Nanofluid Minimum Quantity Lubrication (NMQL): Overview of Nanoparticle Toxicity and Safer-Design Guidelines
Abstract
1. Introduction
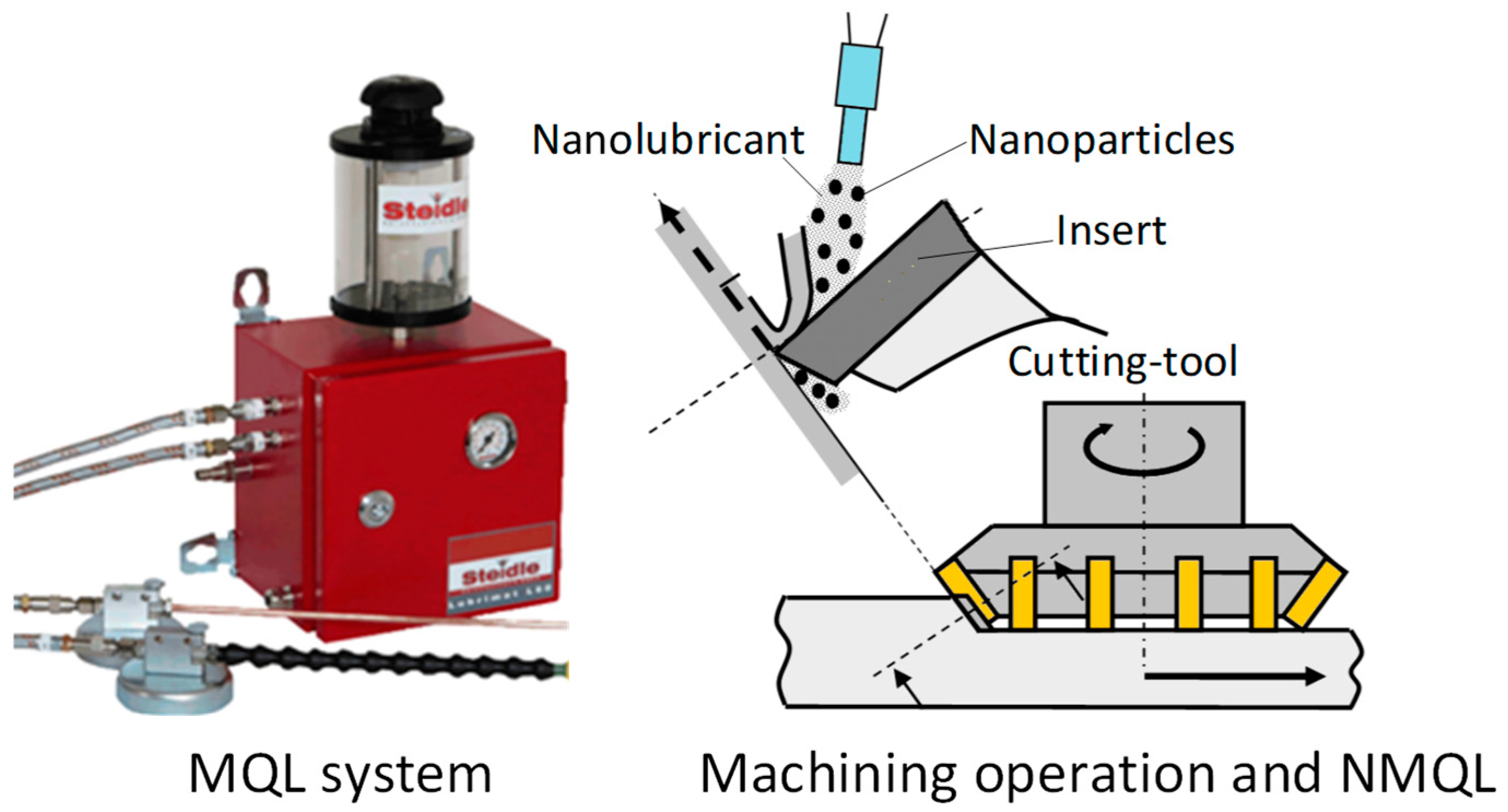
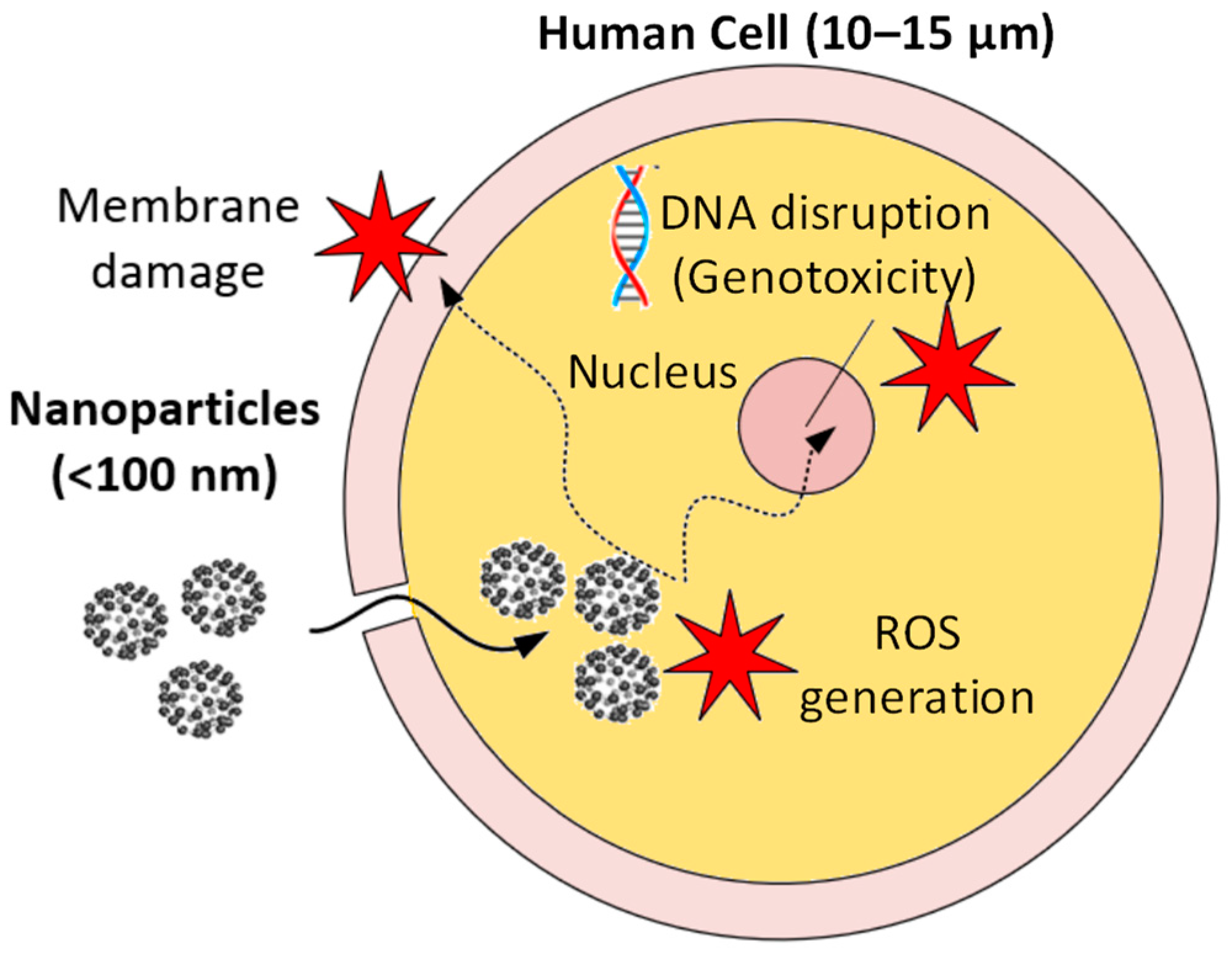
2. Nanoparticle Exposure Pathways in NMQL
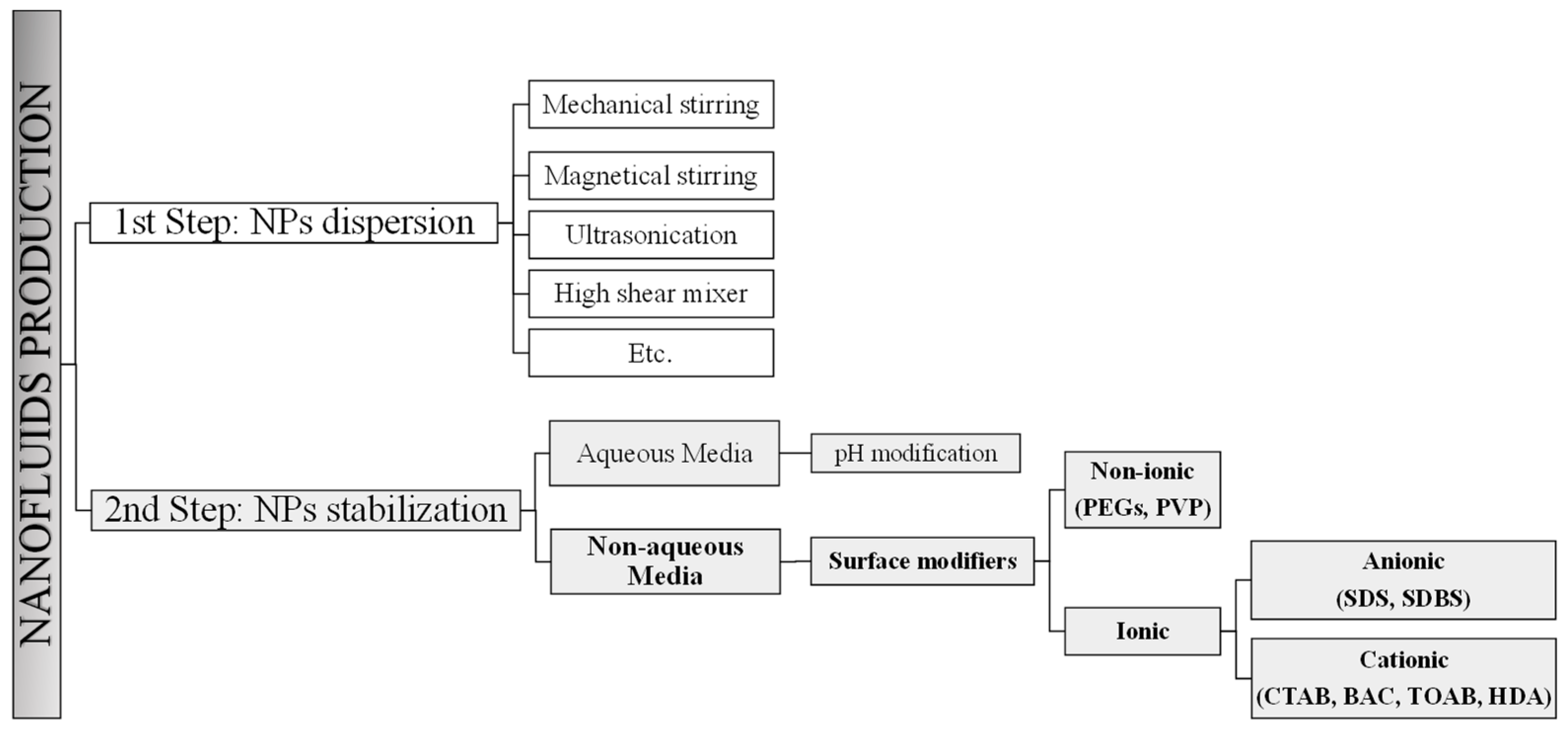
3. Nanoparticle Toxicity Studies: In Vitro, In Vivo and In Silico
4. Toxicity of NMQL Nanoparticles
- Hazard statements: H319 Causes serious eye irritation; H335 May cause respiratory irritation.
