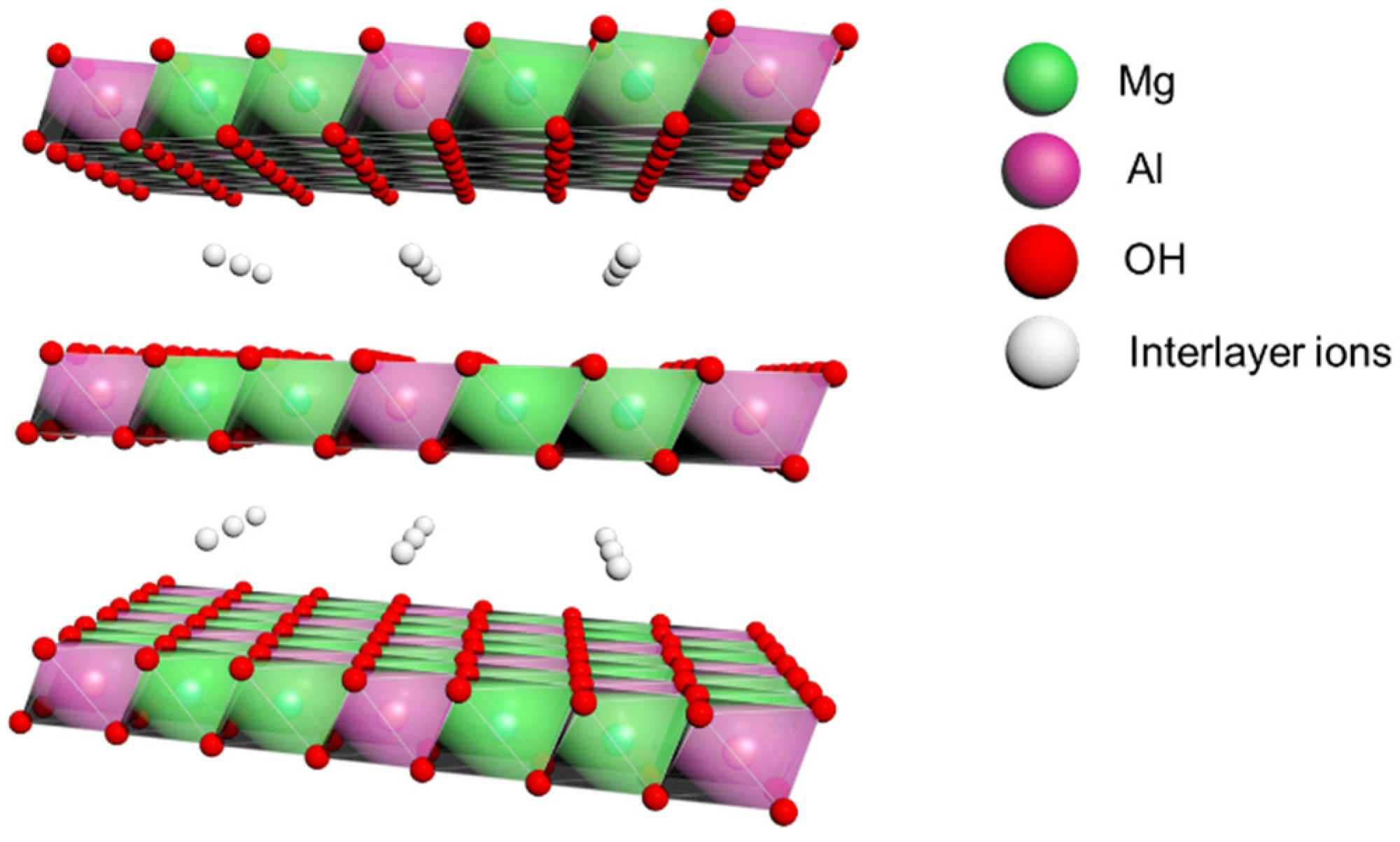
3.2.1. Study on Oxidation Resistance of LDHs in Lithium Base Grease
Over the years, many oxidation bench tests have been developed and proven to be very effective for grease formulation selection, especially in the screening and formulation development of new antioxidants. Due to the limitations of the test site, a single test cannot simulate the real working conditions. Therefore, when examining grease formulation and antioxidant screening, an effective method is to use multiple test methods for comparative evaluation.
Bearing lifetime refers to the ability of grease to lubricate high temperature and high-speed ball bearings for extended periods of time under light load conditions. The test method is ASTM D 3336 (“Life of lubricating greases in ball bearings at elevated temperatures”).
To test the lifetime of the bearing, a set of steps are undertaken. Firstly, a 3.2 mL sample is loaded into the cleaned 6204 bearing, following which, the test bearing, support bearing, and finger spring are installed onto the main shaft, and the thermocouple is positioned and fixed carefully. Secondly, the device is turned on to raise the temperature of the bearing up to the measured temperature. At a speed of 10,000 r/min, the bearing is run continuously until either the grease sample fails or the specific running time is achieved. The running time (in hours) is then recorded as the lifetime of the specimen in the ball bearing.
Analyzing the data presented in
Table 1 and
Table 2, it is evident that, at a temperature of 149 °C, the bearing life of the grease sample is similar when the base grease is added with LDHs and diphenylamine at a concentration of 0.3%. Conversely, when the base grease was added with LDHs and T531 at a concentration of 0.5%, it was observed that the bearing life of the grease sample 4 (with added LDHs) doubled in comparison to sample 3 (with added T531). This indicates that the bearing life is heavily influenced by the addition of different types of antioxidants. Furthermore, these results suggest that LDH could potentially replace traditional organic antioxidants and pave the way towards sustainable, green development.
