Abstract
In order to more accurately characterize the effects of nanoparticles on lubricant viscosity, the effects of copper dialkyl dithiophosphate (HDDP)-modified (CuDDP) nanoparticles on the dynamic viscosity of mineral oils 150N, alkylated naphthalene (AN5), diisooctyl sebacate (DIOS), and polyalphaolefins (PAO4, PAO6, PAO10, PAO40, and PAO100) were investigated at an experimental temperature of 40 °C and additive mass fraction ranging from 0.5% to 2.5%. CuDDP exhibits a viscosity-reducing effect on higher-viscosity base oils, such as PAO40 and PAO100, and a viscosity-increasing effect on lower-viscosity base oils, namely, 150N, AN5, DIOS, PAO4, PAO6, and PAO10. These effects can be attributed to the interfacial slip effect and the shear resistance of the nanoparticles. The experimental dynamic viscosity of the eight base oils containing CuDDP was compared with that calculated by the three classical formulae of nanofluid viscosity, The predicted viscosity values of the formulae deviated greatly from the experimental viscosity values, with the maximum deviation being 7.9%. On this basis, the interface slip effect was introduced into Einstein’s formula, the interface effect was quantified with the aniline point of the base oil, and a new equation was established to reflect the influence of CuDDP nanoparticles on lubricating oil viscosity. It can better reflect the influence of CuDDP on the viscosity of various base oils, and the deviation from the experimental data is less than 1.7%.
1. Introduction
Lubricating oil has a significant impact on the working efficiency and service life of mechanical equipment, since lubricants can reduce the friction and wear between moving parts, as well as the energy loss in a mechanical movement. With the rapid development of nanomaterials, researchers have recently conducted extensive studies on the application of nanomaterials as lubricant additives. It has been found that nanomaterials with unique characteristics (e.g., small dimensions, large surface area, and high surface activity) can improve the friction-reducing and anti-wear abilities of lubricating oil [1,2,3,4]. Among various nanomaterials, nano-copper with low shear strength and grain boundary slip effect might be a promising multifunctional lubricant additive thanks to its synergistic friction reduction, anti-wear, and self-repairing abilities [5,6,7,8]. This new nanoadditive can perform excellently, providing more reliable support for the smooth operation of mechanical equipment. Considering energy and environmental perspectives, lubricating oil can effectively reduce energy loss and improve the energy efficiency of mechanical equipment, thereby relieving energy pressure to a certain extent. In addition, reducing friction and wear also helps to extend the service life of mechanical equipment and reduce resource consumption and waste generation. Therefore, studying nanomaterials as lubricant additives holds significance for the machinery industry. Nanoscale particles are dispersed in a conventional fluid medium (e.g., water, oil, or glycol) to form a homogeneous and stable fluid medium known as a nanofluid, in which the lubricating oil is used as a solvent known as a nanolubricant. In future developments, we can anticipate further optimization and broader application of nanolubricants to meet mechanical equipment’s increasingly stringent performance and environmental requirements. This advancement promises new possibilities for industrial production. Consequently, in-depth investigation and application of nanolubricants possess significant scientific and practical value.
Viscosity is a pivotal property of lubricating oil, acting as a crucial indicator of lubricating oil fluidity and internal friction. Viscosity values that are either too high or too low can detrimentally affect the lubricant’s performance: increased viscosity may lead to heightened frictional forces during fluid lubrication, while reduced viscosity can decrease the lubricant’s load-bearing capacity [9,10]. Breki et al. [11] integrated Einstein’s viscosity equation with dynamic lubrication theory, elucidating the relationship between the friction coefficient and the solid-phase volume fraction, underscoring the significance of viscosity in fluid lubrication. This underscores the importance of investigating the influence of nanoadditives on the viscosity of nanolubricants. However, the existing literature presents divergent results and conclusions concerning the impact of nanoadditives on lubricant viscosity [12,13,14,15,16,17]. Various experimental data show that the addition of nanomaterials may cause complex changes in lubricating oil viscosity, and the specific effect may depend on the type of nanoparticles, mass fraction, and operating conditions. For example, Ma et al. [18] demonstrated that introducing ZnO nanoparticles enhances the viscosity of SAE50 lubricant, likely due to augmented resistance to lubricant flow induced by nanoparticle agglomeration under van der Waals forces. Hemmat et al. [19] found that Al2O3 nanoadditives elevate the viscosity of 10W40 lubricant at 55 °C by 132%, noting that nanolubricant viscosity initially increases and then diminishes with rising temperature, attributed to the augmented shear thermal effect. They [20] further found that MgO nanoparticles reduce the viscosity of 5W30 lubricant, with the spherical nanoparticles functioning as roller balls between fluid layers. Mustafa et al. [21] observed that at low concentrations of TiO2–CuO NPs, the mobility between nano-lubricating oil is facilitated, slightly reducing viscosity. However, at higher concentrations, due to agglomeration or increased particle size, the movement between oil layers is hindered, resulting in elevated viscosity. Sui et al. [22] examined the viscosification effects of four types of SiO2 on PAO100, noting that while nanoparticles modified with different functional groups did not significantly impact PAO at 100 °C, variations in nanoparticle size did affect PAO viscosity at this temperature.