- Precaution statements:
- Prevention: P261 Avoid breathing dust/fume/gas/mist/vapors/spray; P264 Wash skin thoroughly after handling; P271 Use only outdoors or in a well-ventilated area; P280 Wear protective gloves/eye protection/face protection.
- Response: P304 + P340 If inhaled: Remove victim to fresh air and keep at rest in a position comfortable for breathing; P305 + P351 + P338 If in eyes: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.
- Storage: P403 + P233 Store in a well-ventilated place. Keep container tightly closed; P405 Store locked up.
- Disposal: P501 Dispose of contents/container at an approved waste disposal plant.
4.1. Metal-Based Nanoparticles
4.2. Non-Metal-Based Nanoparticles
4.2.1. Carbon-Based Nanoparticles
4.2.2. Metal Sulfides and Boron Nitrides
4.3. Comparison of Nanoparticles’ Toxicity
5. Key Factors of Nanoparticle Toxicity
5.1. Size
5.2. Shape
5.3. Surface Properties
5.4. Aggregation/Agglomeration
5.5. Solubility
6. Regulation
- Soluble nanoparticles: 0.066 × WEL (WEL refers to Workplace Exposure Limit, i.e., the exposure standard) of the corresponding microsized bulk material expressed as mass concentration.
- Fibrous nanoparticles: 0.01 fibers/mL.
- Highly soluble nanomaterials: 0.5 × WEL.
- CMAR nanoparticles: 0.1 × WEL of the corresponding microsized material.
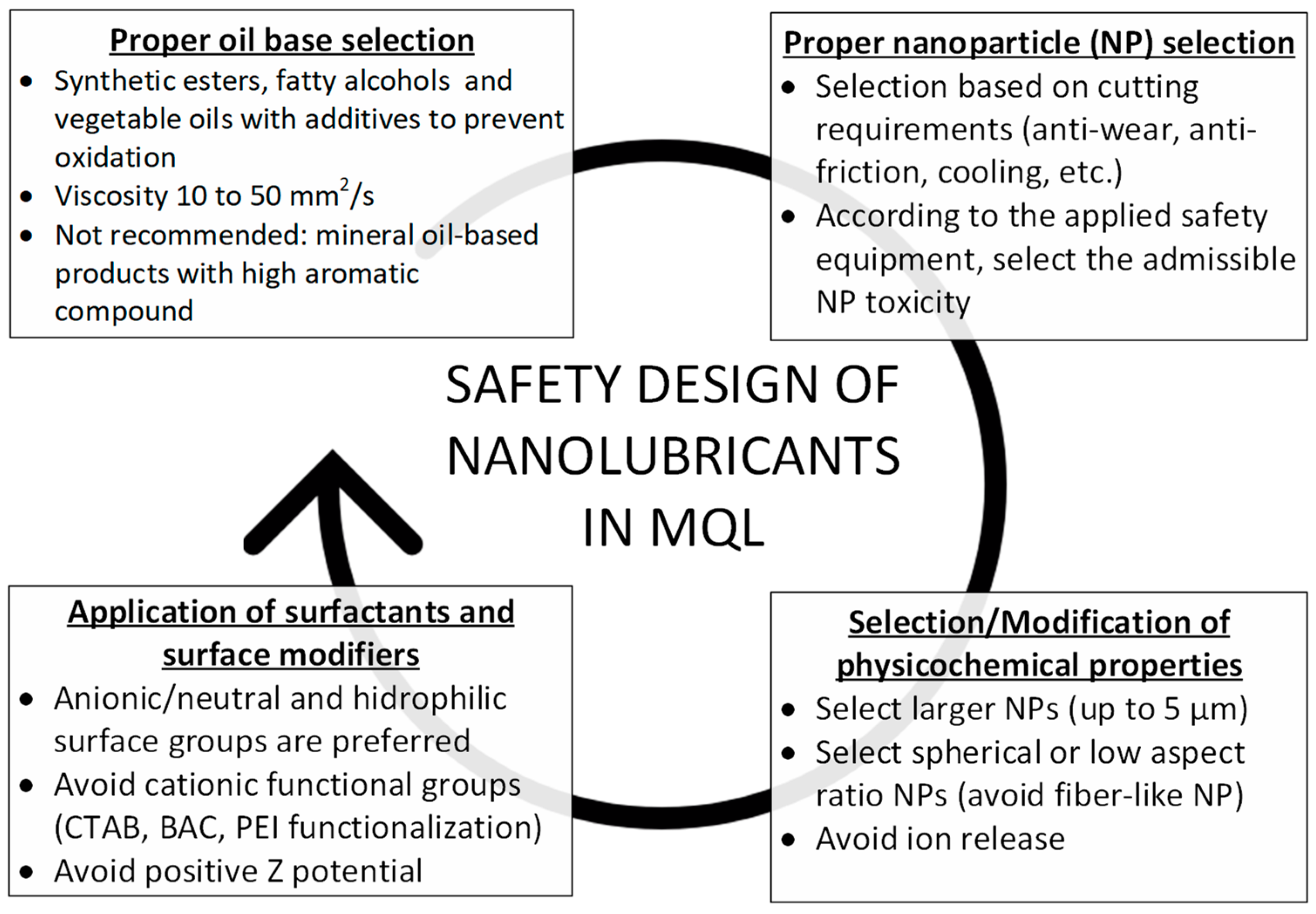
7. Guidelines
7.1. Proper Oil Base Selection
7.2. Proper Nanoparticle Selection
7.3. Selection/Modification of Physicochemical Properties
- Select larger nanoparticles. In general, larger nanoparticles within nanoscale lead to lower toxicity profiles and have reduced translocation capability. However, in case of overlong nanoparticles (fiber-like nanoparticles), e.g., CNTs or graphene nanoplates, length should be lower than 5 µm, which seems a critical value for effective clearance of nanoparticles by macrophages. In some cases, smaller nanoparticles may be preferred since they facilitate dissolution and clearance.
- Reduce the aspect ratio. In general, the lowest toxicity profile is related to spherical shapes. Any increase in the aspect ratio seems to increase the toxicity profile. The worst case scenario is nanoparticles similar to asbestos (fiber-like nanoparticles), which show the worst toxicity.
- Partially soluble nanoparticles are easier to clear and they are preferred if there is no release of toxic ions. Ions release, as occurs with ZnO and CuO nanoparticles, must be avoided.
7.4. Application of Surfactants and Surface Modifiers
- In the case of surface modifiers, covalent functionalization with anionic/neutral and hydrophilic surface groups could potentially decrease their toxicity (e.g., carboxylate, hydroxylate and polyethylene glycol). Avoid cationic functional groups (e.g., CTAB, BAC).
- Avoid positive zeta potential when formulating nanofluids. Nanoparticles with negative zeta potential are less prone to impart toxicity.
