Interaction of Co3O4 Nanocube with Graphene and Reduced Graphene Oxide: Adhesion and Quantum Capacitance
Abstract
:1. Introduction
2. Methods
3. Results
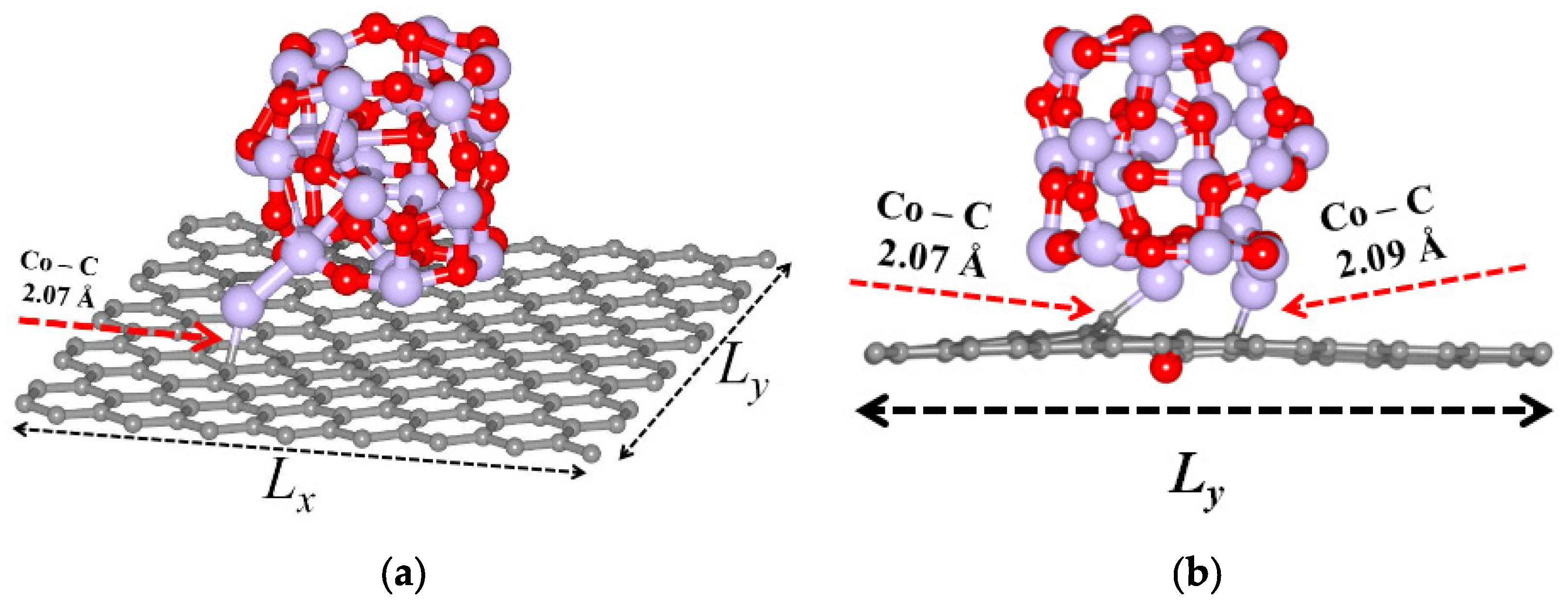
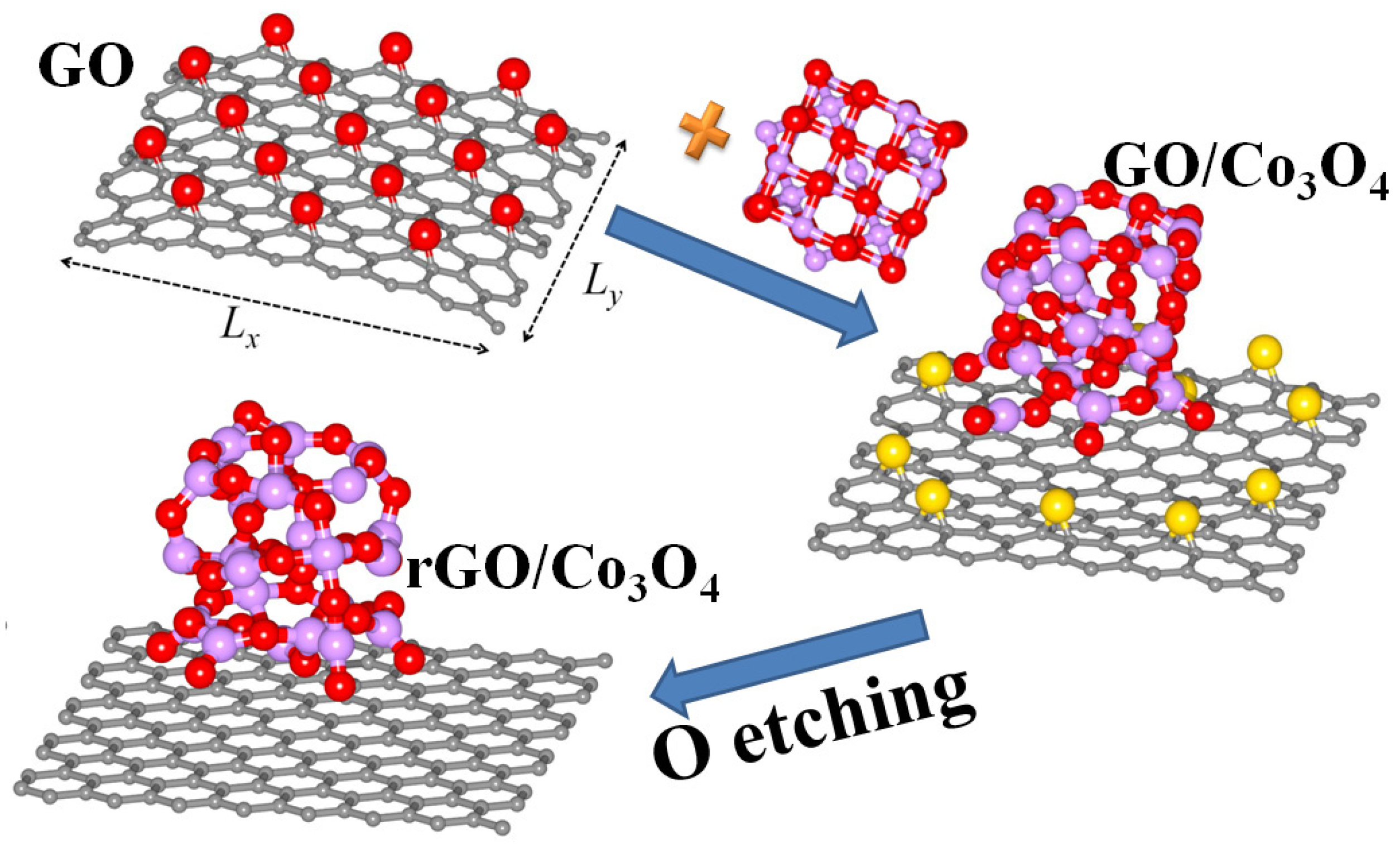
3.1. Adhesion
3.2. Capacitive and Electronic Properties
4. Conclusions and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- An, B.; Shin, J.; Kim, S.-Y.; Kim, J.; Ji, S.; Park, J.; Lee, Y.; Jang, J.; Park, Y.-G.; Cho, E.; et al. Smart sensor systems for wearable electronic devices. Polymers 2017, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Song, Y.; Han, M.; Zhang, H. Portable and wearable self-powered systems based on emerging energy harvesting technology. Microsyst. Nanoeng. 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X. Recent advances of transition metal oxides and chalcogenides in pseudo-capacitors and hybrid capacitors: A review of structures, synthetic strategies, and mechanism studies. J. Energy Storage 2022, 49, 104148. [Google Scholar] [CrossRef]
- Ye, H.; Zheng, G.; Yang, X.; Zhang, D.; Zhang, Y.; Yan, S.; You, L.; Hou, S.; Huanga, Z. Application of different carbon-based transition metal oxide composite materials in lithium-ion batteries. J. Electroanal. Chem. 2021, 898, 115652. [Google Scholar] [CrossRef]
- Hou, C.; Wang, B.; Murugadoss, V.; Vupputuri, S.; Chao, Y.; Guo, Z.; Wang, C.; Du, W. Recent Advances in Co3O4 as Anode Materials for High-Performance Lithium-Ion Batteries. Eng. Sci. 2020, 11, 19–30. [Google Scholar] [CrossRef]
- Meher, S.K.; Rao, G.R. Ultralayered Co3O4 for High-Performance Supercapacitor Applications. J. Phys. Chem. C 2011, 115, 15646–15654. [Google Scholar] [CrossRef]
- Shi, Y.; Pan, X.; Li, B.; Zhao, M.; Pang, H. Co3O4 and its composites for high-performance Li-ion batteries. Chem. Eng. J. 2018, 343, 427–446. [Google Scholar] [CrossRef]
- Li, Q.; Yang, Q.; Yan, Z.; Kang, L.; Lei, Z.; Yang, Z.; Liu, Z. Electrocapacitive performance of graphene/Co3O4 hybrid material prepared by a nanosheet assembly route. Electrochim. Acta 2014, 119, 184–191. [Google Scholar] [CrossRef]