Table 3 and
Table 4 show the results of the bearing lifetime tester test, conducted by a different company, which found that the samples containing LDHs had significantly higher bearing lifetimes compared to the base grease. The base grease had a bearing lifetime of 40 h at 149 °C. However, adding 1% of LDHs increased the grease lifetime by a factor of three, and increasing the LDH content to 3% extended the lifespan by approximately four times. Further, adding 5% of LDHs increased the grease lifetime by nearly five times.
The FE9 method is a testing process that uses angular contact ball bearings in a specialized rolling bearing grease tester to determine the effective service lifetime of grease. This test method is defined by the DIN 51821-2 standard, titled “Testing of lubricants—Test using the FAG roller bearing grease testing apparatus FE9—Part 2: Test method”. The FE9 test steps involve filling five sets of clean test bearings with a certain amount of grease to be tested, and then the test stops involve installing these test bearings onto five test units. The test conditions are set in accordance with specific requirements, including a speed of 6000 r/min, axial force of 1500 N, and a temperature range of 120 °C to 220 °C (set as an integer multiple of 10 °C) under test conditions. The heating system is activated to control the temperature using a temperature controller. During the test, the friction torque of the bearing will increase as a result of poor lubrication. If this results in the power of the driving motor exceeding the limit value and lasting for 6 s to 8 s, the test is ended. After the test, the running times of each of the five test bearings are inputted into the Weibull distribution data evaluation software (version 9) to produce life failure probability distribution diagrams. The effective lifetime of the grease can be determined by reading the running time at the 50% failure probability mark on the diagram.
Through the test results in
Table 3 and
Table 5, it was found that the lifetime of FE9 bearings of the three samples added with LDHs are obviously better than those of the base grease, and with the increase in the amount of LDHs added, the bearing lifetime tends to increase regularly.
Table 6 reveals that samples 1–4 used mineral oil, while samples 5–8 used a higher viscosity semi-synthetic oil, resulting in a significant improvement in the CRC bearing life test.
3.2.2. Study on Oxidation Resistance of LDHs in Grease for Mine Electric Shovel
After the 1930s, electricity replaced other forms of power and became the only power for mining excavators, and the steam shovel was replaced by the electric shovel. At this time, the shovel was mainly used for stripping mining, and the large mining shovel used for stripping is called a “stripping shovel”. With the expansion of the scale of open-pit mining, the stripping shovel developed towards a larger scale. In the 1970s, as surface mining technology improved, smaller pick-up shovels gradually replaced larger stripper shovels for easier matching with mining trucks. So far, this type of electric shovel is still the main equipment for open-pit mining mines.
At the end of 2005, the WK-20 20 m3 electric shovel of Taiyuan Heavy Industry Co., Ltd. (TYHI) (Taiyuan, China) was successfully launched, breaking the long-term monopoly of American companies in the field of large mining electric shovels, with a bucket capacity of more than 20 m3. Since then, TYHI has developed WK-27, WK-35, WK-55, and WK-75 shovels, among which THE WK-75 shovel design standard, with a bucket capacity of 75 m3, is the largest types of shovel independently developed in China.
The shovel is one of the most important parts of equipment in open-pit mining technology. Lubrication of the gear mechanism of the shovel has always been a difficult problem. Improper selection of lubricant often causes serious pitting corrosion, abrasion, scratches, excessive wear, and other phenomena, which makes the gear enter overhaul before its service lifetime, and this increases maintenance costs. It is the bound duty of SINOPEC to prolong the use of grease in mine shovels and to reduce the surface pollution caused by exposed grease in open-pit coal mines.
According to
Table 7 and
Table 8, as well as
Figure 4 and
Figure 5, LDHs added to shovel grease can prolong the service lifetime of grease, while the overall performance of grease is not affected. After LDH is added, the anti-wear performance of grease is slightly improved (
Figure 4), which may be caused by the two-dimensional structure and high crystallinity of LDH, forming a protective film on the surface of the friction pair.
The main premise of DSC to study reaction kinetics is that the degree of reaction is proportional to the thermal effect released or absorbed by the reaction, that is, it is proportional to the area under the DSC curve. This can be expressed by the following formula:
where;
H—enthalpy, heat of reaction at temperature
T;
HT—total enthalpy of the reaction,
—degree of reaction proceeding,
—Total area under the DSC curve
—Reaction process area under DSC curve,
—The difference between the total area under the DSC curve and the area of the reaction process,
T—reaction time. Thus, the basic kinetic equation of the reaction can be rewritten as:
On both sides of the exponential, the following holds:
By comparing DSC scanning curves with the same change rates of the two reactions at different rates, the activation energy
E can be obtained:
If the change rate α is the same at peak temperature, it is further expressed as:
The
Ea can be obtained through Equation (8), and the rate constant
k is dependent on the reaction temperature, according to the Arrhenius Equation (9).
