Antibacterial TaC-(Fe,Cr,Mo,Ni)-(Ag/Cu) Composite Coatings with High Wear and Corrosion Resistance in Artificial Seawater
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrodes
2.2. Deposition of Coatings
2.3. Coatings Characterization
3. Results and Discussion
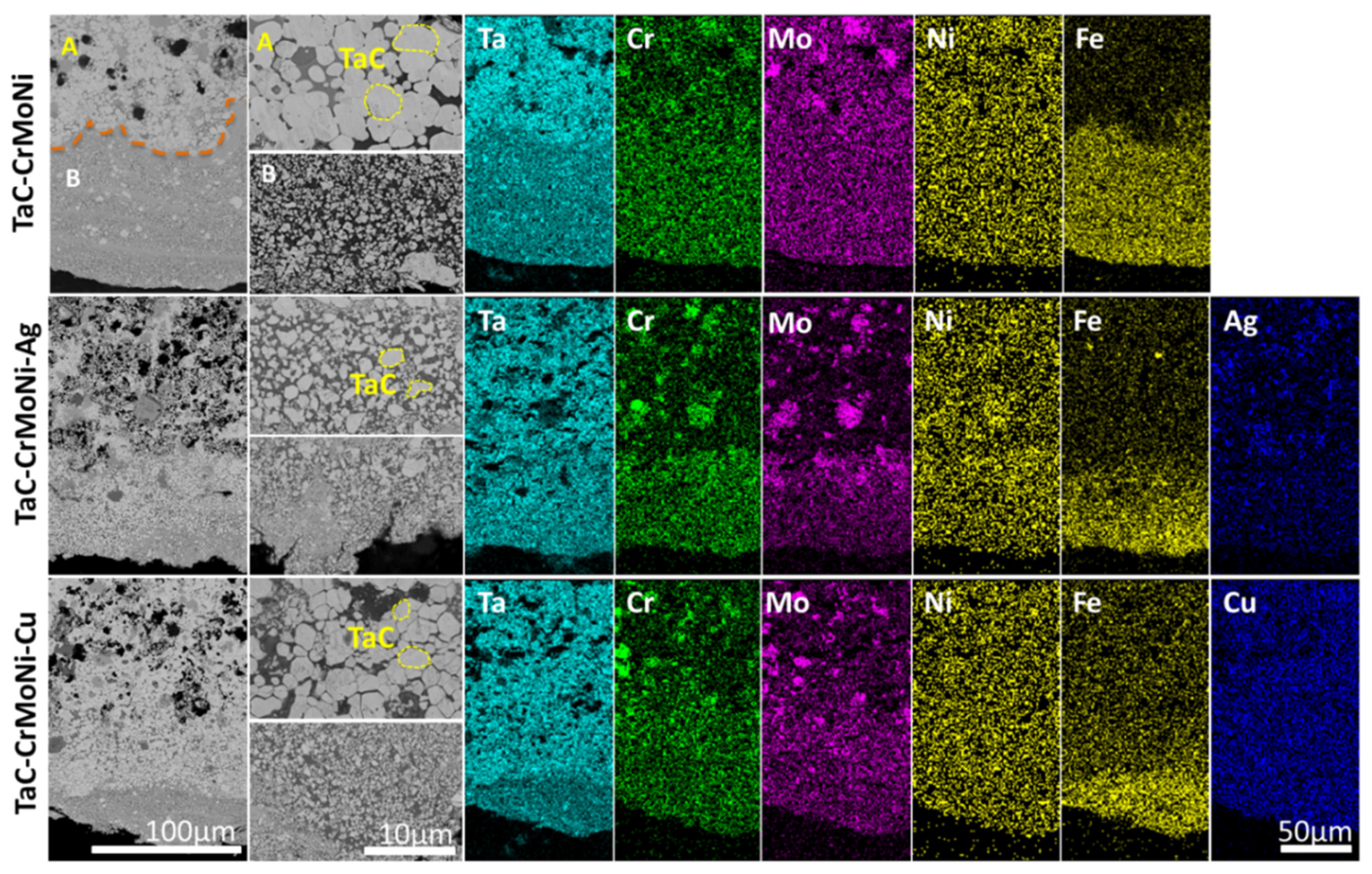
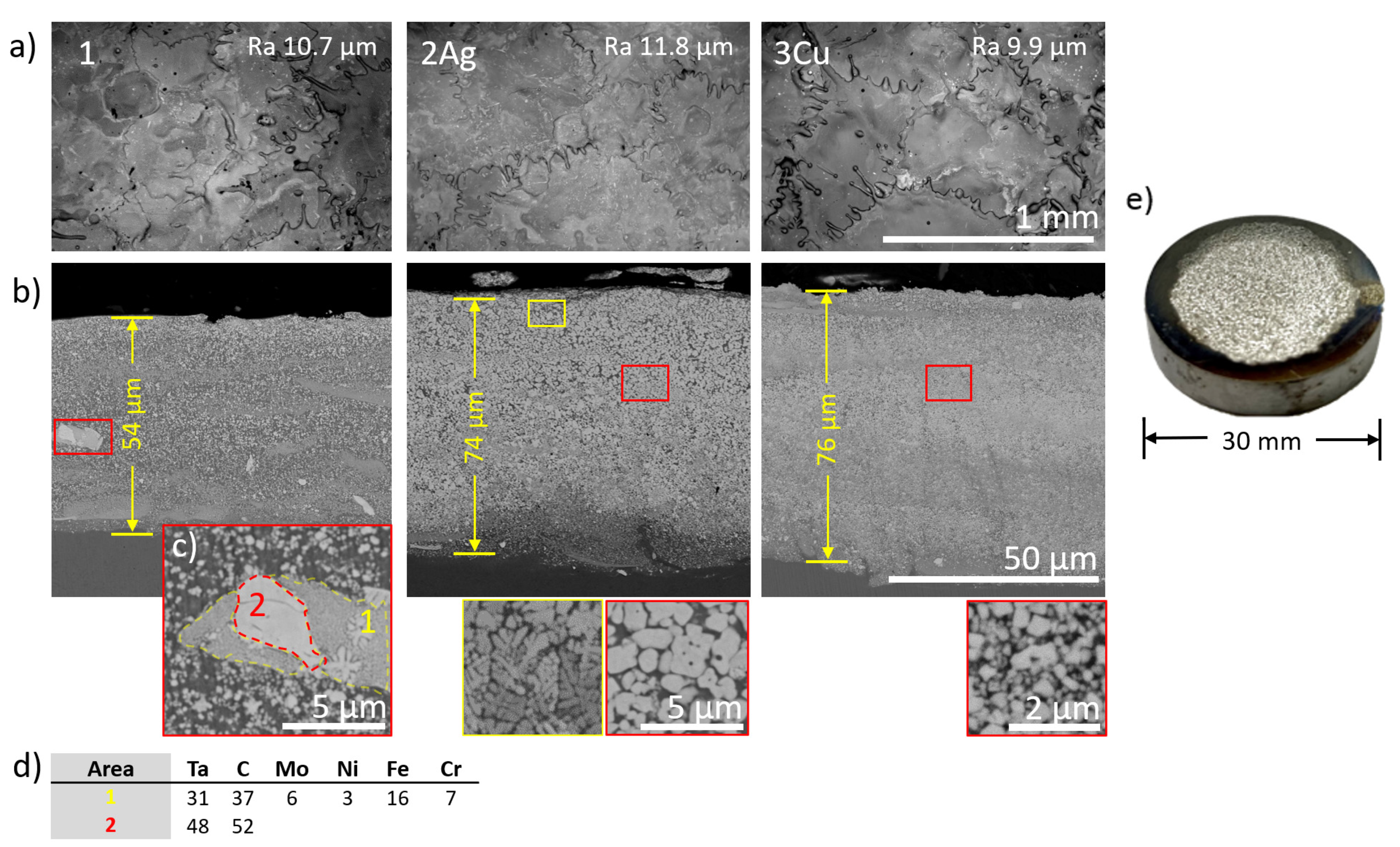
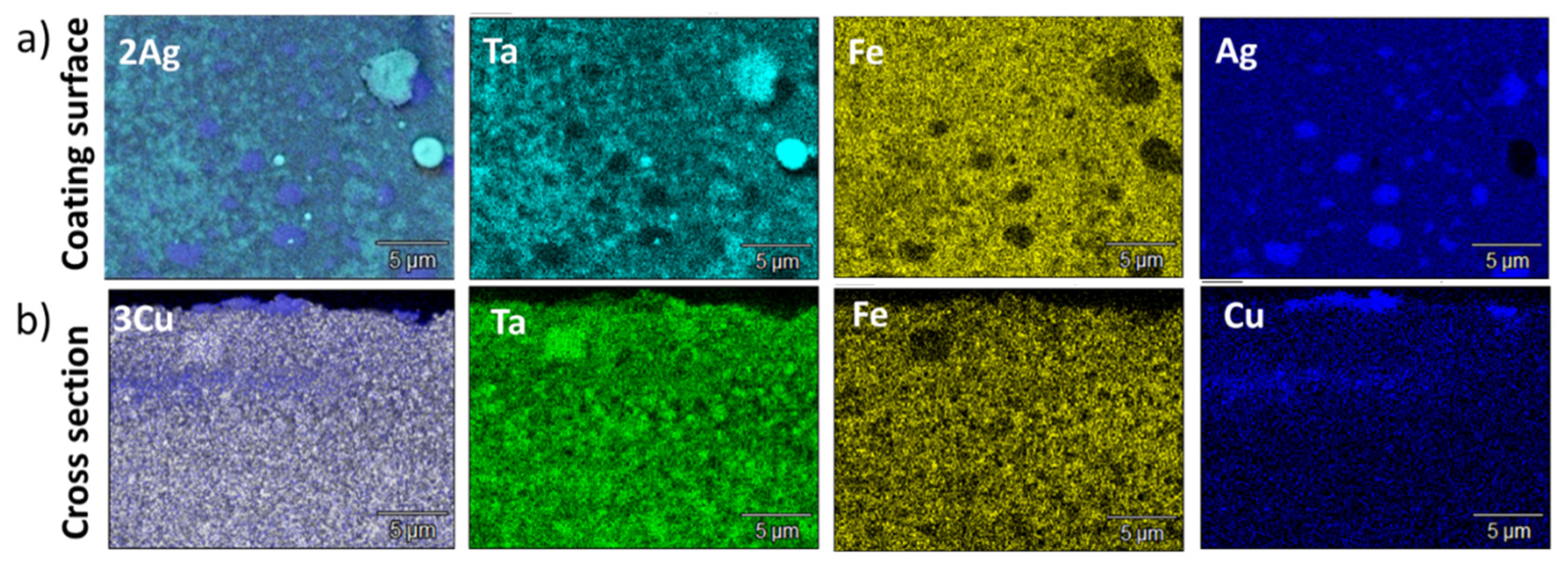
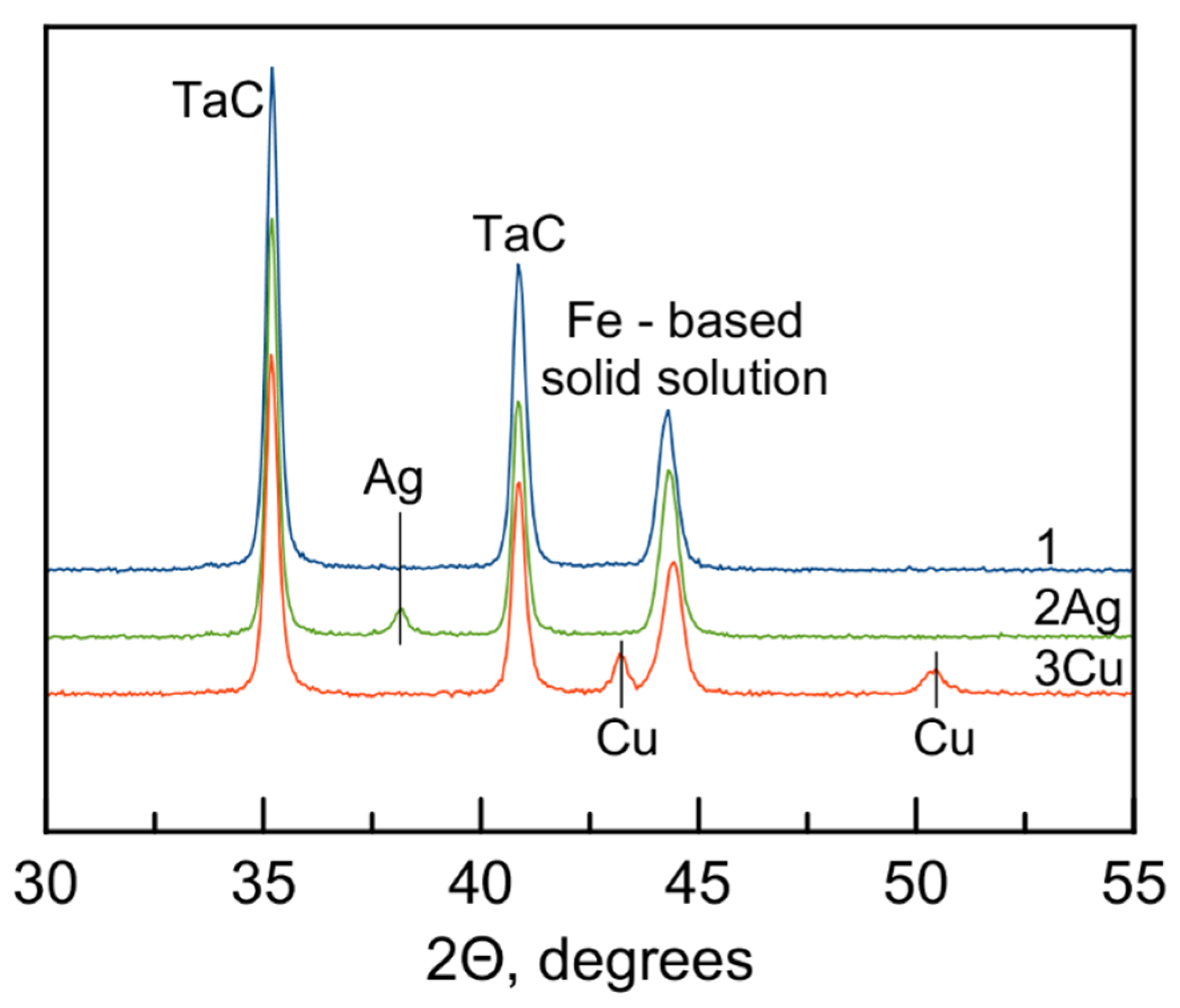
3.1. Structure and Composition of Electrodes and Coatings
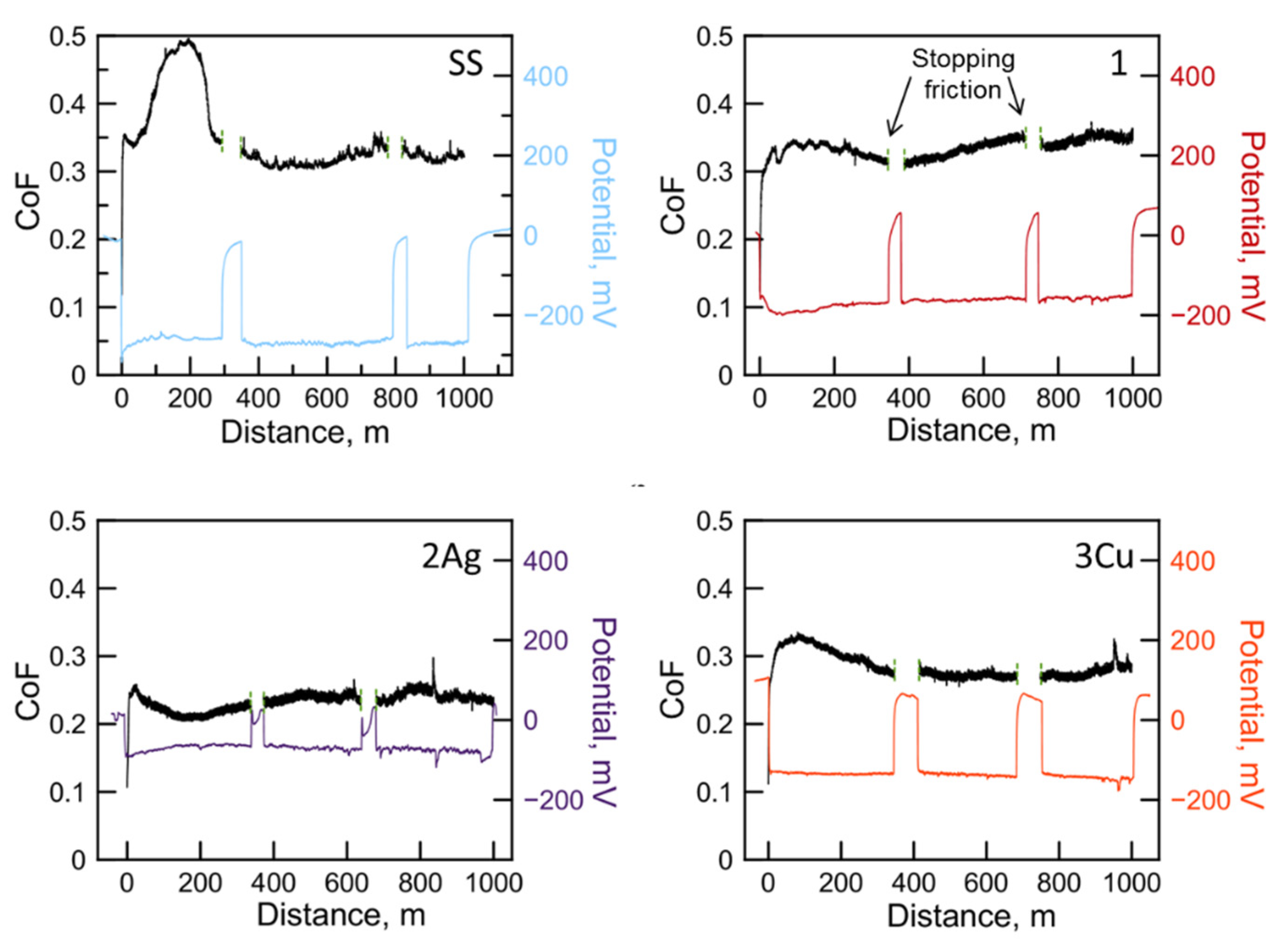
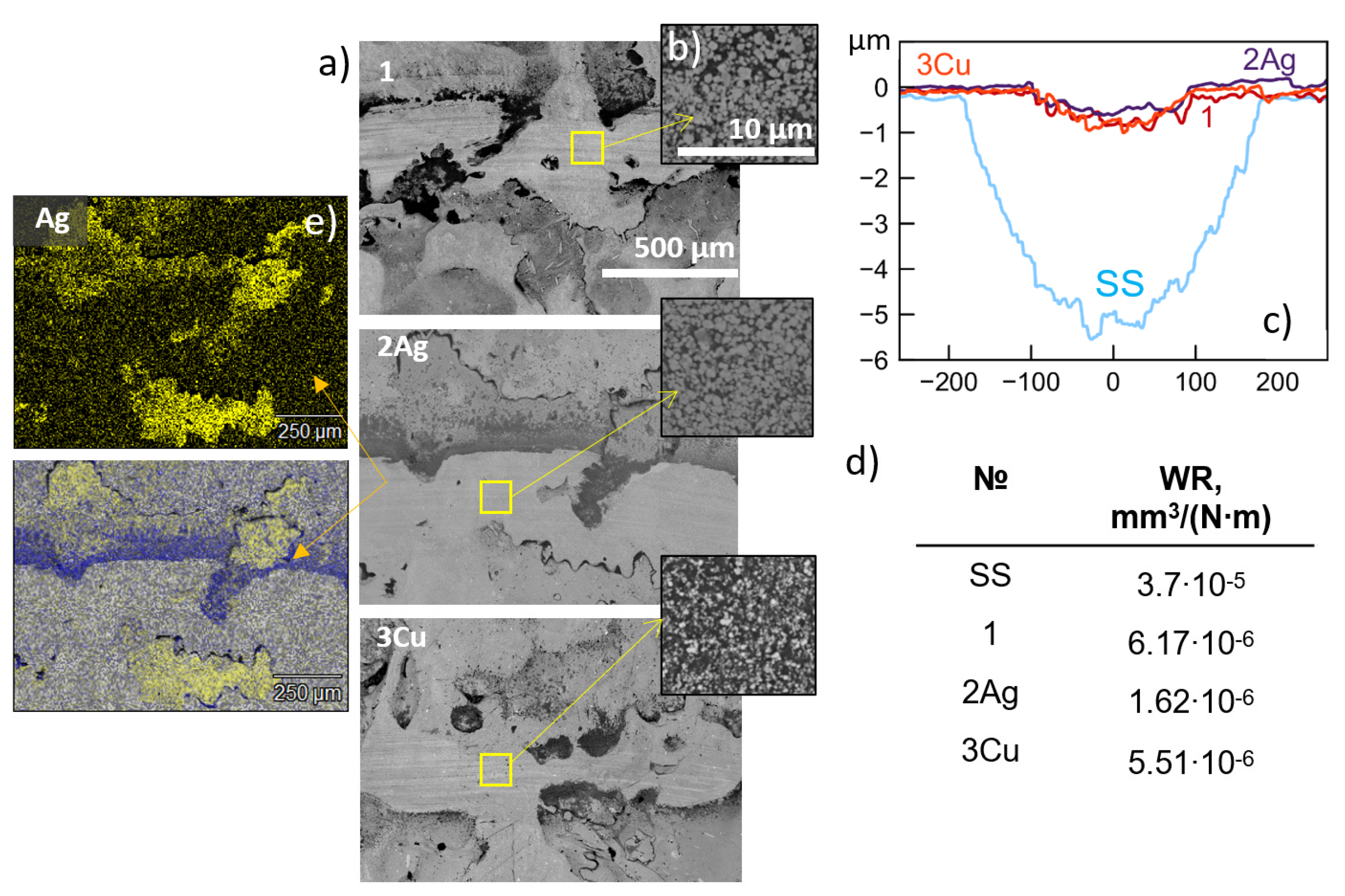
3.2. Tribocorrosion Tests
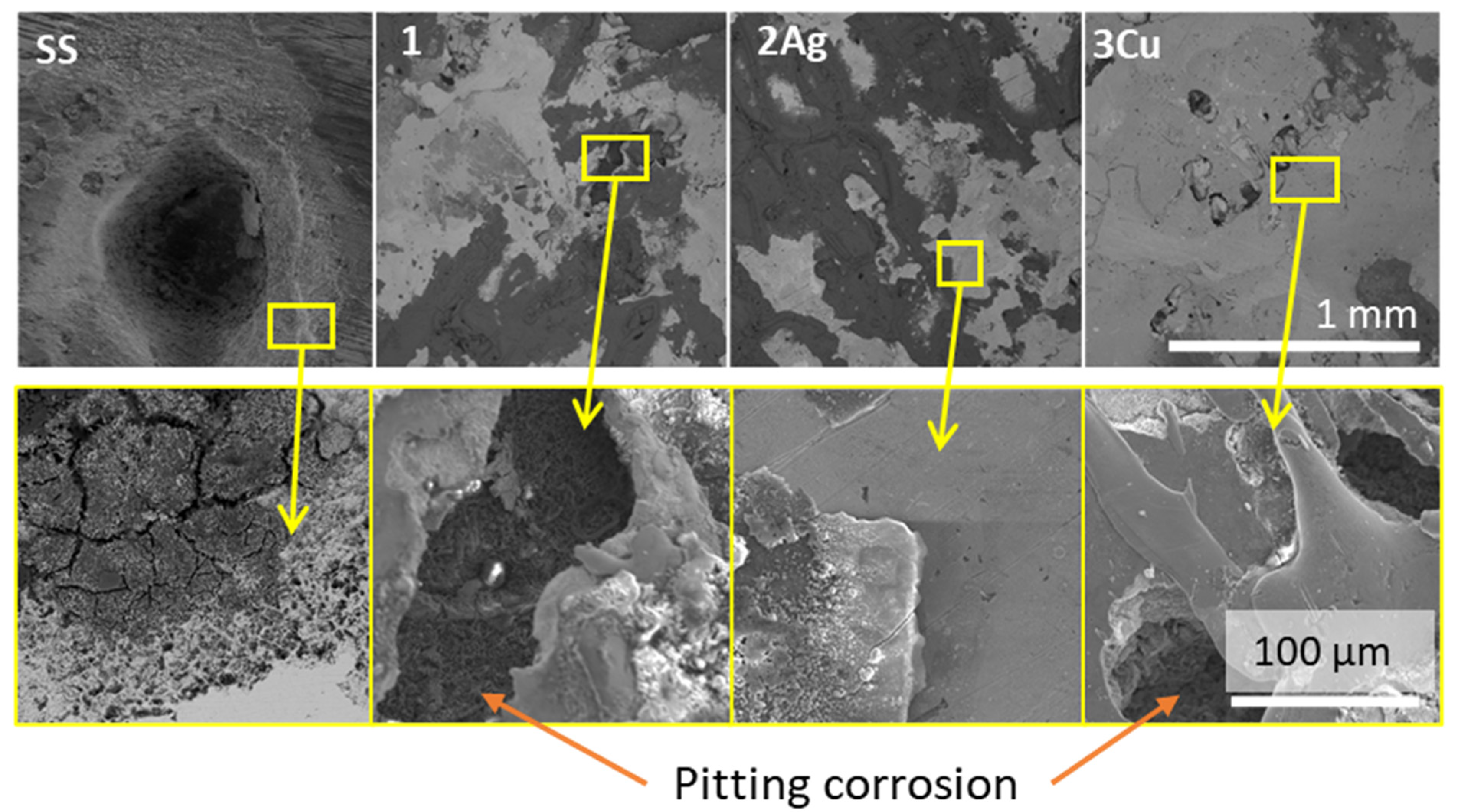
3.3. Corrosion Tests
3.4. Impact Tests
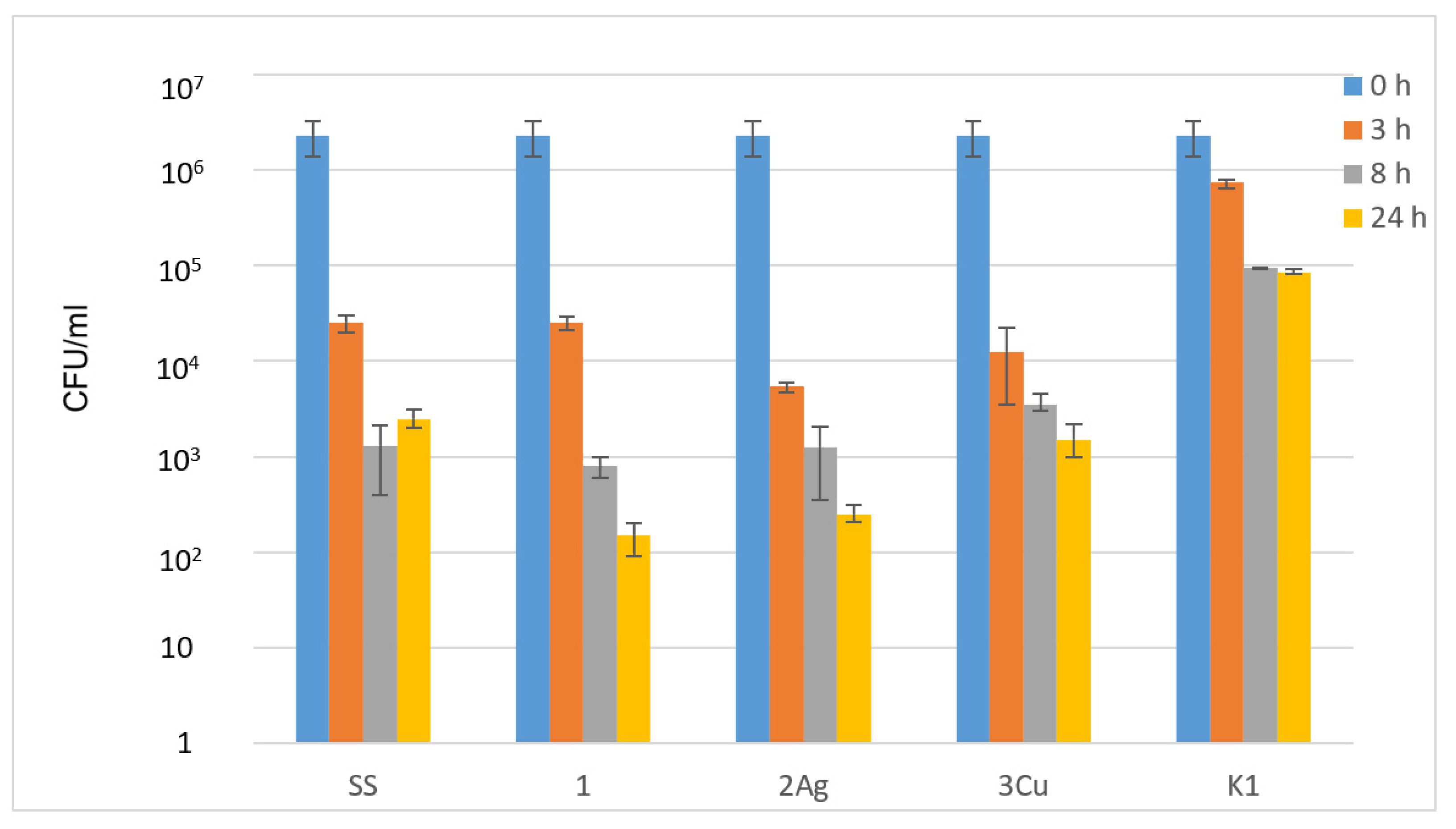
3.5. Antibacterial Tests
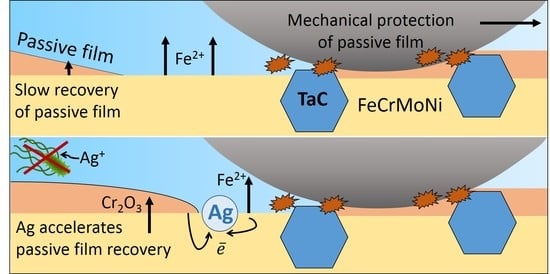
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sui, X.; Xu, R.; Liu, J.; Zhang, S.; Wu, Y.; Yang, J.; Hao, J. Tailoring the Tribocorrosion and Antifouling Performance of (Cr, Cu)-GLC Coatings for Marine Application. ACS Appl. Mater. Interfaces 2018, 10, 36531–36539. [Google Scholar] [CrossRef] [PubMed]
- Melchers, R.E.; Moan, T.; Gao, Z. Corrosion of Working Chains Continuously Immersed in Seawater. J. Mar. Sci. Technol. 2007, 12, 102–110. [Google Scholar] [CrossRef]
- López-Ortega, A.; Bayón, R.; Arana, J.L. Evaluation of Protective Coatings for Offshore Applications. Corrosion and Tribocorrosion Behavior in Synthetic Seawater. Surf. Coat. Technol. 2018, 349, 1083–1097. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Xiao, J.; Chen, W.; Feng, C.; Gan, X.; Zhou, K. The Tribo-Corrosion Behavior of Cu-9 Wt% Ni-6 Wt% Sn Alloy. Tribol. Int. 2016, 94, 260–268. [Google Scholar] [CrossRef]
- Chambers, L.D.; Stokes, K.R.; Walsh, F.C.; Wood, R.J.K. Modern Approaches to Marine Antifouling Coatings. Surf. Coat. Technol. 2006, 201, 3642–3652. [Google Scholar] [CrossRef]
