Development of Doped Carbon Quantum Dot-Based Nanomaterials for Lubricant Additive Applications
Abstract
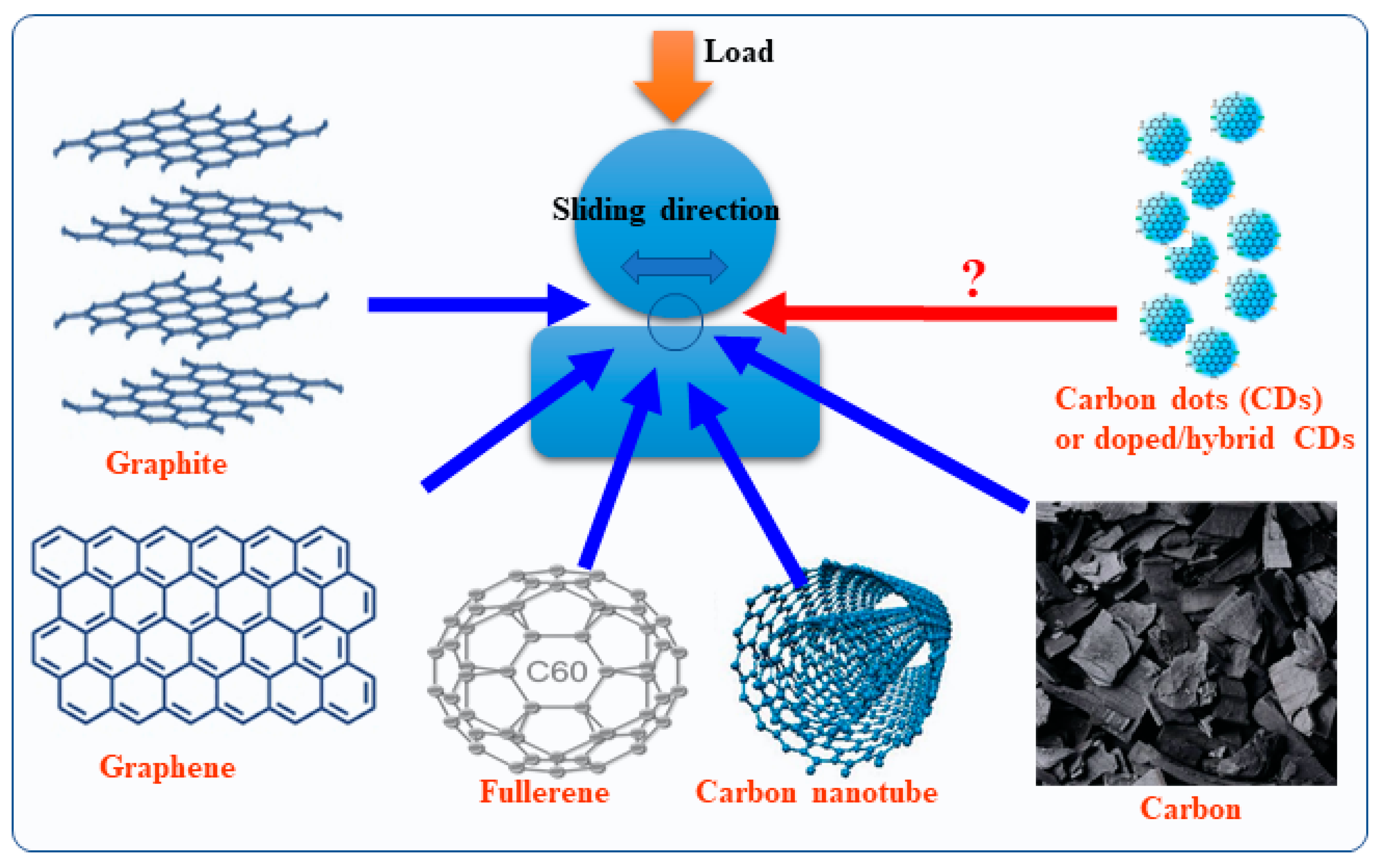
:1. Introduction
2. Synthesis of CDs-Based Material for Tribology
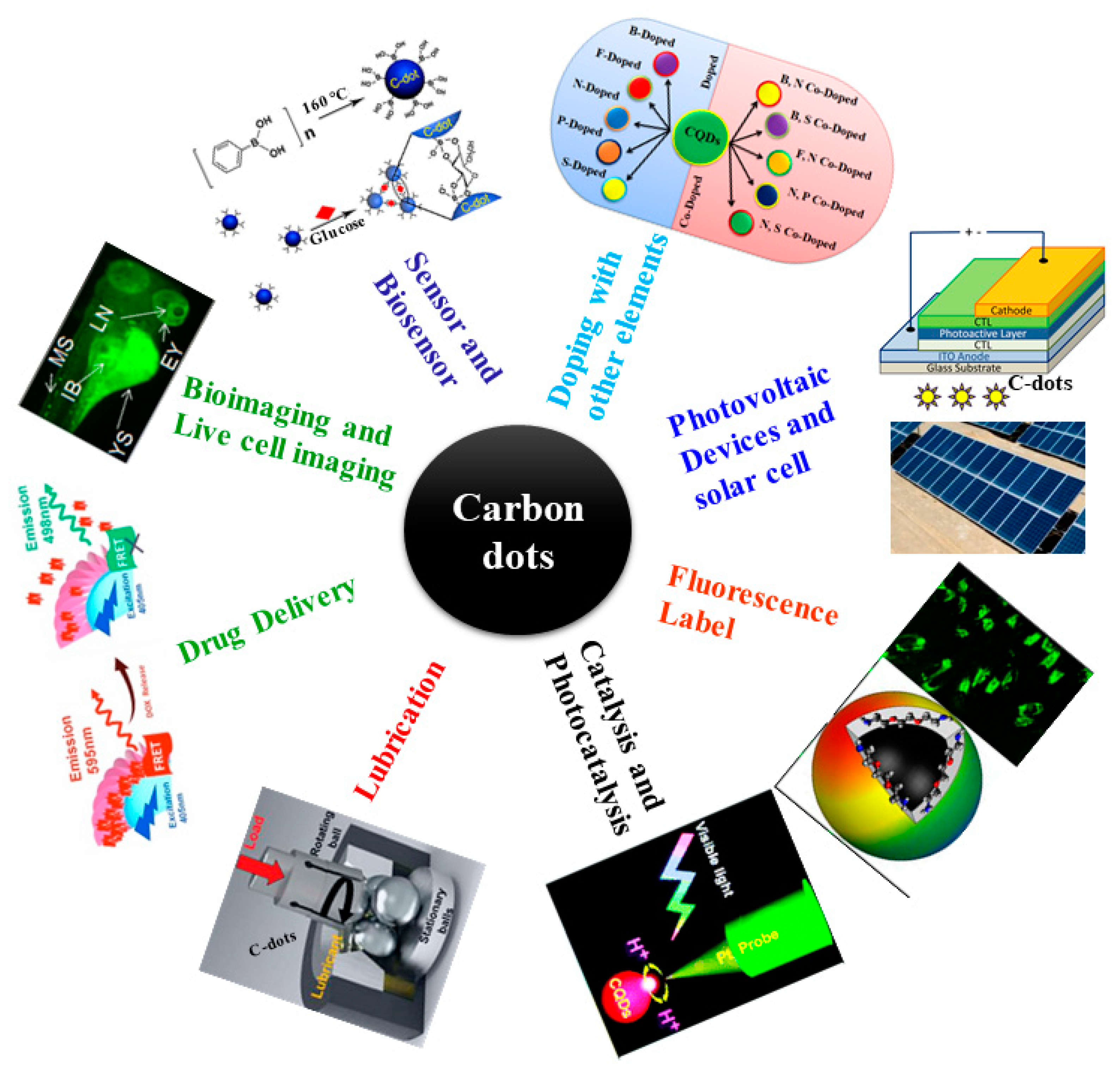
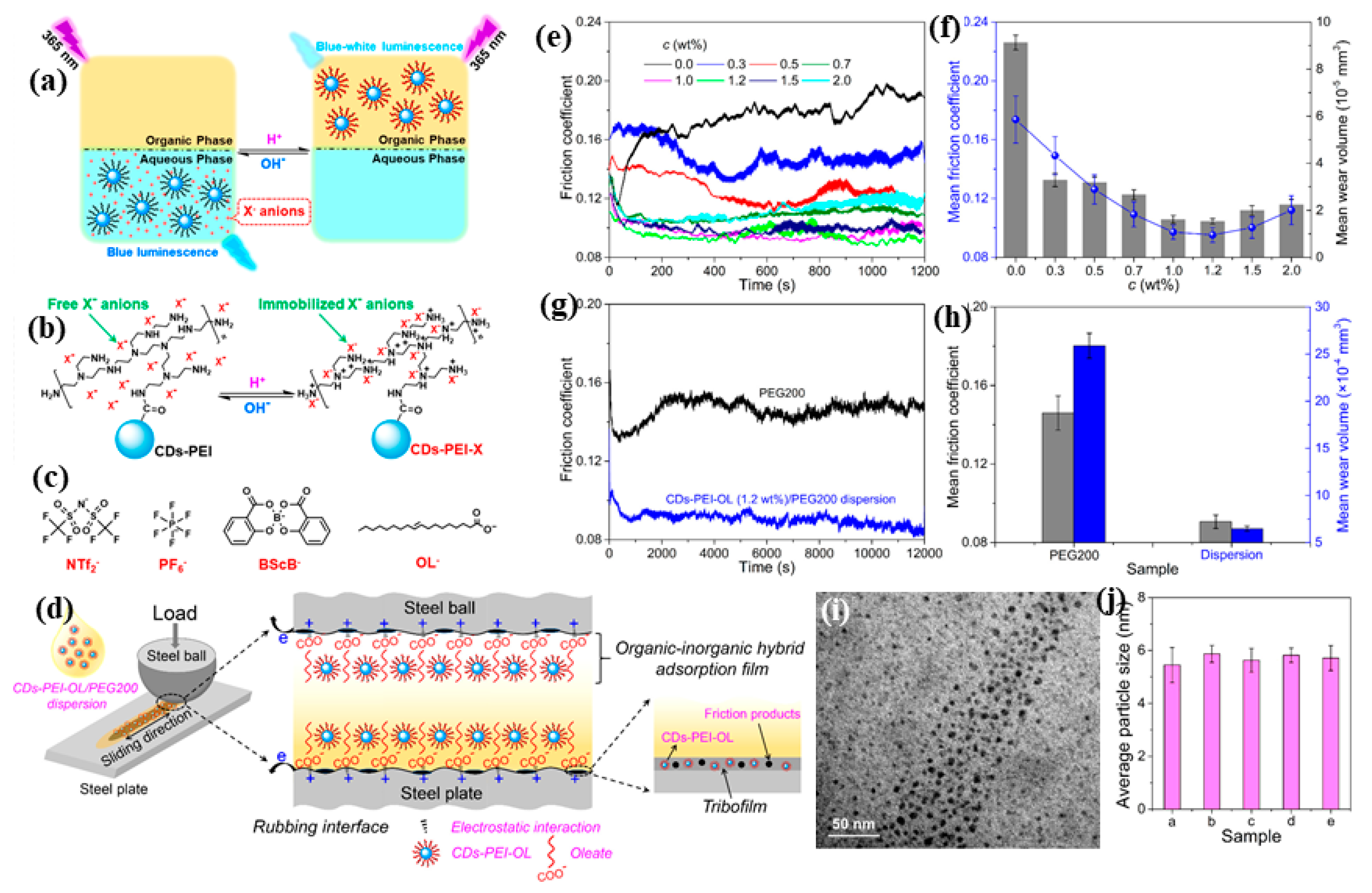
3. Lubricant Properties and Applications of Doped CD-Based Materials
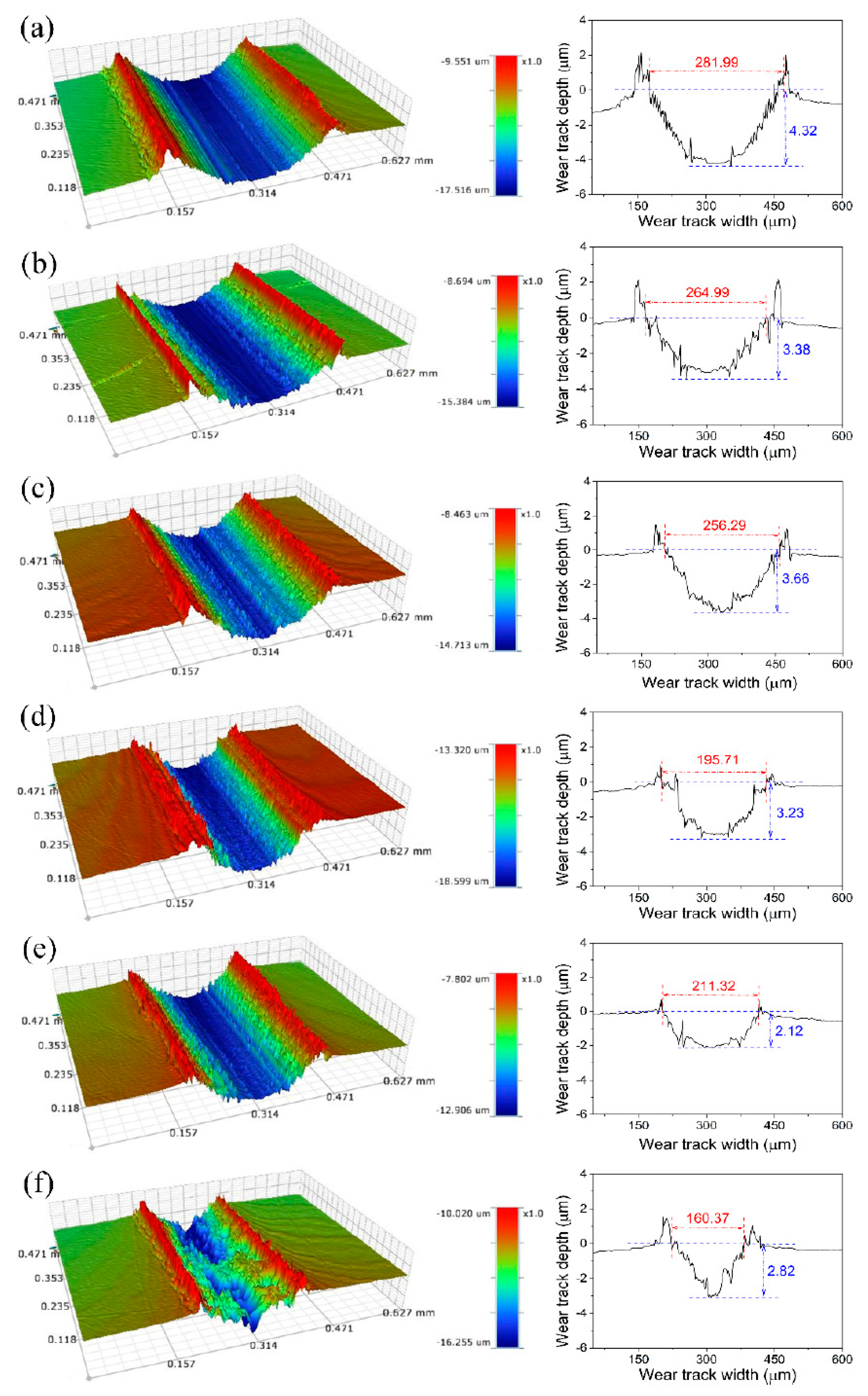