- Han, X.; Huang, Z.; He, C.; Zhang, Q.; Zhang, X.; Yang, Y. Sonochemical synthesis of Co3O4/graphene/Co3O4 sandwich architecture for high-performance supercapacitors. J. Appl. Electrochem. 2019, 49, 1133–1142. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, M.; Chen, Z.; Liu, X.-X. The construction of a sandwich structured Co3O4@C@PPy electrode for improving pseudocapacitive storage. RSC Adv. 2018, 8, 33374. [Google Scholar] [CrossRef] [Green Version]
- Cong, L.; Zhang, S.; Zhu, H.; Chen, W.; Huang, X.; Xing, Y.; Xia, J.; Yang, P.; Lu, X. Structure-design and theoretical-calculation for ultrasmall Co3O4 anchored into ionic liquid modified graphene as anode of flexible lithium-ion batteries. Nano Res. 2022, 15, 2104–2111. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Y.; Hao, Y.; Yang, X.; Goddard, W.A.; Zhang, X.L.; Cao, B. Oxygen-Vacancy Abundant Ultrafine Co3O4/Graphene Composites for High-Rate Supercapacitor Electrodes. Adv. Sci. 2018, 5, 1700659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Wang, R.; Chang, J.; Hu, N.; Xu, C. Self-supporting Co3O4/Graphene Hybrid Films as Binder-free Anode Materials for Lithium Ion Batteries. Sci. Rep. 2018, 8, 3182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Jia, M.; Xu, L.; Gao, J.; Zhang, F.; Jin, X.-J. Graphene and activated carbon-wrapped and Co3O4-intercalated 3D sandwich nanostructure hybrid for high-performance supercapacitance. N. J. Chem. 2018, 42, 10733. [Google Scholar] [CrossRef]
- Jing, M.; Zhou, M.; Li, G.; Chen, Z.; Xu, W.; Chen, X.; Hou, Z. Graphene-Embedded Co3O4 Rose-Spheres for Enhanced Performance in Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 9662–9668. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Jiang, Y.; He, C.; Huang, Z.; Zhanga, X.; Yang, Y. In Situ encapsulation of Co3O4 polyhedra in graphene sheets for high-capacitance supercapacitors. Dalton Trans. 2019, 48, 5773. [Google Scholar] [CrossRef] [PubMed]
- Ramadoss, A.; Yoon, K.-Y.; Kwak, M.-J.; Kim, S.-I.; Ryu, S.-T.; Janga, J.-H. Fully flexible, lightweight, high performance all-solid-state supercapacitor based on 3-Dimensional-graphene/graphite-paper. J. Power Sources 2017, 337, 159–165. [Google Scholar] [CrossRef]
- Chee, W.K.; Lim, H.N.; Zainal, Z.; Huang, N.M.; Harrison, I.; Andou, Y. Flexible Graphene-Based Supercapacitors: A Review. J. Phys. Chem. C 2016, 120, 4153–4172. [Google Scholar] [CrossRef]