- Zhu, H.; Li, X.; Lu, X.; Wang, J.; Hu, Z.; Ma, X. Efficiency of Gemini Surfactant Containing Semi-Rigid Spacer as Microbial Corrosion Inhibitor for Carbon Steel in Simulated Seawater. Bioelectrochemistry 2021, 140, 107809. [Google Scholar] [CrossRef]
- Kwok, C.T.; Lo, K.H.; Cheng, F.T.; Man, H.C. Effect of Processing Conditions on the Corrosion Performance of Laser Surface-Melted AISI 440C Martensitic Stainless Steel. Surf. Coat. Technol. 2003, 166, 221–230. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, T.; Kovacevic, R. Erosion and Corrosion Resistance of Laser Cladded AISI 420 Stainless Steel Reinforced with VC. Appl. Surf. Sci. 2017, 410, 225–240. [Google Scholar] [CrossRef]
- Von der Ohe, C.B.; Johnsen, R.; Espallargas, N. Multi-Degradation Behavior of Austenitic and Super Duplex Stainless Steel–The Effect of 4-Point Static and Cyclic Bending Applied to a Simulated Seawater Tribocorrosion System. Wear 2012, 288, 39–53. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Bhuyan, P.; Bairi, L.R.; Mandal, S. Comprehending the Role of Individual Microstructural Features on Electrochemical Response and Passive Film Behaviour in Type 304 Austenitic Stainless Steel. Corros. Sci. 2021, 180, 109187. [Google Scholar] [CrossRef]
- Olsson, C.O.A.; Landolt, D. Passive Films on Stainless Steels—Chemistry, Structure and Growth. Electrochim. Acta 2003, 48, 1093–1104. [Google Scholar] [CrossRef]
- Kuptsov, K.A.; Antonyuk, M.N.; Bondarev, A.V.; Sheveyko, A.N.; Shtansky, D.V. Electrospark Deposition of Wear and Corrosion Resistant Ta(Zr)C-(Fe,Mo,Ni) Coatings to Protect Stainless Steel from Tribocorrosion in Seawater. Wear 2021, 486–487, 204094. [Google Scholar] [CrossRef]
- Fu, Y.; Zhou, F.; Wang, Q.; Zhang, M.; Zhou, Z. Electrochemical and Tribocorrosion Performances of CrMoSiCN Coating on Ti-6Al-4V Titanium Alloy in Artificial Seawater. Corros. Sci. 2020, 165, 108385. [Google Scholar] [CrossRef]