8. Conclusions
- It is recommended to use vegetable oils as lubricant due to their renewability, low toxicity, and easy biodegradation. Moreover, vegetable oil may cause less serious damage to the human body than mineral oil.
- Regarding nanoparticle selection based on chemical composition, metal sulfides are the best choice with the lowest toxicity. Carbon-based materials present medium to high toxicity while oxides of transition metals are not recommended due to their very high toxicity.
- It is important to pay attention to some physicochemical properties that can reduce toxicity, for example particle size and shape. Smaller particles with lower aspect ratio such as spherical particles are preferred over nanotubes and nanosheets.
- Selection of soluble nanoparticles that do not release ions, which are less cytotoxic than insoluble or partly soluble nanoparticles.
- For nanolubricant production, some surfactant additives may be required to ensure stability. It is recommended to avoid the use of cationic surfactants and cationic functionalization groups and also to avoid positive zeta potential.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schwarz, M.; Dado, M.; Hnilica, R.; Veverková, D. Environmental and Health Aspects of Metalworking Fluid Use. Pol. J. Environ. Stud. 2015, 24, 37–45. [Google Scholar]
- Chinchanikar, S.; Kore, S.S.; Hujare, P. A Review on Nanofluids in Minimum Quantity Lubrication Machining. J. Manuf. Process. 2021, 68, 56–70. [Google Scholar] [CrossRef]
- Tiwari, A.; Singh, D.K.; Mishra, S. A Review on Minimum Quantity Lubrication in Machining of Different Alloys and Superalloys Using Nanofluids. J. Braz. Soc. Mech. Sci. Eng. 2024, 46, 112. [Google Scholar] [CrossRef]
- Ali, S.H.; Yao, Y.; Wu, B.; Zhao, B.; Ding, W.; Jamil, M.; Khan, A.; Baig, A.; Liu, Q.; Xu, D. Recent Developments in MQL Machining of Aeronautical Materials: A Comparative Review. Chin. J. Aeronaut. 2024; in press. [Google Scholar] [CrossRef]
- Kaitwade, N. Metalworking Fluids Market Outlook (2023 to 2033). 2023. Available online: https://www.futuremarketinsights.com/reports/metal-working-fluids-market (accessed on 9 October 2024).
- Najiha, M.S.; Rahman, M.M.; Yusoff, A.R. Environmental Impacts and Hazards Associated with Metal Working Fluids and Recent Advances in the Sustainable Systems: A Review. Renew. Sustain. Energy Rev. 2016, 60, 1008–1031. [Google Scholar] [CrossRef]
- Weinert, K.; Inasaki, I.; Sutherland, J.W.; Wakabayashi, T. Dry Machining and Minimum Quantity Lubrication. CIRP Ann. 2004, 53, 511–537. [Google Scholar] [CrossRef]
- Klocke, F.; Eisenblaetter, G. Dry Cutting. CIRP Ann. Manuf. Technol. 1997, 46, 519–526. [Google Scholar] [CrossRef]
- NIOSH. Occupational Exposure to Metalworking Fluids. Criteria for a Recommended Standard; Education and Information Division of the National Institute for Occupational Safety and Health (NIOSH): Cincinnati, OH, USA, 1998.
- Mirer, F.E. New Evidence on the Health Hazards and Control of Metalworking Fluids since Completion of the OSHA Advisory Committee Report. Am. J. Ind. Med. 2010, 53, 792–801. [Google Scholar] [CrossRef]
- Park, R.M. Risk Assessment for Metalworking Fluids and Respiratory Outcomes. Saf. Health Work 2019, 10, 428–436. [Google Scholar] [CrossRef]
- Simpson, A.T.; Stear, M.; Groves, J.A.; Piney, M.; Bradley, S.D.; Stagg, S.; Crook, B. Occupational Exposure to Metalworking Fluid Mist and Sump Fluid Contaminants. Ann. Occup. Hyg. 2003, 47, 17–30. [Google Scholar] [CrossRef][Green Version]
- Shen, B. Minimum Quantity Lubrication Grinding Using Nanofluids. Doctoral Dissertation, University of Michigan, Ann Arbor, MI, USA, 2008. [Google Scholar]
- Goindi, G.S.; Sarkar, P. Dry Machining: A Step towards Sustainable Machining—Challenges and Future Directions. J. Clean. Prod. 2017, 165, 1557–1571. [Google Scholar] [CrossRef]
- Carter, N. Tribology and Lubrication Technology; STLE: Park Ridge, IL, USA, 2009. [Google Scholar]
- Singh, T.; Singh, P.; Dureja, J.S.; Dogra, M.; Singh, H.; Bhatti, M.S. A Review of near Dry Machining/Minimum Quantity Lubrication Machining of Difficult to Machine Alloys. Int. J. Mach. Mach. Mater. 2016, 18, 213–251. [Google Scholar] [CrossRef]
- Tai, B.L.; Stephenson, D.A.; Furness, R.J.; Shih, A.J. Minimum Quantity Lubrication (MQL) in Automotive Powertrain Machining. Procedia CIRP 2014, 14, 523–528. [Google Scholar] [CrossRef]
- Walker, T. MQL Handbook; Unist Company: Grand Rapids, MI, USA, 2013. [Google Scholar]
- Nouzil, I.; Eltaggaz, A.; Pervaiz, S.; Deiab, I. Toxicity Analysis of Nano-Minimum Quantity Lubrication Machining—A Review. Lubricants 2022, 10, 176. [Google Scholar] [CrossRef]
- Bai, X.; Li, C.; Dong, L.; Yin, Q. Experimental Evaluation of the Lubrication Performances of Different Nanofluids for Minimum Quantity Lubrication (MQL) in Milling Ti-6Al-4V. Int. J. Adv. Manuf. Technol. 2019, 101, 2621–2632. [Google Scholar] [CrossRef]
- Baldin, V.; da Silva, L.R.R.; Gelamo, R.V.; Iglesias, A.B.; da Silva, R.B.; Khanna, N.; Rocha Machado, A. Influence of Graphene Nanosheets on Thermo-Physical and Tribological Properties of Sustainable Cutting Fluids for MQL Application in Machining Processes. Lubricants 2022, 10, 193. [Google Scholar] [CrossRef]
- Duan, Z.; Yin, Q.; Li, C.; Dong, L.; Bai, X.; Zhang, Y.; Yang, M.; Jia, D.; Li, R.; Liu, Z. Milling Force and Surface Morphology of 45 Steel under Different Al2O3 Nanofluid Concentrations. Int. J. Adv. Manuf. Technol. 2020, 107, 1277–1296. [Google Scholar] [CrossRef]
- Marques, A.; Paipa Suarez, M.; Falco Sales, W.; Rocha Machado, Á. Turning of Inconel 718 with Whisker-Reinforced Ceramic Tools Applying Vegetable-Based Cutting Fluid Mixed with Solid Lubricants by MQL. J. Mater. Process. Technol. 2019, 266, 530–543. [Google Scholar] [CrossRef]
- Namlu, R.H.; Lotfi, B.; Kılıç, S.E. Enhancing Machining Efficiency of Ti-6Al-4V through Multi-Axial Ultrasonic Vibration-Assisted Machining and Hybrid Nanofluid Minimum Quantity Lubrication. J. Manuf. Process. 2024, 119, 348–371. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, A.K.; Katiyar, J.K. State-of-the-Art in Sustainable Machining of Different Materials Using Nano Minimum Quality Lubrication (NMQL). Lubricants 2023, 11, 64. [Google Scholar] [CrossRef]
- Dubey, V.; Sharma, A.K. A Short Review on Hybrid Nanofluids in Machining Processes. Adv. Mater. Process. Technol. 2023, 9, 138–151. [Google Scholar] [CrossRef]
- Sharma, A.K.; Tiwari, A.K.; Dixit, A.R. Effects of Minimum Quantity Lubrication (MQL) in Machining Processes Using Conventional and Nanofluid Based Cutting Fluids: A Comprehensive Review. J. Clean. Prod. 2016, 127, 1–18. [Google Scholar] [CrossRef]
- Cui, X.; Li, C.; Ding, W.; Chen, Y.; Mao, C.; Xu, X.; Liu, B.; Wang, D.; Li, H.N.; Zhang, Y.; et al. Minimum Quantity Lubrication Machining of Aeronautical Materials Using Carbon Group Nanolubricant: From Mechanisms to Application. Chin. J. Aeronaut. 2022, 35, 85–112. [Google Scholar] [CrossRef]
- Patole, P.B.; Kulkarni, V.V.; Bhatwadekar, S.G. MQL Machining with Nano Fluid: A Review. Manuf. Rev. 2021, 8, 13. [Google Scholar] [CrossRef]
- Balasuadhakar, A.; Thirumalai Kumaran, S.; Ahmed, F. A Review on the Role of Nanoparticles in MQL Machining. Mater. Today Proc. 2023, 72, 2828–2832. [Google Scholar] [CrossRef]
- Hamed Adibi, O.H.; Rezaei, S.M. Effects of Minimum Quantity Lubrication (MQL) on Grinding Processes Using Eco-Friendly Nanofluids: A Review. Adv. Mater. Process. Technol. 2024, 10, 1666–1707. [Google Scholar] [CrossRef]
- Chu, A.; Li, C.; Zhou, Z.; Liu, B.; Zhang, Y.; Yang, M.; Gao, T.; Liu, M.; Zhang, N.; Dambatta, Y.S.; et al. Nanofluids Minimal Quantity Lubrication Machining: From Mechanisms to Application. Lubricants 2023, 11, 422. [Google Scholar] [CrossRef]
- Mondragón, R.; Abellán-Nebot, J.V.; Habib, K.; Serrano, J. Tribological Analysis and Operation Issues of SiO2 Nanolubricants for MQL Machining Operations. Key Eng. Mater. 2023, 960, 11–20. [Google Scholar] [CrossRef]
- Wang, Y.; Murga, A.; Long, Z.; Yoo, S.J.; Ito, K. Experimental Study of Oil Mist Characteristics Generated from Minimum Quantity Lubrication and Flood Cooling. Energy Built Environ. 2021, 2, 45–55. [Google Scholar] [CrossRef]
- Ben Said, L.; Kolsi, L.; Ghachem, K.; Almeshaal, M.; Maatki, C. Application of Nanofluids as Cutting Fluids in Machining Operations: A Brief Review. Appl. Nanosci. 2022, 13, 4247–4278. [Google Scholar] [CrossRef]
- Krajnik, P.; Pusavec, F.; Rashid, A. Nanofluids: Properties, Applications and Sustainability Aspects in Materials Processing Technologies. In Advances in Sustainable Manufacturing; Springer: Berlin/Heidelberg, Germany, 2011; pp. 107–113. [Google Scholar]
- Seyedmahmoudi, S.H.; Harper, S.L.; Weismiller, M.C.; Haapala, K.R. Evaluating the Use of Zinc Oxide and Titanium Dioxide Nanoparticles in a Metalworking Fluid from a Toxicological Perspective. J. Nanoparticle Res. 2015, 17, 104. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry. Guidance for Inhalation Exposures to Particulate Matter; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2020.