In the realm of nanofluid viscosity, comprehensive research has elucidated the influence of nanomaterials on the viscosity attributes of fluids. The viscosity of nanofluids is intricately linked to the nanomaterials’ size, density, ultrasonic treatment time, and interfacial interactions. For example, Abdelhalim et al. [23] observed an increase in the viscosity of nanofluids with the incorporation of larger Au nanoparticles, reinforcing the notion that nanoparticle size is a critical determinant of nanofluid viscosity. Dehghani et al. [24] compared the viscosities of WO3 and Al2O3 nanoparticles dispersed in deionized water and liquid paraffin, noting higher viscosities in the former, which may be attributed to WO3’s greater density and reduced Brownian motion velocity, underscoring density’s role in influencing lubrication characteristics. Zhang et al. [25] conducted an experimental investigation into the viscosity of hydrophilic TiO2–water and hydrophobic TiO2–water nanofluids, discovering that hydrophilic nanoparticles form water-attracting layers more swiftly than hydrophobic ones, leading to higher viscosities in nanofluids with hydrophilic nanoparticles.
These investigations enhance our comprehension of how nanoparticles affect fluid viscosity and offer empirical data elucidating the mechanisms by which nanoparticles modulate viscosity. Nonetheless, a consolidated consensus on the impact of nanoparticles on viscosity and the associated mechanisms remains elusive. Therefore, to gain a deeper understanding of nanomaterials’ effects on lubricant viscosity, further research is imperative. In this study, we examine the impact of dialkyl dithiophosphate (HDDP) and copper HDDP-modified (CuDDP) nanoparticles on the kinematic viscosity of various base oils. Diverging from prior research, we introduce the aniline point as a metric of lubricant polarity. By integrating the aniline point, we formulate a novel equation intended to characterize the viscosity of base oils infused with nanoparticles across different volume fractions. This innovative methodology offers a fresh lens through which to view the impact of nanoparticles on lubricant viscosity. Through rigorous analysis of the correlations between predicted dynamic viscosity and measured data, we aim to uncover regularity and determinants. This endeavor enhances our understanding of how nanoparticles influence lubricant viscosity. Finally, we undertake a thorough comparison of the experimental outcomes with the computational results to validate the accuracy of the newly developed formulae. This comparison is instrumental in ascertaining the practical applicability of our theoretical model and establishes a groundwork for future inquiries.
2. Materials and Methods
2.1. Material Characterization
Fourier-transform infrared (FTIR) spectroscopy (Tensor II, Bruker, Billerica, MS, USA) covering a wavelength range of 400 cm−1 to 4000 cm−1 was employed to ascertain the composition of the modifier. The thermal stability and modifier content of the sample were examined using a thermogravimetric analyzer (TGA/DSC3+, Mettler Toledo, Greifensee, Switzerland). The thermal analysis was conducted in a nitrogen atmosphere, with a heating rate of 10 °C per minute, spanning from 25 °C to 900 °C. To eliminate impurities, the sample was maintained at 100 °C for 5 min before the analysis. Additionally, the morphology and size of the CuDDP nanoparticles were characterized using transmission electron microscopy (TEM, JEM-F200, JEOL, Tokyo, Japan). This technique provided detailed insights into the nanostructure of CuDDP nanoparticles, which is crucial for understanding its interactions and performance in lubricant applications.
2.2. Sample Preparation
In our experiments, oil-soluble copper nanoparticles (CuNPs) and CuNPs surface-coated with oil-soluble dialkyl dithiophosphate (CuDDP) prepared by the Nanomaterials Engineering and Technology Research Center of Henan University (Kaifeng, China) were used as the nanoadditives. CuDDP nanoparticles were synthesized by means of a redox surface modification technique [26]. They were dispersed in base oils 150N, alkylated naphthalene (AN5), and diisooctyl sebacate (DIOS), as well as polyalphaolefins (PAO4, PAO6, PAO10, PAO40, and PAO100) at mass fractions of 0.5%, 1.0%, 1.5%, 2.0% and 2.5%. HDPP was dispersed in base oils 150N, AN5, DIOS and PAO6 at mass fractions of 0.5%, 1.0%, 1.5%, 2.0% and 2.5%, respectively. The eight base oils employed in the experiment were obtained from Qingdao Lubemater Group (Shandong, China). The typical physical properties of these oils are delineated in Table 1. The lubricant sample was mixed ultrasonically for 15 min to achieve uniform dispersion of the nanoadditives CuDDP and HDDP. CuDDP had good dispersion stability in the base oil used in the experiment, and no samples showed obvious precipitation after standing for 7 days.

Table 1.
Physical properties of HDDP and various base oils at 40 °C.
2.3. Viscosity and Density Tests
A viscometer (SVM3001, Anton Paar, Styria, Austria) was employed to determine the kinematic viscosity of the lubricants. It uses the oscillating piston method to measure the density and dynamic viscosity of the sample and can adjust the temperature and calculate the kinematic viscosity automatically. At the end of the measurement, the measuring cell was fully washed with petroleum ether and anhydrous ethanol and dried by blowing. During the experimental procedure, the thermometer showed an expanded (k = 2) uncertainty of 0.03 °C. Relative expanded (k = 2) uncertainty of 0.35% was estimated for the kinematic viscosity.
