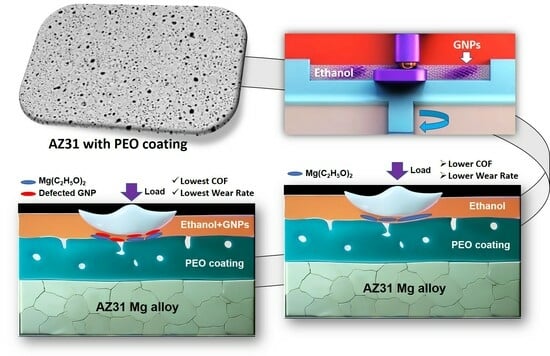
Tribological Performance of a Plasma Electrolytic Oxidation-Coated Mg Alloy in Graphene-Incorporated Ethanol
Abstract
:1. Introduction
2. Experimental Procedure
3. Results
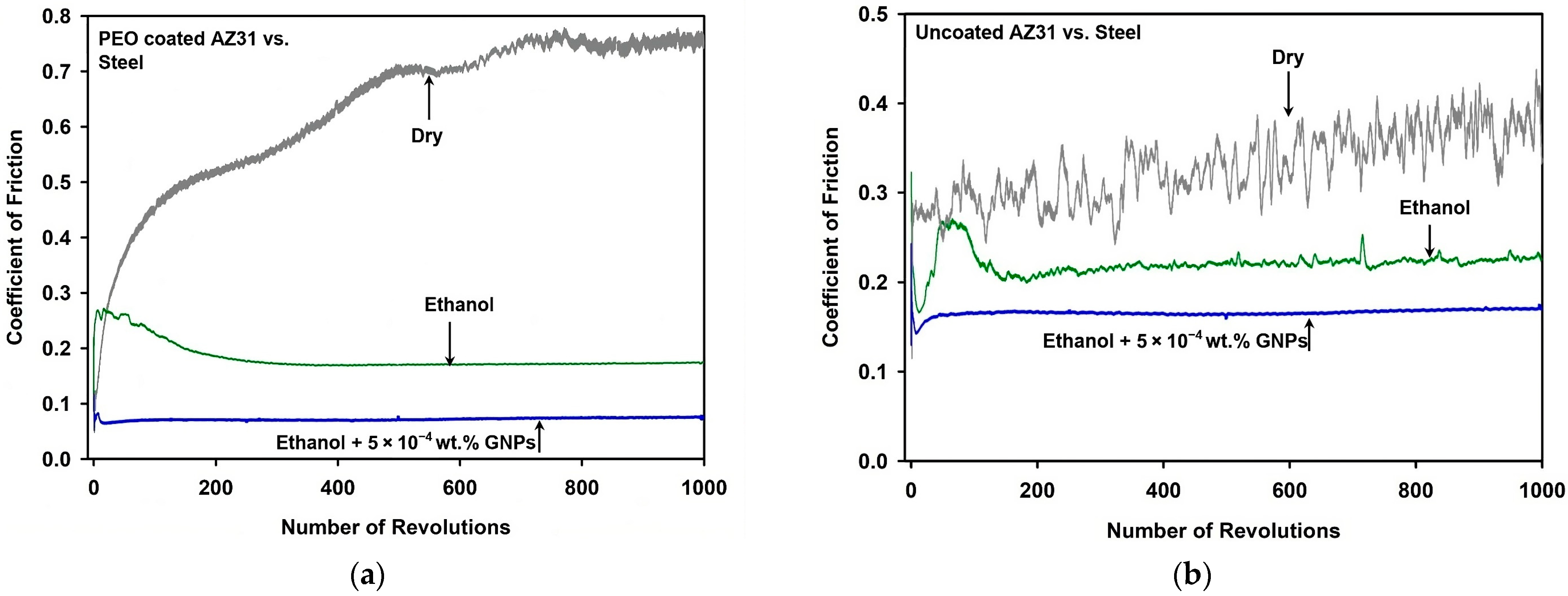
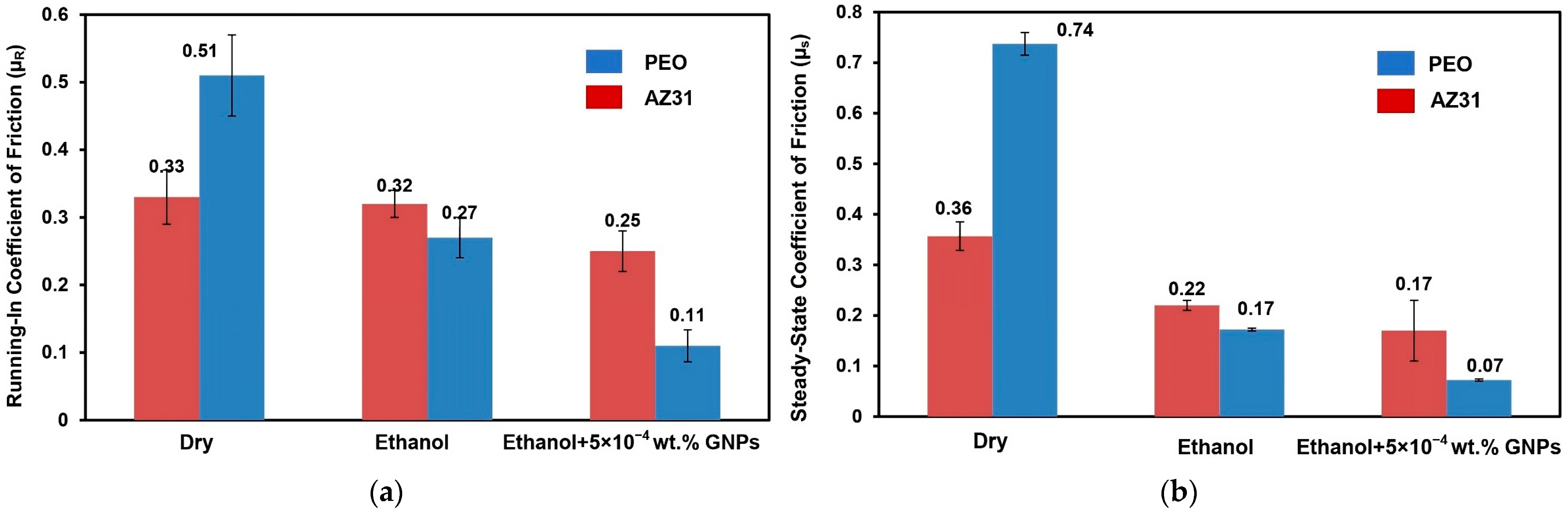