- Kumah, E.A.; Fopa, R.D.; Harati, S.; Boadu, P.; Zohoori, F.V.; Pak, T. Human and Environmental Impacts of Nanoparticles: A Scoping Review of the Current Literature. BMC Public Health 2023, 23, 1059. [Google Scholar] [CrossRef] [PubMed]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and Nanoparticles: Sources and Toxicity. Biointerphases 2007, 2, MR17. [Google Scholar] [CrossRef] [PubMed]
- Kermanizadeh, A.; Gosens, I.; MacCalman, L.; Johnston, H.; Danielsen, P.H.; Jacobsen, N.R.; Lenz, A.-G.; Fernandes, T.; Schins, R.P.F.; Cassee, F.R.; et al. A Multilaboratory Toxicological Assessment of a Panel of 10 Engineered Nanomaterials to Human Health–ENPRA Project–The Highlights, Limitations, and Current and Future Challenges. J. Toxicol. Environ. Health B Crit. Rev. 2016, 19, 1126210. [Google Scholar] [CrossRef]
- Ramanathan, A. Toxicity of Nanoparticles_Challenges and Opportunities. Appl. Microsc. 2019, 49, 2. [Google Scholar] [CrossRef]
- Bierkandt, F.S.; Leibrock, L.; Wagener, S.; Laux, P.; Luch, A. The Impact of Nanomaterial Characteristics on Inhalation Toxicity. Toxicol. Res. 2018, 7, 321–346. [Google Scholar] [CrossRef]
- Gangwa, S.; Brown, J.S.; Wang, A.; Houck, K.A.; Dix, D.J.; Kavlock, R.J.; Cohen Hubal, E.A. Informing Selection of Nanomaterial Concentrations for ToxCast in Vitro Testing Based on Occupational Exposure Potential. Environ. Health Perspect. 2011, 119, 1539–1546. [Google Scholar] [CrossRef]
- Raies, A.B.; Bajic, V.B. In Silico Toxicology: Computational Methods for the Prediction of Chemical Toxicity. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2016, 6, 147–172. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Yue, L.; Chen, F.; Cao, X.; Wang, Z. Nano-QSAR Modeling for Predicting the Cytotoxicity of Metallic and Metal Oxide Nanoparticles: A Review. Ecotoxicol. Environ. Saf. 2022, 243, 113955. [Google Scholar] [CrossRef]
- Subramanian, N.A.; Palaniappan, A. NanoTox: Development of a Parsimonious in Silico Model for Toxicity Assessment of Metal-Oxide Nanoparticles Using Physicochemical Features. ACS Omega 2021, 6, 11729–11739. [Google Scholar] [CrossRef]
- Hernández Battez, A.; González, R.; Viesca, J.L.; Fernández, J.E.; Díaz Fernández, J.M.; Machado, A.; Chou, R.; Riba, J. CuO, ZrO2 and ZnO Nanoparticles as Antiwear Additive in Oil Lubricants. Wear 2008, 265, 422–428. [Google Scholar] [CrossRef]
- Sengul, A.B.; Asmatulu, E. Toxicity of Metal and Metal Oxide Nanoparticles: A Review. Environ. Chem. Lett. 2020, 18, 1659–1683. [Google Scholar] [CrossRef]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2021, 22, 385. [Google Scholar] [CrossRef]
- Sharifi, S.; Behzadi, S.; Laurent, S.; Forrest, M.L.; Stroeve, P.; Mahmoudi, M. Toxicity of Nanomaterials. Chem. Soc. Rev. 2012, 41, 2323–2343. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sharma, N.; Maitra, S.S. In Vitro and in Vivo Toxicity Assessment of Nanoparticles. Int. Nano Lett. 2017, 7, 243–256. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Yin, L.H.; Tang, M.; Pu, Y.P. ZnO, TiO2, SiO2, and Al2O3 Nanoparticles-Induced Toxic Effects on Human Fetal Lung Fibroblasts. Biomed. Environ. Sci. 2011, 24, 661–669. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Möller, L. Copper Oxide Nanoparticles Are Highly Toxic: A Comparison between Metal Oxide Nanoparticles and Carbon Nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef]
- Kim, I.-S.; Baek, M.; Choi, S.-J. Comparative Cytotoxicity of Al2O3, CeO2, TiO2 and ZnO Nanoparticles to Human Lung Cells. J. Nanosci. Nanotechnol. 2010, 10, 3453–3458. [Google Scholar] [CrossRef]
- Lee, J.H.; Ju, J.E.; Kim, B.I.; Pak, P.J.; Choi, E.K.; Lee, H.S.; Chung, N. Rod-Shaped Iron Oxide Nanoparticles Are More Toxic than Sphere-Shaped Nanoparticles to Murine Macrophage Cells. Environ. Toxicol. Chem. 2014, 33, 2759–2766. [Google Scholar] [CrossRef]
- Remzova, M.; Zouzelka, R.; Brzicova, T.; Vrbova, K.; Pinkas, D.; Rőssner, P.; Topinka, J.; Rathousky, J. Toxicity of TiO2, ZnO, and SiO2 Nanoparticles in Human Lung Cells: Safe-by-Design Development of Construction Materials. Nanomaterials 2019, 9, 968. [Google Scholar] [CrossRef]
- Wei, W.; Yan, Z.; Liu, X.; Qin, Z.; Tao, X.; Zhu, X.; Song, E.; Chen, C.; Ke, P.C.; Leong, D.T.; et al. Brain Accumulation and Toxicity Profiles of Silica Nanoparticles: The Influence of Size and Exposure Route. Environ. Sci. Technol. 2022, 56, 8319–8325. [Google Scholar] [CrossRef]
- Brown, D.M.; Johnston, H.J.; Gaiser, B.; Pinna, N.; Caputo, G.; Culha, M.; Kelestemur, S.; Altunbek, M.; Stone, V.; Roy, J.C.; et al. A Cross-Species and Model Comparison of the Acute Toxicity of Nanoparticles Used in the Pigment and Ink Industries. NanoImpact 2018, 11, 20–32. [Google Scholar] [CrossRef]
- Ma-Hock, L.; Strauss, V.; Treumann, S.; Küttler, K.; Wohlleben, W.; Hofmann, T.; Gröters, S.; Wiench, K.; van Ravenzwaay, B.; Landsiedel, R. Comparative Inhalation Toxicity of Multi-Wall Carbon Nanotubes, Graphene, Graphite Nanoplatelets and Low Surface Carbon Black. Part. Fibre Toxicol. 2013, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Izumi, H.; Morimoto, Y. Review of Toxicity Studies of Carbon Nanotubes. J. Occup. Health 2017, 59, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Mollá, F.A.; Alvaro, J.A.H.; Sánchez, O.A.; Fito-López, C.; González, I.C. Nanosafety Analysis of Graphene-Based Polyester Resin Composites on a Life Cycle Perspective. Nanomaterials 2022, 12, 2036. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.R.; Mercer, R.R.; Stefaniak, A.B.; Seehra, M.S.; Geddam, U.K.; Chaudhuri, I.S.; Kyrlidis, A.; Kodali, V.K.; Sager, T.; Kenyon, A.; et al. Evaluation of Pulmonary and Systemic Toxicity Following Lung Exposure to Graphite Nanoplates: A Member of the Graphene-Based Nanomaterial Family. Part. Fibre Toxicol. 2016, 13, 34. [Google Scholar] [CrossRef]
- Chng, E.L.K.; Chua, C.K.; Pumera, M. Graphene Oxide Nanoribbons Exhibit Significantly Greater Toxicity than Graphene Oxide Nanoplatelets. Nanoscale 2014, 6, 10792–10797. [Google Scholar] [CrossRef]