The densities of CuDDP and PAO4 dispersions at various concentrations were quantified employing SVM3001. Subsequently, the densities of CuDDP were extrapolated utilizing the equations derived from curve fitting. The expanded (k = 2) uncertainty of density measurements performed with the SVM3001 was 0.0005 g·cm−3.
2.4. Aniline Point Test
The aniline point of the eight base oils used in the experiment was tested with a petroleum product aniline point tester (DZY-013A, Dalian Instruments and Meters Co., Ltd., Dalian, China), and details about the test method are described in GB/T262 “Determination of Aniline Point of Petroleum Products”.
3. Results and Discussion
3.1. Characterization of CuDDP
Figure 1a presents the thermogravimetric analysis (TGA) curve of CuDDP, where the weight loss observed below 100 °C is attributed to the removal of impurities. In the temperature range of 100 °C to 900 °C, CuDDP exhibits a weight loss of approximately 72% (mass fraction), suggesting that the organic modifier constitutes around 72% of its composition. Figure 1b illustrates the FTIR spectrum of CuDDP, with distinct C-H characteristic peaks (including CH3 and CH2) at 1380 cm−1. Pertinent to this study are two pronounced absorption peaks near 1000 cm−1, attributed to P-O-C at 636 cm−1 and P=S, indicative of the HDDP modifier’s presence [27,28]. Figure 1c displays a TEM image and particle size distribution of CuDDP, revealing that the nanoparticles are spherical with an average diameter of approximately 5 nm, as shown in Figure 1d. These observations provide critical insights into the structural and compositional attributes of CuDDP, essential for understanding its behavior and efficacy as a lubricant additive.
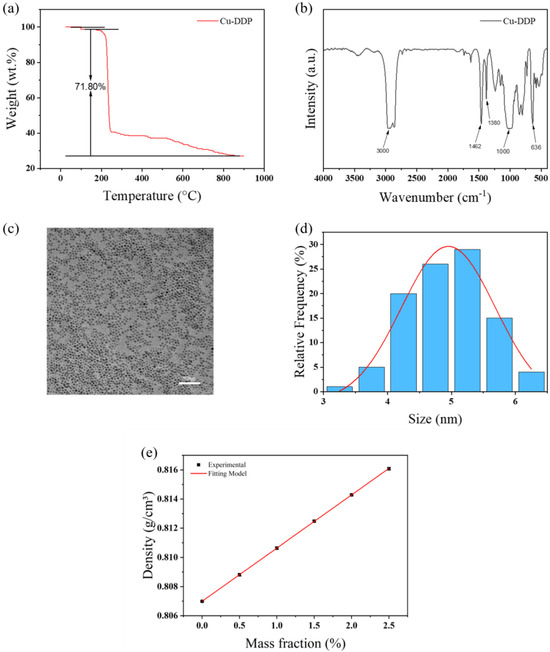
Figure 1.
(a) TGA curves, (b) FTIR spectra of CuDDP, (c) TEM image of CuDDP, (d) and particle size distribution of CuDDP. (e) Density of CuDDP and PAO4 mixture changes with CuDDP mass fraction.
Table 2 enumerates the densities of the PAO4 and CuDDP mixtures. Figure 1e illustrates the dependency of the mixture density on the CuDDP mass fraction, which has been subjected to a fitting procedure to derive Equation (1), where ρnf represents the density of the mixture, ρbf denotes the density of the base oil, ω symbolizes the mass fraction of CuDDP, and k is the fitting factor. This equation exhibits a correlation coefficient R2 exceeding 0.99. Inserting a 100% mass fraction into Equation (1) results in a calculated density of CuDDP of 1.171 g/m3, which is designated in this context the nanoparticle fitting density. It is pertinent to highlight that the nanoparticle fitting density (NFD) is a conceptualized density, formulated to precisely ascertain the volume fraction of nanoparticles within a dispersion, and its applicability is confined to scenarios of low concentration.

Table 2.
Density of PAO4 and CuDDP mixtures at 40 °C.
3.2. Kinematic Viscosity
In the study of lubricating oils, kinematic viscosity is a critical performance parameter that significantly influences the efficacy of lubricating oil in mechanical systems. Arrhenius proposed the following expression for calculating the viscosity of a mixed solution [29]:
In the equation, νm represents the kinematic viscosity of the oil blend, while ν1 and ν2 denote the kinematic viscosities of component 1 and component 2 and ω1 and ω2 represent the mass fractions of component 1 and component 2. The kinematic viscosity of lubricating oils containing HDDP and CuDDP was calculated using Equation (2) and compared with experimental values. Table 3 shows the experimental kinematic viscosity of four base oils containing HDDP. The empirical outcomes indicate that the discrepancy between the measured kinematic viscosity of HDDP base oils and the theoretical values is minimal. This deviation is ascribed to the lipophilic nature of HDDP, which does not significantly affect the flow characteristics of the lubricating oil. Since HDDP and the base oil are merely physically mixed without substantial interactions, the kinematic viscosity of the samples with added HDDP closely align with the values predicted by Equation (2).

Table 3.
