A Study on the Corrosion Resistance of a Coating Prepared by Electrical Explosion of 321 Metal Wire
Abstract
1. Introduction
2. Experimental Procedures
2.1. Coating System Design
2.2. Characterizations
3. Results
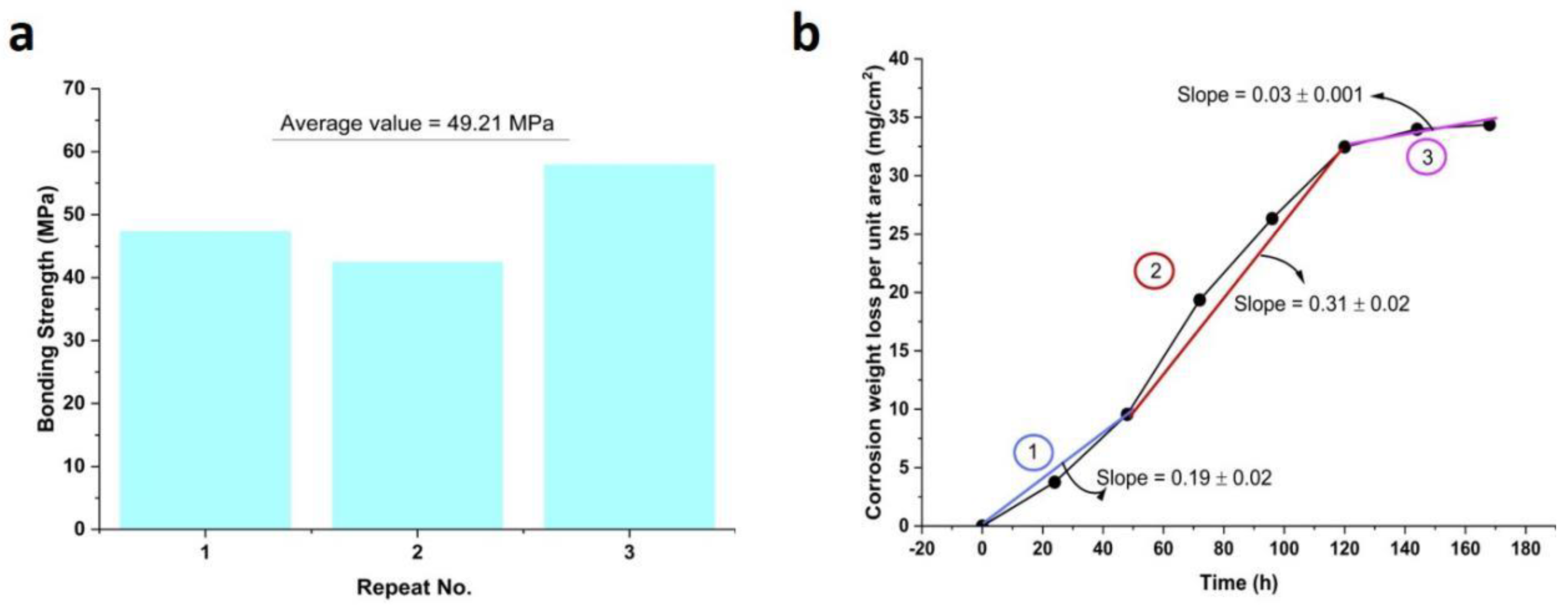
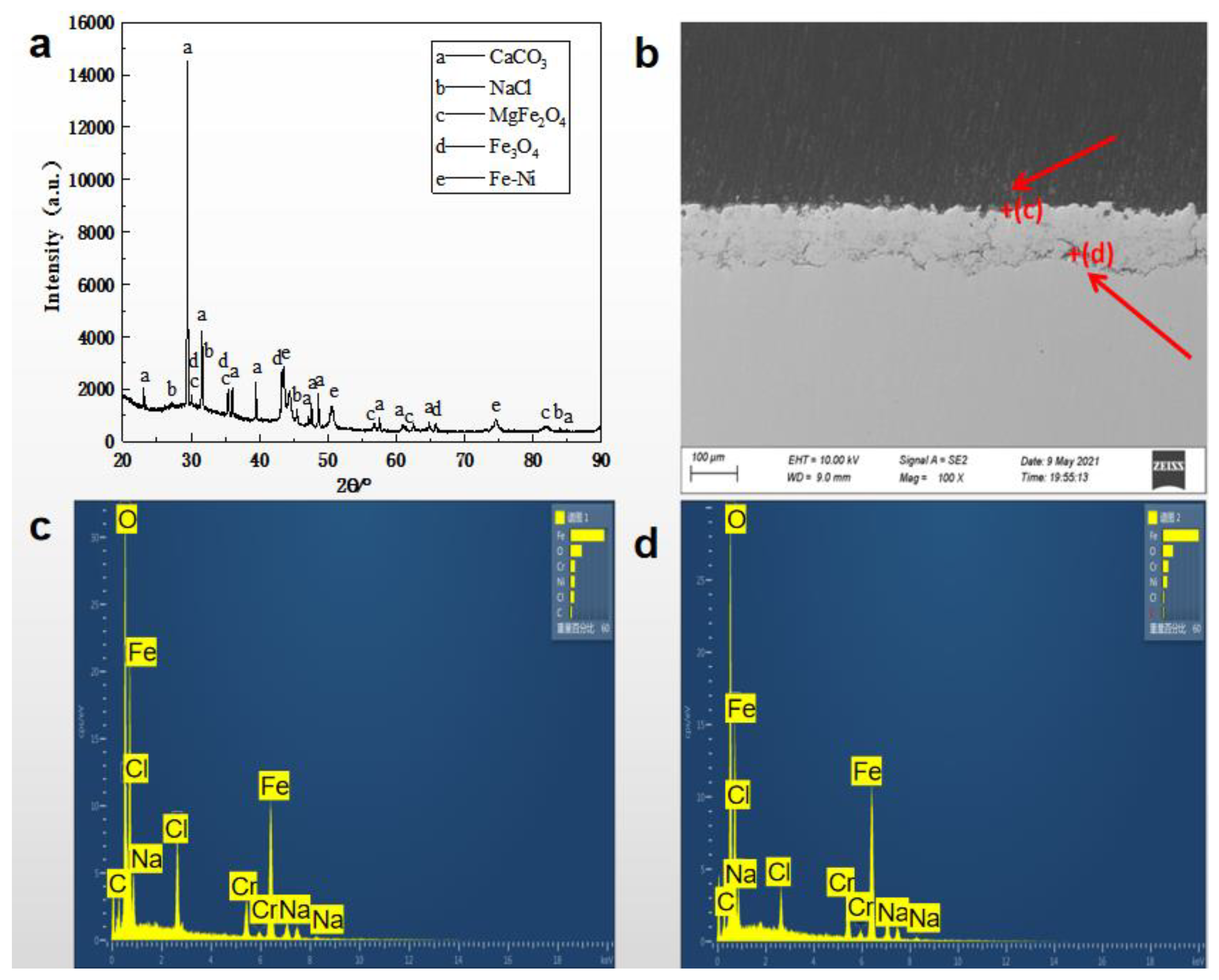
3.1. Physiochemical Characteristics of the Coating Layer
3.2. Corrosion Behavior of Coatings Prepared by Electric Explosion
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaczmarek, J.; Kolegowicz, K.; Szymla, W. Restructuring of the coal mining industry and the challenges of energy transition in Poland (1990–2020). Energies 2022, 15, 3518. [Google Scholar] [CrossRef]
- Alamri, A.H. Localized corrosion and mitigation approach of steel materials used in oil and gas pipelines—An overview. Eng. Fail. Anal. 2020, 116, 104735. [Google Scholar] [CrossRef]
- Bharatiya, U.; Gal, P.; Agrawal, A.; Shah, M.; Sircar, A. Effect of corrosion on crude oil and natural gas pipeline with emphasis on prevention by ecofriendly corrosion inhibitors: A comprehensive review. J. Bio. Tribo-Corros. 2019, 5, 1–12. [Google Scholar] [CrossRef]
- Cicek, V.; Al-Numan, B. Corrosion Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- McCafferty, E. Introduction to Corrosion Science; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Raoelison, R.; Verdy, C.; Liao, H. Cold gas dynamic spray additive manufacturing today: Deposit possibilities, technological solutions and viable applications. Mater. Des. 2017, 133, 266–287. [Google Scholar] [CrossRef]
- Shtepliuk, I. 2D noble metals: Growth peculiarities and prospects for hydrogen evolution reaction catalysis. Phys. Chem. Chem. Phys. 2023, 25, 8281–8292. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, H.; Zhang, D.; Lou, Y.; Huang, L.; Ma, L.; Hao, X.; Dong, L.; Rosei, F.; Lau, W.M. Ultrafast and high-efficient self-healing epoxy coatings with active multiple hydrogen bonds for corrosion protection. Corros. Sci. 2021, 187, 109485. [Google Scholar] [CrossRef]