3.1. Tribological Properties of the PEO-Coated AZ31 and Uncoated AZ31
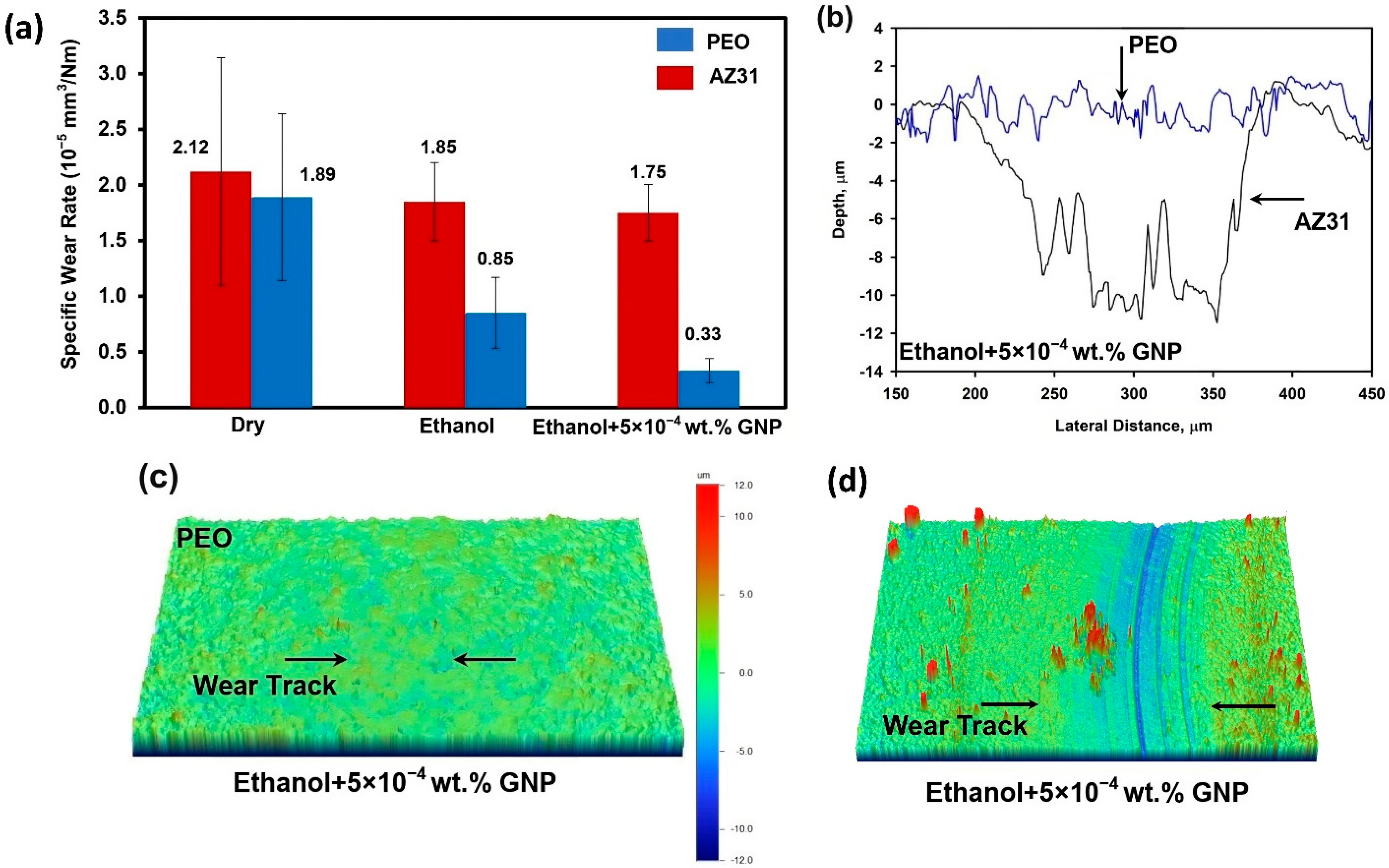
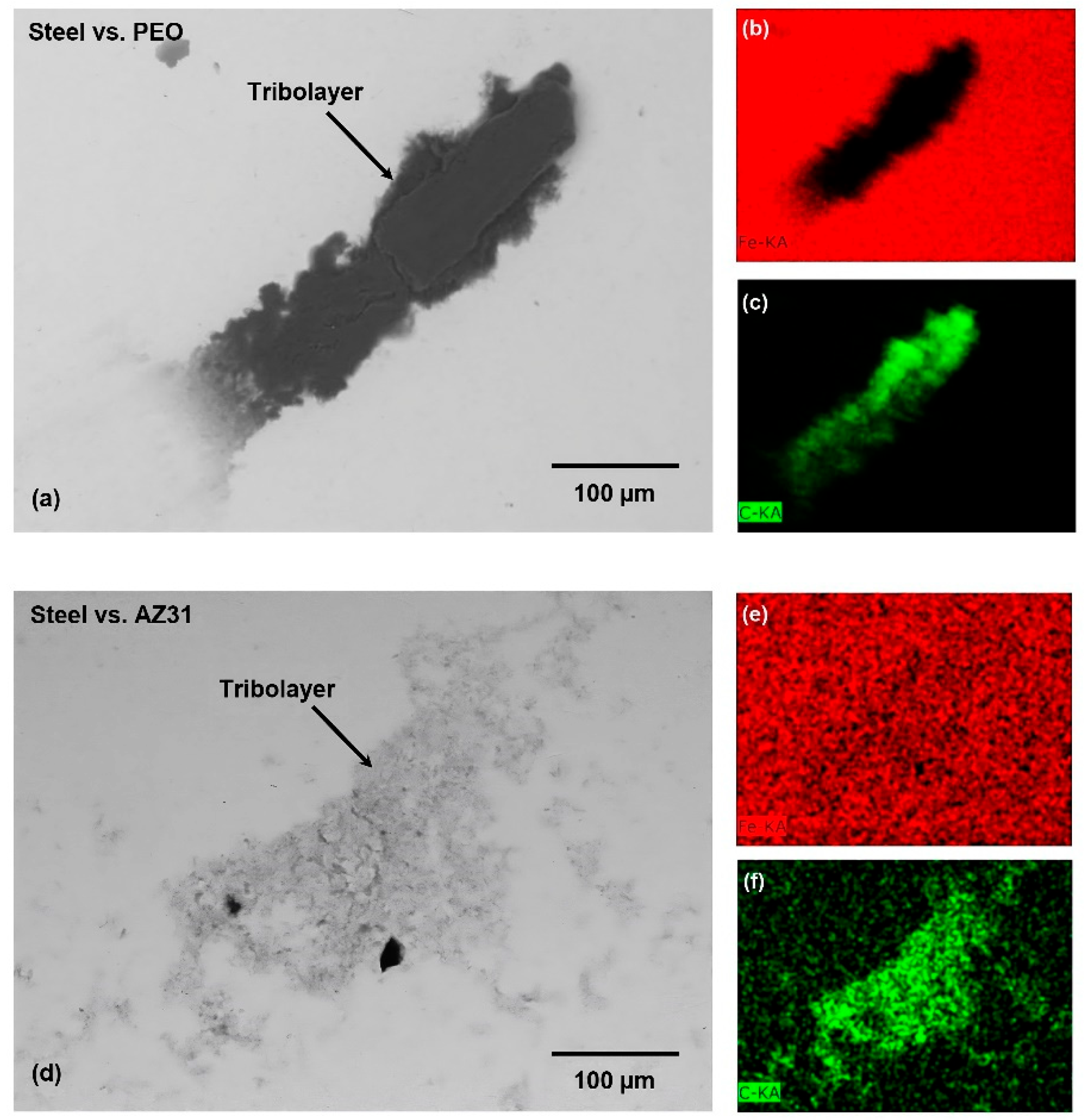
3.2. Worn Surfaces and Transfer Layer Formed during Testing in Ethanol and GNP- Added Ethanol
3.3. Wear and Surface Characterization of Counterface Balls Used in Sliding Wear Tests