- Feng, Q.; Zeng, Y.; Xu, P.; Lin, S.; Feng, C.; Li, X.; Wang, J. Tuning the electrical conductivity of amorphous carbon/reduced graphene oxide wrapped-Co3O4 ternary nanofibers for highly sensitive chemical sensors. J. Mater. Chem. A 2019, 7, 27522. [Google Scholar] [CrossRef]
- Beatty, J.; Cheng, T.; Cao, Y.; Sky Driver, M.; Goddard, W.A.; Kelber, J.A. Nucleation of Graphene Layers on Magnetic Oxides: Co3O4(111) and Cr2O3(0001) from Theory and Experiment. J. Phys. Chem. Lett. 2017, 8, 188–192. [Google Scholar] [CrossRef] [Green Version]
- Odedairo, T.; Yan, X.; Ma, J.; Jiao, Y.; Yao, X.; Du, A.; Zhu, Z. Nanosheets Co3O4 Interleaved with Graphene for Highly Efficient Oxygen Reduction. ACS Appl. Mater Interfaces 2015, 7, 21373–21380. [Google Scholar] [CrossRef] [PubMed]
- Hofer, C.; Skákalová, V.; Görlich, T.; Tripathi, M.; Mittelberger, A.; Mangler, C.; Reza, M.; Monazam, A.; Susi, T.; Kotakoski, J.; et al. Direct imaging of light-element impurities in graphene reveals triple-coordinated oxygen. Nat. Commun. 2019, 10, 4570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackenzie, D.; Galbiati, M.; de Cerio, X.; Sahalianov, I.; Radchenko, T.; Sun, J.; Peña, D.; Gammelgaard, L.; Jessen, B.; Thomsen, J. Unraveling the electronic properties of graphene with substitutional oxygen. 2D Mater. 2021, 8, 045035. [Google Scholar] [CrossRef]
- Elstner, M.; Porezag, D.; Jungnickel, G.; Elsner, J.; Haugk, M.; Frauenheim, T. Self-consistent-charge density-functional tight-binding method for simulations of complex materials properties. Phys. Rev. B 1998, 58, 7260–7268. [Google Scholar] [CrossRef]
- Lennard-Jones, J.E. On the determination of molecular fields.—II. From the equation of state of a gas. Proc. R. Soc. 1924, 106, 463. [Google Scholar]
- Luriy, S. Quantum capacitance devices. Appl. Phys. Lett. 1988, 52, 501–503. [Google Scholar] [CrossRef]
- Shunaev, V.V.; Ushakov, A.V.; Glukhova, O.E. Increase of γ-Fe2O3/CNT composite quantum capacitance by structural design for performance optimization of electrode materials. Int. J. Quantum Chem. 2020, 120, e26165. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, G.; Fan, X.; Zheng, W. Improving the Quantum Capacitance of Graphene-Based Supercapacitors by the Doping and Co-Doping: First-Principles Calculations. ACSOmega 2019, 4, 13209–13217. [Google Scholar] [CrossRef] [Green Version]