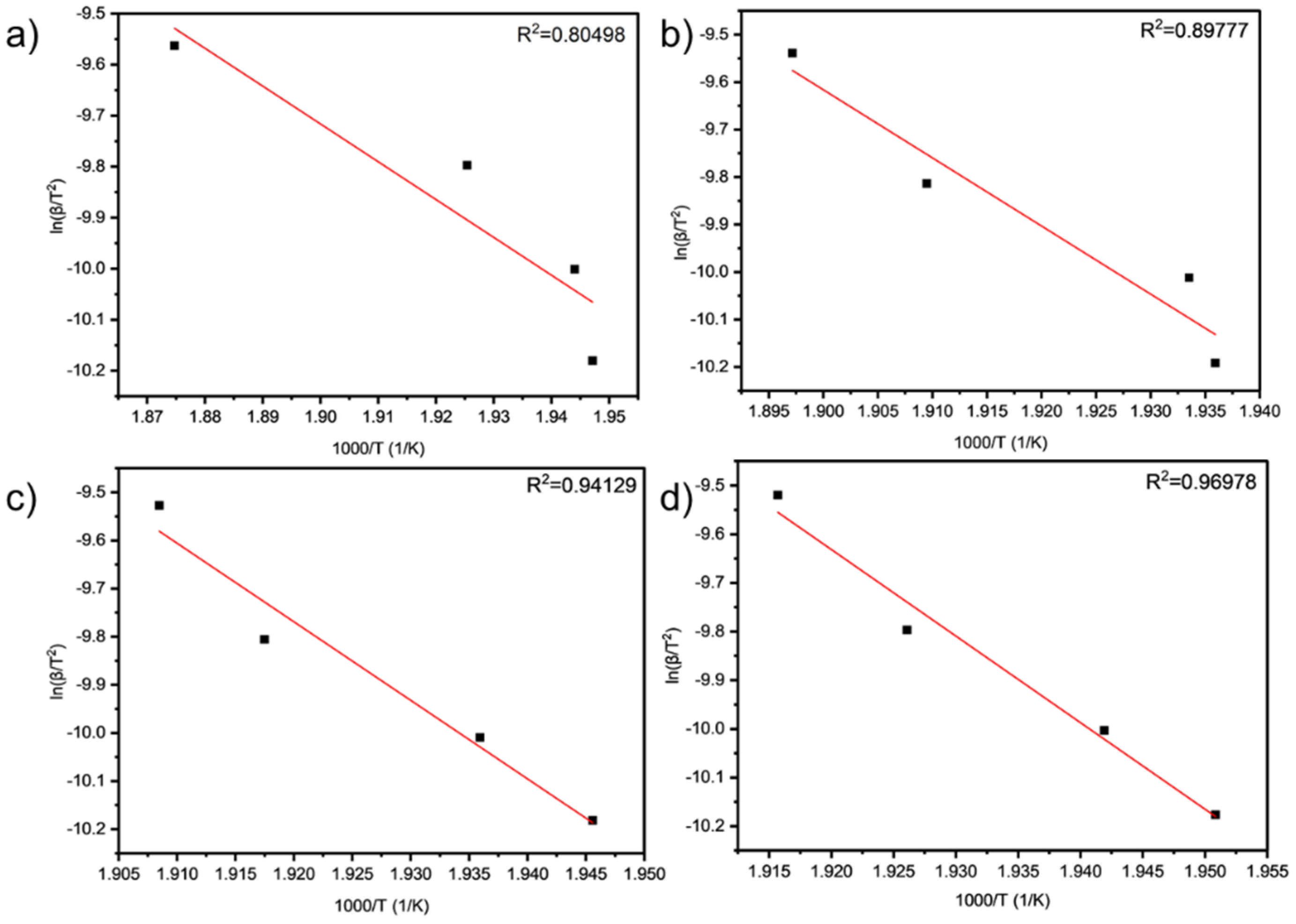
In these equations, β is the heating rate (K min−1), Tp is the temperature corresponding to the inflection point of the thermodegradation curves, which correspond to the maximum reaction rate (K), A is the pre-exponential factor (min−1), Ea is the activation energy (kJ mol−1), and R is the gas constant (8.314 J mol−1 K−1). The activation energy and pre-exponential factor can be obtained from the slope.