- Ma, F.; Li, J.; Zeng, Z.; Gao, Y. Tribocorrosion Behavior in Artificial Seawater and Anti-Microbiologically Influenced Corrosion Properties of TiSiN-Cu Coating on F690 Steel. J. Mater. Sci. Technol. 2019, 35, 448–459. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, F.; Wang, Q.; Fu, Y.; Zhou, Z. Tribocorrosion Characteristics of CrMoSiCN/Ag Coatings on Ti6Al4V Alloys in Seawater. Ceram. Int. 2021, 47, 31780–31797. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, F.; Fu, Y.; Wang, Q.; Kong, J. Electrochemical Corrosion and Tribological Properties of CrMoCN Coatings Sliding against Al2O3 Balls in Artificial Seawater. Surf. Coat. Technol. 2021, 417, 127225. [Google Scholar] [CrossRef]
- Joy, N.; Sai Venkatesh, B.; Dilip Kumar, S. Microstructure and Corrosion Behavior of Cr-Cu and Cr-Ag Composite Coatings for Industrial Applications. Mater. Today Proc. 2021, 44, 3697–3700. [Google Scholar] [CrossRef]
- Lee, J.B. Effects of Alloying Elements, Cr, Mo and N on Repassivation Characteristics of Stainless Steels Using the Abrading Electrode Technique. Mater. Chem. Phys. 2006, 99, 224–234. [Google Scholar] [CrossRef]
- Dalmau, A.; Richard, C.; Igual-Muñoz, A. Degradation Mechanisms in Martensitic Stainless Steels: Wear, Corrosion and Tribocorrosion Appraisal. Tribol. Int. 2018, 121, 167–179. [Google Scholar] [CrossRef]
- Calabrese, C.; La Parola, V.; Testa, M.L.; Liotta, L.F. Antifouling and Antimicrobial Activity of Ag, Cu and Fe Nanoparticles Supported on Silica and Titania. Inorganica Chim. Acta 2022, 529, 120636. [Google Scholar] [CrossRef]
- Hsieh, J.H.; Li, C.; Lin, Y.C.; Chiu, C.H.; Hu, C.C.; Chang, Y.H. Antibacteria and Anti-Wear TaN–(Ag,Cu) Nanocomposite Thin Films Deposited on Polyether Ether Ketone. Thin Solid Films 2015, 584, 277–282. [Google Scholar] [CrossRef]
- Dang, C.; Li, J.; Wang, Y.; Chen, J. Structure, Mechanical and Tribological Properties of Self-Toughening TiSiN/Ag Multilayer Coatings on Ti6Al4V Prepared by Arc Ion Plating. Appl. Surf. Sci. 2016, 386, 224–233. [Google Scholar] [CrossRef]