- Li, X.; Kang, B.; Eom, Y.; Zhong, J.; Lee, H.K.; Kim, H.M.; Song, J.S. Comparison of Cytotoxicity Effects Induced by Four Different Types of Nanoparticles in Human Corneal and Conjunctival Epithelial Cells. Sci. Rep. 2022, 12, 155. [Google Scholar] [CrossRef]
- Yang, H.; Liu, C.; Yang, D.; Zhang, H.; Xi, Z. Comparative Study of Cytotoxicity, Oxidative Stress and Genotoxicity Induced by Four Typical Nanomaterials: The Role of Particle Size, Shape and Composition. J. Appl. Toxicol. 2009, 29, 69–78. [Google Scholar] [CrossRef]
- Angoth, B.; Lingabathula, H.; Gandamalla, D.; Yellu, N.R. Cytotoxicity Evaluation of Carbon Nanomaterials on Human Cell Lines Using MTT Assay. Int. J. Pharm. Pharm. Sci. 2014, 2014, 379–382. [Google Scholar]
- Lam, C.W.; James, J.T.; McCluskey, R.; Arepalli, S.; Hunter, R.L. A Review of Carbon Nanotube Toxicity and Assessment of Potential Occupational and Environmental Health Risks. Crit. Rev. Toxicol. 2006, 36, 189–217. [Google Scholar] [CrossRef] [PubMed]
- Raja, I.S.; Song, S.J.; Kang, M.S.; Bin Lee, Y.; Kim, B.; Hong, S.W.; Jeong, S.J.; Lee, J.C.; Han, D.W. Toxicity of Zero-and One-Dimensional Carbon Nanomaterials. Nanomaterials 2019, 9, 1214. [Google Scholar] [CrossRef] [PubMed]
- Appel, J.H.; Li, D.O.; Podlevsky, J.D.; Debnath, A.; Green, A.A.; Wang, Q.H.; Chae, J. Low Cytotoxicity and Genotoxicity of Two-Dimensional MoS2 and WS2. ACS Biomater. Sci. Eng. 2016, 2, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Pardo, M.; Shuster-Meiseles, T.; Levin-Zaidman, S.; Rudich, A.; Rudich, Y. Low Cytotoxicity of Inorganic Nanotubes and Fullerene-like Nanostructures in Human Bronchial Epithelial Cells: Relation to Inflammatory Gene Induction and Antioxidant Response. Environ. Sci. Technol. 2014, 48, 3457–3466. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.Z.; Chng, E.L.K.; Sofer, Z.; Pumera, M. Cytotoxicity of Exfoliated Transition-Metal Dichalcogenides (MoS2, WS2, and WSe2) Is Lower than That of Graphene and Its Analogues. Chem.—Eur. J. 2014, 20, 9627–9632. [Google Scholar] [CrossRef]
- Zapór, L. Rocznik Ochrona Środowiska Cytotoxicity Elicited by Molybdenum Disulphide in Different Size of Particles in Human Airway Cells. Rocz. Ochr. Sr. 2019, 21, 794–809. [Google Scholar]
- Hao, J.; Song, G.; Liu, T.; Yi, X.; Yang, K.; Cheng, L.; Liu, Z. In Vivo Long-Term Biodistribution, Excretion, and Toxicology of PEGylated Transition-Metal Dichalcogenides MS2 (M = Mo, W, Ti) Nanosheets. Adv. Sci. 2017, 4, 1600160. [Google Scholar] [CrossRef]
- Chen, X.; Wu, P.; Rousseas, M.; Okawa, D.; Gartner, Z.; Zettl, A.; Bertozzi, C.R. Boron Nitride Nanotubes Are Noncytotoxic and Can Be Functionalized for Interaction with Proteins and Cells. J. Am. Chem. Soc. 2009, 131, 890–891. [Google Scholar] [CrossRef]
- Horváth, L.; Magrez, A.; Golberg, D.; Zhi, C.; Bando, Y.; Smajda, R.; Horváth, E.; Forró, L.; Schwaller, B. In Vitro Investigation of the Cellular Toxicity of Boron Nitride Nanotubes. ACS Nano 2011, 5, 3800–3810. [Google Scholar] [CrossRef]
- Mao, Y.; Guo, Q.; Geng, X.; Zeng, H.; Liu, S.; Yin, X.; Yang, Z. Hexagonal Boron Nitride Nanodots Inhibit Cell Proliferation of HUVECs and the Underlying Mechanism. Colloid Interface Sci. Commun. 2023, 56, 100738. [Google Scholar] [CrossRef]
- Lanone, S.; Rogerieux, F.; Geys, J.; Dupont, A.; Maillot-Marechal, E.; Boczkowski, J.; Lacroix, G.; Hoet, P. Comparative Toxicity of 24 Manufactured Nanoparticles in Human Alveolar Epithelial and Macrophage Cell Lines. Part. Fibre Toxicol. 2009, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Maynard, A.; Donaldson, K.; Castranova, V.; Fitzpatrick, J.; Ausman, K.; Carter, J.; Karn, B.; Kreyling, W.; Lai, D.; et al. Principles for Characterizing the Potential Human Health Effects from Exposure to Nanomaterials: Elements of a Screening Strategy. Part. Fibre Toxicol. 2005, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Xiong, G.; Liu, Z. Toxicity of Metal-Based Nanoparticles: Challenges in the Nano Era. Front. Bioeng. Biotechnol. 2022, 10, 1001572. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Kim, H.; An, S.S.A.; Maeng, E.H.; Kim, M.K.; Song, Y.J. In Vitro Cytotoxicity of SiO2 or ZnO Nanoparticles with Different Sizes and Surface Charges on U373MG Human Glioblastoma Cells. Int. J. Nanomed. 2014, 9, 235–241. [Google Scholar] [CrossRef]
- Sahu, D.; Kannan, G.M.; Tailang, M.; Vijayaraghavan, R. In Vitro Cytotoxicity of Nanoparticles: A Comparison between Particle Size and Cell Type. J. Nanosci. 2016, 2016, 4023852. [Google Scholar] [CrossRef]
- Hsiao, I.L.; Huang, Y.J. Effects of Various Physicochemical Characteristics on the Toxicities of ZnO and TiO2 Nanoparticles toward Human Lung Epithelial Cells. Sci. Total Environ. 2011, 409, 1219–1228. [Google Scholar] [CrossRef]
- Gurr, J.R.; Wang, A.S.S.; Chen, C.H.; Jan, K.Y. Ultrafine Titanium Dioxide Particles in the Absence of Photoactivation Can Induce Oxidative Damage to Human Bronchial Epithelial Cells. Toxicology 2005, 213, 66–73. [Google Scholar] [CrossRef]
- De Jong, W.H.; Hagens, W.I.; Krystek, P.; Burger, M.C.; Sips, A.J.A.M.; Geertsma, R.E. Particle Size-Dependent Organ Distribution of Gold Nanoparticles after Intravenous Administration. Biomaterials 2008, 29, 1912–1919. [Google Scholar] [CrossRef]
- Raftis, J.B.; Miller, M.R. Nanoparticle Translocation and Multi-Organ Toxicity: A Particularly Small Problem. Nano Today 2019, 26, 8–12. [Google Scholar] [CrossRef]
- Semmler-Behnke, M.; Kreyling, W.G.; Lipka, J.; Fertsch, S.; Wenk, A.; Takenaka, S.; Schmid, G.; Brandau, W. Biodistribution of 1.4-and 18-Nm Gold Particles in Rats. Small 2008, 4, 2108–2111. [Google Scholar] [CrossRef]
- Oberdorster, G.; Ferin, J.; Lehnert, B.E. Correlation between Particle Size, In Vivo Particle Persistence, and Lung Injury. Environ. Health Perspect. 1994, 102 (Suppl. S5), 173–179. [Google Scholar] [PubMed]
- Hadji, H.; Bouchemal, K. Effect of Micro- and Nanoparticle Shape on Biological Processes. J. Control Release 2022, 342, 93–110. [Google Scholar] [CrossRef] [PubMed]
- WHO. Determination of Airborne Fibre Number Concentrations: A Recommended Method, by Phase-Contrast Optical Microscopy (Membrane Filter Method); World Health Organization: Geneva, Switzerland, 1997.