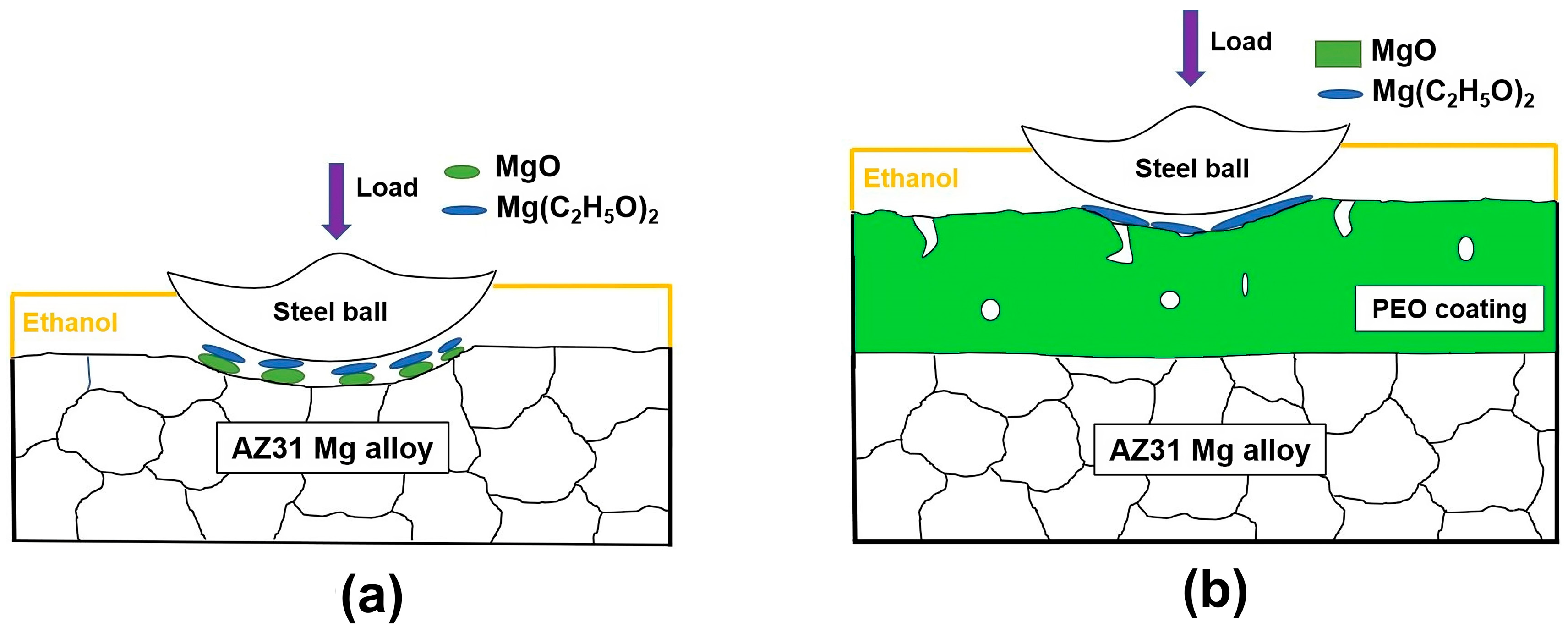
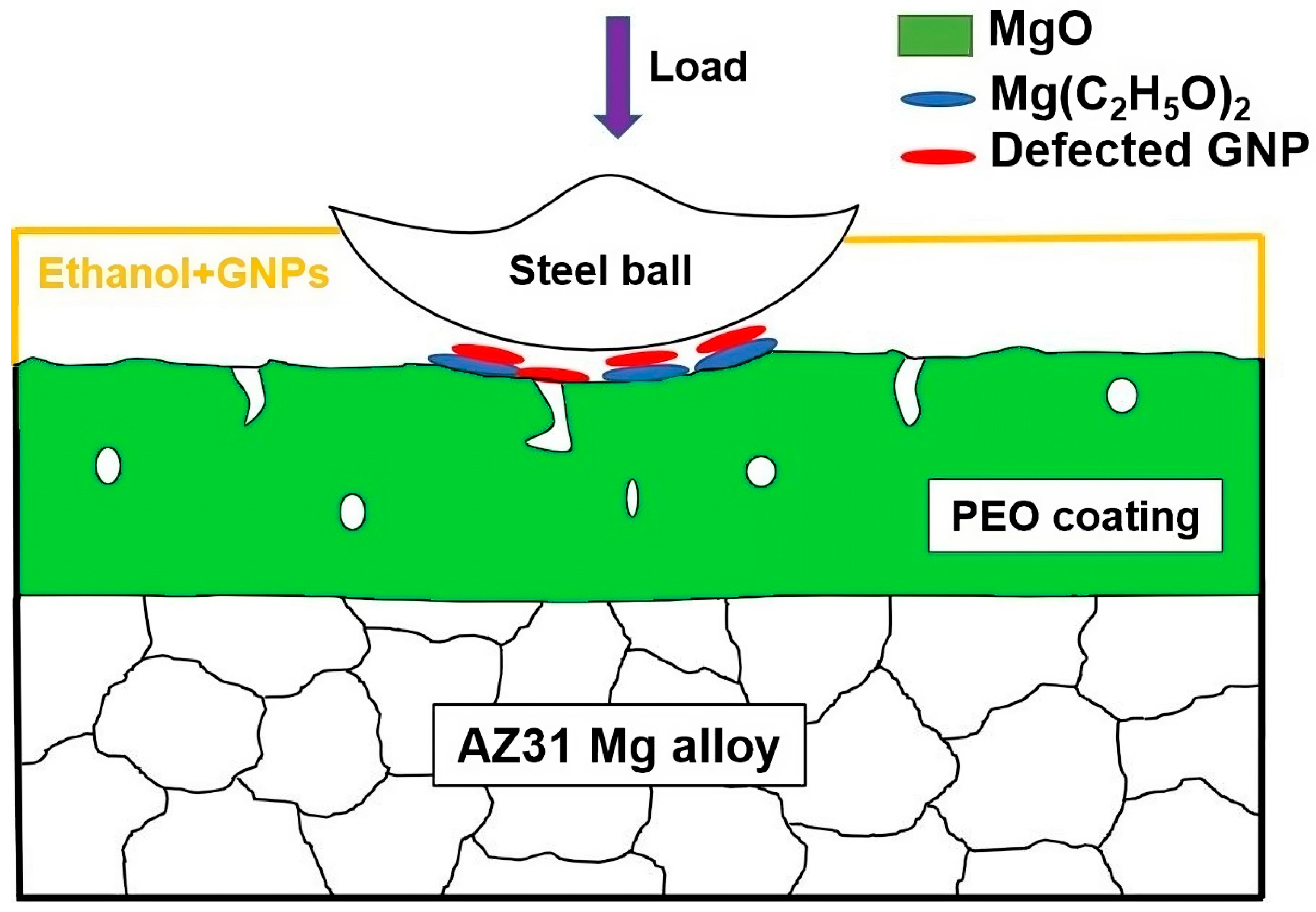
4. Discussion
5. Conclusions
- The PEO-coated AZ31 surfaces maintained a steady-state COF of 0.18 in ethanol, a value lower than that observed in dry sliding (0.74), and closely matched that of uncoated AZ31.