Effect of HDDP on kinematic viscosity of four base oils at 40 °C: comparison of experimental values with computed ones (frac., Exp., Calc., and Devi. refer to fraction, experimental, calculated, and deviation).
Furthermore, the data illustrate a declining trend in the kinematic viscosity of the blend comprising HDDP and PAO6, 150N, and AN5 as the mass fraction of HDDP escalates, as depicted in Figure 2. Conversely, under identical conditions, the kinematic viscosity of DIOS exhibits an increasing trend. Thus, the addition of HDDP predictably influences the kinematic viscosity of lubricating oils. This observation is logically consistent with the fact that HDDP’s viscosity is lower than that of PAO6, 150N, and AN5, but higher than DIOS. In contrast, CuDDP is observed to increase the viscosity across all tested base oils, attributable to its stronger shear resistance compared to an equivalent amount of HDDP. This enhanced resistance significantly alters the lubricant’s fluidity, resulting in notable viscosity increases in the base oils.
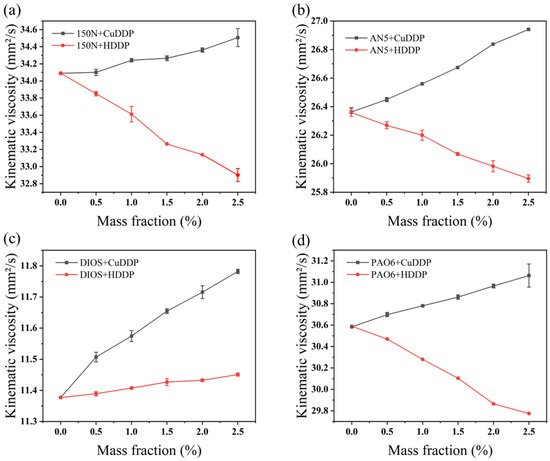
Figure 2.
Effects of CuDDP and HDDP on kinematic viscosity of different base oils at 40 °C: (a) 150N, (b) AN5, (c) DIOS, (d) PAO6.
3.3. Dynamic Viscosity
In the investigation of nanofluid viscosity, the impact of nanoparticles on the fluid’s viscosity is predominantly linked to the shear resistance associated with nanoparticle volume. Consequently, the correlation between the volume fraction of nanoparticles and the dynamic viscosity is frequently employed to illustrate how nanoparticles affect viscosity. The mathematical expression for converting the mass fraction of nanoparticles to their volume fraction is provided below:
In 1905, Einstein focused on elucidating the dimensions of atoms and molecules, resulting in a seminal publication that delineated the relationship between the viscosity of dispersions (including suspensions and colloids) or dilute mixtures consisting of a liquid phase and small dispersed solid particles, and their volume concentration. This relationship was established under the premise of rigid spherical particles moving in an incompressible fluid, leading to the derivation of the subsequent equation [30]:
Batchelor [31] refined Einstein’s equation integrating the influence of Brownian motion and addressing the rotational dynamics of nanoparticles. This augmentation resulted in the derivation of Equation (5):
Brinkman [32] developed an expression to characterize the viscosity of solutions and suspensions at finite concentrations, considering the impact of solute molecule addition to the solution and treating the system as a continuum, as shown in Equation (6):
In Equations (3)–(6), the symbol ω represents the mass fraction, while φ denotes the volume fraction, which is derived from Equation (3). The variables mnp and mnf correspond to the mass of nanoparticle and base oil, ρnp is the density of nanoparticle, ρnf signifies the density of the nanolubricant, μnf refers to the viscosity of the nanofluid, and μbf denotes the viscosity of the base fluid.
The theoretical viscosity values of different base oils with CuDDP were calculated with Equations (4)–(6) and compared with the experimental values shown in Table 4. Pronounced discrepancies were observed between the predicted viscosity values and those obtained experimentally. Notably, the greatest deviations forecast by Einstein’s formula, Batchelor’s formula, and Brinkman’s formula were 7.7%, 7.9%, and 7.9%, respectively. As can be seen in Figure 3, the predicted viscosity values of different base oils tend to increase with the increase in volume fraction of the nanomaterial. The experimental values are lower than predicted ones, which implies that—aside from the shear resistance of the nanoadditive—there are also other factors that cause decreased experimental viscosity values of the lubricants. In addition, the nanoadditive CuDDP leads to increased viscosity in low-viscosity base oils 150N, AN5, DIOS, PAO4, PAO6, and PAO10, as well as decreased viscosity in high-viscosity base oils PAO40 and PAO100. The reason might lie in that the nanoadditive mainly affects the viscosity of the base oil by an anti-shear effect [30], which is more significant in low-viscosity base oils. In high-viscosity base oils, the shear resistance of CuDDP is relatively small, which corresponds to its reduced anti-shear effect and the viscosity of the base oils.

Table 4.
Effects of CuDDP on the dynamic viscosity of diverse base oils at 40 °C: experimental results.
