Mechanical and Tribological Behaviour of Surface-Graphitised Al-1100 Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Setup
2.3. Characterization
3. Results and Discussions
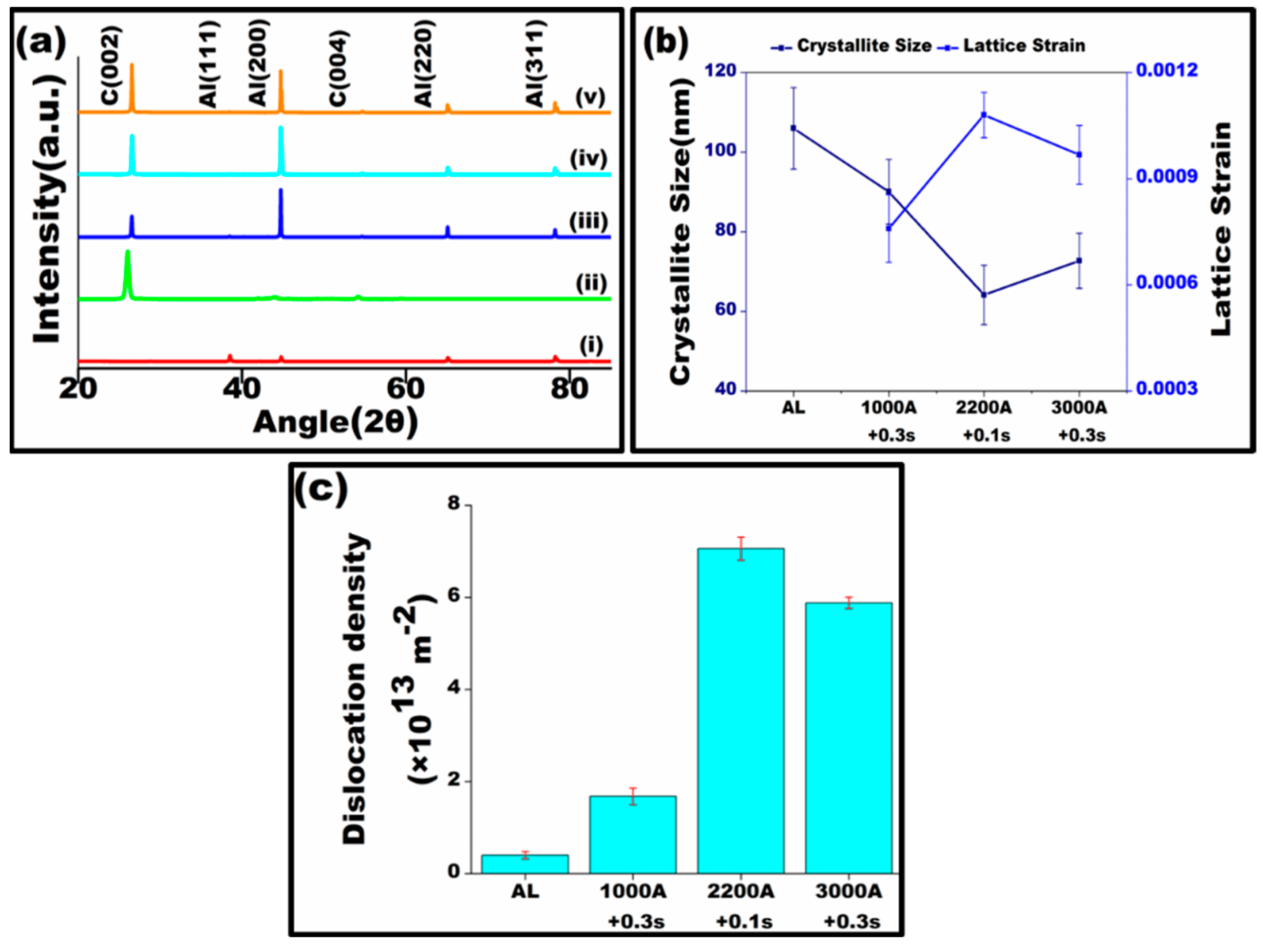
3.1. Microstructural Characterization
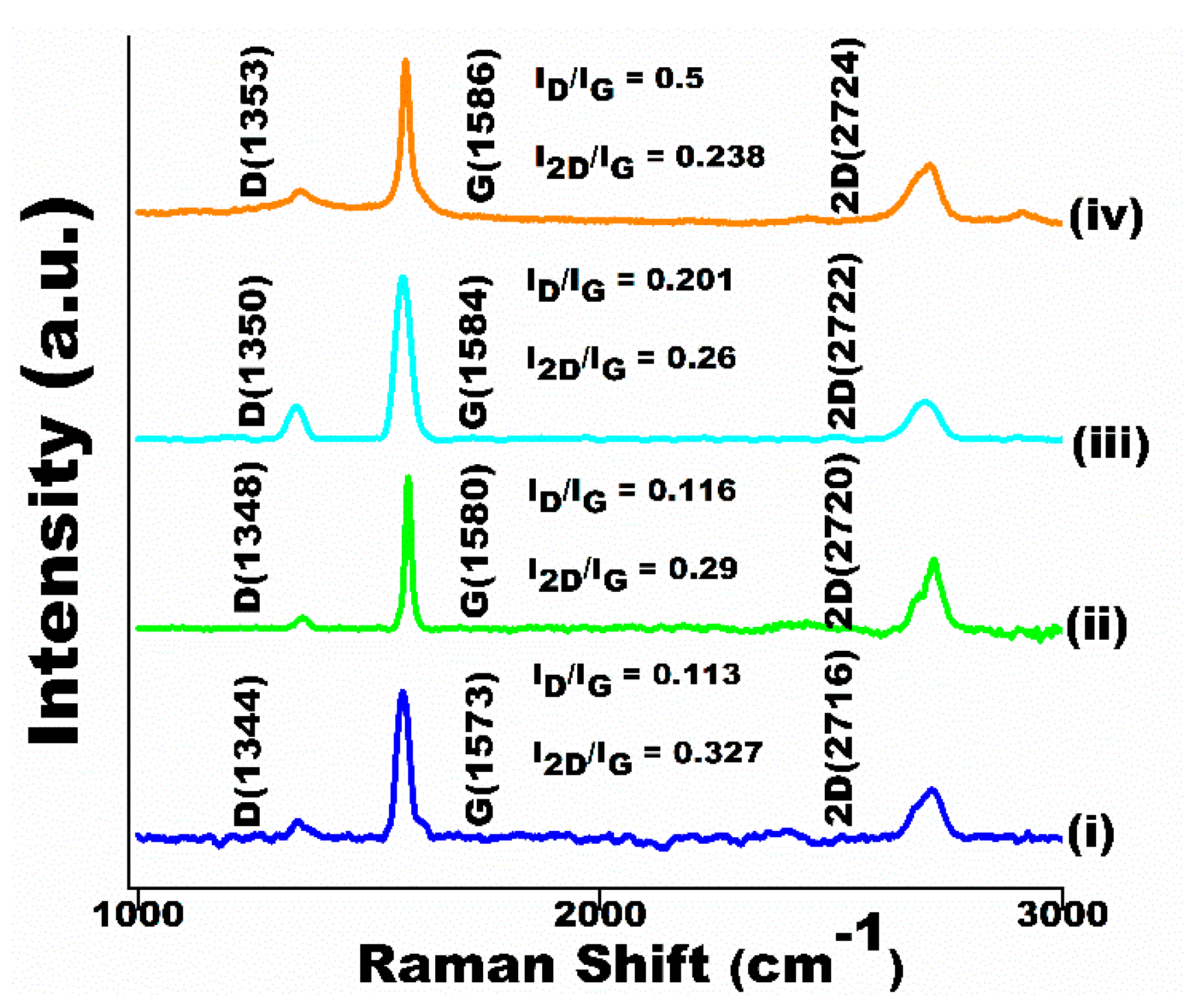
3.2. Spectroscopic Studies
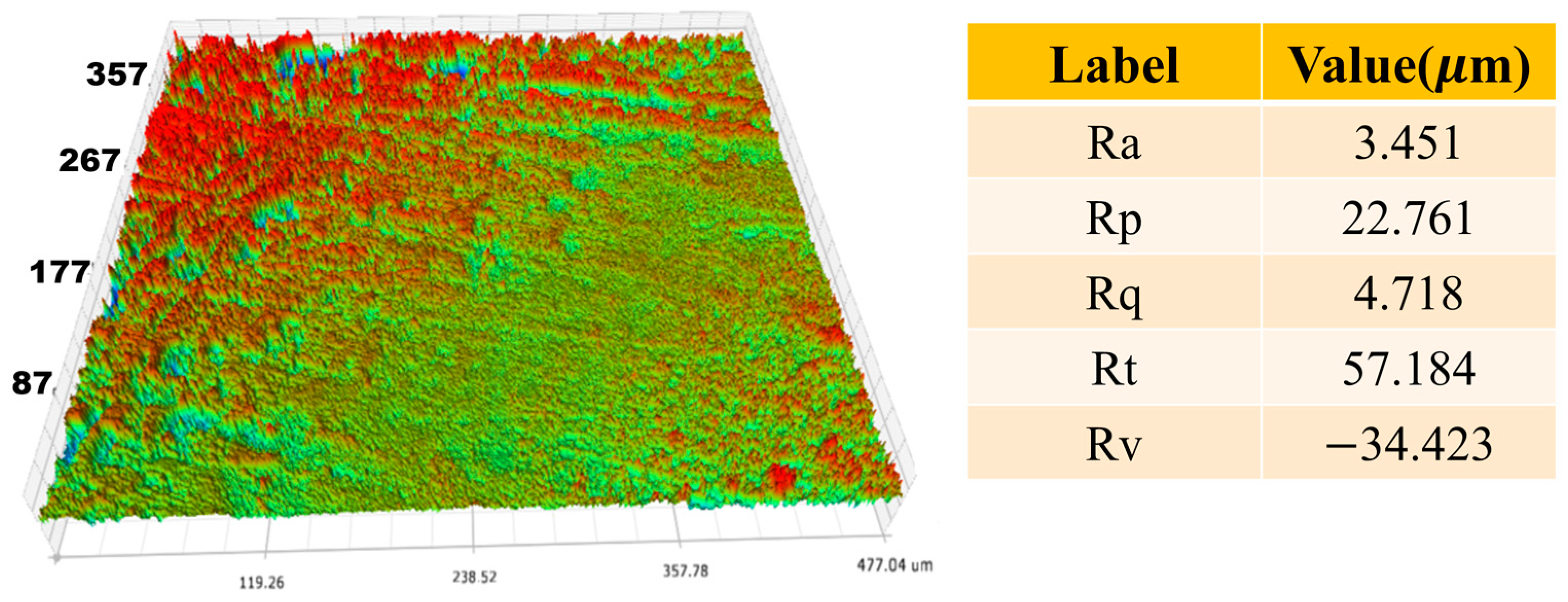
3.3. Surface Mechanical Characterization