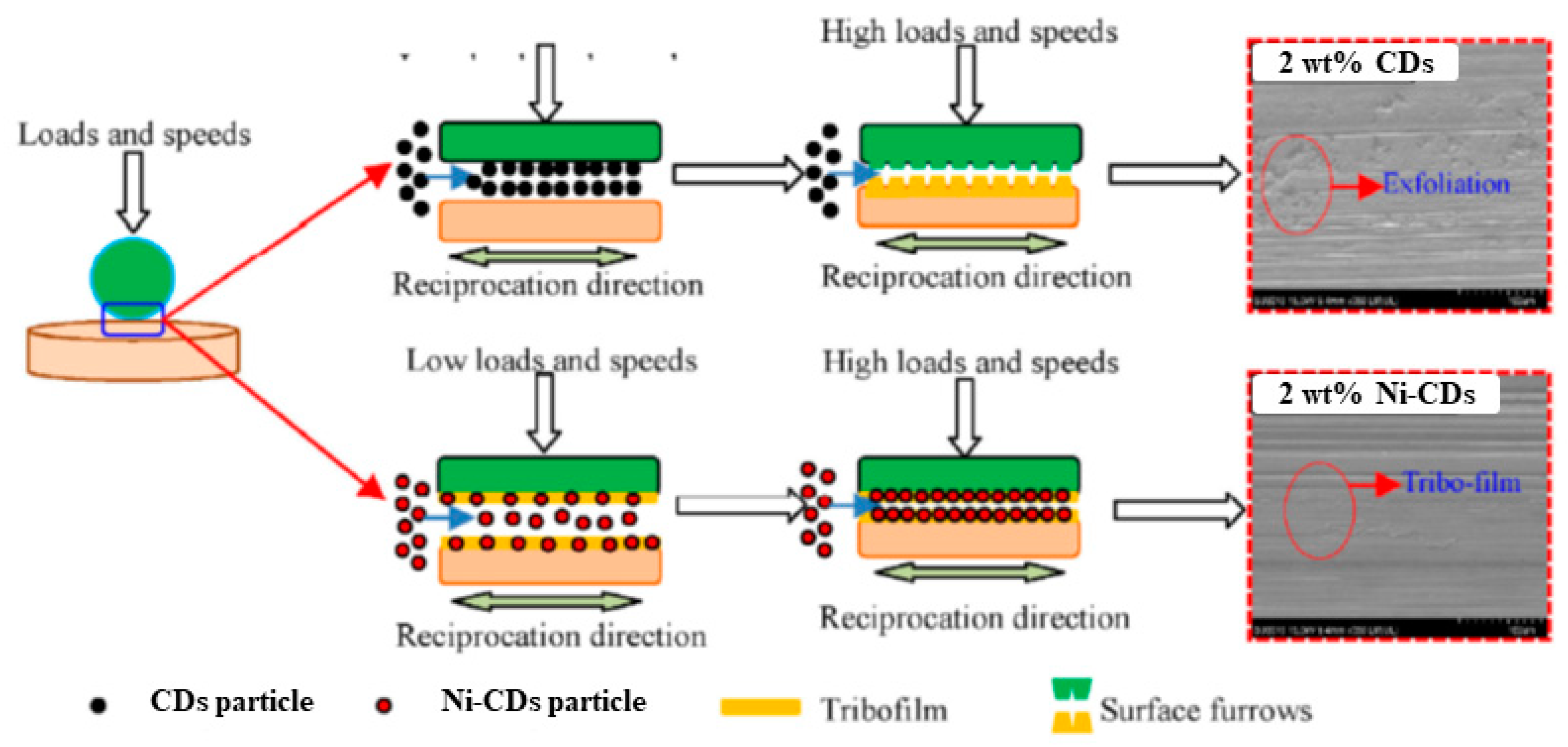
4. The Friction and Possible Wear Mechanisms of the Doped CD-Based Lubricants
5. The Limits of CD-Based Lubricants and Their Future Prospects
6. Conclusions and Summary
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Q.J.; Chung, Y.W. Encyclopedia of Tribology; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Lenard, J.G. Tribology, Primer on Flat Rolling, 2nd ed.; Elsevier, 2014; Chapter 9; pp. 193–266. [Google Scholar] [CrossRef]
- Dunn, A.C.; Krick, B.A.; Liechti, K.M.; DelRio, F.W. Special Issue on Tribology of Advanced Materials. Exp. Mech. 2021, 61, 1503–1505. [Google Scholar] [CrossRef]
- Yoo, S.-S.; Kim, D.-E. Minimum lubrication technique using silicone oil for friction reduction of stainless steel. Int. J. Precis. Eng. Manuf. 2013, 14, 875–880. [Google Scholar] [CrossRef]
- Stork, K.; U.S. Vehiche Tehnologies Office US Department of Energy. 2013 Fuel & Lubricant Technologies; 1000 Independence Avenue, S.W. Washington, D.C. 20585-0121: 2014. Available online: https://www.energy.gov/sites/prod/files/2014/07/f17/fy2013_fuels_technologies.pdf (accessed on 1 May 2022).