- Bin Humam, S.; Gyawali, G.; Amanov, A.; Kim, T.H.; Lee, S.W. Microstructure, Interface, and Nanostructured Surface Modifications to Improve Mechanical and Tribological Performance of Electrodeposited Ni-W-TaC Composite Coating. Surf. Coat. Technol. 2021, 419, 127293. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, S.; Wang, S.; Wang, H.; Ramachandran, C.S. Wear, Erosion and Corrosion Resistance of HVOF-Sprayed WC and Cr3C2 Based Coatings for Electrolytic Hard Chrome Replacement. Int. J. Refract. Met. Hard Mater. 2019, 81, 242–252. [Google Scholar] [CrossRef]
- Santaella-González, J.B.; Hernández-Torres, J.; Morales-Hernández, J.; Flores-Ramírez, N.; Ferreira-Palma, C.; Rodríguez-Jiménez, R.C.; García-González, L. Effect of the Number of Bilayers in Ti/TiN Coatings on AISI 316L Deposited by Sputtering on Their Hardness, Adhesion, and Wear. Mater. Lett. 2022, 316, 132037. [Google Scholar] [CrossRef]
- Fang, J.; Piliptsou, D.G.; Bekarevich, R.; Rogachev, A.V.; Jiang, X.; Kulesh, E. Effect of the Alloying Elements in TiN Sublayer on the Structure and Mechanical Properties of Carbon Coatings. Thin Solid Films 2022, 755, 139324. [Google Scholar] [CrossRef]
- Özkan, D.; Yılmaz, M.A.; Erdem, C.; Türküz, C.; Sulukan, E.; Yağcı, M.B. Wear and Friction Behavior of CrTiN/TiCN and CrTiN/CrCN Multilayer Composite Coatings. Ceram. Int. 2022, 48, 13732–13747. [Google Scholar] [CrossRef]
- Kim, D.; Jeon, K.; Lee, Y.; Seo, J.; Seo, K.; Han, H.; Khan, S. Preparation and Characterization of UV-Cured Polyurethane Acrylate/ZnO Nanocomposite Films Based on Surface Modified ZnO. Prog. Org. Coatings 2012, 74, 435–442. [Google Scholar] [CrossRef]
- Choudhury, D.; Rincon, C.; Wei, R.; Benamara, M.; Zou, M. Polydopamine + SiO2 Nanoparticle Underlayer for Improving DLC Coating Adhesion and Durability. Surf. Coat. Technol. 2022, 429, 127964. [Google Scholar] [CrossRef]