- Yazdani, S.; Prince, L.; Vitry, V. Optimization of electroless Ni–B-nanodiamond coating corrosion resistance and understanding the nanodiamonds role on pitting corrosion behavior using shot noise theory and molecular dynamic simulation. Diam. Relat. Mater. 2023, 134, 109793. [Google Scholar] [CrossRef]
- Wortelen, D.; Frieling, R.; Bracht, H.; Graf, W.; Natrup, F. Impact of zinc halide addition on the growth of zinc-rich layers generated by sherardizing. Surf. Coat. Technol. 2015, 263, 66–77. [Google Scholar] [CrossRef]
- Dickinson, H. A study of galvanised and corrugated sheet metal. Trans. Newcom. Soc. 1943, 24, 27–36. [Google Scholar] [CrossRef]
- Rojas-Molano, H.F.; Olaya-Flórez, J.J.; Guzmán-Pardo, M.A.; José, E. Effect of the Surface Chemical Composition on the Corrosion Resistance in the Mixture of FeCrMoNbB (140MXC) and FeCMnSi (530AS) Coatings Produced with the Electric Wire Arc Spraying Technique: Part I. Materials 2023, 16, 4182. [Google Scholar] [CrossRef]
- Hasanbeigi, S.; Tabaian, S.H.; Yazdani, S. Effect of Manufacturing Parameters on the Corrosion Behavior of AZ31 Coated by Mg–Al Layered Double Hydroxide. Met. Mater. Int. 2021, 27, 4441–4454. [Google Scholar] [CrossRef]
- Govindharajan, S.; Raman, S.; Viswapriya, S.; Rathanasamy, R.; Kaliyannan, G.V.; Palaniappan, S.K. Wet corrosion behavior of copper exposed to recycled groundnut oil as biofuel. Mater. Test. 2019, 61, 131–135. [Google Scholar] [CrossRef]
- Govindharajan, S.; Raman, S.; Shanmugam, V.; Rathanasamy, R.; Palaniappan, S.K. Corrosion of brass subjected to cast-off cooking oil blended with diesel. Mater. Test. 2021, 63, 1032–1040. [Google Scholar] [CrossRef]