3.4. Tribological Properties
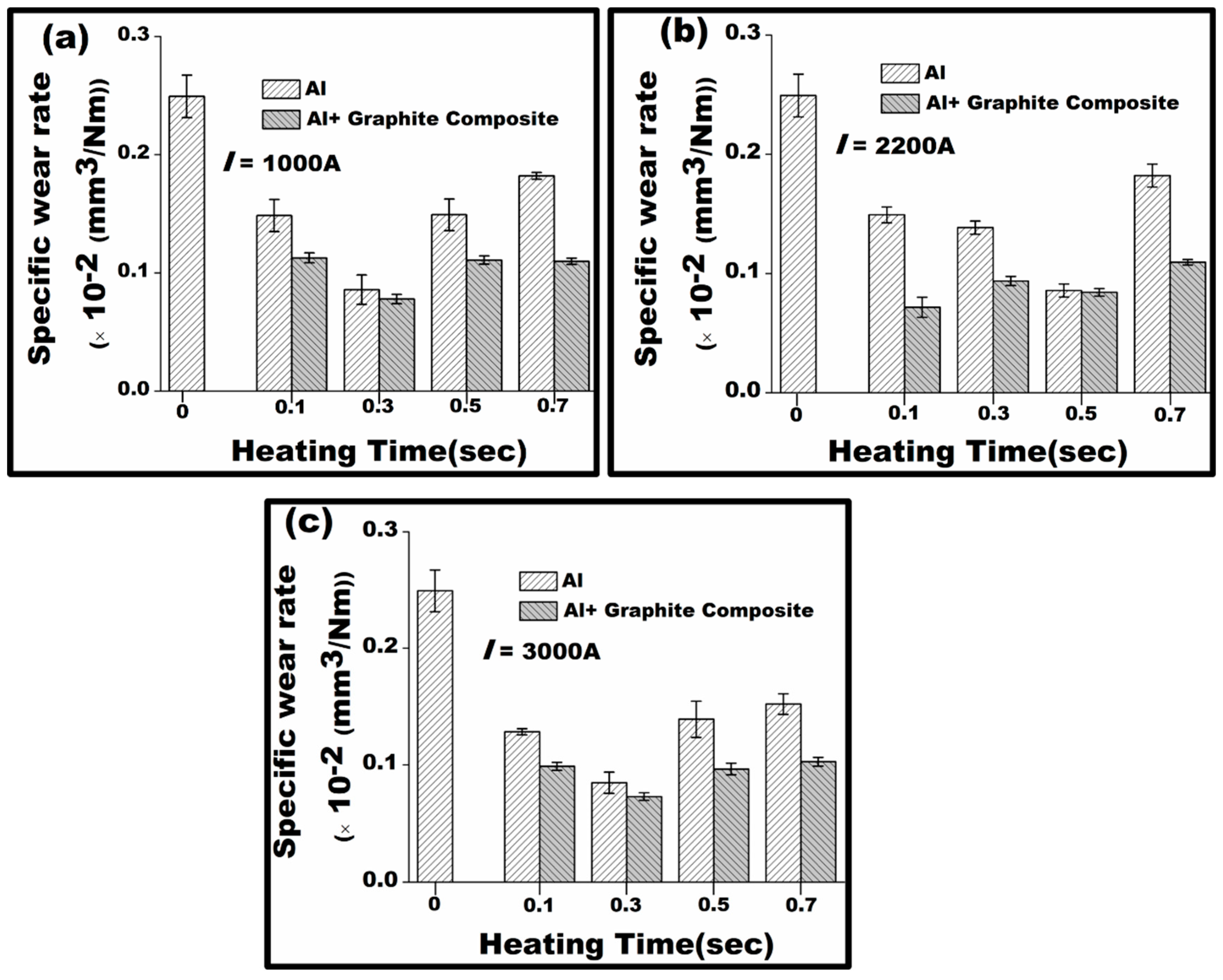
3.4.1. Specific Wear Rate Analysis
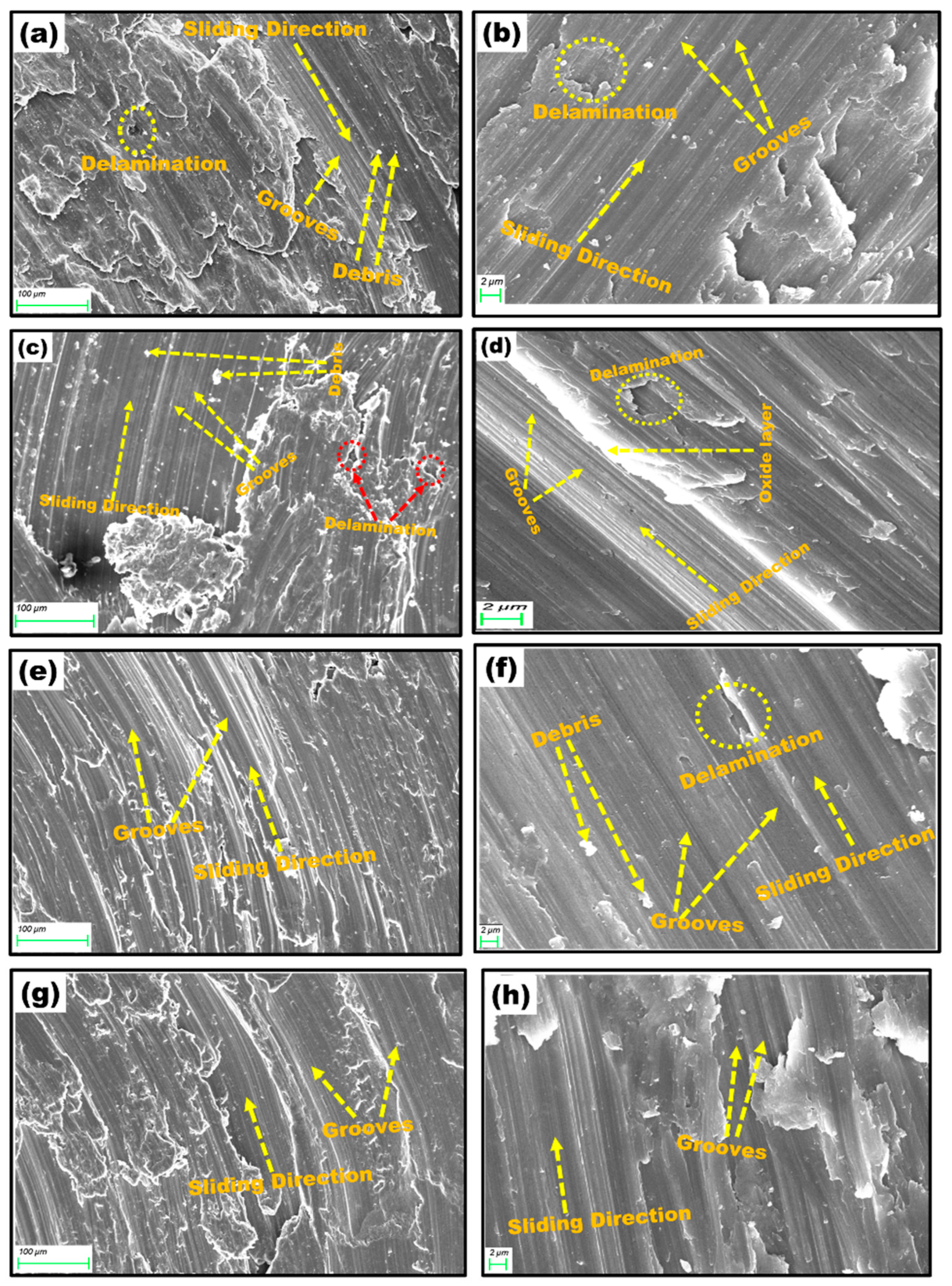
3.4.2. SEM Analysis of Worn Surfaces
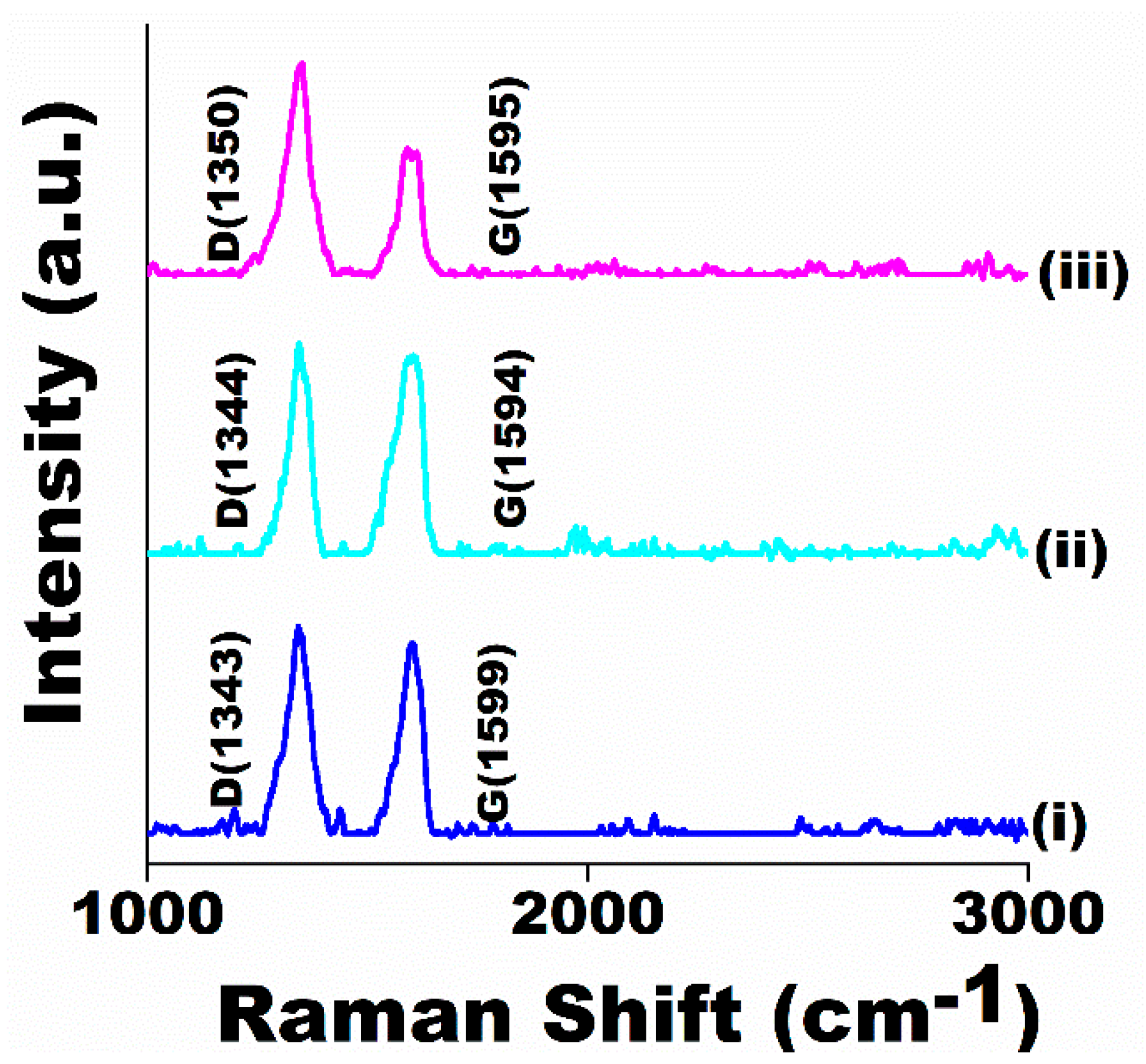
3.4.3. Raman Analysis of Worn Surfaces
3.4.4. Coefficient of Friction (COF)
4. Conclusions
- The process of surface heating and infusion of graphite particles into the Al matrix primarily depends on key factors such as current and time. The recommended optimal range is 1–3 kA for current and 0.1–0.7 s for time.
- Microstructural examination confirms the effective incorporation of graphite particles into the Al matrix along with observed oxidation. Other chemical responses and carbide materialisation are less expected due to minimal involvement of processing temperatures, indicating that the bonding amid reinforcement and matrix is mainly mechanical.
- Raman analysis reveals upward shifts in band frequencies and increased intensity, indicating compressive stress on graphite particles due to elevated heat. XRD investigation demonstrates alterations in grain structure and enhanced lattice strain, a consequence of varied heat exposure.
- Under optimal processing conditions, graphite-infused aluminium exhibits 2.1 times increase in microhardness due to various strengthening mechanisms from the presence of graphite particles. However, nanoindentation experiments suggest that the surface mechanical properties of graphite-infused aluminium are poorer to those of unprocessed aluminium.