- Persson, K. Materials Data on Co3O4 (SG:227) by Materials Project. 2016. Available online: https://doi.org/10.17188/1193429 (accessed on 23 March 2022).
- Mu, J.-C.; Wang, E.-Q.; Zhang, Y.-L.; Zhang, L.-P. Sandwich-Like Co3O4/Graphene Nanocomposites as Anode Material for Lithium Ion Batteries. J. Nanosci. Nanotechnol. 2019, 19, 7819–7825. [Google Scholar] [CrossRef]
- Shunaev, V.V.; Glukhova, O.E. Graphene/Fe3O4 Nanocomposite as a Promising Material for Chemical Current Sources: A Theoretical Study. Membranes 2021, 11, 642. [Google Scholar] [CrossRef]
- Zhang, J.; Li, P.; Wang, Z.; Qiao, J.; Rooney, D.; Sun, W.; Sun, K. Three-dimensional graphene–Co3O4 cathodes for rechargeable Li–O2 batteries. J. Mater. Chem. A 2015, 3, 1504. [Google Scholar] [CrossRef]


| Structure | Fermi Level, eV | Charge, e | QC (0 V), F/g |
|---|---|---|---|
| G | −4.67 | 20.78 | |
| G* | −4.41 | 111.5 | |
| 2D G/Co3O4 | −3.50 | 1.15 | 837.55 |
| 2D G*/Co3O4 | −3.57 | 1.32 | 832.50 |
| 2D GO/Co3O4 | −3.57 | 1.38 | 644.25 |
| 2D rGO/Co3O4 | −3.48 | 0.88 | 652.17 |
| 3D G/Co3O4 | −3.41 | 1.73 | 880.34 |
| 3D G*/Co3O4 | −3.40 | 2.25 | 862.81 |
| 3D GO/Co3O4 | −3.65 | 1.99 | 692.13 |
| 3D rGO/Co3O4 | −3.26 | 1.13 | 691.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shunaev, V.; Glukhova, O. Interaction of Co3O4 Nanocube with Graphene and Reduced Graphene Oxide: Adhesion and Quantum Capacitance. Lubricants 2022, 10, 79. https://doi.org/10.3390/lubricants10050079
Shunaev V, Glukhova O. Interaction of Co3O4 Nanocube with Graphene and Reduced Graphene Oxide: Adhesion and Quantum Capacitance. Lubricants. 2022; 10(5):79. https://doi.org/10.3390/lubricants10050079
Chicago/Turabian StyleShunaev, Vladislav, and Olga Glukhova. 2022. "Interaction of Co3O4 Nanocube with Graphene and Reduced Graphene Oxide: Adhesion and Quantum Capacitance" Lubricants 10, no. 5: 79. https://doi.org/10.3390/lubricants10050079
APA StyleShunaev, V., & Glukhova, O. (2022). Interaction of Co3O4 Nanocube with Graphene and Reduced Graphene Oxide: Adhesion and Quantum Capacitance. Lubricants, 10(5), 79. https://doi.org/10.3390/lubricants10050079