- Huang, K.; Song, Q.; Chen, P.; Liu, Y. Preparation and Performance of Mo/Cu/Fe Multi-Layer Composite Coating with Staggered Spatial Structure by Electro-Explosive Spraying Technology. Materials 2022, 15, 3552. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Song, Q.; Chen, P.; Liu, Y.; Jing, Y. Wear Mechanism of Fe/Cu Self-Lubricating Composite Coatings Fabricated by Electro-Explosive Spraying under Dry Friction. Metals 2023, 13, 390. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, H.; Wei, Y.; Zhang, A.; Zhu, L. Electrical explosion spray of Ag/C composite coating and its deposition behavior. Ceram. Int. 2022, 48, 4497–4504. [Google Scholar] [CrossRef]
- Billon, H. The Protection of Electro-Explosive Devices (EEDs) and Electronics from Electrostatic Discharge (ESD) Hazards; Defence Science and Technology Organization: Canberra, Australia, 1994. [Google Scholar]
- Ramachandran, R. Reaction Tuning of Selectively Deposited Nano-Thermite Inks for Thrust and Heat Deposition; Purdue University: West Lafayette, IN, USA, 2016. [Google Scholar]
- Willis, K.; Willis, K. Methods of protecting electro-explosive devices from the effects of RF radiation and ESD. In Proceedings of the 33rd Joint Propulsion Conference and Exhibit, Seattle, WA, USA, 6–9 July 1997; p. 2695. [Google Scholar]
- Wang, J.; Zhou, B.; Ye, S.; Chen, H. Novel electro-explosive device incorporating a planar transient suppression diode. IEEE Electron Device Lett. 2020, 41, 1416–1419. [Google Scholar] [CrossRef]
- Szala, M.; Łatka, L.; Walczak, M.; Winnicki, M. Comparative study on the cavitation erosion and sliding wear of cold-sprayed Al/Al2O3 and Cu/Al2O3 coatings, and stainless steel, aluminium alloy, copper and brass. Metals 2020, 10, 856. [Google Scholar] [CrossRef]
- Sova, A.; Grigoriev, S.; Okunkova, A.; Smurov, I. Cold spray deposition of 316L stainless steel coatings on aluminium surface with following laser post-treatment. Surf. Coat. Technol. 2013, 235, 283–289. [Google Scholar] [CrossRef]