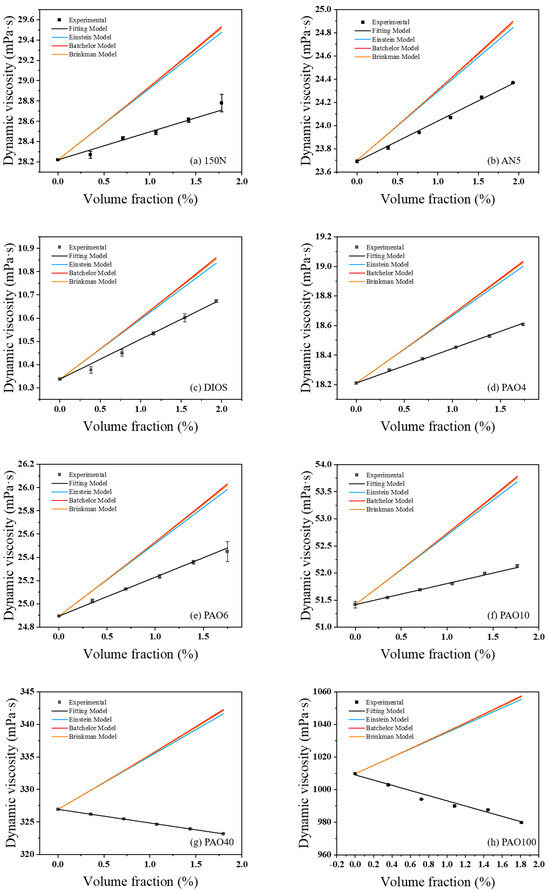
Figure 3.
Comparison of experimental and predicted values of dynamic viscosity of various base oils with CuDDP.
3.4. Fitting Formula
To better understand the impact of CuDDP on the viscosity of base oils, we modified the relationship between the dynamic viscosity of nanomaterial additives and their volume fraction, based on Einstein’s viscosity equation, as follows:
In Equation (7), α is the fitting coefficient, and its value is presented in Table 5. The discrepancy between the dynamic viscosity value derived from Equation (6) and the empirically measured value is observed to be less than 0.5%, and the correlation coefficient R2 is greater than 0.99, which indicates that the fitting equation can better describe the influence of CuDDP on the viscosity of the base oils. Furthermore, based on molecular dynamic simulations and experimental studies, we also established a mechanistic model to describe the solid–liquid interfacial slip behavior and shear viscosity of lubricants in relation to nanomodulation [33]. With the assumption that a fluid is a Newtonian fluid with constant viscosity, a rigid solid would not be deflected upon insertion into the liquid, nor would it be subjected to any inertial force in the flow field. In this case, the shear slip motion is only concentrated on the upper surface of the solid. As seen in Figure 4a, the velocity of the upper shear plate is vx1, and the lower shear plate is static. In a pure liquid, the flow velocity distribution is V0(z), the shear stress is τ0, and the viscosity is μ0. When no solid is added, the shear stress in the Zh0 height range of the liquid would be τh1 = μ0v1(Zh0)/Zh0 = μ0vs1/Zh0, and the shear stress in its Zh0 height range would be τh0 = μ0v0(Zh0)/Zh0. When solids are added to the liquid in the absence of interface slip (Figure 4b), both the upper-surface velocity and the lower-surface velocity of the solids are Vs1. In this case, we have Vs1 > V0(Zh0) and τh1 > τh0, as well as a total stress of τ1 > τ0 and a total viscosity of μ0 < μ1, which corresponds to the Einstein model. When the interface slip effect is significant (Figure 4c), we would have vs2 < v0(Zh0) and a total viscosity of μ2 < μ0, which corresponds to the experimental value in Figure 3a–f. When the interface slip is less than the shear resistance caused by the solid (Figure 4d), we would have vs1 > vs3 > v0, a total stress of τ1 > τ3 > τ0, and a total viscosity of μ1 > μ3 > μ0, which corresponds to the experimental values in Figure 3g,h.

Table 5.
Values of coefficient α for different base oils.
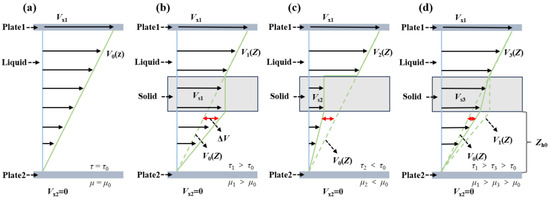
Figure 4.
The influence of solids on fluid viscosity of (a) pure liquid, (b) liquid–solid blend without interface slip, (c) low-viscosity base oils containing CuDDP with a large interface slip, and (d) high-viscosity base oils containing CuDDP with a weak interface slip.