- Kuptsov, K.A.; Sheveyko, A.N.; Sidorenko, D.A.; Shtansky, D.V. Electro-Spark Deposition in Vacuum Using Graphite Electrode at Different Electrode Polarities: Peculiarities of Microstructure, Electrochemical and Tribological Properties. Appl. Surf. Sci. 2021, 566, 150722. [Google Scholar] [CrossRef]
- Siddiqui, A.R.; Maurya, R.; Katiyar, P.K.; Balani, K. Superhydrophobic, Self-Cleaning Carbon Nanofiber CVD Coating for Corrosion Protection of AISI 1020 Steel and AZ31 Magnesium Alloys. Surf. Coat. Technol. 2020, 404, 126421. [Google Scholar] [CrossRef]
- Triana, A.; Olaya, J.J.; Prieto, C. Effect of the Electrolyte Composition on the Corrosion Resistance of Single-Layer CVD-Graphene. Surfaces and Interfaces 2022, 31, 102025. [Google Scholar] [CrossRef]
- Liao, L.; Gao, R.; Yang, Z.H.; Wu, S.T.; Wan, Q. A Study on the Wear and Corrosion Resistance of High-Entropy Alloy Treated with Laser Shock Peening and PVD Coating. Surf. Coat. Technol. 2022, 437, 128281. [Google Scholar] [CrossRef]
- Shan, L.; Zhang, Y.R.; Wang, Y.X.; Li, J.L.; Jiang, X.; Chen, J.M. Corrosion and Wear Behaviors of PVD CrN and CrSiN Coatings in Seawater. Trans. Nonferrous Met. Soc. China 2016, 26, 175–184. [Google Scholar] [CrossRef]
- Aubry, P.; Blanc, C.; Demirci, I.; Gorny, C.; Maskrot, H. Analysis of a Ni-Fe-Cr-Mo-Si Hardfacing Alloy Manufactured by Laser Cladding: Influence of the Iron Content on the Wear Resistance Properties. Procedia CIRP 2018, 74, 210–213. [Google Scholar] [CrossRef]
- Wen, X.; Cui, X.; Jin, G.; Liu, Y.; Zhang, Y.; Zhang, X.; Liu, E.; Tian, H.; Fang, Y. Corrosion and Tribo-Corrosion Behaviors of Nano-Lamellar Ni1.5CrCoFe0.5Mo0.1Nbx Eutectic High-Entropy Alloy Coatings: The Role of Dual-Phase Microstructure. Corros. Sci. 2022, 201, 110305. [Google Scholar] [CrossRef]
- Sriraman, K.R.; Brahimi, S.; Szpunar, J.A.; Osborne, J.H.; Yue, S. Tribocorrosion Behavior of Zn, Zn–Ni, Cd and Cd–Ti Electrodeposited on Low Carbon Steel Substrates. Surf. Coat. Technol. 2013, 224, 126–137. [Google Scholar] [CrossRef]