- Donaldson, K.; Poland, C.A. Inhaled Nanoparticles and Lung Cancer—What We Can Learn from Conventional Particle Toxicology. Swiss Med. Wkly. 2012, 142, w13547. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Murphy, F.; Schinwald, A.; Duffin, R.; Poland, C.A. Identifying the Pulmonary Hazard of High Aspect Ratio Nanoparticles to Enable Their Safety-by-Design. Nanomedicine 2011, 6, 143–153. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, G.H.; Shim, J.H.; Cho, M.H.; Lee, B.S.; Kim, Y.B.; Kim, J.H.; Kim, Y.; Kim, D.W. Comparison of the Toxicity of Aluminum Oxide Nanorods with Different Aspect Ratio. Arch. Toxicol. 2015, 89, 1771–1782. [Google Scholar] [CrossRef]
- Huang, Y.-W.; Cambre, M.; Lee, H.-J. The Toxicity of Nanoparticles Depends on Multiple Molecular and Physicochemical Mechanisms. Int. J. Mol. Sci. 2017, 18, 2702. [Google Scholar] [CrossRef]
- Cohignac, V.; Landry, M.J.; Ridoux, A.; Pinault, M.; Annangi, B.; Gerdil, A.; Herlin-Boime, N.; Mayne, M.; Haruta, M.; Codogno, P.; et al. Carbon Nanotubes, but Not Spherical Nanoparticles, Block Autophagy by a Shape-Related Targeting of Lysosomes in Murine Macrophages. Autophagy 2018, 14, 1323–1334. [Google Scholar] [CrossRef]
- Jiang, T.; Amadei, C.A.; Gou, N.; Lin, Y.; Lan, J.; Vecitis, C.D.; Gu, A.Z. Toxicity of Single-Walled Carbon Nanotubes (SWCNTs): Effect of Lengths, Functional Groups and Electronic Structures Revealed by a Quantitative Toxicogenomics Assay. Environ. Sci. Nano 2020, 7, 1348–1364. [Google Scholar] [CrossRef]
- Cui, X.; Wan, B.; Yang, Y.; Ren, X.; Guo, L.H. Length Effects on the Dynamic Process of Cellular Uptake and Exocytosis of Single-Walled Carbon Nanotubes in Murine Macrophage Cells. Sci. Rep. 2017, 7, 1518. [Google Scholar] [CrossRef]
- Rodrigues, A.F.; Newman, L.; Jasim, D.; Mukherjee, S.P.; Wang, J.; Vacchi, I.A.; Ménard-Moyon, C.; Bianco, A.; Fadeel, B.; Kostarelos, K.; et al. Size-Dependent Pulmonary Impact of Thin Graphene Oxide Sheets in Mice: Toward Safe-by-Design. Adv. Sci. 2020, 7, 1903200. [Google Scholar] [CrossRef]
- Oberdörster, G.; Castranova, V.; Asgharian, B.; Sayre, P. Inhalation Exposure to Carbon Nanotubes (CNT) and Carbon Nanofibers (CNF): Methodology and Dosimetry. J. Toxicol. Environ. Health B Crit. Rev. 2015, 18, 121–212. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.S.; Duffin, R.; Thielbeer, F.; Bradley, M.; Megson, I.L.; MacNee, W.; Poland, C.A.; Tran, C.L.; Donaldson, K. Zeta Potential and Solubility to Toxic Ions as Mechanisms of Lung Inflammation Caused by Metal/Metal Oxide Nanoparticles. Toxicol. Sci. 2012, 126, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, X.; Ji, Z.; Sun, B.; Zhang, H.; Chang, C.H.; Lin, S.; Meng, H.; Liao, Y.P.; Wang, M.; et al. Surface Charge and Cellular Processing of Covalently Functionalized Multiwall Carbon Nanotubes Determine Pulmonary Toxicity. ACS Nano 2013, 7, 2352–2368. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, L.M.; Melnikov, F.; Wehmas, L.C.; Anastas, P.T.; Tanguay, R.L.; Zimmerman, J.B. Toward Safer Multi-Walled Carbon Nanotube Design: Establishing a Statistical Model That Relates Surface Charge and Embryonic Zebrafish Mortality. Nanotoxicology 2016, 10, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.; Kim, M.K.; Cho, H.J.; Lee, J.A.; Yu, J.; Chung, H.E.; Choi, S.J. Factors Influencing the Cytotoxicity of Zinc Oxide Nanoparticles: Particle Size and Surface Charge. In Proceedings of the Journal of Physics: Conference Series; Institute of Physics Publishing: Bristol, UK, 2011; Volume 304. [Google Scholar]
- Nagarajan, R. Nanoparticles: Building Blocks for Nanotechnology; UTC: Greenwich, UK, 2023; Volume 16. [Google Scholar]
- Oleszczuk, P.; Jośko, I.; Skwarek, E. Surfactants Decrease the Toxicity of ZnO, TiO2 and Ni Nanoparticles to Daphnia Magna. Ecotoxicology 2015, 24, 1923–1932. [Google Scholar] [CrossRef]
- Zhang, Y.; Newton, B.; Lewis, E.; Fu, P.P.; Kafoury, R.; Ray, P.C.; Yu, H. Cytotoxicity of Organic Surface Coating Agents Used for Nanoparticles Synthesis and Stability. Toxicology In Vitro 2015, 29, 762–768. [Google Scholar] [CrossRef]
- Wang, S.; Lu, W.; Tovmachenko, O.; Rai, U.S.; Yu, H.; Ray, P.C. Challenge in Understanding Size and Shape Dependent Toxicity of Gold Nanomaterials in Human Skin Keratinocytes. Chem. Phys. Lett. 2008, 463, 145–149. [Google Scholar] [CrossRef]
- Wan, J.; Wang, J.H.; Liu, T.; Xie, Z.; Yu, X.F.; Li, W. Surface Chemistry but Not Aspect Ratio Mediates the Biological Toxicity of Gold Nanorods in Vitro and in Vivo. Sci. Rep. 2015, 5, 11398. [Google Scholar] [CrossRef]
- Miyazawa, T.; Itaya, M.; Burdeos, G.C.; Nakagawa, K.; Miyazawa, T. A Critical Review of the Use of Surfactant-Coated Nanoparticles in Nanomedicine and Food Nanotechnology. Int. J. Nanomed. 2021, 16, 3937–3999. [Google Scholar] [CrossRef]
- Fröhlich, E. The Role of Surface Charge in Cellular Uptake and Cytotoxicity of Medical Nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Vranic, S.; Watanabe, E.; Miyakawa, K.; Takeuchi, S.; Osada, Y.; Ichihara, S.; Zong, C.; Wu, W.; Sakurai, T.; Sato, A.; et al. Impact of Surface Modication on Cellular Uptake and Cytotoxicity of Silica Nanoparticles. Res. Sq. 2020, 10, 1–20. [Google Scholar] [CrossRef]
- Zhang, S.; Chu, Q.; Zhang, Z.; Xu, Y.; Mao, X.; Zhang, M. Responses of Caenorhabditis Elegans to Various Surface Modifications of Alumina Nanoparticles. Environ. Pollut. 2021, 271, 116335. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.M.; McCusker, C.D.; Yilmaz, T.; Rotello, V.M. Toxicity of Gold Nanoparticles Functionalized with Cationic and Anionic Side Chains. Bioconjug Chem. 2004, 15, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, Y.; Li, J.; Zhu, Z.; Li, J.; Li, W.; Huang, Q. Tuning the Cellular Uptake and Cytotoxicity of Carbon Nanotubes by Surface Hydroxylation. J. Nanopart. Res. 2011, 13, 6941–6952. [Google Scholar] [CrossRef]
- Zhou, L.; Forman, H.J.; Ge, Y.; Lunec, J. Multi-Walled Carbon Nanotubes: A Cytotoxicity Study in Relation to Functionalization, Dose and Dispersion. Toxicol. In Vitro 2017, 42, 292–298. [Google Scholar] [CrossRef]
- Dong, X.; Liu, L.; Zhu, D.; Zhang, H.; Li, Y.; Leng, X. Effects of Carboxylated Multiwalled Carbon Nanotubes on the Function of Macrophages. J. Nanomater. 2015, 2015, 638760. [Google Scholar] [CrossRef]
- Bruinink, A.; Wang, J.; Wick, P. Effect of Particle Agglomeration in Nanotoxicology. Arch. Toxicol. 2015, 89, 659–675. [Google Scholar] [CrossRef]
- Cheng, J.; Cheng, S.H. Influence of Carbon Nanotube Length on Toxicity to Zebrafish Embryos. Int. J. Nanomed. 2012, 7, 3731–3739. [Google Scholar] [CrossRef]
- Murugadoss, S.; Brassinne, F.; Sebaihi, N.; Petry, J.; Cokic, S.M.; Van Landuyt, K.L.; Godderis, L.; Mast, J.; Lison, D.; Hoet, P.H.; et al. Agglomeration of Titanium Dioxide Nanoparticles Increases Toxicological Responses in Vitro and in Vivo. Part. Fibre Toxicol. 2020, 17, 10. [Google Scholar] [CrossRef]
- Mutlu, G.M.; Budinger, G.R.S.; Green, A.A.; Urich, D.; Soberanes, S.; Chiarella, S.E.; Alheid, G.F.; McCrimmon, D.R.; Szleifer, I.; Hersam, M.C. Biocompatible Nanoscale Dispersion of Single-Walled Carbon Nanotubes Minimizes in Vivo Pulmonary Toxicity. Nano Lett. 2010, 10, 1664–1670. [Google Scholar] [CrossRef]
- Lim, C.-H.; Kang, M.; Han, J.-H.; Yang, J.-S. Effect of Agglomeration on the Toxicity of Nano-Sized Carbon Black in Sprague-Dawley Rats. Environ. Health Toxicol. 2012, 27, e2012015. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chang, Y.N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The Toxic Effects and Mechanisms of CuO and ZnO Nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef]
- Keller, J.G.; Persson, M.; Müller, P.; Ma-Hock, L.; Werle, K.; Arts, J.; Landsiedel, R.; Wohlleben, W. Variation in Dissolution Behavior among Different Nanoforms and Its Implication for Grouping Approaches in Inhalation Toxicity. NanoImpact 2021, 23, 100341. [Google Scholar] [CrossRef] [PubMed]
- Hadrup, N.; Saber, A.T.; Kyjovska, Z.O.; Jacobsen, N.R.; Vippola, M.; Sarlin, E.; Ding, Y.; Schmid, O.; Wallin, H.; Jensen, K.A.; et al. Pulmonary Toxicity of Fe2O3, ZnFe2O4, NiFe2O4 and NiZnFe4O8 Nanomaterials: Inflammation and DNA Strand Breaks. Environ. Toxicol. Pharmacol. 2020, 74, 103303. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadis, S.H.; Chrissopoulou, K.; Stratakis, E.; Kavatzikidou, P.; Kaklamani, G.; Ranella, A. How the Physicochemical Properties of Manufactured Nanomaterials Affect Their Performance in Dispersion and Their Applications in Biomedicine: A Review. Nanomaterials 2022, 12, 552. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.K.; Dybowska, A.; Berhanu, D.; Croteau, M.N.; Luoma, S.N.; Boccaccini, A.R.; Valsami-Jones, E. Isotopically Modified Nanoparticles for Enhanced Detection in Bioaccumulation Studies. Environ. Sci. Technol. 2012, 46, 1216–1222. [Google Scholar] [CrossRef]
- Utembe, W.; Potgieter, K.; Stefaniak, A.B.; Gulumian, M. Dissolution and Biodurability: Important Parameters Needed for Risk Assessment of Nanomaterials. Part. Fibre Toxicol. 2015, 12, 11. [Google Scholar] [CrossRef]
- Wang, D.; Lin, Z.; Wang, T.; Yao, Z.; Qin, M.; Zheng, S.; Lu, W. Where Does the Toxicity of Metal Oxide Nanoparticles Come from: The Nanoparticles, the Ions, or a Combination of Both? J. Hazard. Mater. 2016, 308, 328–334. [Google Scholar] [CrossRef]
- Deveau, M.; Chen, C.P.; Johanson, G.; Krewski, D.; Maier, A.; Niven, K.J.; Ripple, S.; Schulte, P.A.; Silk, J.; Urbanus, J.H.; et al. The Global Landscape of Occupational Exposure Limits—Implementation of Harmonization Principles to Guide Limit Selection. J. Occup. Environ. Hyg. 2015, 12, S127–S144. [Google Scholar] [CrossRef]
- OSHA. OSHA Fact Sheet. Working Safely with Nanomaterials; OSHA: Washington, DC, USA, 2013.