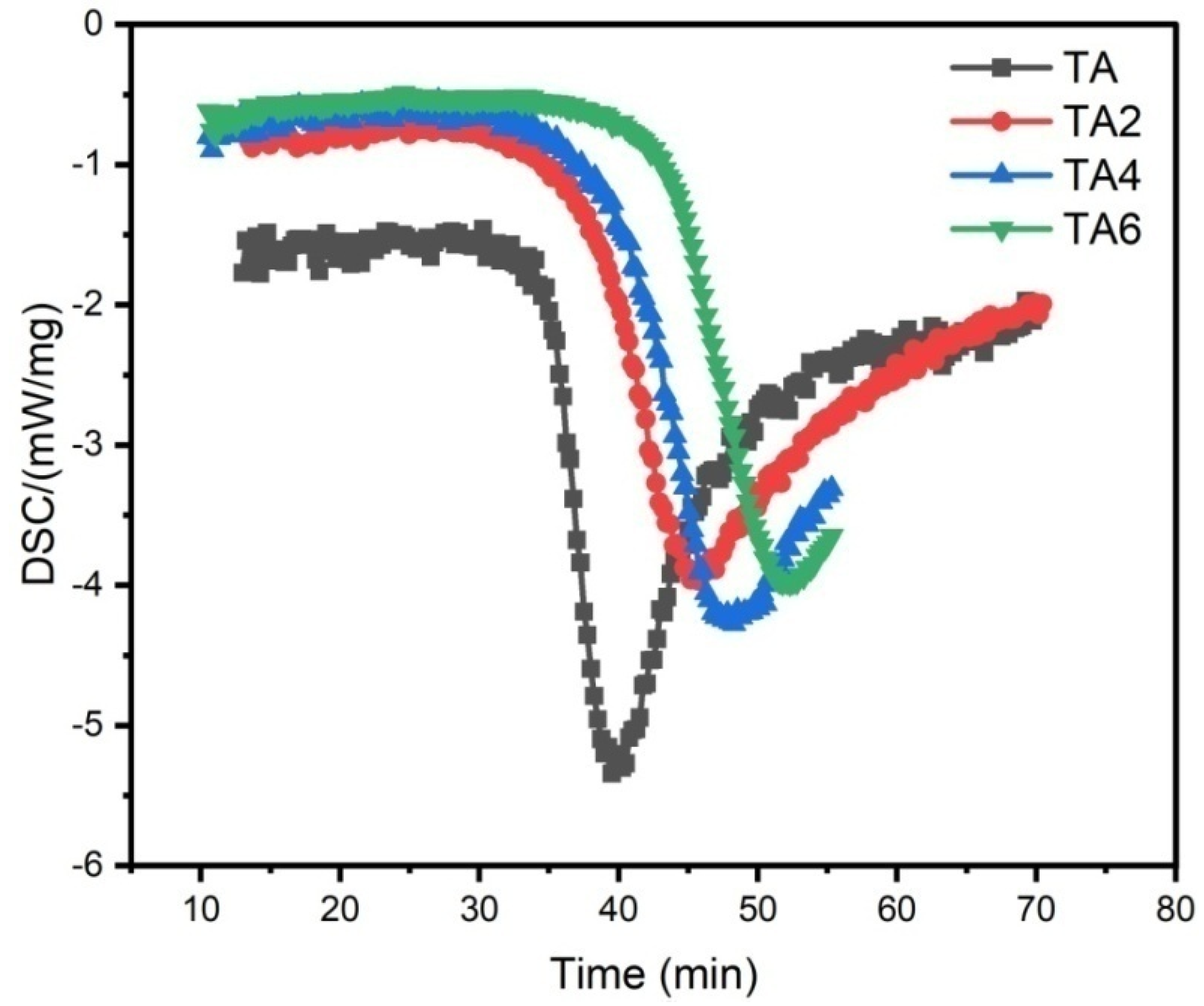
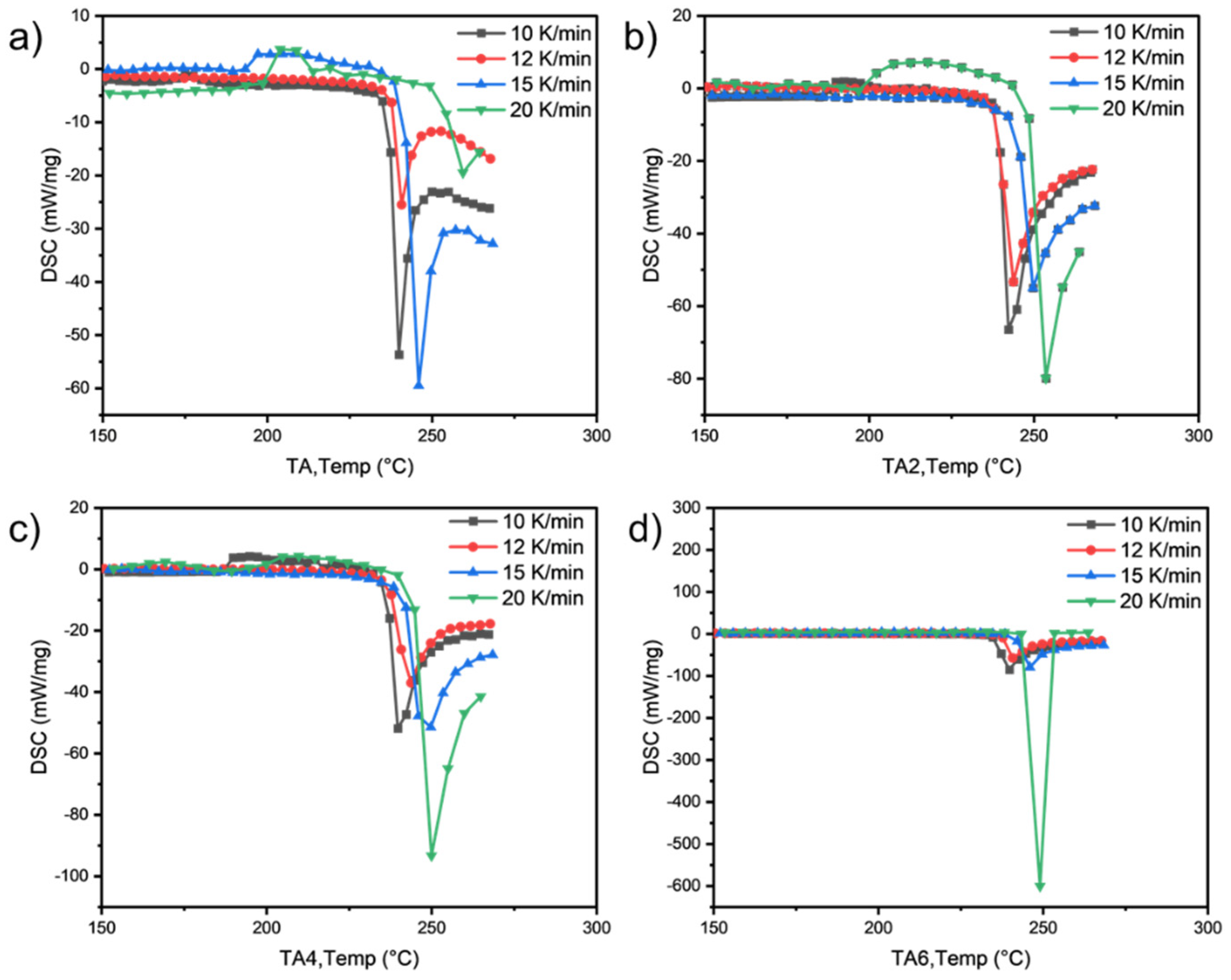
As can be seen from
Table 9 and
Table 10 and
Figure 6 and
Figure 7, the activation energies of the four samples all meet the increasing law of E
TA < E
TA2 < E
TA4 < E
TA6, indicating that, with the increase in the amount of LDHs added, the larger reaction activation energy of grease indicates the stronger that its thermal anti-oxidation and decomposition ability are.