- Wei, S.-C.; Xu, B.-S.; Wang, H.-D.; Jin, G.; Lv, H. Comparison on corrosion-resistance performance of electro-thermal explosion plasma spraying FeAl-based coatings. Surf. Coat. Technol. 2007, 201, 5294–5297. [Google Scholar] [CrossRef]
- Romanov, D.; Moskovskii, S.; Sosnin, K. Structure and Electrical Erosion Resistance of An Electro-Explosive Coating of the ZnO-Ag System. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; p. 012006. [Google Scholar]
- Wang, J.; Su, H.; Chen, K.; Du, D. Effect of δ-ferrite on the stress corrosion cracking behavior of 321 stainless steel. Corros. Sci. 2019, 158, 108079. [Google Scholar] [CrossRef]
- Li, Q.; Song, Q.-Z.; Wang, J.-Z.; Duo, Y.-X. Effect of Charging Energy on Droplet Diameters and Properties of High-carbon Steel Coatings Sprayed by Wire Explosion Spraying. Surf. Coat. Technol. 2011, 206, 202–207. [Google Scholar] [CrossRef]
- Ahmad, Z. Principles of Corrosion Engineering and Corrosion Control, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Zhang, X.-M.; Chen, Z.-Y.; Luo, H.-F.; Zhou, T.; Zhao, Y.-L.; Ling, Z.-C. Corrosion resistances of metallic materials in environments containing chloride ions: A review. Trans. Nonferrous Met. Soc. China 2022, 32, 377–410. [Google Scholar] [CrossRef]
- Tiamiyu, A.; Eduok, U.; Szpunar, J.; Odeshi, A. Corrosion behavior of metastable AISI 321 austenitic stainless steel: Investigating the effect of grain size and prior plastic deformation on its degradation pattern in saline media. Sci. Rep. 2019, 9, 12116. [Google Scholar] [CrossRef]
- Rezaei, H.A.; Ghazani, M.S.; Eghbali, B. Effect of post deformation annealing on the microstructure and mechanical properties of cold rolled AISI 321 austenitic stainless steel. Mater. Sci. Eng. A 2018, 736, 364–374. [Google Scholar] [CrossRef]
- Fischer, H.; Vogel, B.; Welle, A. Applications of shape memory alloys in medical instruments. Minim. Invasive Ther. Allied Technol. 2004, 13, 248–253. [Google Scholar] [CrossRef]