- NIOSH. Current Intelligence Bulletin: Occupational Exposure to Titanium Dioxide; NIOSH: Washington, DC, USA, 2011.
- NIOSH. Current Intelligence Bulletin: Occupational Exposure to Carbon Nanotubes and Nanofibers, Draft for Public Comment; NIOSH: Washington, DC, USA, 2010.
- NIOSH. Current Intelligence Bulletin 65: Occupational Exposure to Carbon Nanotubes and Nanofibers; NIOSH: Washington, DC, USA, 2013.
- Safety, O.; Administration, H. Silver, Metal & Soluble Compounds (as Ag). Available online: https://www.osha.gov/chemicaldata/519 (accessed on 9 October 2024).
- NIOSH. Current Intelligence Bulletin: Health Effects of Occupational Exposure to Silver Nanomaterials; NIOSH: Washington, DC, USA, 2021.
- Katsnelson, B.A.; Privalova, L.I.; Kuzmin, S.V.; Gurvich, V.B.; Sutunkova, M.P.; Kireyeva, E.P.; Minigalieva, I.A. An Approach to Tentative Reference Levels Setting for Nanoparticles in the Workroom Air Based on Comparing Their Toxicity with That of Their Micrometric Counterparts: A Case Study of Iron Oxide Fe3O4. ISRN Nanotechnol. 2012, 2012, 143613. [Google Scholar] [CrossRef]
- OSHA E.U. Workplace Exposure to Nanoparticles; EU: Bilbao, Spain, 2008.
- BSI. Nanotechnologies–Part 2: Guide to Safe Handling and Disposal of Manufactured Nanomaterials; BSI: London, UK, 2007. [Google Scholar]
- NIOSH. Oil Mist (Mineral); NIOSH: Washington, DC, USA, 2019.
- NIOSH. Vegetable Oil Mist; NIOSH: Washington, DC, USA, 2019.
- Lin, S.; Yu, T.; Yu, Z.; Hu, X.; Yin, D. Nanomaterials Safer-by-Design: An Environmental Safety Perspective. Adv. Mater. 2018, 30, e1705691. [Google Scholar] [CrossRef] [PubMed]
- Hwang, R.; Mirshafiee, V.; Zhu, Y.; Xia, T. Current Approaches for Safer Design of Engineered Nanomaterials. Ecotoxicol. Environ. Saf. 2018, 166, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Morose, G. The 5 Principles of “Design for Safer Nanotechnology”. J. Clean. Prod. 2010, 18, 285–289. [Google Scholar] [CrossRef]
- Brown, N.J. Health Hazard Manual for Cutting Oils, Coolants, and Metalworking Fluids. 1991. Available online: https://ecommons.cornell.edu/items/e567278d-18f7-412b-9661-3a20ac4b0bd1 (accessed on 9 October 2024).
- Zhang, X.; Li, C.; Zhou, Z.; Liu, B.; Zhang, Y.; Yang, M.; Gao, T.; Liu, M.; Zhang, N.; Said, Z.; et al. Vegetable Oil-Based Nanolubricants in Machining: From Physicochemical Properties to Application. Chin. J. Mech. Eng. (Engl. Ed.) 2023, 36, 76. [Google Scholar] [CrossRef]
- Cabanettes, F.; Faverjon, P.; Sova, A.; Dumont, F.; Rech, J. MQL Machining: From Mist Generation to Tribological Behavior of Different Oils. Int. J. Adv. Manuf. Technol. 2017, 90, 1119–1130. [Google Scholar] [CrossRef]
- Fachausschuss Information Sheet No. 006 Low-Emission Metalworking with Minimum Quantity Lubrication (MQL); Deutsche Gesetzliche Unfallversicherung e.V. (DGUV): Berlin, Germany, 2021; Available online: https://publikationen.dguv.de/widgets/pdf/download/article/4329 (accessed on 9 October 2024).
- Abellán-Nebot, J.V.; Habib, K.; Mondragón, R.; Khan, A.M. Application of Hybrid Nanofluids in MQL Assisted Machining Operations. Exploring Synergies and Establishing Guidelines. Int. J. Precis. Eng. Manuf.-Green Technol. 2024; to be published. [Google Scholar]
- Makhesana, M.A.; Patel, K.M.; Krolczyk, G.M.; Danish, M.; Singla, A.K.; Khanna, N. Influence of MoS2 and Graphite-Reinforced Nanofluid-MQL on Surface Roughness, Tool Wear, Cutting Temperature and Microhardness in Machining of Inconel 625. CIRP J. Manuf. Sci. Technol. 2023, 41, 225–238. [Google Scholar] [CrossRef]
- Lv, T.; Huang, S.; Liu, E.; Ma, Y.; Xu, X. Tribological and Machining Characteristics of an Electrostatic Minimum Quantity Lubrication (EMQL) Technology Using Graphene Nano-Lubricants as Cutting Fluids. J. Manuf. Process. 2018, 34, 225–237. [Google Scholar] [CrossRef]
- Xu, X.; Lv, T.; Luan, Z.; Zhao, Y.; Wang, M.; Hu, X. Capillary Penetration Mechanism and Oil Mist Concentration of Al2O3 Nanoparticle Fluids in Electrostatic Minimum Quantity Lubrication (EMQL) Milling. Int. J. Adv. Manuf. Technol. 2019, 104, 1937–1951. [Google Scholar] [CrossRef]




| Type of Study | Advantages | Limitations |
|---|---|---|
| In vitro | Cost-effective approach. Allows a controlled environment for deriving effective conclusions. Some efforts have been made in this regard with some recommendations about in vitro testing concentrations for specific occupational exposures to nanomaterials given particle size distribution, aerosol concentration, nanoparticle aspect ratio and exposure duration. | Concentrations of substances tested in vitro might not accurately reflect real exposure levels in vivo. In vitro studies may not fully replicate the complexity of a living organism. Biological processes such as absorption, distribution, metabolism and excretion (ADME) are often absent or misrepresented. Chronic effects might not be adequately assessed. |
| In vivo | Provides a more holistic view of toxicity. The real effect on the biological system can be derived. For testing respirable substances, inhalation exposures are preferred to intratracheal instillation since it resembles real-life situations. | Expensive and ethically challenging, currently limited by the Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. |
| In silico | Cost-effective alternative to in vitro and in vivo approaches. | Limited applicability due to the current scarcity of experimental data related to nanotoxicity. |
| NP | Typical Shape | Thermal Conductivity (W/mK) 1 | Mohs Hardness 2 | Density (kg/m3) | Hazards & Precautions 3 |
|---|---|---|---|---|---|
| Metal oxides | |||||
| Al2O3 | Spherical | 36 | 9 | 3975 | H319, H335 P261, P264, P271, P280, P304 + P340 P305 + P351 + P338 P403 + P233 P405 P501 |
| SiO2 | Spherical | 1.34 | 7 | 2400 | |
| CuO | Spherical, rod, platelet | 18 | 3.5 | 6400 | |
| ZrO2 | Spherical, rod | 1.85 | 6.5 | 5560 | |
| Fe2O3 | Spherical, rod | 12.55 | 5.5–6.5 | 5240 | |
| TiO2 | Spherical, rod, tubular | 8.79–13.39 | 6.5 | 4230 | |
| ZnO | Spherical, rod | 27.20 | 4.5 | 5630 | |
| Carbon-based structures | |||||