The aniline point is the lowest temperature required for the oil and an equal volume of aniline to dissolve into a single liquid phase with each other. It serves as a measure of the aromatic content in oil and can also reflect the polarity of lubricating oils [34]. Here, we use the aniline point to represent polarity and reflect the interfacial interactions. Table 6 displays the aniline point test outcomes for the analyzed base oils, and there are discernible correlations between the viscosity of polyalphaolefins and their aniline points. Through fitting the experimental data presented in Table 5 with the aniline point, the coefficient α can be articulated as follows:
where A is the aniline point of the base oil and e is the natural logarithmic base. The correlation coefficient is more than 0.98, which indicates that Equation (8) can be used to accurately express the relationship between the base oil aniline point and the coefficient α. When the aniline point is low (Figure 5), α basically remains unchanged with varying polarity of the high-polarity base oil, and in this case, the nanoadditive CuDDP would have a weak interfacial slip as well as an enhanced viscosity-increasing effect therein. When the base oil polarity decreases to a certain degree, α decreases dramatically therewith, and in this case, the interfacial slip is enhanced, while the viscosity-increasing effect of the nanoadditive would be negligible. When the base oil polarity decreases to a certain degree, α decreases dramatically therewith, and in this case, the interfacial slip is enhanced, while the viscosity-increasing effect of the nanoadditive would be negligible. When the base oil polarity continues to decrease, α will drop to 0 and even become negative. In this case, the viscosity-reduction effect caused by interfacial slip is greater than the viscosity-enhancement effect caused by the anti-shear effect of the nanoparticles, and the overall outcome would be a viscosity-reduction effect.

Table 6.
Aniline point of various base oils.
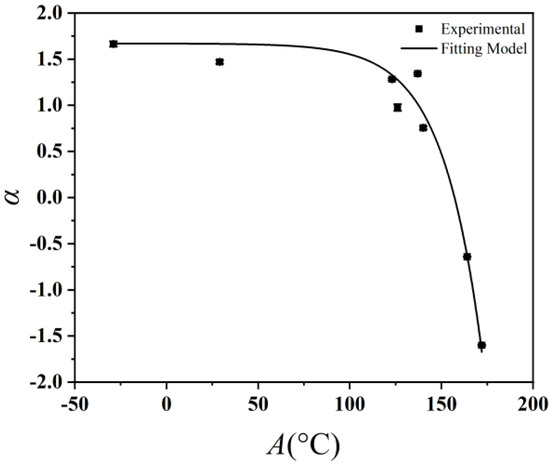
Figure 5.
Variation in coefficient α of base oils with their aniline point.
By bringing Equation (8) into Equation (7), we have the final equation to describe the effect of CuDDP on the viscosity of different base oils:
Equation (9), which incorporates the viscosity and aniline point of the base oil, facilitates the computation of the viscosity for base oils infused with a specific concentration of CuDDP nanoadditive at 40 °C, yielding an error margin of less than 1.7%. This level of precision marks a notable enhancement over traditional nanofluid viscosity estimation models. It is important to note that viscosity is influenced by additional parameters, such as the surface polarity and geometric shape of the nanoparticles. Consequently, Equation (8) is tailored specifically for the viscosity determination of CuDDP nanolubricants. further investigative efforts are necessary to establish applicable predictive models.
4. Conclusions
Under the condition that the experimental temperature was 40 °C and the additive mass fraction ranged from 0.5% to 2.5%, we studied the effects of dialkyl dithiophosphate (HDDP) copper-modified (CuDDP) nanoparticles on the dynamic kinematic viscosity of mineral oils 150N, alkylated naphthalene (AN5), diisooctyl sebacate (DIOS), and polyalphaolefins (PAO4, PAO6, PAO10, PAO40, and PAO100). Based on classical formulae and experimental data, a novel equation was developed to quantify the interfacial interaction between the nanoparticles and base oil using aniline points, elucidating the impact of CuDDP nanoparticles on the viscosity of lubricating oil. The main findings are as follows.
CuDDP reduces the viscosity of higher-viscosity base oils, such as PAO40 and PAO100, and can increase the viscosity of lower-viscosity base oils, such as 150N, AN5, DIOS, PAO4, PAO6, and PAO10. The influence of CuDDP on the viscosity of base oils is governed by the interfacial slip effect and the nanoparticles’ shear resistance. When the interfacial slip effect predominates, it can lead to a more significant decrease in viscosity compared to the viscosity-increasing anti-shear effect of the nanoparticles. Conversely, when shear resistance is more pronounced, an increase in viscosity occurs.
Experimental dynamic viscosity of eight base oils containing CuDDP was compared with values calculated using three classical nanofluid viscosity formulae. A significant deviation was observed between the predicted and experimental viscosity values, with the maximum deviation reaching 7.9%. This discrepancy indicates that traditional nanofluid viscosity equations cannot accurately characterize the effect of CuDDP on the viscosity of base oils.
A modified equation was developed by incorporating specific interfacial effects into Einstein’s viscosity formula and quantifying these effects using aniline points. This new equation elucidates the relationship between the base oil’s aniline points and the viscosity of CuDDP nanoparticles, yielding more accurate viscosity predictions for nanolubricants. The deviation in the predicted values from the experimental data is less than 1.7%, marking a significant improvement over traditional nanofluid viscosity models.