- Rizvi, S.Q.A. History of automotive lubrication. J. FUELS Lubr. 1996, 105, 1420–1434. [Google Scholar] [CrossRef]
- Rahman, M.H.; Warneke, H.; Webbert, H.; Rodriguez, J.; Austin, E.; Tokunaga, K.; Rajak, D.K.; Menezes, P.L. Water-Based Lubricants: Development, Properties, and Performances. Lubricants 2021, 9, 73. [Google Scholar] [CrossRef]
- Hájek, M.; Vávra, A.; Carmona, H.D.P.; Kocík, J. The Catalysed Transformation of Vegetable Oils or Animal Fats to Biofuels and Bio-Lubricants: A Review. Catalysts 2021, 11, 1118. [Google Scholar] [CrossRef]
- Masjuki, H.; Maleque, A.; Kubo, A.; Nonaka, T. Palm oil and mineral oil based lubricants—Their tribological and emission performance. Tribol. Int. 1999, 32, 305–314. [Google Scholar] [CrossRef]
- Willing, A. Lubricants based on renewable resources—An environmentally compatible alternative to mineral oil products. Chemosphere 2001, 43, 89–98. [Google Scholar] [CrossRef]
- Khan, A.; Gusain, R.; Sahai, M.; Khatri, O.P. Fatty acids-derived protic ionic liquids as lubricant additive to synthetic lube base oil for enhancement of tribological properties. J. Mol. Liq. 2019, 293, 111444. [Google Scholar] [CrossRef]
- Briscoe, W.H.; Titmuss, S.; Tiberg, F.; Thomas, R.K.; McGillivray, D.J.; Klein, J. Boundary lubrication under wate. Nature 2006, 444, 191–194. [Google Scholar] [CrossRef]
- Anand, M.; Hadfield, M.; Viesca, J.-L.; Thomas, B.; González, R.; Cantrill, R.; Battez, A.H. Assessing Boundary Film Forming Behavior of Phosphonium Ionic Liquids as Engine Lubricant Additives. Lubricants 2016, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Perez-Martinez, C.S.; Perkin, S. Interfacial Structure and Boundary Lubrication of a Dicationic Ionic Liquid. Langmuir 2019, 35, 15444–15450. [Google Scholar] [CrossRef] [PubMed]
- Khanmohammadi, H.; Wijanarko, W.; Cruz, S.; Evaristo, M.; Espallargas, N. Triboelectrochemical friction control of W- and Ag-doped DLC coatings in water–glycol with ionic liquids as lubricant additives. RSC Adv. 2022, 12, 3573–3583. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, W.; Li, J. Lubrication and Anti-Rust Properties of Jeffamine-Triazole Derivative as Water-Based Lubricant Additive. Coatings 2021, 11, 679. [Google Scholar] [CrossRef]
- Minami, I. Molecular Science of Lubricant Additives. Appl. Sci. 2017, 7, 445. [Google Scholar] [CrossRef]
- Kumara, C.; Luo, H.; Leonard, D.N.; Meyer, H.M.; Qu, J. Organic-Modified Silver Nanoparticles as Lubricant Additives. ACS Appl. Mater. Interfaces 2017, 9, 37227–37237. [Google Scholar] [CrossRef]
- Waqas, M.; Zahid, R.; Bhutta, M.U.; Khan, Z.A.; Saeed, A. A Review of Friction Performance of Lubricants with Nano Additives. Materials 2021, 14, 6310. [Google Scholar] [CrossRef]
- Uflyand, I.E.; Zhinzhilo, V.A.; Burlakova, V.E. Metal-containing nanomaterials as lubricant additives: State-of-the-art and future development. Friction 2019, 7, 93–116. [Google Scholar] [CrossRef] [Green Version]
- Ali, I.; Basheer, A.A.; Kucherova, A.; Memetov, N.; Pasko, T.; Ovchinnikov, K.; Pershin, V.; Kuznetsov, D.; Galunin, E.; Grachev, V.; et al. Advances in carbon nanomaterials as lubricants modifiers. J. Mol. Liq. 2019, 279, 251–266. [Google Scholar] [CrossRef]
- Zhai, W.; Srikanth, N.; Kong, L.B.; Zhou, K. Carbon nanomaterials in tribology. Carbon 2017, 119, 150–171. [Google Scholar] [CrossRef]
- Hou, X.; Ma, Y.; Bhandari, G.; Yin, Z.; Dai, L.; Liao, H.; Wei, Y. Preparation and Tribological Properties of Graphene Lubricant Additives for Low-Sulfur Fuel by Dielectric Barrier Discharge Plasma-Assisted Ball Milling. Processes 2021, 9, 272. [Google Scholar] [CrossRef]