| Fullerene (C60) | Spherical | 0.4 | 3–4 | 1650 | H319, H335 P261, P264, P271, P280, P304 + P340 P305 + P351 + P338 P403 + P233 P405 P501 |
| Nanodiamond (ND) | Spherical | 2300 | 10 | 3500 | |
| Single-walled carbon nanotube (SWCNT) | Tubular | 3000–5000 | 1–3 | 2100 | |
| Multi-walled carbon nanotube (MWCNT) | Tubular | 3000–5000 | 1–3 | 2100 | |
| Graphite (GR) | Platelet, rod | 167.36 | 1–2 | 2260 | |
| Graphene (GNP) | Sheets, platelet | 3000–5000 | 2–3 | 2267 | |
| Graphene oxide (GO) | Sheets, platelet | 600–5000 | 2 | 1360 | |
| SiC | Spherical, rod | 370 | 9 | 3216 | |
| Metal Sulfides and Boron Nitrides | |||||
| MoS2 | Sheets, rods, spherical | 138 | 1–1.5 | 4800 | H319, H335 P261, P264, P271, P280, P304 + P340 P305 + P351 + P338 P403 + P233 P405 P501 |
| WS2 | 53 | 1–1.5 | 7500 | ||
| hBN | 27 | 2–4 | 2300 | ||
| Very High | High | Moderate | Low | Very Low |
|---|---|---|---|---|
| CuO ZnO | MWCNTs SWCNTs Graphene oxide hBN SiO2 (crystalline) | Graphene SiO2 (amorphous) ZrO2 Fullerene (C60) Nanodiamond (ND) Carbon Quantum Dot (QD) Carbon Black | SiC Al2O3 Graphite TiO2 | WS2 MoS2 Fe2O3 Fe3O4 |
| Physicochemical Properties | Concluding Remarks |
|---|---|
| Size | Low nanoparticle size increases biodistribution and accumulation/retention leading to higher cytotoxicity. |
| Shape | Nanoparticles with higher aspect ratios for a given mass present higher specific surface area which is associated with higher cell reactivity and cytotoxicity. Special care is given when dealing with nanofibers which present a toxicity similar to asbestos, proven highly cytotoxic and genotoxic particles. |
| Surface properties | Positive surface charge (zeta potential) increases nanotoxicity while surfactants modify nanoparticle interactions with the following toxicity order: strong cationic (CATB, BAC, PEI functionalization) > weakly cationic (amine functionalization) > anionic (PEG, PVP, carboxyl functionalization) > non-ionic (hydroxyl functionalization). Anionic/non-ionic surfactants and anionic/non-ionic functionalized groups seems to reduce the potential nanoparticle toxicity. Anionic surfactants and hydroxyl functionalization typically increase hydrophilicity reducing nanoparticles’ toxicity profile. |
| Aggregation/Agglomeration | Agglomerated nanoparticles are larger and may not penetrate cells as easily as individual, smaller nanoparticles. Agglomerates might be less reactive than dispersed nanoparticles, although their accumulation after crossing primary barriers can cause more severe toxicity. |
| Solubility | Nanoparticle solubility affects potential toxicity. Soluble nanoparticles that do not release ions are less cytotoxic than insoluble or partly soluble nanoparticles. |
| Particles | Regulatory Entity | |||||
|---|---|---|---|---|---|---|
| OSHA | NIOSH | OSHA | NIOSH | According to Note 1 | According to Note 2 | |
| Particle Size | Microscale (dust) | nanoscale | ||||
| Exposure Limit | PEL + | REL ^ | PEL + | REL ^ | OEL | OEL |
| Carbon black | 3.5 mg/m3 | 3.5 mg/m3 | - | - | 0.44 mg/m3 | 0.23 mg/m3 |
| Carbon nanotubes | - | - | 1 µg/m3 | 1 µg/m3 | - | - |
| TiO2 | 15 mg/m3 (total dust) | 2.4 mg/m3 | 0.3 mg/m3 | 0.3 mg/m3 | 0.3 mg/m3 | 1 mg/m3 |
| SiO2 * | 80 mg/m3/%SiO2 | 6 mg/m3 | - | - | 0.75 mg/m3 | 0.4 mg/m3 |
| CuO | 1 mg/m3 (dust) 0.1 mg/m3 (fume) | 1 mg/m3 (dust) 0.1 mg/m3 (fume) | - | - | 13 µg/m3 | 7 µg/m3 |
| ZnO | 15 mg/m3 (total dust) 5 mg/m3 (fume) | 15 mg/m3 (dust) 10 mg/m3 (fume) | - | - | 1.25 mg/m3 | 0.66 mg/m3 |
| Al2O3 | 15 mg/m3 (total dust) 5 mg/m3 (resp. fraction) | 10 mg/m3 (total dust) 5 mg/m3 (resp. fraction) | - | - | 0.63 mg/m3 | 0.33 mg/m3 |
| Fe2O3 | 10 mg/m3 | 5 mg/m3 | - | - | 0.63 mg/m3 | 0.33 mg/m3 |
| Graphite | 15 mg/m3 (total dust) 5 mg/m3 (resp. fraction) | 2.5 mg/m3 | - | - | 0.31 mg/m3 | 0.16 mg/m3 |
| MoS2 | 15 mg/m3 (total dust) 5 mg/m3 (resp. dust) | 5 mg/m3 | - | - | 0.63 mg/m3 | 0.33 mg/m3 |
| SiC | 15 mg/m3 (total dust) 5 mg/m3 (resp. fraction) | 10 mg/m3 (total dust) 5 mg/m3 (resp. fraction) | - | - | 0.63 mg/m3 | 0.33 mg/m3 |
| WS2 | 5 mg/m3 | 10 mg/m3 (total dust) 5 mg/m3 (resp. fraction) | - | - | 0.63 mg/m3 | 0.33 mg/m3 |
| Graphene/Graphene oxide | - | - | - | - | - | - |
| hBN | - | - | - | - | - | - |
| Nanoparticles | Anti-Friction | Anti-Wear | Cooling | Mending | Polishing | Toxicity |
|---|---|---|---|---|---|---|
| WS2 | + | +++ | + | ++ | + | + |
| MoS2 | ++ | +++ | ++ | ++ | + | + |
| Fe2O3 | +++ | ++ | + | +++ | ++ | + |
| Al2O3 | +++ | + | + | +++ | +++ | + |
| SiC | +++ | + | ++ | +++ | +++ | + |
| Graphite | + | ++ | ++ | ++ | + | + |
| TiO2 | +++ | ++ | + | +++ | ++ | + |
| SiO2 | +++ | + | + | +++ | ++ | ++ |
| Graphene | + | + | + | + | + | ++ |
| ZrO2 | +++ | + | + | +++ | ++ | ++ |
| Fullerene (C60) | +++ | + | + | +++ | + | ++ |
| Nanodiamond | +++ | + | +++ | +++ | +++ | ++ |
| Graphene Oxide | + | ++ | +++ | ++ | + | +++ |
| SWCNT | ++ | + | +++ | + | + | +++ |
| MWCNT | ++ | + | +++ | + | + | +++ |
| hBN | +++ | ++ | + | +++ | + | +++ |
| ZnO | +++ | ++ | + | +++ | ++ | +++ |
| CuO | +++ | ++ | + | +++ | + | +++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abellán-Nebot, J.V.; Andreu-Sánchez, O.; Fito-López, C.; Mondragón, R. Nanofluid Minimum Quantity Lubrication (NMQL): Overview of Nanoparticle Toxicity and Safer-Design Guidelines. Lubricants 2024, 12, 359. https://doi.org/10.3390/lubricants12100359
Abellán-Nebot JV, Andreu-Sánchez O, Fito-López C, Mondragón R. Nanofluid Minimum Quantity Lubrication (NMQL): Overview of Nanoparticle Toxicity and Safer-Design Guidelines. Lubricants. 2024; 12(10):359. https://doi.org/10.3390/lubricants12100359
Chicago/Turabian StyleAbellán-Nebot, José V., Oscar Andreu-Sánchez, Carlos Fito-López, and Rosa Mondragón. 2024. "Nanofluid Minimum Quantity Lubrication (NMQL): Overview of Nanoparticle Toxicity and Safer-Design Guidelines" Lubricants 12, no. 10: 359. https://doi.org/10.3390/lubricants12100359
APA StyleAbellán-Nebot, J. V., Andreu-Sánchez, O., Fito-López, C., & Mondragón, R. (2024). Nanofluid Minimum Quantity Lubrication (NMQL): Overview of Nanoparticle Toxicity and Safer-Design Guidelines. Lubricants, 12(10), 359. https://doi.org/10.3390/lubricants12100359