- Tests conducted in ethanol with the addition of 5 × 10−4 wt.% GNPs significantly decreased the COF to 0.07 and the wear rate of PEO-coated AZ31 from 18.36 × 10−4 mm3 (when tested in ethanol) to 11.02 × 10−4 mm3, demonstrating superior performance compared to the uncoated AZ31.
- XPS, Raman spectroscopy, and SEM analyses revealed the formation of a stable tribolayer on the PEO-coated AZ31 and the steel counterface during sliding in ethanol with dispersed GNPs.
- GNPs in ethanol were less effective as a lubricant for the uncoated AZ31. The enhanced performance of the PEO-coated AZ31 in ethanol with GNPs was due to a combination of the formation of graphene-incorporated tribolayers and their chemical interactions within the ethanol environment.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pesode, P.; Barve, S. Surface Modification of Titanium and Titanium Alloy by Plasma Electrolytic Oxidation Process for Biomedical Applications: A Review. Mater. Today Proc. 2021, 46, 594–602. [Google Scholar] [CrossRef]
- Clyne, T.W.; Troughton, S.C. A Review of Recent Work on Discharge Characteristics during Plasma Electrolytic Oxidation of Various Metals. Int. Mater. Rev. 2019, 64, 127–162. [Google Scholar] [CrossRef]
- Yao, W.; Wu, L.; Wang, J.; Jiang, B.; Zhang, D.; Serdechnova, M.; Shulha, T.; Blawert, C.; Zheludkevich, M.L.; Pan, F. Micro-arc Oxidation of Magnesium Alloys: A Review. J. Mater. Sci. Technol. 2022, 118, 158–180. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, W.; Wang, X.; Wang, Y.; Yue, H.; Li, Q.; Yang, X.; Guo, C.; Li, C. Micro-Arc Oxidation of Al Alloys: Mechanism, Microstructure, Surface Properties, and Fatigue Damage Behavior. J. Mater. Res. Technol. 2023, 23, 4307–4333. [Google Scholar] [CrossRef]
- Schwartz, A.; Kossenko, A.; Zinigrad, M.; Danchuk, V.; Sobolev, A. Cleaning Strategies of Synthesized Bioactive Coatings by PEO on Ti-6Al-4V Alloys of Organic Contaminations. Materials 2023, 16, 4624. [Google Scholar] [CrossRef]
- Liang, J.; Guo, B.; Tian, J.; Liu, H.; Zhou, J.; Xu, T. Effect of Potassium Fluoride in Electrolytic Solution on the Structure and Properties of Microarc Oxidation Coatings on Magnesium Alloy. Appl. Surf. Sci. 2005, 252, 345–351. [Google Scholar] [CrossRef]
- Liang, J.; Wang, P.; Hu, L.; Hao, J. Tribological Properties of Duplex MAO/DLC Coatings on Magnesium Alloy Using Combined Microarc Oxidation and Filtered Cathodic Arc Deposition. Mater. Sci. Eng. A 2007, 454–455, 164–169. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Chaharmahali, R. Enhancing Corrosion and Wear Performance of PEO Coatings on Mg Alloys Using Graphene and Graphene Oxide Additions: A Review. FlatChem 2021, 27, 100241. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Y.; Zhang, Y. Effect of Graphene on Micro-Structure and Properties of MAO Coating Prepared on Mg-Li Alloy. Int. J. Electrochem. Sci. 2017, 12, 6081–6091. [Google Scholar] [CrossRef]