- Pourpasha, H.; Heris, S.Z.; Mohammadfam, Y. Comparison between multi-walled carbon nanotubes and titanium dioxide nanoparticles as additives on performance of turbine meter oil nano lubricant. Sci. Rep. 2021, 11, 11064. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Li, H.; Guo, Y.; Zhang, B.; Li, J.; Zeng, X. Water lubrication of graphene oxide-based materials. Friction 2021, 10, 977–1004. [Google Scholar] [CrossRef]
- Liu, Y.; Shin, D.-G.; Xu, S.; Kim, C.-L.; Kim, D.-E. Understanding of the lubrication mechanism of reduced graphene oxide coating via dual in-situ monitoring of the chemical and topographic structural evolution. Carbon 2020, 173, 941–952. [Google Scholar] [CrossRef]
- Tang, J.; Chen, S.; Jia, Y.; Ma, Y.; Xie, H.; Quan, X.; Ding, Q. Carbon dots as an additive for improving performance in water-based lubricants for amorphous carbon (a-C) coatings. Carbon 2019, 156, 272–281. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, Z.; Li, Y. Applications of carbon quantum dots in lubricant additives: A review. J. Mater. Sci. 2021, 56, 12061–12092. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, V.B.; Gedanken, A. Sonochemical synthesis of carbon dots, mechanism, effect of parameters, and catalytic, energy, biomedical and tissue engineering applications. Ultrason. Sonochem. 2020, 64, 105009. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.B.; Kumar, R.; Friedman, O.; Golan, Y.; Gedanken, A.; Shefi, O. One-Pot Hydrothermal Synthesis of Elements (B, N, P)-Doped Fluorescent Carbon Dots for Cell Labelling, Differentiation and Outgrowth of Neuronal Cells. ChemistrySelect 2019, 4, 4222–4232. [Google Scholar] [CrossRef]
- Kumar, V.B.; Kumar, R.; Gedanken, A.; Shefi, O. Fluorescent metal-doped carbon dots for neuronal manipulations. Ultrason. Sonochem. 2018, 52, 205–213. [Google Scholar] [CrossRef]
- Kumar, V.B.; Porat, Z.E.; Gedanken, A. Synthesis of Doped/Hybrid Carbon Dots and Their Biomedical Application. Nanomaterials 2022, 12, 898. [Google Scholar] [CrossRef]
- Kim, A.; Dash, J.K.; Kumar, P.; Patel, R. Carbon-Based Quantum Dots for Photovoltaic Devices: A Review. ACS Appl. Electron. Mater. 2021, 4, 27–58. [Google Scholar] [CrossRef]
- Mao, L.-H.; Tang, W.-Q.; Deng, Z.-Y.; Liu, S.-S.; Wang, C.-F.; Chen, S. Facile Access to White Fluorescent Carbon Dots toward Light-Emitting Devices. Ind. Eng. Chem. Res. 2014, 53, 6417–6425. [Google Scholar] [CrossRef]
- Sun, H.; Wu, L.; Wei, W.; Qu, X. Recent advances in graphene quantum dots for sensing. Mater. Today 2013, 16, 433–442. [Google Scholar] [CrossRef]
- Li, X.; Zhao, S.; Li, B.; Yang, K.; Lan, M.; Zeng, L. Advances and perspectives in carbon dot-based fluorescent probes: Mechanism, and application. Coord. Chem. Rev. 2020, 431, 213686. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Wang, K.; Gao, Z.; Gao, G.; Wo, Y.; Wang, Y.; Shen, G.; Cui, D. Systematic safety evaluation on photoluminescent carbon dots. Nanoscale Res. Lett. 2013, 8, 122. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.B.; Sheinberger, J.; Porat, Z.; Shav-Tal, Y.; Gedanken, A. A hydrothermal reaction of an aqueous solution of BSA yields highly fluorescent N doped C-dots used for imaging of live mammalian cells. J. Mater. Chem. B 2016, 4, 2913–2920. [Google Scholar] [CrossRef]
- Ji, C.; Zhou, Y.; Leblanc, R.M.; Peng, Z. Recent Developments of Carbon Dots in Biosensing: A Review. ACS Sens. 2020, 5, 2724–2741. [Google Scholar] [CrossRef]
- Molaei, M.J. Carbon quantum dots and their biomedical and therapeutic applications: A review. RSC Adv. 2019, 9, 6460–6481. [Google Scholar] [CrossRef]
- Gao, N.; Huang, L.; Li, T.; Song, J.; Hu, H.; Liu, Y.; Ramakrishna, S. Application of carbon dots in dye-sensitized solar cells: A review. J. Appl. Polym. Sci. 2019, 137, 48443. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.B.; Borenstein, A.; Markovsky, B.; Aurbach, D.; Gedanken, A.; Talianker, M.; Porat, Z. Activated Carbon Modified with Carbon Nanodots as Novel Electrode Material for Supercapacitors. J. Phys. Chem. C 2016, 120, 13406–13413. [Google Scholar] [CrossRef]