- The wear test results indicate that graphite impregnation enhances the wear resistance of aluminium. Both the specific wear rate and the coefficient of friction (COF) of the graphite-infused aluminium decrease by over 50% as a result of graphite particles smearing across the wear track.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alpas, A.T.; Zhang, J. Effect of SiC particulate reinforcement on the dry sliding wear of aluminium-silicon alloys (A356). Wear 1992, 155, 83–104. [Google Scholar] [CrossRef]
- Kok, M. Production and mechanical properties of Al2O3 particle-reinforced 2024 aluminium alloy composites. J. Mater. Process. Technol. 2005, 161, 381–387. [Google Scholar] [CrossRef]
- Walker, J.C.; Rainforth, W.M.; Jones, H. Lubricated sliding wear behaviour of aluminium alloy composites. Wear 2005, 259, 577–589. [Google Scholar] [CrossRef]
- Garg, H.K.; Sharma, S.; Kumar, R.; Manna, A.; Dwivedi, S.P.; Abbas, M.; Kumar, A.; Khan, M.I.; Bisht, Y.S. Mechanical, tribological, and morphological properties of SiC and Gr reinforced Al-0.7Fe-0.6Si-0.375Cr-0.25Zn based stir-casted hybrid metal matrix composites for automotive applications: Fabrication and characterizations. J. Mater. Res. Technol. 2024, 28, 3267–3285. [Google Scholar] [CrossRef]
- Liu, Y.; Rohatgi, P.K.; Ray, S. Tribological characteristics of aluminum-50 Vol Pct graphite composite. Metall. Trans. A 1993, 24, 151–159. [Google Scholar] [CrossRef]
- Sharma, P.; Paliwal, K.; Garg, R.K.; Sharma, S.; Khanduja, D. A study on wear behaviour of Al/6101/graphite composites. J. Asian Ceram. Soc. 2017, 5, 42–48. [Google Scholar] [CrossRef]
- Baradeswaran, A.; Perumal, A.E. Wear and mechanical characteristics of Al 7075/graphite composites. Compos. Part B Eng. 2014, 56, 472–476. [Google Scholar] [CrossRef]
- Akhlaghi, F.; Zare-Bidaki, A. Influence of graphite content on the dry sliding and oil impregnated sliding wear behavior of Al 2024—Graphite composites produced by in situ powder metallurgy method. Wear 2009, 266, 37–45. [Google Scholar] [CrossRef]
- El-Sayed Seleman, M.M.; Ahmed, M.M.Z.; Ataya, S. Microstructure and mechanical properties of hot extruded 6016 aluminum alloy/graphite composites. J. Mater. Sci. Technol. 2018, 34, 1580–1591. [Google Scholar] [CrossRef]
- Akhil, M.G.; Manoj, V.; Suja, P.; Rajan, T.P.D. Squeeze infiltration processing of lightweight smart aluminum graphite functionally graded composite for enhanced wear resistance. J. Manuf. Process. 2023, 104, 177–188. [Google Scholar] [CrossRef]
- Hasan, M.S.; Kordijazi, A.; Rohatgi, P.K.; Nosonovsky, M. Machine learning models of the transition from solid to liquid lubricated friction and wear in aluminum-graphite composites. Tribol. Int. 2022, 165, 107326. [Google Scholar] [CrossRef]
- Yi, L.-F.; Noguchi, K.; Liu, L.; Otsu, A.; Onda, T.; Chen, Z.-C. Deformation behavior of graphite and its effect on microstructure and thermal properties of aluminum/graphite composites. J. Alloys Compd. 2023, 933, 167752. [Google Scholar] [CrossRef]
- Mohamad, S.; Liza, S.; Yaakob, Y. Strengthening of the mechanical and tribological properties of composite oxide film formed on aluminum alloy with the addition of graphite. Surf. Coat. Technol. 2020, 403, 126435. [Google Scholar] [CrossRef]
- Deaquino-Lara, R.; Gutiérrez-Castañeda, E.; Estrada-Guel, I.; Hinojosa-Ruiz, G.; García-Sánchez, E.; Herrera-Ramírez, J.M.M.; Pérez-Bustamante, R.; Martínez-Sánchez, R. Structural characterization of aluminium alloy 7075–graphite composites fabricated by mechanical alloying and hot extrusion. Mater. Des. 2014, 53, 1104–1111. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Z.Q. Structural characterization of a mechanical alloyed Al–C mixture. J. Alloys Compd. 2006, 414, 107–112. [Google Scholar] [CrossRef]
- Son, H.T.; Kim, T.S.; Suryanarayana, C.; Chun, B.S. Homogeneous dispersion of graphite in a 6061 aluminum alloy by ball milling. Mater. Sci. Eng. A 2003, 348, 163–169. [Google Scholar] [CrossRef]