- Nasiri Vatan, H.; Adabi, M. Investigation of Tribological Behavior of Ceramic–Graphene Composite Coating Produced by Plasma Electrolytic Oxidation. Trans. Indian Inst. Met. 2018, 71, 1643–1652. [Google Scholar] [CrossRef]
- Bhowmick, S.; Muhaffel, F.; Sun, G.; Cimenoglu, H.; Alpas, A.T. Role of Counterfaces with DLC and N-Based Coatings on Frictional Behaviour of AZ31 Magnesium Alloy Subjected to Plasma Electrolytic Oxidation (PEO) Process. Surf. Coatings Technol. 2020, 397, 125997. [Google Scholar] [CrossRef]
- Bhowmick, S.; Muhaffel, F.; Eskandari, B.; Cimenoglu, H.; Alpas, A.T. Low Friction and High Wear Resistance of Plasma Electrolytic Oxidation (PEO)-Coated AZ31 Mg Alloy Sliding against Hydrogenated DLC (a-C-H) at Elevated Temperatures. Coatings 2022, 12, 607. [Google Scholar] [CrossRef]
- Tokgoz, S.; Traoré, F. Understanding E10 Markets in the U.S.: Evidence from Spatial Data. Econ. Anal. Policy 2023, 78, 1267–1281. [Google Scholar] [CrossRef]
- Kubo, T.; Nanao, H.; Mori, S.; Enomoto, Y.; Nie, H.; Nomura, H. Chemical Analysis of Boundary Lubrication Film Formed on Metal Nitride Coatings with Ethanol by Means of TOF-SIMS. Wear 2010, 268, 1225–1229. [Google Scholar] [CrossRef]
- Bandeira, A.L.; Trentin, R.; Aguzzoli, C.; Maia da Costa, M.E.H.; Michels, A.F.; Baumvol, I.J.R.; Farias, M.C.M.; Figueroa, C.A. Sliding Wear and Friction Behavior of CrN-Coating in Ethanol and Oil-Ethanol Mixture. Wear 2013, 301, 786–794. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, H.J.; Lee, C.; Lee, S.K.; Jang, H.; Ahn, J.H.; Kim, J.H.; Lee, H.J. Chemical Vapor Deposition-Grown Graphene: The Thinnest Solid Lubricant. ACS Nano 2011, 5, 5107–5114. [Google Scholar] [CrossRef] [PubMed]
- Eswaraiah, V.; Sankaranarayanan, V.; Ramaprabhu, S. Graphene-Based Engine Oil Nanofluids for Tribological Applications. ACS Appl. Mater. Interfaces 2011, 3, 4221–4227. [Google Scholar] [CrossRef]
- Huang, J.; Tan, J.; Fang, H.; Gong, F.; Wang, J. Tribological and Wear Performances of Graphene-Oil Nanofluid under Industrial High-Speed Rotation. Tribol. Int. 2019, 135, 112–120. [Google Scholar] [CrossRef]
- Gan, C.; Liang, T.; Chen, D.; Li, W.; Fan, X.; Tang, G.; Lin, B.; Zhu, M. Phosphonium-Organophosphate Modified Graphene Gel towards Lubrication Applications. Tribol. Int. 2020, 145, 106180. [Google Scholar] [CrossRef]
- Kong, S.; Wang, J.; Hu, W.; Li, J. Effects of Thickness and Particle Size on Tribological Properties of Graphene as Lubricant Additive. Tribol. Lett. 2020, 68, 112. [Google Scholar] [CrossRef]