- Kumar, V.B.; Perkas, N.; Porat, Z.; Gedanken, A. Solar-Light-Driven Photocatalytic Activity of Novel Sn@C-Dots-Modified TiO2 Catalyst. ChemistrySelect 2017, 2, 6683–6688. [Google Scholar] [CrossRef]
- Wu, H.; Lu, S.; Yang, B. Carbon-Dot-Enhanced Electrocatalytic Hydrogen Evolution. Acc. Mater. Res. 2022, 3, 319–330. [Google Scholar] [CrossRef]
- He, C.; Shuang, E.; Yan, H.; Li, X. Structural engineering design of carbon dots for lubrication. Chin. Chem. Lett. 2021, 32, 2693–2714. [Google Scholar] [CrossRef]
- Tomala, A.M.; Kumar, V.B.; Porat, Z.; Michalczewski, R.; Gedanken, A. Tribological Anti-Wear and Extreme-Pressure Performance of Multifunctional Metal and Nonmetal Doped C-based Nanodots. Lubricants 2019, 7, 36. [Google Scholar] [CrossRef] [Green Version]
- Manikandan, V.; Lee, N.Y. Green synthesis of carbon quantum dots and their environmental applications. Environ. Res. 2022, 212, 113283. [Google Scholar] [CrossRef]
- Mou, Z.; Zhao, B.; Wang, B.; Xiao, D. Integration of functionalized polyelectrolytes onto carbon dots for synergistically improving the tribological properties of polyethylene glycol. ACS Appl. Mater. Interfaces 2021, 13, 8794–8807. [Google Scholar] [CrossRef]
- Koutsogiannis, P.; Thomou, E.; Stamatis, H.; Gournis, D.; Rudolf, P. Advances in fluorescent carbon dots for biomedical applications. Adv. Phys. X 2020, 5, 1758592. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Ji, M.; Wang, B.; Yin, S.; Xu, H.; Chen, Z.; Li, H. Carbon Quantum Dots Induced Ultrasmall BiOI Nanosheets with Assembled Hollow Structures for Broad Spectrum Photocatalytic Activity and Mechanism Insight. Langmuir 2016, 32, 2075–2084. [Google Scholar] [CrossRef]
- Muthamma, K.; Sunil, D.; Shetty, P. Carbon dots as emerging luminophores in security inks for anti-counterfeit applications—An up-to-date review. Appl. Mater. Today 2021, 23, 101050. [Google Scholar] [CrossRef]
- Xu, J.; Tao, J.; Su, L.; Wang, J.; Jiao, T. A Critical Review of Carbon Quantum Dots: From Synthesis toward Applications in Electrochemical Biosensors for the Determination of a Depression-Related Neurotransmitter. Materials 2021, 14, 3987. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, S.; Xu, Q.; Zhang, H. Carbon quantum dots: An innovative additive for water lubrication. Sci. China Technol. Sci. 2018, 62, 587–596. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, H.; Hu, K.; Nie, G.; Yang, Y.; Wang, Y.; Duan, X.; Wang, S. Carbon dots based photocatalysis for environmental applications. J. Environ. Chem. Eng. 2022, 10, 107336. [Google Scholar] [CrossRef]
- Tang, W.; Zhu, X.; Li, Y. Tribological performance of various metal-doped carbon dots as water-based lubricant additives and their potential application as additives of poly(ethylene glycol). Friction 2021, 10, 688–705. [Google Scholar] [CrossRef]
- Struchkova, T.S.; Vasilev, A.P.; Okhlopkova, A.A.; Danilova, S.N.; Alekseev, A.G. Mechanical and Tribological Properties of Polytetrafluoroethylene Composites Modified by Carbon Fibers and Zeolite. Lubricants 2021, 10, 4. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Hu, H.; Qiao, S.; Bai, L.; Han, M.; Liu, Y.; Kang, Z. Carbon quantum dot/CuSxnanocomposites towards highly efficient lubrication and metal wear repair. Nanoscale 2015, 7, 11321–11327. [Google Scholar] [CrossRef]
- Zhang, W.; Li, T.; An, R.; Wang, J.; Tian, Y. Delivering quantum dots to lubricants: Current status and prospect. Friction 2022, 1–21. [Google Scholar] [CrossRef]