3.2.3. Analysis of Rheological Properties of Electric Shovel Grease
Grease is a viscoelastic non-Newtonian fluid. The elastic part of viscoelasticity is represented by the energy storage modulus G′, which indicates that the stress energy can be temporarily stored and recovered during the experiment. The viscous part is represented by the loss modulus G″, which indicates thatthe grease loses energy during the initial flow, which is converted into shear heat, and the loss is irreversible. When rheological experiments are performed, the shear stress increases, and the point where the energy storage modulus G′ begins to decrease is usually defined as the end of the linear viscoelastic zone (LVE), called the yield point, and the shear stress at this point is also called the yield stress τy. The maximum elastic deformation that the grease can withstand can be determined by this point. Shear stress and deformation continue to increase, G′ line and G″ line intersect, the intersection point is called the “flow point”, the shear stress at this point is called the “flow stress”, τf, and, at this time, the grease thickener structure is more damaged, and the grease also begins to flow.
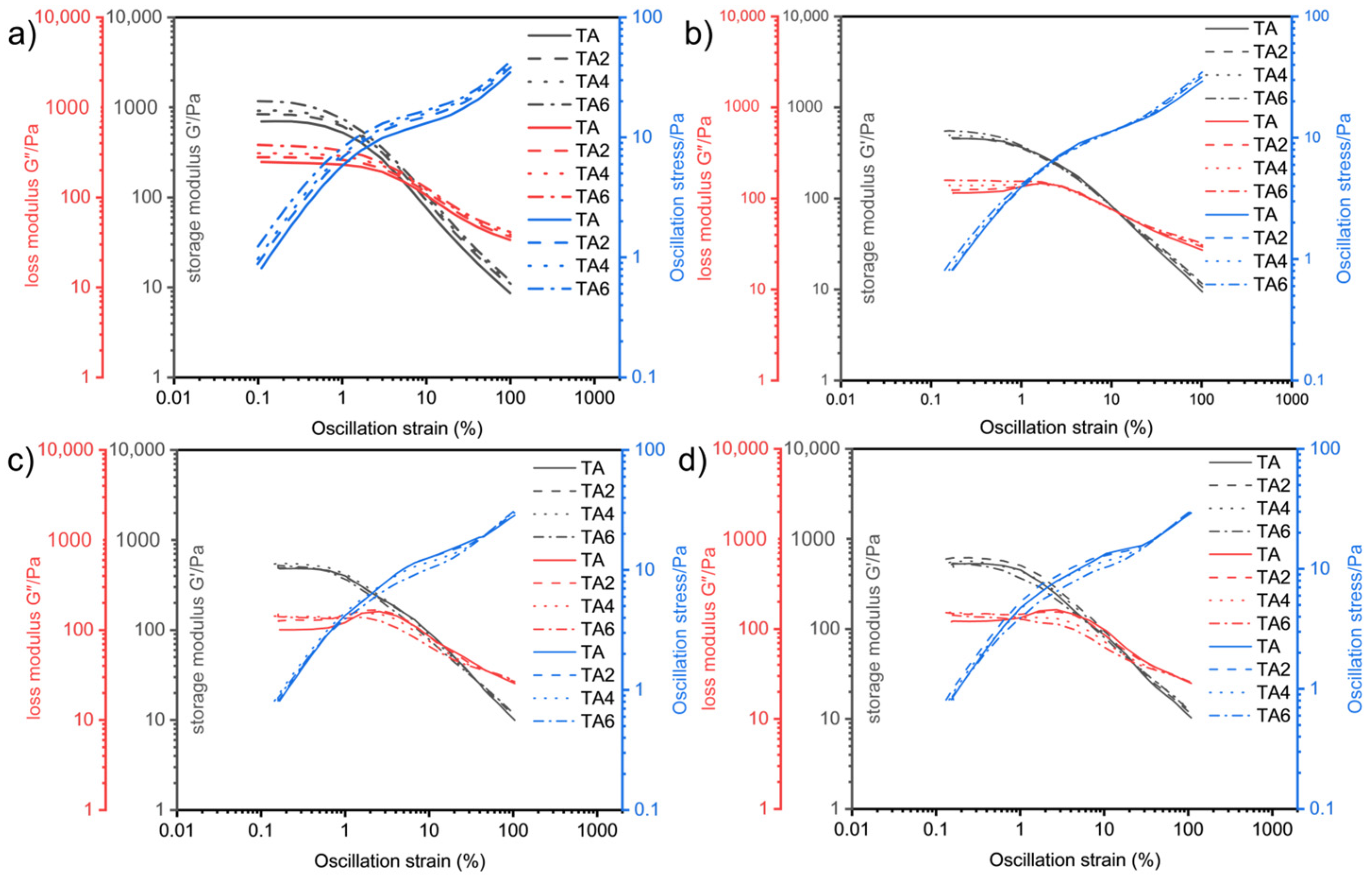
The oscillatory rheological experiments were performed on four grease samples using this method.
Figure 8 shows the relationship between the storage modulus G′ and loss modulus G″, with the variation in shear deformation γ at different temperatures measured by changing the experimental temperatures (60, 80, 100, and 120 °C).