- Ren, J.; Gong, K.; Zhao, G.; Lou, W.; Wu, X.; Wang, X. Investigation of the Tribological Performances of Graphene and WS2 Nanosheets as Additives for Perfluoroalkylpolyethers Under Simulated Space Environment. Tribol. Lett. 2021, 69, 45. [Google Scholar] [CrossRef]
- Nasser, K.I.; Liñeira del Río, J.M.; Mariño, F.; López, E.R.; Fernández, J. Double Hybrid Lubricant Additives Consisting of a Phosphonium Ionic Liquid and Graphene Nanoplatelets/Hexagonal Boron Nitride Nanoparticles. Tribol. Int. 2021, 163, 107189. [Google Scholar] [CrossRef]
- Bertolini, R.; Ghiotti, A.; Bruschi, S. Graphene Nanoplatelets as Additives to MQL for Improving Tool Life in Machining Inconel 718 Alloy. Wear 2021, 476, 203656. [Google Scholar] [CrossRef]
- Zhang, D.; Du, X.; Bai, A.; Wang, L. The Synergistic Effect of MAO-Treated and PAO–Graphene Oil on Tribological Properties of Ti6Al4V Alloys. Wear 2022, 510–511, 204494. [Google Scholar] [CrossRef]
- Wang, L.; Gong, P.; Li, W.; Luo, T.; Cao, B. Mono-Dispersed Ag/Graphene Nanocomposite as Lubricant Additive to Reduce Friction and Wear. Tribol. Int. 2020, 146, 106228. [Google Scholar] [CrossRef]
- Liu, Y.; Mateti, S.; Li, C.; Liu, X.; Glushenkov, A.M.; Liu, D.; Li, L.H.; Fabijanic, D.; Chen, Y. Synthesis of Composite Nanosheets of Graphene and Boron Nitride and Their Lubrication Application in Oil. Adv. Eng. Mater. 2018, 20, 1700488. [Google Scholar] [CrossRef]
- Sikdar, S.; Rahman, M.H.; Ralls, A.M.; Menezes, P.L. Enhancement in Tribological Performance of Plastic Oil by Solid Lubricant Additives. Lubr. Sci. 2023, 35, 420–437. [Google Scholar] [CrossRef]
- Berman, D.; Erdemir, A.; Sumant, A.V. Graphene: A New Emerging Lubricant. Mater. Today 2014, 17, 31–42. [Google Scholar] [CrossRef]
- Fan, X.; Xia, Y.; Wang, L.; Li, W. Multilayer Graphene as a Lubricating Additive in Bentone Grease. Tribol. Lett. 2014, 55, 455–464. [Google Scholar] [CrossRef]
- Mungse, H.P.; Kumar, N.; Khatri, O.P. Synthesis, Dispersion and Lubrication Potential of Basal Plane Functionalized Alkylated Graphene Nanosheets. RSC Adv. 2015, 5, 25565–25571. [Google Scholar] [CrossRef]
- Sanes, J.; Avilés, M.D.; Saurín, N.; Espinosa, T.; Carrión, F.J.; Bermúdez, M.D. Synergy between Graphene and Ionic Liquid Lubricant Additives. Tribol. Int. 2017, 116, 371–382. [Google Scholar] [CrossRef]
- Bordignon, R.; Salvaro, D.; Binder, C.; Klein, A.N.; Drago, V.; de Mello, J.D.B. Tribological Behaviour of Plasma-Functionalized Graphene as Low-Viscosity Oil Additive. Tribol. Lett. 2018, 66, 114. [Google Scholar] [CrossRef]
- Ali, M.K.A.; Xianjun, H.; Abdelkareem, M.A.A.; Gulzar, M.; Elsheikh, A.H. Novel Approach of the Graphene Nanolubricant for Energy Saving via Anti-Friction/Wear in Automobile Engines. Tribol. Int. 2018, 124, 209–229. [Google Scholar] [CrossRef]