Author Contributions
X.W., conceptualization, methodology, and writing—original draft preparation; S.F. and N.S., investigation and data curation; L.Y., writing—review and editing; Y.Z. and S.Z., resources, writing—review and editing, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge the financial support provided by the National Key Research Development Plan (grant 2023YFB3812104), National Natural Science Foundation of China (grants 52305189 and 52105180), and Henan Province Key Research and Development Project (grant 231111230600).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (S.Z.) upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Nomenclature
| νm | kinematic viscosity of oil blend (mm2/s) |
| ν1 | kinematic viscosity of oil component 1 (mm2/s) |
| ν2 | kinematic viscosity of oil component 2 (mm2/s) |
| ω1 | mass fraction of oil component 1 |
| ω2 | mass fraction of oil component 2 |
| φ | volume fraction |
| mnp | mass of nanoparticle (g) |
| mbf | mass of base oil (g) |
| ρnp | density of nanoparticle (g/m3) |
| ρnf | density of nanoparticle (g/m3) |
| μnf | nanofluid dynamic viscosity (mPa·s) |
| μbf | base fluid dynamic viscosity (mPa·s) |
| vx1 | plate 1 speed (m/s) |
| vx2 | plate 2 speed (m/s) |
| V0(z) | flow velocity (m/s) |
| V1(z) | flow velocity (no interface slip) (m/s) |
| V2(z) | flow velocity (large interface slip) (m/s) |
| V3(z) | flow velocity (weak interface slip) (m/s) |
| Vs | solid velocity (m/s) |
| τ0–τ3 | shear stress (Pa) |
| μ0–μ3 | nanofluid dynamic viscosity (Pa·s) |
| A | aniline point (°C) |
| α | coefficient |
References
- Du, F.; Li, C.; Li, D.; Sa, X.; Yu, Y.; Li, C.; Yang, Y.; Wang, J. Research Progress Regarding the Use of Metal and Metal Oxide Nanoparticles as Lubricant Additives. Lubricants 2022, 10, 196. [Google Scholar] [CrossRef]
- Lu, Z.; Cao, Z.; Hu, E.; Hu, K.; Hu, X. Preparation and tribological properties of WS2 and WS2/TiO2 nanoparticles. Tribol. Int. 2019, 130, 308–316. [Google Scholar] [CrossRef]
- Padgurskas, J.; Rukuiza, R.; Prosyčevas, I.; Kreivaitis, R. Tribological properties of lubricant additives of Fe, Cu and Co nanoparticles. Tribol. Int. 2013, 60, 224–232. [Google Scholar] [CrossRef]
- Duan, L.; Jia, D.; Zhan, S.; Zhang, W.; Yang, T.; Tu, J.; Liu, J.; Li, J.; Duan, H. Copper phosphate nanosheets as high-performance oil-based nanoadditives: Tribological properties and lubrication mechanism. Tribol. Int. 2023, 179, 108077. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Y.; Wang, J.; Gao, C.; Zhang, S.; Zhang, P.; Zhang, Z. Interactions of Cu nanoparticles with conventional lubricant additives on tribological performance and some physicochemical properties of an ester base oil. Tribol. Int. 2020, 141, 105941. [Google Scholar] [CrossRef]
- Zhu, M.; Song, N.; Zhang, S.; Zhang, Y.; Yu, L.; Yang, G.; Zhang, P. Effect of micro nano-structured copper additives with different morphology on tribological properties and conductivity of lithium grease. Tribol. Trans. 2022, 65, 686–694. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Sun, D.; Yang, G.; Gao, C.; Zhou, C.; Zhang, C.; Zhang, P. Wide adaptability of Cu nano-additives to the hardness and composition of DLC coatings in DLC /PAO solid-liquid composite lubricating system. Tribol. Int. 2019, 138, 184–195. [Google Scholar] [CrossRef]
- Shen, M.x.; Rong, K.j.; Li, C.h.; Xu, B.; Xiong, G.y.; Zhang, R.h. In situ Friction-Induced Copper Nanoparticles at the Sliding Interface Between Steel Tribo-Pairs and their Tribological Properties. Tribol. Lett. 2020, 68, 98. [Google Scholar] [CrossRef]
- Martini, A.; Ramasamy, U.S.; Len, M. Review of Viscosity Modifier Lubricant Additives. Tribol. Lett. 2018, 66, 58. [Google Scholar] [CrossRef]
- Froböse, E.; Murr, T.; Vdi. Viscosity reduction of driveline lubricants. In Proceedings of the VDI Congress on Drivetrain for Vehicles: Light—Compact—Efficient, Friedrichshafen, Germany, 19–20 June 2012; pp. 213–226. [Google Scholar]
- Breki, A.; Nosonovsky, M. Einstein’s Viscosity Equation for Nanolubricated Friction. Langmuir 2018, 34, 12968–12973. [Google Scholar] [CrossRef]
- Dolatabadi, N.; Rahmani, R.; Rahnejat, H.; Garner, C.P.; Brunton, C. Performance of Poly Alpha Olefin Nanolubricant. Lubricants 2020, 8, 17. [Google Scholar] [CrossRef]
- Esfe, M.H.; Motallebi, S.M.; Toghraie, D.; Hatami, H. Experimental study and viscosity modeling by adding oxide nanoparticles to oil to improve the performance. Tribol. Int. 2023, 190, 109031. [Google Scholar] [CrossRef]