- Hamlaoui, Y.; Tifouti, L.; Pedraza, F. On the Corrosion Resistance of Porous Electroplated Zinc Coatings in Different Corrosive Media. Corros. Sci. 2010, 52, 1883–1888. [Google Scholar] [CrossRef]
- Enrique, P.D.; Marzbanrad, E.; Mahmoodkhani, Y.; Jiao, Z.; Toyserkani, E.; Zhou, N.Y. Surface Modification of Binder-Jet Additive Manufactured Inconel 625 via Electrospark Deposition. Surf. Coat. Technol. 2019, 362, 141–149. [Google Scholar] [CrossRef]
- Sheveyko, A.N.; Kuptsov, K.A.; Kiryukhantsev-Korneev, P.V.; Kaplansky, Y.Y.; Orekhov, A.S.; Levashov, E.A. Protective Coatings for LPBF Ni-Based Superalloys Using a Combination of Electrospark Deposition and Pulsed Arc Evaporation Methods. Appl. Surf. Sci. 2022, 581, 152357. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, J.; Peng, S. Synthesis and Evaluation of TaC Nanocrystalline Coating with Excellent Wear Resistance, Corrosion Resistance, and Biocompatibility. Ceram. Int. 2021, 47, 20032–20044. [Google Scholar] [CrossRef]
- Kuptsov, K.A.; Kiryukhantsev-Korneev, P.V.; Sheveyko, A.N.; Shtansky, D.V. Comparative Study of Electrochemical and Impact Wear Behavior of TiCN, TiSiCN, TiCrSiCN, and TiAlSiCN Coatings. Surf. Coat. Technol. 2013, 216, 273–281. [Google Scholar] [CrossRef]
- Hu, J.; Li, H.; Li, J.; Wu, Q.; Huang, J.; Kong, J.; Shi, Y.; Zhang, G.; Xiong, D. Effect of Ag Target Power on Structure, Mechanical Properties of TaC–Ag Films. Ceram. Int. 2022, 48, 11718–11728. [Google Scholar] [CrossRef]
- Bondarev, A.V.; Kiryukhantsev-Korneev, P.V.; Levashov, E.A.; Shtansky, D.V. Tribological Behavior and Self-Healing Functionality of TiNbCN-Ag Coatings in Wide Temperature Range. Appl. Surf. Sci. 2017, 396, 110–120. [Google Scholar] [CrossRef]
- Sukhorukova, I.V.; Sheveyko, A.N.; Manakhov, A.; Zhitnyak, I.Y.; Gloushankova, N.A.; Denisenko, E.A.; Filippovich, S.Y.; Ignatov, S.G.; Shtansky, D.V. Synergistic and Long-Lasting Antibacterial Effect of Antibiotic-Loaded TiCaPCON-Ag Films against Pathogenic Bacteria and Fungi. Mater. Sci. Eng. C 2018, 90, 289–299. [Google Scholar] [CrossRef]