- Paul, G.; Shit, S.; Hirani, H.; Kuila, T.; Murmu, N.C. Tribological Behavior of Dodecylamine Functionalized Graphene Nanosheets Dispersed Engine Oil Nanolubricants. Tribol. Int. 2019, 131, 605–619. [Google Scholar] [CrossRef]
- Kogovšek, J.; Kalin, M. Lubrication Performance of Graphene-Containing Oil on Steel and DLC-Coated Surfaces. Tribol. Int. 2019, 138, 59–67. [Google Scholar] [CrossRef]
- Elomaa, O.; Singh, V.K.; Iyer, A.; Hakala, T.J.; Koskinen, J. Graphene Oxide in Water Lubrication on Diamond-like Carbon vs. Stainless Steel High-Load Contacts. Diam. Relat. Mater. 2015, 52, 43–48. [Google Scholar] [CrossRef]
- Wu, L.; Zhong, Y.; Yuan, H.; Liang, H.; Wang, F.; Gu, L. Ultra-Dispersive Sulfonated Graphene as Water-Based Lubricant Additives for Enhancing Tribological Performance. Tribol. Int. 2022, 174, 107759. [Google Scholar] [CrossRef]
- Berman, D.; Erdemir, A.; Sumant, A.V. Few Layer Graphene to Reduce Wear and Friction on Sliding Steel Surfaces. Carbon N. Y. 2013, 54, 454–459. [Google Scholar] [CrossRef]
- Bhowmick, S.; Yang, Z.; Banerji, A.; Alpas, A.T. Effect of Graphene Nanoplates Dispersed in Ethanol on Frictional Behaviour of Tool Steel Running Against Uncoated and DLC-Coated Tool Steel. Tribol. Lett. 2019, 67, 32. [Google Scholar] [CrossRef]
- Bhushan, B. Principles and Applications of Tribology, 2nd ed.; John Wiley & Sons, Ltd.: Columbus, OH, USA, 2013; ISBN 3527305521. [Google Scholar]
- Bhowmick, S.; Banerji, A.; Alpas, A.T. Role of Humidity in Reducing Sliding Friction of Multilayered Graphene. Carbon N. Y. 2015, 87, 374–384. [Google Scholar] [CrossRef]
- Kim, B.I.; Lee, S.; Guenard, R.; Fernandez Torres, L.C.; Perry, S.S.; Frantz, P.; Didziulis, S.V. Chemical Modification of the Interfacial Frictional Properties of Vanadium Carbide through Ethanol Adsorption. Surf. Sci. 2001, 481, 185–197. [Google Scholar] [CrossRef]
- Zhu, T.; Yu, Y.; Shen, Y.; Xiong, Y. Wear Behavior of Extruded ZK60 Magnesium Alloy in Simulated Body Fluid with Different PH Values. Mater. Chem. Phys. 2021, 262, 124292. [Google Scholar] [CrossRef]
- Takezawa, N.; Hanamaki, C.; Kobayashi, H. The Mechanism of Dehydrogenation of Ethanol on Magnesium Oxide. J. Catal. 1975, 38, 101–109. [Google Scholar] [CrossRef]
- Liu, D.B.; Wu, B.; Wang, X.; Chen, M.F. Corrosion and Wear Behavior of an Mg–2Zn–0.2Mn Alloy in Simulated Body Fluid. Rare Met. 2015, 34, 553–559. [Google Scholar] [CrossRef]
- Dai, J.; Zhang, X.; Yin, Q.; Ni, S.; Ba, Z.; Wang, Z. Friction and Wear Behaviors of Biodegradable Mg-6Gd-0.5Zn-0.4Zr Alloy under Simulated Body Fluid Condition. J. Magnes. Alloys 2017, 5, 448–453. [Google Scholar] [CrossRef]