It can be seen, from
Figure 8, that the overall pattern of the energy storage modulus G′ of the four greases entering the rheological transition zone at 60, 80, 100, and 120 °C is gradually decreasing with the increase in temperature. From the previous principle analysis, it is known that the larger the G′ is, the better the structure of the grease that is maintained before it is in gel state, and the lubricating performance of the grease inside the electric shovel gear is often carried out within a few seconds after the start of operation. Therefore, it is beneficial to improve the lubrication of gears by appropriately improving the energy storage modulus and fluidity performance of grease. At 60 °C, as the proportion of LDHs increases, the energy storage modulus of TA6 grease sample is the largest, and TA2 is the second-largest at 120 °C, and, as the proportion of LDHs increases, the energy storage modulus of TA6 grease sample is the largest, and TA4 is the second-largest; at 100 °C, the energy storage modulus of TA4 grease sample is the largest, and TA2 is the second-largest; at 120 °C, the energy storage modulus of TA2 grease sample is the largest, TA is the second-largest, and TA6 is the smallest; it means that, at low temperature, the energy storage modulus of grease increases simultaneously with the increase in LDH ratio. When there is an increase in temperature, the larger the LDHs ratio is, the larger the system elastic deformation damage is, so it is necessary to consider the effect of suitable ratio of LDHs on energy storage modulus. Combined with the flow transition index and energy storage moduli in different linear viscoelastic intervals, it can be seen that adding two-percent LDH is the best for the system’s elastic deformation and energy storage modulus. This indicates that the internal structure of sample TA2 is more stable under shear, and the fiber structure is better maintained, which is better for gear support and lubrication, and this is conducive to reducing gear wear.
The values of the energy storage modulus and the loss modulus, as well as shear stress and the corresponding strain values of the four greases at yield point and flow point at the two sets of experimental temperatures, can be calculated, and the numerical results are listed in
Table 11.
Table 11 shows that, at 60 °C, the yield point energy storage modulus G′ is ranked as G′ (TA6) > G′ (TA2) > G′ (TA4) > G′ (TA); at 80 °C, G′ (TA6) > G′ (TA4) > G′ (TA) > G′ (TA2); at 100 °C, G′ (TA4) > G′ (TA2) > G′ (TA) > G′ (TA6); at 120 °C, G′ (TA2) > G′ (TA) > G′ (TA4) > G′ (TA6).
At the flow point, the strain amplitude γ is ordered as follows: 60 °C, γ (TA6) > γ (TA4) > γ (TA2) > γ (TA); 80 °C, γ (TA4) > γ (TA2) > γ (TA6) > γ (TA); 100 °C, γ (TA4) > γ (TA6) > γ (TA2) > γ (TA); 120 °C, γ (TA2) > γ (TA6) > γ (TA4) > γ (TA). The energy storage modulus, G′, is ordered as follows: 60 °C, G′ (TA2) > G′ (TA4) > G′ (TA) > G′ (TA6); 80 °C, G′ (TA) > G′ (TA6) > G′ (TA2) > G′ (TA4); 100 °C, G′ (TA) >G′ (TA2) > G′ (TA4) > G′ (TA6); and, at 120 °C, G′ (TA) > G′ (TA4) > G′ (TA2) > G′ (TA6). The shear stress, τf, is sorted as follows: 60 °C, τf (TA6) > τf (TA4) > τf (TA2) > τf (TA); 80 °C, τf (TA4) > τf (TA2) > τf (TA6) > τf (TA); and, at 100 °C, τf (TA4) > τf (TA2) > τf (TA6) > τf (TA); finally, at 120 °C, τf (TA2) > τf (TA4) > τf (TA6) > τf (TA).
At the flow point, the flow transition index τf/τy is ordered as follows: τf/τy (TA2) > τf/τy (TA) > τf/τy (TA4) > τf/τy (TA6) for 60 °C; τf/τy (TA2) > τf/τy (TA4) > τf/τy (TA6) > τf/τy (TA) for 80 °C; τf/τy (TA2) > τf/τy (TA6) > τf/τy (TA4) > τf/τy (TA) for 100 °C; and τf/τy (TA2) > τf/τy (TA6) > τf/τy (TA4) > τf/τy (TA) for 120 °C.
The maximum value of τf/τy for sample TA2 at the test temperature indicates that, at this temperature, TA2 grease-like soap fibers have the best structural stability, and the soap fibers are less likely to fracture or break. Comparing the storage modulus and flow transition index at different temperatures, it can be seen that the addition of the appropriate amount of LDHs for the system oxidation resistance and viscoelasticity requires close attention. For the electric shovel grease system, adding 2% LDH can achieve the best oxidation resistance and rheological properties.