- Liu, X.; Xu, N.; Li, W.; Zhang, M.; Lou, W.; Wang, X. Viscosity modification of lubricating oil based on high-concentration silica nanoparticle colloidal system. J. Dispers. Sci. Technol. 2017, 38, 1360–1365. [Google Scholar] [CrossRef]
- Wan, Q.; Jin, Y.; Sun, P.; Ding, Y. Rheological and tribological behaviour of lubricating oils containing platelet MoS2 nanoparticles. J. Nanoparticle Res. 2014, 16, 2386. [Google Scholar] [CrossRef]
- Mackay, M.E.; Dao, T.T.; Tuteja, A.; Ho, D.L.; van Horn, B.; Kim, H.C.; Hawker, C.J. Nanoscale effects leading to non-Einstein-like decrease in viscosity. Nat. Mater. 2003, 2, 762–766. [Google Scholar] [CrossRef]
- Kotia, A.; Kumar, R.; Haldar, A.; Deval, P.; Ghosh, S.K. Characterization of Al2O3-SAE 15W40 engine oil nanolubricant and performance evaluation in 4-stroke diesel engine. J. Braz. Soc. Mech. Sci. Eng. 2018, 40, 38. [Google Scholar] [CrossRef]
- Ma, J.; Shahsavar, A.; Al-Rashed, A.A.; Karimipour, A.; Yarmand, H.; Rostami, S. Viscosity, cloud point, freezing point and flash point of zinc oxide/SAE50 nanolubricant. J. Mol. Liq. 2020, 298, 112045. [Google Scholar] [CrossRef]
- Hemmat Esfe, M.; Afrand, M.; Gharehkhani, S.; Rostamian, H.; Toghraie, D.; Dahari, M. An experimental study on viscosity of alumina-engine oil: Effects of temperature and nanoparticles concentration. Int. Commun. Heat Mass Transf. 2016, 76, 202–208. [Google Scholar] [CrossRef]
- Hemmat Esfe, M.; Mosaferi, M. Effect of MgO nanoparticles suspension on rheological behavior and a new correlation. J. Mol. Liq. 2020, 309, 112632. [Google Scholar] [CrossRef]
- Fahad, M.R.; Abdulmajeed, B.A. Experimental investigation of base oil properties containing modified TiO2/CuO nanoparticles additives. J. Phys. Conf. Ser. 2021, 1973, 012089. [Google Scholar] [CrossRef]
- Sui, T.; Ding, M.; Ji, C.; Yan, S.; Wei, J.; Wang, A.; Zhao, F.; Fei, J. Dispersibility and rheological behavior of functionalized silica nanoparticles as lubricant additives. Ceram. Int. 2018, 44, 18438–18443. [Google Scholar] [CrossRef]
- Abdelhalim, M.A.; Mady, M.M.; Ghannam, M.M. Rheological and dielectric properties of different gold nanoparticle sizes. Lipids Health Dis. 2011, 10, 208. [Google Scholar] [CrossRef]
- Dehghani, Y.; Abdollahi, A.; Karimipour, A. Experimental investigation toward obtaining a new correlation for viscosity of WO3 and Al2O3 nanoparticles-loaded nanofluid within aqueous and non-aqueous basefluids. J. Therm. Anal. Calorim. 2018, 135, 713–728. [Google Scholar] [CrossRef]
- Zhang, S.; Han, X. Effect of different surface modified nanoparticles on viscosity of nanofluids. Adv. Mech. Eng. 2018, 10, 1687814018762011. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, J.; Zhang, Z.; Liu, W.; Xue, Q. Study on the structure and tribological properties of surface-modified Cu nanoparticles. Mater. Res. Bull. 1999, 34, 1361–1367. [Google Scholar] [CrossRef]
- Piwoński, I.; Kisielewska, A. Dialkyldithiophosphate Acids (HDDPs) as Effective Lubricants of Sol–Gel Titania Coatings in Technical Dry Friction Conditions. Tribol. Lett. 2011, 45, 237–249. [Google Scholar] [CrossRef][Green Version]
- Čoga, L.; Akbari, S.; Kovač, J.; Kalin, M. Differences in nano-topography and tribochemistry of ZDDP tribofilms from variations in contact configuration with steel and DLC surfaces. Friction 2021, 10, 296–315. [Google Scholar] [CrossRef]
- Grunberg, L.; Nissan, A.H. Mixture law for viscosity. Nature 1949, 164, 799–800. [Google Scholar] [CrossRef]
- Einstein, A. Eine neue Bestimmung der Moleküldimensionen. Ann. Phys. 1906, 324, 289–306. [Google Scholar] [CrossRef]
- Batchelor, G.K. The effect of Brownian motion on the bulk stress in a suspension of spherical particles. J. Fluid Mech. 2006, 83, 97–117. [Google Scholar] [CrossRef]
- Brinkman, H.C. The Viscosity of Concentrated Suspensions and Solutions. J. Chem. Phys. 1952, 20, 571. [Google Scholar] [CrossRef]
- Yue, P.; Zhang, Y.; Zhang, S.; Jia, J.; Han, K.; Song, N. The key role of interfacial non-bonding interactions in regulating lubricant viscosity using nanoparticles. Tribol. Int. 2023, 187, 108716. [Google Scholar] [CrossRef]
- Marie, H.; Rigol, S.; Deeg, H.P.; Philipp, H. Impact of Aniline Octane Booster on Lubricating Oil. In Proceedings of the SAE 2016 International Powertrains, Fuels & Lubricants Meeting, Baltimore, MD, USA, 24–26 October 2016; Volume 2273, p. 8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).