- Konicek, A.R.; Grierson, D.S.; Sumant, A.V.; Friedmann, T.A.; Sullivan, J.P.; Gilbert, P.U.P.A.; Sawyer, W.G.; Carpick, R.W. Influence of Surface Passivation on the Friction and Wear Behavior of Ultrananocrystalline Diamond and Tetrahedral Amorphous Carbon Thin Films. Phys. Rev. B Condens. Matter Mater. Phys. 2012, 85, 155448. [Google Scholar] [CrossRef]
- Barthel, A.J.; Al-Azizi, A.; Surdyka, N.D.; Kim, S.H. Effects of Gas or Vapor Adsorption on Adhesion, Friction, and Wear of Solid Interfaces. Langmuir 2014, 30, 2977–2992. [Google Scholar] [CrossRef]
- Dilks, A.; Kay, E. Plasma Polymerization of Ethylene and the Series of Fluoroethylenes: Plasma Effluent Mass Spectrometry and ESCA Studies. Macromolecules 1981, 14, 855–862. [Google Scholar] [CrossRef]
- Rietsch, J.C.; Brender, P.; Dentzer, J.; Gadiou, R.; Vidal, L.; Vix-Guterl, C. Evidence of Water Chemisorption during Graphite Friction under Moist Conditions. Carbon N. Y. 2013, 55, 90–97. [Google Scholar] [CrossRef]
- Egberts, P.; Han, G.H.; Liu, X.Z.; Johnson, A.T.C.; Carpick, R.W. Frictional Behavior of Atomically Thin Sheets: Hexagonal-Shaped Graphene Islands Grown on Copper by Chemical Vapor Deposition. ACS Nano 2014, 8, 5010–5021. [Google Scholar] [CrossRef]
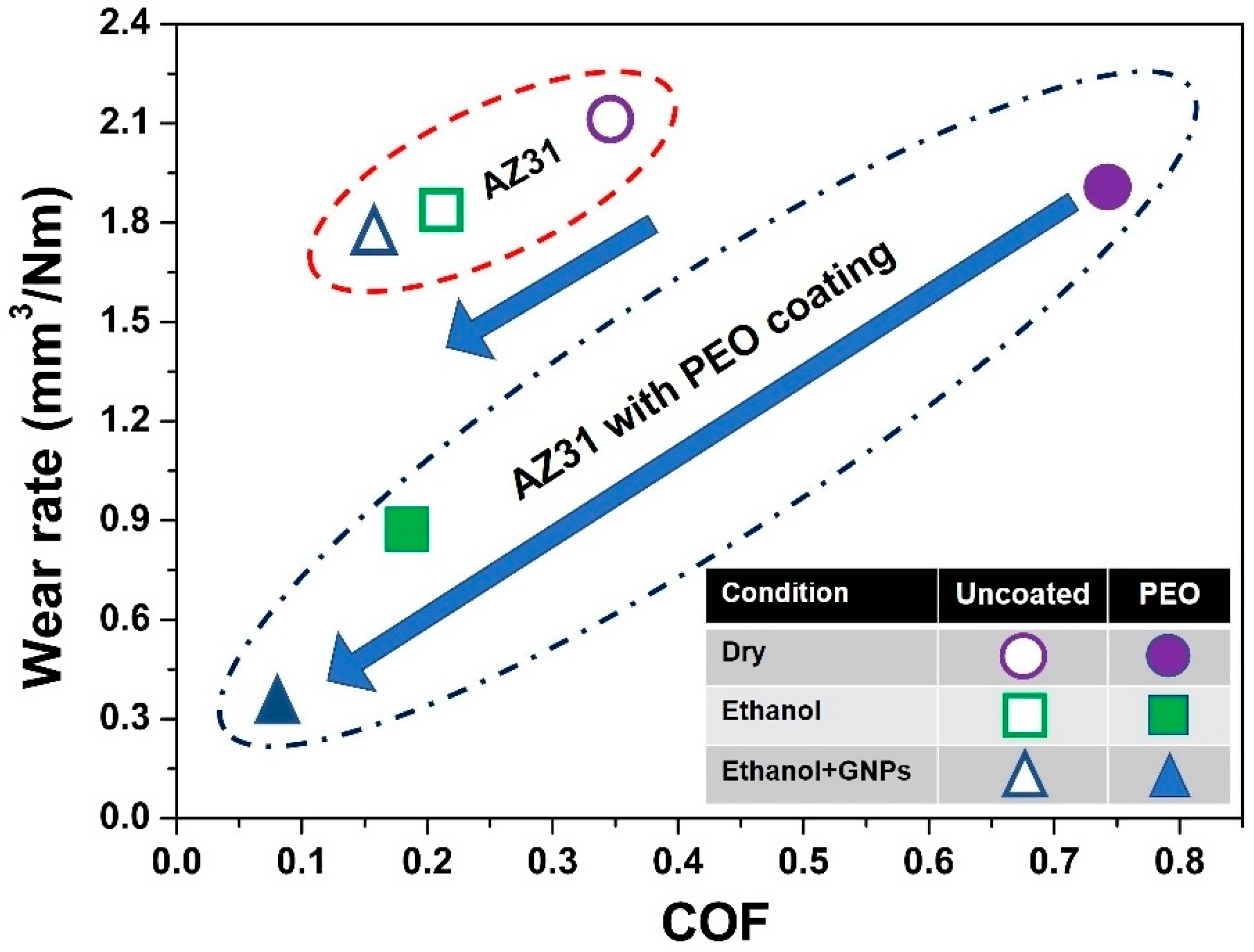
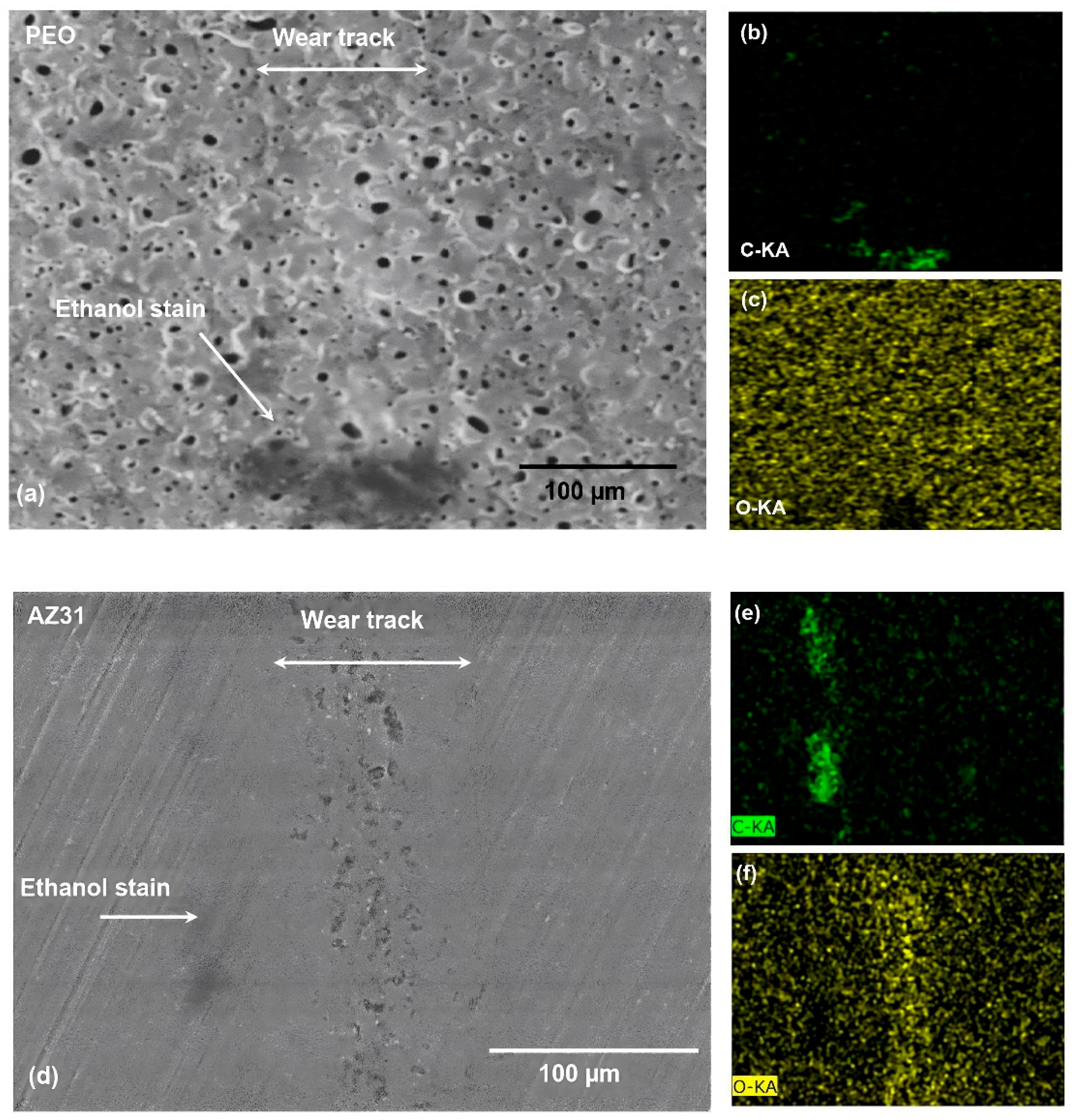
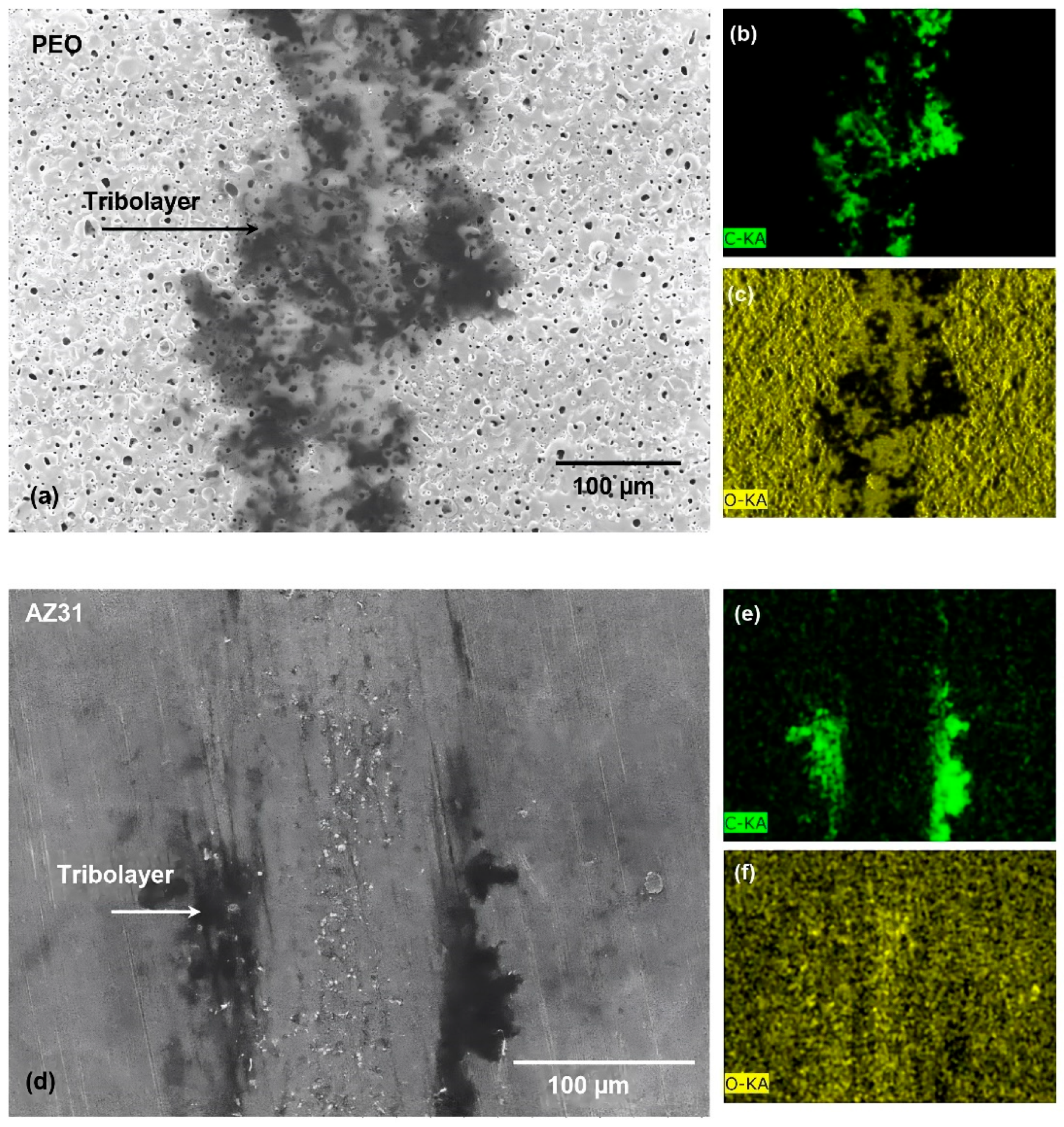

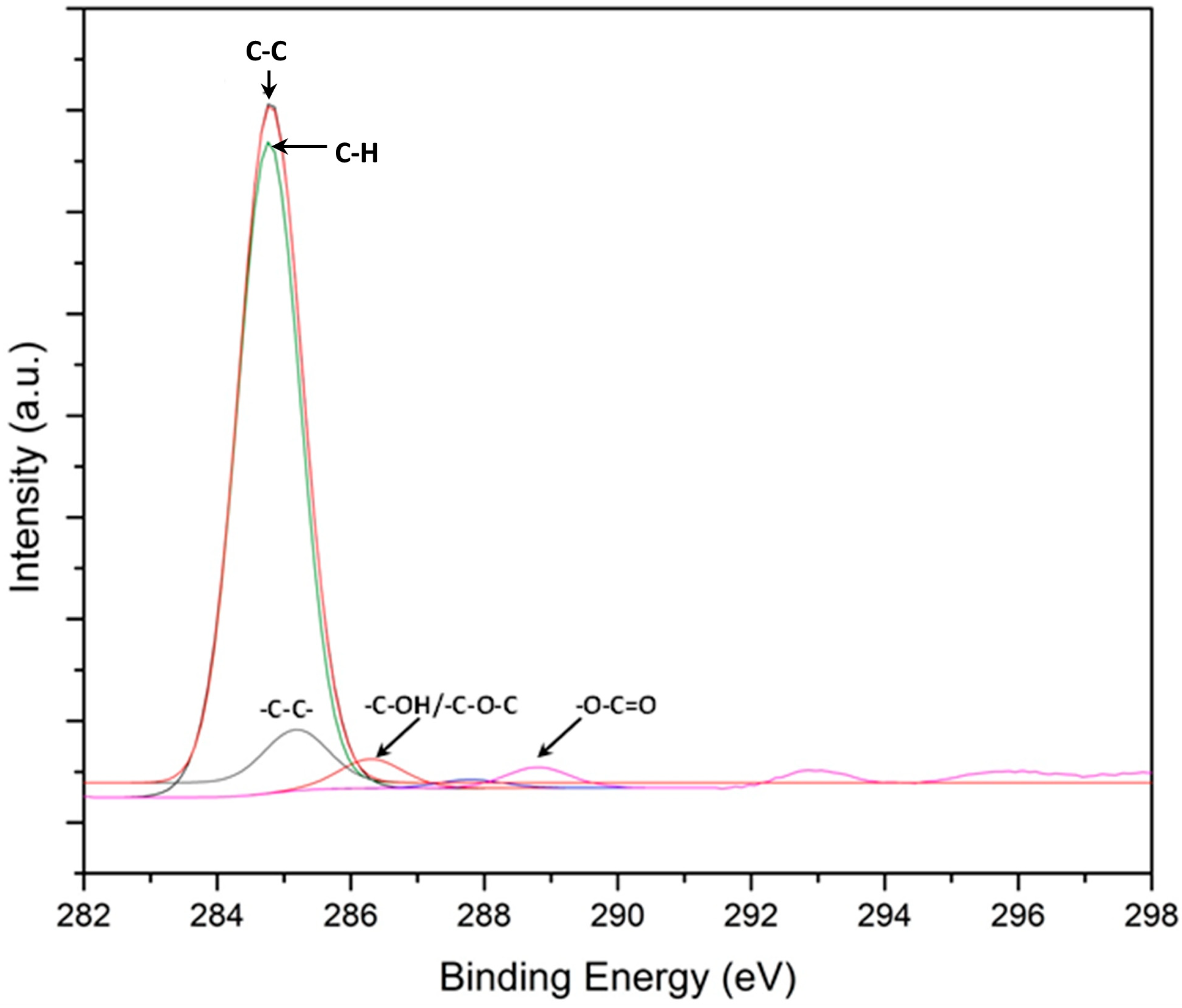
| Materials | Dry (×10−4 mm3) | Ethanol (×10−4 mm3) | Ethanol with GNPs (×10−4 mm3) |
|---|---|---|---|
| Uncoated AZ31 | 33.41 | 29.32 | 22.34 |
| PEO-coated AZ31 | 37.29 | 18.36 | 11.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhowmick, S.; Muhaffel, F.; Shirzadian, S.; Cimenoglu, H.; Alpas, A.T. Tribological Performance of a Plasma Electrolytic Oxidation-Coated Mg Alloy in Graphene-Incorporated Ethanol. Lubricants 2024, 12, 9. https://doi.org/10.3390/lubricants12010009
Bhowmick S, Muhaffel F, Shirzadian S, Cimenoglu H, Alpas AT. Tribological Performance of a Plasma Electrolytic Oxidation-Coated Mg Alloy in Graphene-Incorporated Ethanol. Lubricants. 2024; 12(1):9. https://doi.org/10.3390/lubricants12010009
Chicago/Turabian StyleBhowmick, Sukanta, Faiz Muhaffel, Shayan Shirzadian, Huseyin Cimenoglu, and Ahmet T. Alpas. 2024. "Tribological Performance of a Plasma Electrolytic Oxidation-Coated Mg Alloy in Graphene-Incorporated Ethanol" Lubricants 12, no. 1: 9. https://doi.org/10.3390/lubricants12010009
APA StyleBhowmick, S., Muhaffel, F., Shirzadian, S., Cimenoglu, H., & Alpas, A. T. (2024). Tribological Performance of a Plasma Electrolytic Oxidation-Coated Mg Alloy in Graphene-Incorporated Ethanol. Lubricants, 12(1), 9. https://doi.org/10.3390/lubricants12010009