- Song, S.-J.; Kim, J.-G. Influence of magnesium ions in the seawater environment on the improvement of the corrosion resistance of low-chromium-alloy steel. Materials 2018, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Chen, L.; Liu, H.; Han, L.; Gong, N.; Misra, R. Precipitation behavior of Laves phase in the vicinity of oxide film of ferritic stainless steel: Selective oxidation-induced precipitation. Oxid. Met. 2020, 93, 195–213. [Google Scholar] [CrossRef]
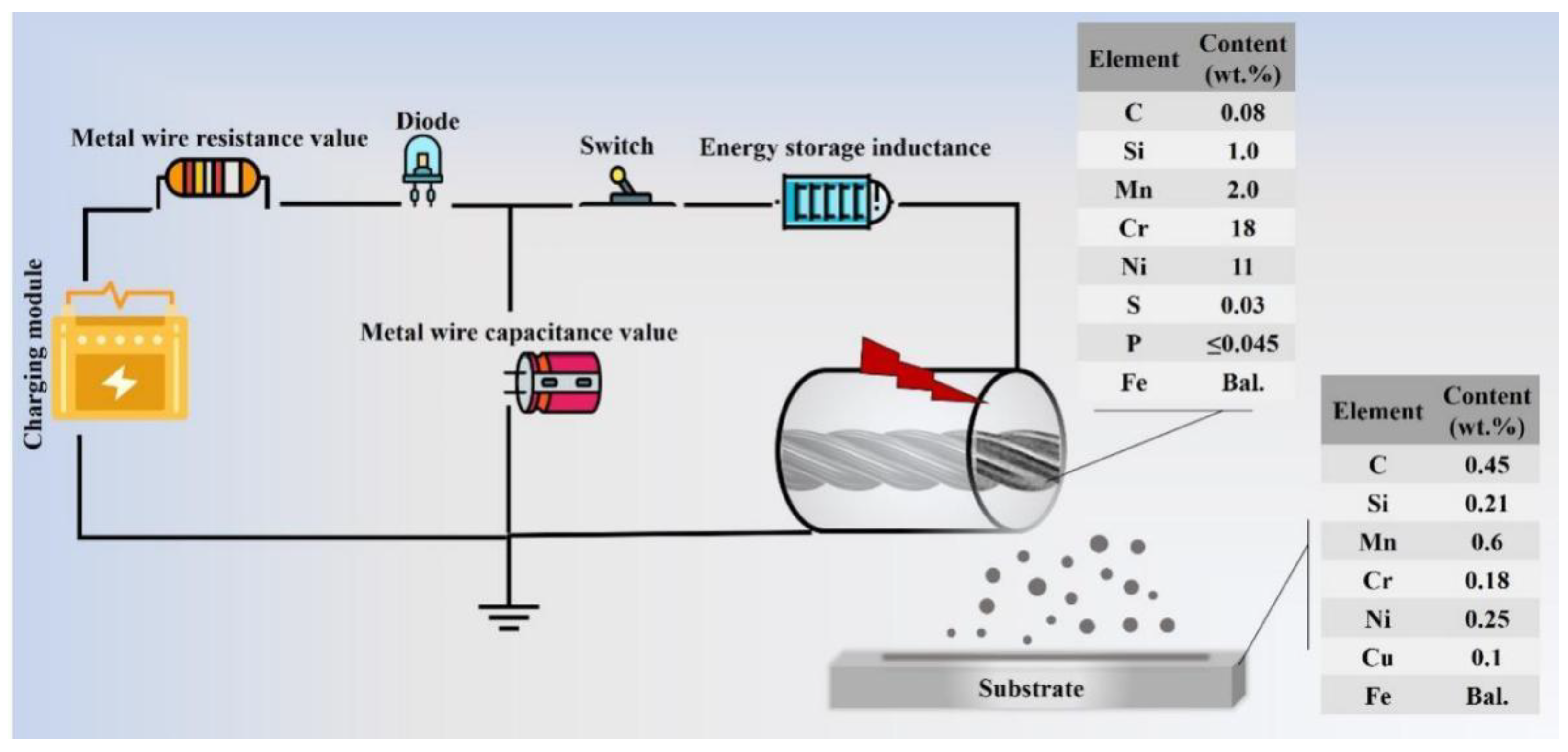



| Compound | Concentration (mol/L) | Ion Species | Content (g/L) |
|---|---|---|---|
| CaCl2 | 1.665 | Ca2+ | 0.6 |
| NaHCO3 | 0.826 | 0.6 | |
| NaCl | 30.715 | Cl− | 20 |
| Na2SO4 | 1.775 | 1.2 | |
| MgCl2·6H2O | 0.846 | Mg2+ | 0.1 |
| Coating | Bonding Strength (MPa) |
|---|---|
| Sample 1 | 53.825 |
| Sample 2 | 60.005 |
| Sample 3 | 47.369 |
| Sample 4 | 51.757 |
| Average | 53.239 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Song, Q.; Deng, H.; Liu, Y.; Chen, P.; Huang, K. A Study on the Corrosion Resistance of a Coating Prepared by Electrical Explosion of 321 Metal Wire. Lubricants 2023, 11, 309. https://doi.org/10.3390/lubricants11070309
Liu Y, Song Q, Deng H, Liu Y, Chen P, Huang K. A Study on the Corrosion Resistance of a Coating Prepared by Electrical Explosion of 321 Metal Wire. Lubricants. 2023; 11(7):309. https://doi.org/10.3390/lubricants11070309
Chicago/Turabian StyleLiu, Ye, Qiuzhi Song, Hongbin Deng, Yali Liu, Pengwan Chen, and Kun Huang. 2023. "A Study on the Corrosion Resistance of a Coating Prepared by Electrical Explosion of 321 Metal Wire" Lubricants 11, no. 7: 309. https://doi.org/10.3390/lubricants11070309
APA StyleLiu, Y., Song, Q., Deng, H., Liu, Y., Chen, P., & Huang, K. (2023). A Study on the Corrosion Resistance of a Coating Prepared by Electrical Explosion of 321 Metal Wire. Lubricants, 11(7), 309. https://doi.org/10.3390/lubricants11070309