- Mejía, H.D.; Echavarría, A.M.; Bejarano G., G. Influence of Ag-Cu Nanoparticles on the Microstructural and Bactericidal Properties of TiAlN(Ag,Cu) Coatings for Medical Applications Deposited by Direct Current (DC) Magnetron Sputtering. Thin Solid Films 2019, 687, 137460. [Google Scholar] [CrossRef]
- Zhu, S.; Yu, Y.; Cheng, J.; Qiao, Z.; Yang, J.; Liu, W. Solid/Liquid Lubrication Behavior of Nickel Aluminum-Silver Alloy under Seawater Condition. Wear 2019, 420–421, 9–16. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of Corrosion Rates Measured by the Tafel Extrapolation Method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Zhong, L.; Luo, Y.; Li, Y.; Yang, Y.; Wang, X.; Zhou, T.; Liu, M.; Zhao, Y.; Lai, X.; Bi, J.; et al. Realization of Improved Electrochemical Performance for ZnCo2O4/C Nanosheets through Ag Coating. Ceram. Int. 2022, 48, 16206–16214. [Google Scholar] [CrossRef]
- Bhaumik, S.; Datta, S.; Pathak, S.D. Analyses of Tribological Properties of Castor Oil with Various Carbonaceous Microand Nano-Friction Modifiers. J. Tribol. 2017, 139, 061802. [Google Scholar] [CrossRef]

| NaCl | MgCl2 | Na2SO4 | CaCl2 | KCL | NaHCO3 | KBr | H3BO3 | SrCl2 | NaF | |
|---|---|---|---|---|---|---|---|---|---|---|
| Concentration, g/L | 24.53 | 5.20 | 4.09 | 1.16 | 0.695 | 0.201 | 0.10 | 0.027 | 0.003 | 0.003 |
| Electrode | Structure | Ta | C | Mo | Ni | Fe | Cr | Ag | Cu |
|---|---|---|---|---|---|---|---|---|---|
| TaC-CrMoNi | Initial | 37 | 38 | 9 | 5 | - | 11 | - | - |
| Secondary | 21 | 22 | 4 | 4 | 38 | 11 | - | - | |
| TaC-CrMoNi-Ag | Initial | 36 | 37 | 4 | 4 | - | 9 | 10 | - |
| Secondary | 23 | 24 | 4 | 3 | 30 | 8 | 8 | - | |
| TaC-CrMoNi-Cu | Initial | 36 | 37 | 8 | 4 | - | 9 | - | 6 |
| Secondary | 21 | 23 | 5 | 4 | 28 | 7 | - | 12 |
| Ta | C | Mo | Ni | Fe | Cr | Cu | Ag | |
|---|---|---|---|---|---|---|---|---|
| 1 | 16 | 18 | 3 | 2 | 50 | 11 | - | - |
| 2Ag | 15 | 16 | 2 | 2 | 48 | 10 | - | 7 |