- Tu, Z.; Hu, E.; Wang, B.; David, K.D.; Seeger, P.; Moneke, M.; Stengler, R.; Hu, K.; Hu, X. Tribological behaviors of Ni-modified citric acid carbon quantum dot particles as a green additive in polyethylene glycol. Friction 2019, 8, 182–197. [Google Scholar] [CrossRef] [Green Version]
- Chimeno-Trinchet, C.; Pacheco, M.; Fernández-González, A.; Díaz-García, M.; Badía-Laíño, R. New metal-free nanolubricants based on carbon-dots with outstanding antiwear performance. J. Ind. Eng. Chem. 2020, 87, 152–161. [Google Scholar] [CrossRef]
- Wolk, A.; Rosenthal, M.; Neuhaus, S.; Huber, K.; Brassat, K.; Lindner, J.K.N.; Grothe, R.; Grundmeier, G.; Bremser, W.; Wilhelm, R. A Novel Lubricant Based on Covalent Functionalized Graphene Oxide Quantum Dots. Sci. Rep. 2018, 8, 5843. [Google Scholar] [CrossRef] [Green Version]
- Shang, W.; Cai, T.; Zhang, Y.; Liu, D.; Liu, S. Facile one pot pyrolysis synthesis of carbon quantum dots and graphene oxide nanomaterials: All carbon hybrids as eco-environmental lubricants for low friction and remarkable wear-resistance. Tribol. Int. 2018, 118, 373–380. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y. Synthesis of polyethylene glycol modified carbon dots as a kind of excellent water-based lubricant additives. Fuller. Nanotub. Carbon Nanostruct. 2019, 27, 400–409. [Google Scholar] [CrossRef]
- Pham, S.T.; Wan, S.; Tieu, K.A.; Ma, M.; Zhu, H.; Nguyen, H.H.; Mitchell, D.R.G.; Nancarrow, M.J. Unusual Competitive and Synergistic Effects of Graphite Nanoplates in Engine Oil on the Tribofilm Formation. Adv. Mater. Interfaces 2019, 6, 1901081. [Google Scholar] [CrossRef]
- Huynh, K.; Tieu, K.; Pham, S. Synergistic and Competitive Effects between Zinc Dialkyldithiophosphates and Modern Generation of Additives in Engine Oil. Lubricants 2021, 9, 35. [Google Scholar] [CrossRef]
- Zhou, Y.; Leonard, D.N.; Meyer III, H.M.; Luo, H.; Qu, J. Does the Use of Diamond-Like Carbon Coating and Organophosphate Lubricant Additive Together Cause Excessive Tribochemical Material Removal? Adv. Mater. Interfaces 2015, 2, 1–6. [Google Scholar] [CrossRef]
- Rabaso, P.; Dassenoy, F.; Ville, F.; Diaby, M.; Vacher, B.; Le Mogne, T.; Belin, M.; Cavoret, J. An investigation on the reduced ability of IF-MoS2 nanoparticles to reduce friction and wear in the presence of dispersants. Tribol. Lett. 2014, 55, 503–516. [Google Scholar] [CrossRef]
- Hu, E.; Hu, X.; Liu, T.; Fang, L.; Dearn, K.D.; Xu, H. The role of soot particles in the tribological behavior of engine lubricating oils. Wear 2013, 304, 152–161. [Google Scholar] [CrossRef] [Green Version]
- Cui, L.; Ren, X.; Sun, M.; Liu, H.; Xia, L. Carbon Dots: Synthesis, Properties and Applications. Nanomaterials 2021, 11, 3419. [Google Scholar] [CrossRef]
- Anuar, N.K.K.; Tan, H.L.; Lim, Y.P.; So’Aib, M.S.; Abu Bakar, N.F. A Review on Multifunctional Carbon-Dots Synthesized From Biomass Waste: Design/Fabrication, Characterization and Applications. Front. Energy Res. 2021, 9, 626549. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, V.B.; Sahu, A.K.; Rao, K.B.S. Development of Doped Carbon Quantum Dot-Based Nanomaterials for Lubricant Additive Applications. Lubricants 2022, 10, 144. https://doi.org/10.3390/lubricants10070144
Kumar VB, Sahu AK, Rao KBS. Development of Doped Carbon Quantum Dot-Based Nanomaterials for Lubricant Additive Applications. Lubricants. 2022; 10(7):144. https://doi.org/10.3390/lubricants10070144
Chicago/Turabian StyleKumar, Vijay Bhooshan, Amit Kumar Sahu, and Kota Bhanu Sankara Rao. 2022. "Development of Doped Carbon Quantum Dot-Based Nanomaterials for Lubricant Additive Applications" Lubricants 10, no. 7: 144. https://doi.org/10.3390/lubricants10070144
APA StyleKumar, V. B., Sahu, A. K., & Rao, K. B. S. (2022). Development of Doped Carbon Quantum Dot-Based Nanomaterials for Lubricant Additive Applications. Lubricants, 10(7), 144. https://doi.org/10.3390/lubricants10070144