- Pai, B.C.; Rohatgi, P.K. Production of cast aluminium-graphite particle composites using a pellet method. J. Mater. Sci. 1978, 13, 329–335. [Google Scholar] [CrossRef]
- Zamani, N.A.B.N.; Asif Iqbal, A.K.M.; Nuruzzaman, D.M. Mechanical and Tribological Behavior of Powder Metallurgy Processed Aluminum–Graphite Composite. Russ. J. Non-Ferrous Met. 2019, 60, 274–281. [Google Scholar] [CrossRef]
- Krishnan, B.P.; Surappa, M.K.; Rohatgi, P.K. The UPAL process: A direct method of preparing cast aluminium alloy-graphite particle composites. J. Mater. Sci. 1981, 16, 1209–1216. [Google Scholar] [CrossRef]
- Hassan, A.M.; Tashtoush, G.M.; Al-Khalil, J.A. Effect of Graphite and/or Silicon Carbide Particles Addition on the Hardness and Surface Roughness of Al-4 wt% Mg Alloy. J. Compos. Mater. 2007, 41, 453–465. [Google Scholar] [CrossRef]
- Akhlaghi, F.; Pelaseyyed, S.A. Characterization of aluminum/graphite particulate composites synthesized using a novel method termed “in-situ powder metallurgy”. Mater. Sci. Eng. A 2004, 385, 258–266. [Google Scholar] [CrossRef]
- Sahoo, B.; Kumar, R.; Joseph, J.; Sharma, A.; Paul, J. Preparation of aluminium 6063-graphite surface composites by an electrical resistance heat assisted pressing technique. Surf. Coat. Technol. 2017, 309, 563–572. [Google Scholar] [CrossRef]
- Radhika, N.; Subramanian, R.; Venkat Prasat, S.; Anandavel, B. Dry sliding wear behaviour of aluminium/alumina/graphite hybrid metal matrix composites. Ind. Lubr. Tribol. 2012, 64, 359–366. [Google Scholar] [CrossRef]
- Sahoo, B.; Paul, J. Solid state processed Al-1100 alloy/MWCNT surface nanocomposites. Materialia 2018, 2, 196–207. [Google Scholar] [CrossRef]
- Sahoo, B.; Joseph, J.; Sharma, A.; Paul, J. Particle size and shape effects on the surface mechanical properties of aluminium coated with carbonaceous materials. J. Compos. Mater. 2019, 53, 261–270. [Google Scholar] [CrossRef]
- Sahoo, B.; Joseph, J.; Sharma, A.; Paul, J. Surface modification of aluminium by graphene impregnation. Mater. Des. 2017, 116, 51–64. [Google Scholar] [CrossRef]
- Wilson, W.E.; Angadi, S.V.; Jackson, R.L. Surface separation and contact resistance considering sinusoidal elastic–plastic multi-scale rough surface contact. Wear 2010, 268, 190–201. [Google Scholar] [CrossRef]
- ASTM G99-23; Standard Test Method for Wear and Friction Testing with a Pin-on-Disk or Ball-on-Disk Apparatus 1. ASTM: West Conshohocken, PA, USA, 2023; pp. 1–6. [CrossRef]
- Pelleg, J.; Ashkenazi, D.; Ganor, M. The influence of a third element on the interface reactions in metal-matrix composites (MMC): Al-graphite system. Mater. Sci. Eng. A 2000, 281, 239–247. [Google Scholar] [CrossRef]
- Sahoo, B.; Narsimhachary, D.; Paul, J. Tribological Behavior of Solid-State Processed Al-1100/GNP Surface Nanocomposites. J. Mater. Eng. Perform. 2018, 27, 6529–6544. [Google Scholar] [CrossRef]
- Ungár, T.; Leoni, M.; Scardi, P. The dislocation model of strain anisotropy in whole powder-pattern fitting: The case of an Li–Mn cubic spinel. J. Appl. Crystallogr. 1999, 32, 290–295. [Google Scholar] [CrossRef]
- Kishor, R.; Sahu, L.; Dutta, K.; Mondal, A.K. Assessment of dislocation density in asymmetrically cyclic loaded non-conventional stainless steel using X-ray diffraction profile analysis. Mater. Sci. Eng. A 2014, 598, 299–303. [Google Scholar] [CrossRef]
- Adachi, H.; Miyajima, Y.; Sato, M.; Tsuji, N. Evaluation of Dislocation Density for 1100 Aluminum with Different Grain Size during Tensile Deformation by Using In-Situ X-ray Diffraction Technique. Mater. Trans. 2015, 56, 671–678. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1291. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Wang, Y.; Alsmeyer, D.C.; McCreery, R.L. Raman spectroscopy of carbon materials: Structural basis of observed spectra. Chem. Mater. 1990, 2, 557–563. [Google Scholar] [CrossRef]
- del Corro, E.; Kavan, L.; Kalbac, M.; Frank, O. Strain Assessment in Graphene Through the Raman 2D′ Mode. J. Phys. Chem. C 2015, 119, 25651–25656. [Google Scholar] [CrossRef]