| 3Cu | 11 | 13 | 3 | 3 | 51 | 13 | 6 | - |
| 420S | - | - | 1 | 86 | 13 | - | - |
| H, GPa | E, GPa | |
|---|---|---|
| 420S | 3.7 ± 0.1 | 210 ± 3.2 |
| 1 | 10.3 ± 0.7 | 278 ± 4.3 |
| 2Ag | 5.9 ± 0.6 | 244 ± 2.2 |
| 3Cu | 8.6 ± 0.8 | 250 ± 3.4 |
| Sample | OCP, mV | Corrosion Potential (i = 0), mV | Corrosion Current Density, µA/cm2 | Pitting Breakdown Potential, mV |
|---|---|---|---|---|
| 420S | −56 ± 5 | −63 ± 5 | 1.03 ± 0.07 | 200 |
| 1 | −82 ± 6 | −87 ± 7 | 0.72 ± 0.06 | - |
| 2Ag | −35 ± 4 | −43 ± 5 | 0.17 ± 0.04 | 260 |
| 3Cu | −96 ± 9 | −97 ± 8 | 0.75 ± 0.05 | 210 |
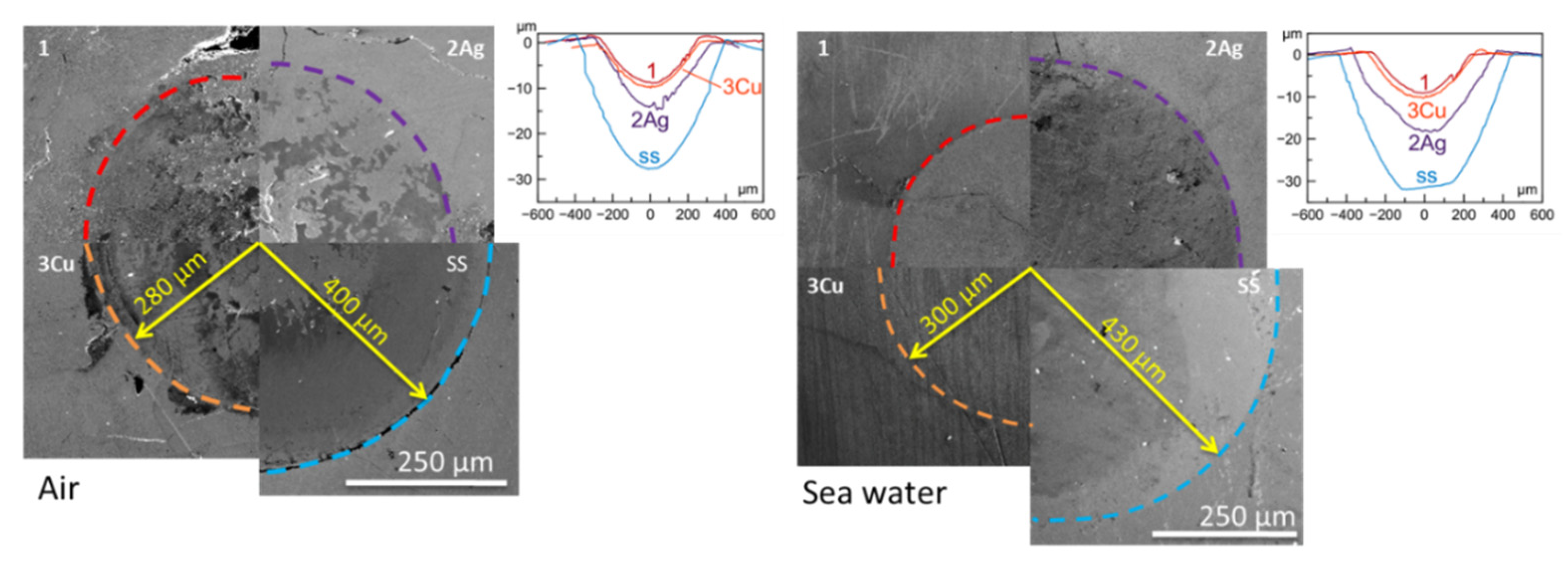
| Coating | Air | Sea Water | ||
|---|---|---|---|---|
| R, µm | h, µm | R, µm | h, µm | |
| 420S | 400 | 28 | 430 | 32 |
| 1 | 280 | 8.5 | 270 | 9 |
| 2Ag | 300 | 14 | 340 | 18 |
| 3Cu | 290 | 9.5 | 300 | 10.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonyuk, M.N.; Kuptsov, K.A.; Sheveyko, A.N.; Shtansky, D.V. Antibacterial TaC-(Fe,Cr,Mo,Ni)-(Ag/Cu) Composite Coatings with High Wear and Corrosion Resistance in Artificial Seawater. Lubricants 2022, 10, 320. https://doi.org/10.3390/lubricants10110320
Antonyuk MN, Kuptsov KA, Sheveyko AN, Shtansky DV. Antibacterial TaC-(Fe,Cr,Mo,Ni)-(Ag/Cu) Composite Coatings with High Wear and Corrosion Resistance in Artificial Seawater. Lubricants. 2022; 10(11):320. https://doi.org/10.3390/lubricants10110320
Chicago/Turabian StyleAntonyuk, Mariya N., Konstantin A. Kuptsov, Alexander N. Sheveyko, and Dmitry V. Shtansky. 2022. "Antibacterial TaC-(Fe,Cr,Mo,Ni)-(Ag/Cu) Composite Coatings with High Wear and Corrosion Resistance in Artificial Seawater" Lubricants 10, no. 11: 320. https://doi.org/10.3390/lubricants10110320
APA StyleAntonyuk, M. N., Kuptsov, K. A., Sheveyko, A. N., & Shtansky, D. V. (2022). Antibacterial TaC-(Fe,Cr,Mo,Ni)-(Ag/Cu) Composite Coatings with High Wear and Corrosion Resistance in Artificial Seawater. Lubricants, 10(11), 320. https://doi.org/10.3390/lubricants10110320