; Karpasas, M.; Golan, R.; Golan, Y. Superior biolubricant from a species of red microalga. Langmuir 2006, 22, 7313–7317. [Google Scholar] [CrossRef]
- Tan, S.; Shi, H.; Du, X.; Wang, K.; Xu, H.; Wan, J.; Deng, K.; Zeng, Q.; Liu, Y. Electric field controlled superlubricity of fullerene-based host—Guest assembly. Nano Res. 2023, 16, 583–588. [Google Scholar] [CrossRef]
- Li, H.; Wood, R.J.; Rutland, M.W.; Atkin, R. An ionic liquid lubricant enables superlubricity to be “switched on” in situ using an electrical potential. Chem. Commun. 2014, 50, 4368–4370. [Google Scholar] [CrossRef]
- Zhang, Y.; Rutland, M.W.; Luo, J.; Atkin, R.; Li, H. Potential-dependent superlubricity of ionic liquids on a graphite surface. J. Phys. Chem. C 2021, 125, 3940–3947. [Google Scholar] [CrossRef]
- Cai, H.; Zhang, C.; Li, F.; Liu, M.; Zhang, T.; Chu, H.; Liu, Z. Study on the microcosmic superlubricity mechanism of PVPA affected by metal cations. Friction 2023, 11, 1150–1164. [Google Scholar] [CrossRef]
- Ye, C.F.; Liu, W.M.; Chen, Y.X.; Yu, L.G. Room-temperature ionic liquids: A novel versatile lubricant. Chem. Commun. 2001, 21, 2244–2245. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Qu, J. Ionic liquids as lubricant additives: A review. ACS Appl. Mater. Interfaces 2017, 9, 3209–3222. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, M.; Ripin, Z.M.; Jiang, C.-P.; Pasang, T. Superlubricity of materials: Progress, potential, and challenges. Materials 2023, 16, 5145. [Google Scholar] [CrossRef]
- Lebedeva, O.; Kultin, D.; Kustov, L. Polymeric ionic liquids: Here, there and everywhere. Eur. Polym. J. 2023, 203, 112657. [Google Scholar] [CrossRef]
- Cao, Z.; Xia, Y.; Liu, L.; Feng, X. Study on the conductive and tribological properties of copper sliding electrical contacts lubricated by ionic liquids. Tribol. Int. 2019, 130, 27–35. [Google Scholar] [CrossRef]
- Gatti, F.; Amann, T.; Kailer, A.; Baltes, N.; Rühe, J.; Gumbsch, P. Towards programmable friction: Control of lubrication with ionic liquid mixtures by automated electrical regulation. Sci. Rep. 2020, 10, 17634. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Meng, Y.; Tian, Y. Effect of imidazolium ionic liquid additives on lubrication performance of propylene carbonate under different electrical potentials. Tribol. Lett. 2014, 56, 161–169. [Google Scholar] [CrossRef]
- Ge, X.; Wu, X.; Shi, Q.; Song, S.; Liu, Y.; Wang, W. Study on the superlubricity behavior of ions under external electric fields at steel interfaces. Langmuir 2023, 39, 18757–18767. [Google Scholar] [CrossRef]
- Song, Z.; Fan, M.; Liang, Y.; Zhou, F.; Liu, W. Lithium-based ionic liquids: In situ-formed lubricant additive only by blending. Tribol. Lett. 2013, 49, 127–133. [Google Scholar] [CrossRef]
- Fan, M.; Liang, Y.; Zhou, F.; Liu, W. Dramatically improved friction reduction and wear resistance by in situ formed ionic liquids. RSC Adv. 2012, 2, 6824–6830. [Google Scholar] [CrossRef]
- Lv, F.; Wang, W.; Zhang, B.; Jia, Q.; Gao, Y.; Wang, K. Black phosphorus quantum dots in aqueous environments as lubricants. ACS Appl. Nano Mater. 2023, 6, 13637–13645. [Google Scholar] [CrossRef]
- Zeng, D.W.; Yung, K.C.; Xie, C.S. XPS investigation of the chemical characteristics of Kapton films ablated by a pulsed TEA CO2 laser. Surf. Coat. Technol. 2002, 153, 210–216. [Google Scholar] [CrossRef]