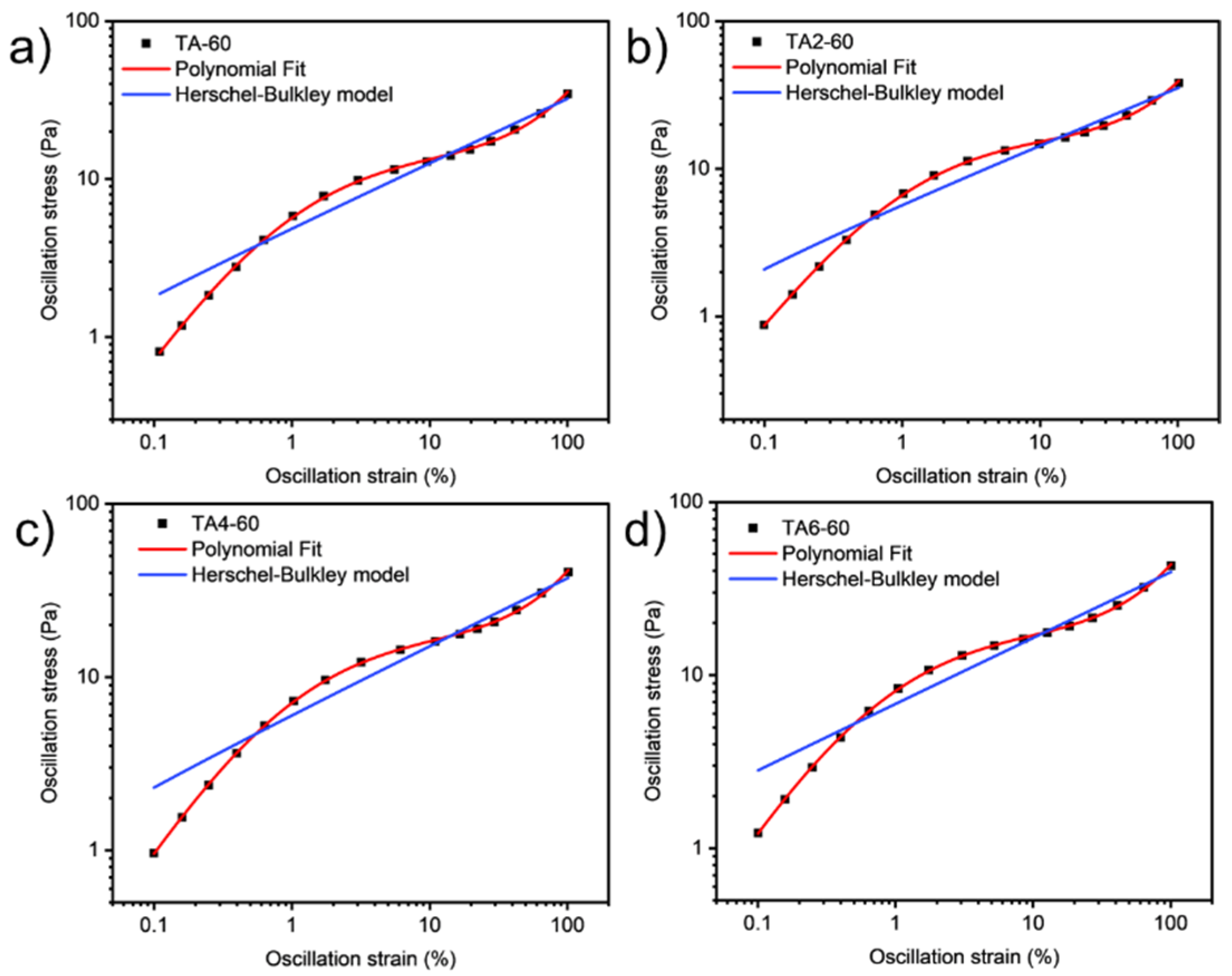
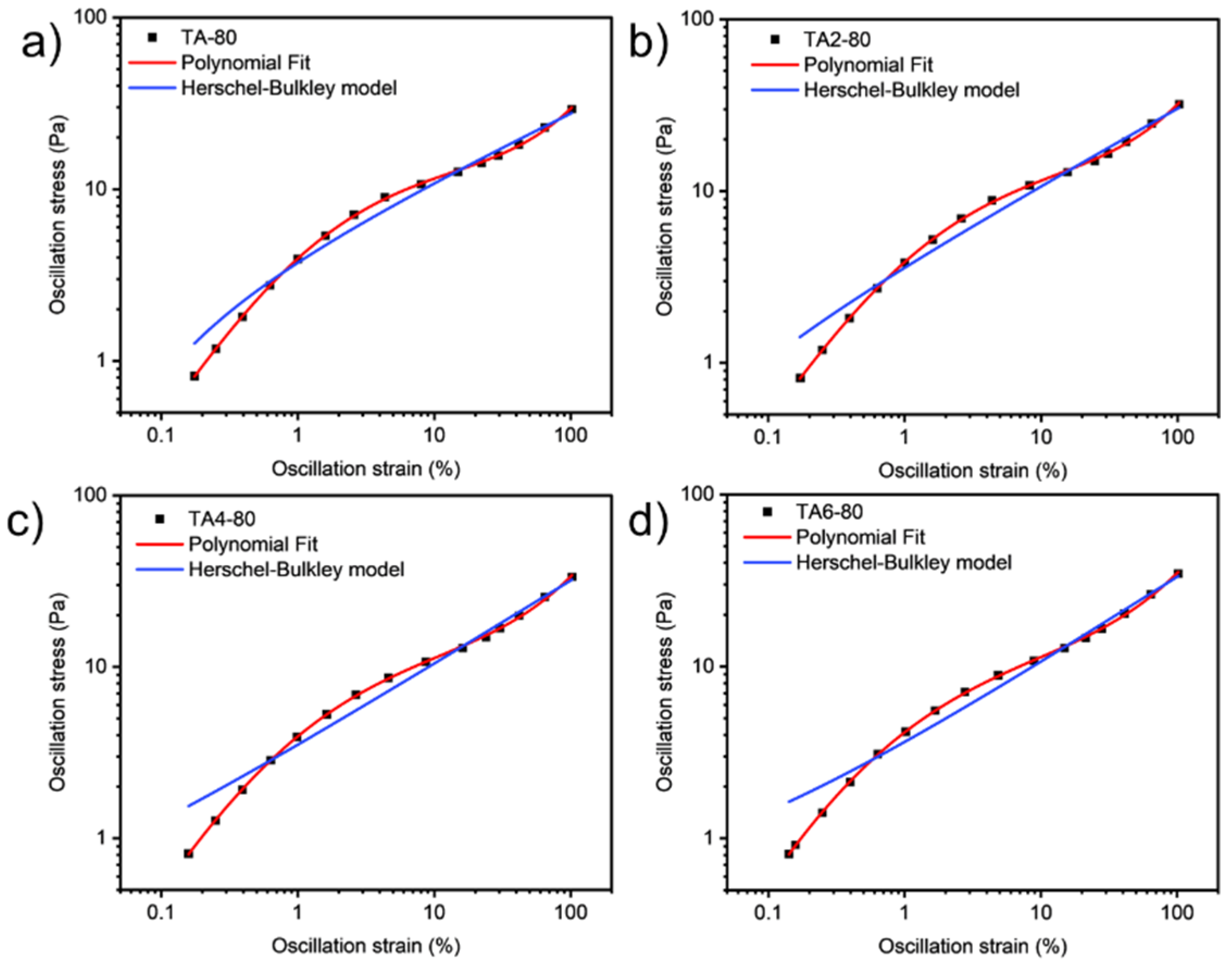
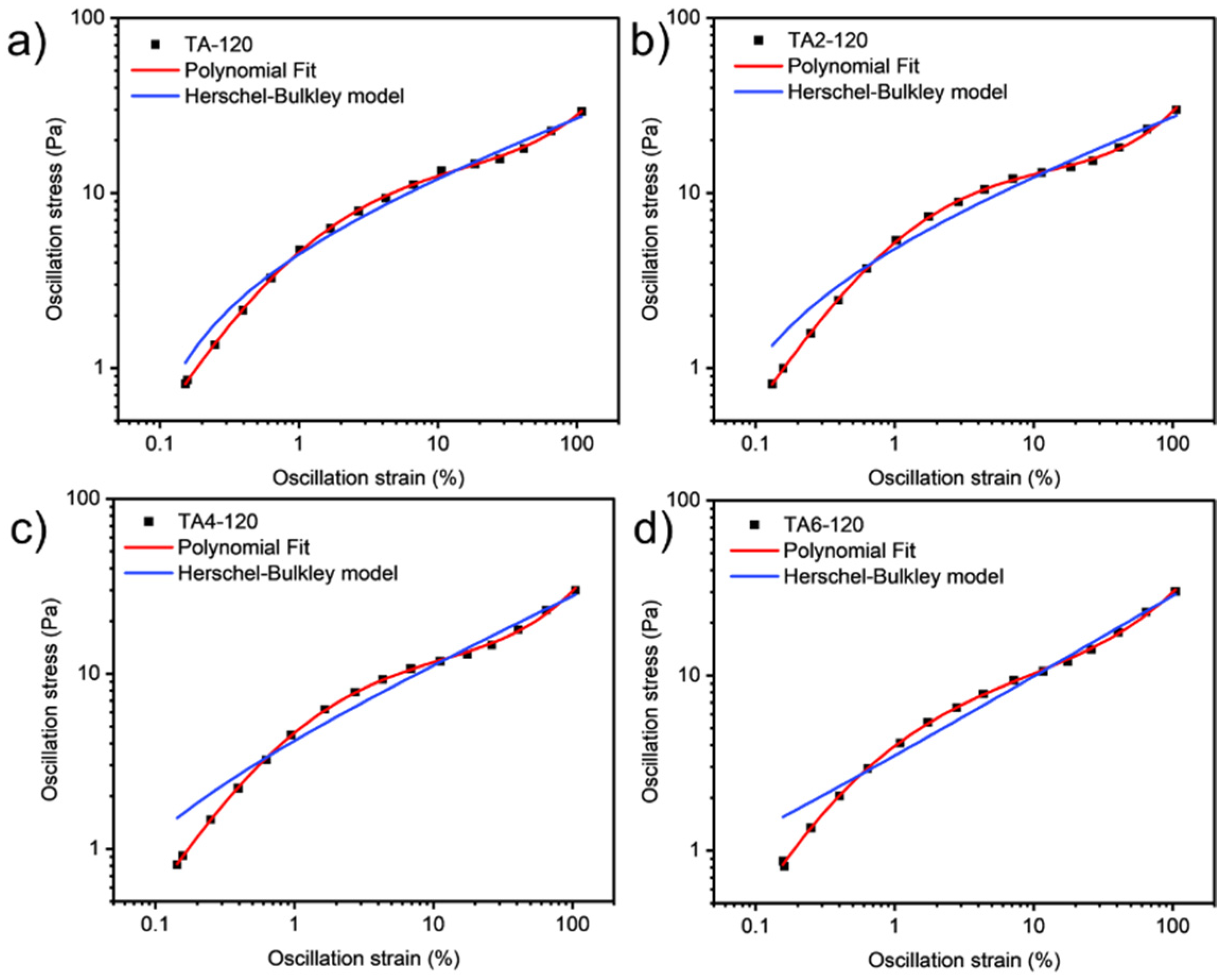
Since grease is a non-Newtonian fluid, curve fitting in this paper refers to the streamlining of flow curves of varying graphical complexity into an equation with two, three, or four coefficients. Curve fitting has the following advantages: in quality control, it is easier to mathematically set the tolerance range with the help of standard regression coefficients, and it is more difficult to visually compare the shape difference between the unique flow curve formed by the measured test point and the standard curve. After completing the procedural processing of the experimental data, the second step is obviously to continue the automatic evaluation of the samples for compliance with the technical specifications. Regression calculations help to solve this problem.
These data were not fitted to the Ostwald-de-Waele model (Equation (10)) because of R
2. These presented a value of n not close to 1, as was expected for non-Newtonian fluids. The existence of a yield stress value can be recognized in
Figure 9,
Figure 10,
Figure 11 and
Figure 12, and the data were not satisfactorily fitted to a Herschel-Bulkley model (Equation (11)), and the results are collected in
Table 12. The data were satisfactorily fitted to a polynomial model (Equation (12), R
2 > 0.999), and the results are collected in
Table 12.