- Sahoo, B.; Narsimhachary, D.; Paul, J. Tribological characteristics of aluminium-CNT/graphene/graphite surface nanocomposites: A comparative study. Surf. Topogr. Metrol. Prop. 2019, 7, 034001. [Google Scholar] [CrossRef]
- Katagiri, G.; Ishida, H.; Ishitani, A. Raman spectra of graphite edge planes. Carbon 1988, 26, 565–571. [Google Scholar] [CrossRef]
- Arsenault, R.J.; Fisher, R.M. Microstructure of fiber and particulate SiC in 6061 Al composites. Scr. Metall. 1983, 17, 67–71. [Google Scholar] [CrossRef]
- Sahoo, B.; Das, T.; Paul, J. Effect of ball milling and subsequent size reduction of graphite flakes on the surface mechanical properties of graphitized Al-1100 alloy. Results Surf. Interfaces 2023, 12, 100134. [Google Scholar] [CrossRef]
- Kestursatya, M.; Kim, J.K.; Rohatgi, P.K. Friction and wear behavior of a centrifugally cast lead-free copper alloy containing graphite particles. Metall. Mater. Trans. A 2001, 32, 2115–2125. [Google Scholar] [CrossRef]
- Imada, Y.; Nakajima, K. Effect of Humidity on the Friction and Wear Properties of Sn. J. Tribol. 1995, 117, 737. [Google Scholar] [CrossRef]
- El-Ghazaly, A.; Anis, G.; Salem, H.G. Effect of graphene addition on the mechanical and tribological behavior of nanostructured AA2124 self-lubricating metal matrix composite. Compos. Part A Appl. Sci. Manuf. 2017, 95, 325–336. [Google Scholar] [CrossRef]
- Jafari, M.; Enayati, M.H.; Abbasi, M.H.; Karimzadeh, F. Compressive and wear behaviors of bulk nanostructured Al2024 alloy. Mater. Des. 2010, 31, 663–669. [Google Scholar] [CrossRef]
- Suh, N.P. The delamination theory of wear. Wear 1973, 25, 111–124. [Google Scholar] [CrossRef]
- Singh, J. Fabrication characteristics and tribological behavior of Al/SiC/Gr hybrid aluminum matrix composites: A review. Friction 2016, 4, 191–207. [Google Scholar] [CrossRef]
- Iqbal, M.W.; Singh, A.K.; Iqbal, M.Z.; Eom, J. Raman fingerprint of doping due to metal adsorbates on graphene. J. Phys. Condens. Matter 2012, 24, 335301. [Google Scholar] [CrossRef]
- Tsoukleri, G.; Parthenios, J.; Papagelis, K.; Jalil, R.; Ferrari, A.C.; Geim, A.K.; Novoselov, K.S.; Galiotis, C. Subjecting a Graphene Monolayer to Tension and Compression. Small 2009, 5, 2397–2402. [Google Scholar] [CrossRef]
- Park, J.G.; Keum, D.H.; Lee, Y.H.; Gil, J.; Hoon, D.; Hee, Y. Strengthening mechanisms in carbon nanotube-reinforced aluminum composites. Carbon 2015, 95, 690–698. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, S.M.; Bae, D.H. Wear characteristic of aluminum-based composites containing multi-walled carbon nanotubes. Wear 2010, 270, 12–18. [Google Scholar] [CrossRef]






| Constituent | Si | Fe | Others | Al |
|---|---|---|---|---|
| Quantity (wt.%) | 0.748 | 0.828 | 0.224 | 98.2 |
| G Band (cm−1) | ID/IG | I2D/IG | |
|---|---|---|---|
| Graphite | 1573 | 0.113 | 0.327 |
| 1000 A + 0.3 s | 1580 | 0.116 | 0.29 |
| 2200 A + 0.1 s | 1584 | 0.201 | 0.26 |
| 3000 A + 0.3 s | 1586 | 0.5 | 0.238 |
| Before Wear Test | After Wear Test | |||
|---|---|---|---|---|
| G Band (cm−1) | ID/IG | G Band (cm−1) | ID/IG | |
| 1000 A + 0.3 s | 1580 | 0.116 | 1599 | 1.09 |
| 2200 A + 0.1 s | 1584 | 0.201 | 1594 | 1.07 |
| 3000 A + 0.3 s | 1586 | 0.5 | 1595 | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahoo, B.; Paul, J.; Sharma, A. Mechanical and Tribological Behaviour of Surface-Graphitised Al-1100 Alloy. Lubricants 2024, 12, 139. https://doi.org/10.3390/lubricants12040139
Sahoo B, Paul J, Sharma A. Mechanical and Tribological Behaviour of Surface-Graphitised Al-1100 Alloy. Lubricants. 2024; 12(4):139. https://doi.org/10.3390/lubricants12040139
Chicago/Turabian StyleSahoo, Baidehish, Jinu Paul, and Abhishek Sharma. 2024. "Mechanical and Tribological Behaviour of Surface-Graphitised Al-1100 Alloy" Lubricants 12, no. 4: 139. https://doi.org/10.3390/lubricants12040139
APA StyleSahoo, B., Paul, J., & Sharma, A. (2024). Mechanical and Tribological Behaviour of Surface-Graphitised Al-1100 Alloy. Lubricants, 12(4), 139. https://doi.org/10.3390/lubricants12040139