- Tan, H.; Zhai, D.; Kang, F.; Zhang, B. Synergistic PF6− and FSI− intercalation enables stable graphite cathode for potassium-based dual ion battery. Carbon 2021, 178, 363–370. [Google Scholar] [CrossRef]
- Strohmeier, B.R. Evaluation of polymeric standard reference materials for monitoring the performance of X-ray photoelectron spectrometers. Appl. Surf. Sci. 1991, 47, 225–234. [Google Scholar] [CrossRef]
- Sleigh, C.; Pijpers, A.; Jaspers, A.; Coussens, B.; Meier, R.J. On the determination of atomic charge via ESCA including application to organometallics. J. Electron Spectrosc. Relat. Phenom. 1996, 77, 41–57. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High resolution monochromated X-ray photoelectron spectroscopy of organic polymers: A comparison between solid state data for organic polymers and gas phase data for small molecules. Mol. Phys. 1992, 76, 919–936. [Google Scholar] [CrossRef]
- Glaser, T.; Meinecke, J.; Länger, C.; Luy, J.; Tonner, R.; Koert, U.; Dürr, M. Combined XPS and DFT investigation of the adsorption modes of methyl enol ether functionalized cyclooctyne on Si(001). Chemphyschem 2021, 22, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Wang, Y.; Xia, W.; Muhler, M. Thermal stability and reducibility of oxygen-containing functional groups on multiwalled carbon nanotube surfaces: A quantitative high-resolution XPS and TPD/TPR study. J. Phys. Chem. C 2008, 112, 16869–16878. [Google Scholar] [CrossRef]
- Zhao, G.; Wu, X.; Li, W.; Wang, X. Hydroquinone bis(diphenyl phosphate) as an antiwear/extreme pressure additive in polyalkylene glycol for steel/steel contacts at elevated temperature. Ind. Eng. Chem. Res. 2013, 52, 7419–7424. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy Database. Available online: https://srdata.nist.gov/xps/ (accessed on 26 March 2024).
- Ge, X.; Wu, X.; Shi, Q.; Wang, W. Lubrication performances of polyalkylene glycols at steel interface under external electric fields. Nanomaterials 2022, 12, 2067. [Google Scholar] [CrossRef]



| Voltages (V) | −0.8 | −0.1 | 0.0 | +0.1 |
|---|---|---|---|---|
| Fluid film thickness (hc/μm) | 150 | 61 | 139 | 114 |
| Film thickness ratio (λ) | 2.3 | 1.1 | 2.3 | 2.9 |
| Lubrication state | ML | ML | ML | ML |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, X.; Wu, X.; Shi, Q.; Liu, Y.; Liang, H. Influence of Electrical Stimulation on the Friction Performance of LiPF6-Based Ionic Liquids. Lubricants 2024, 12, 167. https://doi.org/10.3390/lubricants12050167
Ge X, Wu X, Shi Q, Liu Y, Liang H. Influence of Electrical Stimulation on the Friction Performance of LiPF6-Based Ionic Liquids. Lubricants. 2024; 12(5):167. https://doi.org/10.3390/lubricants12050167
Chicago/Turabian StyleGe, Xiangyu, Xiaodong Wu, Qiuyu Shi, Yanfei Liu, and He Liang. 2024. "Influence of Electrical Stimulation on the Friction Performance of LiPF6-Based Ionic Liquids" Lubricants 12, no. 5: 167. https://doi.org/10.3390/lubricants12050167
APA StyleGe, X., Wu, X., Shi, Q., Liu, Y., & Liang, H. (2024). Influence of Electrical Stimulation on the Friction Performance of LiPF6-Based Ionic Liquids. Lubricants, 12(5), 167. https://doi.org/10.3390/lubricants12050167







