Abstract
Millimeter-waveband spectra of Venus from both the James Clerk Maxwell
Telescope (JCMT) and the Atacama Large Millimeter/submillimeter Array
(ALMA) seem to indicate there may be evidence (signal-to-noise ratio of
about 15σ) of a phosphine absorption-line profile against the thermal background from deeper,
hotter layers of the atmosphere. Phosphine is an important biomarker; e.g.,
the trace of phosphine in
the Earth’s atmosphere is unequivocally associated with anthropogenic
activity and microbial life (which produces this highly reducing gas
even in an overall oxidizing environment). Motivated by the JCMT and ALMA
tantalizing observations, we reexamine whether Venus
could accommodate Earthly life. More concretely, we hypothesize that the
microorganisms populating the Venusian atmosphere are not free
floating but confined to the liquid environment inside cloud
aerosols or droplets. Armed with this hypothesis, we generalize a
study of airborne germ transmission to
constrain the maximum size of droplets that could be floating in the
Venusian atmosphere by demanding that their
Stokes fallout times to reach moderately high
temperatures are pronouncedly larger than the microbe’s replication
time.
We also comment on the effect of cosmic ray
showers on the evolution of aerial microbial life.
1. Introduction
It has long been known that the surface of Venus is too harsh an environment for life [1,2]. Observations from Mariner 2 and Venus 4 spacecraft seem to indicate that the surface of Venus is a hot dielectric carrying temperatures of roughly 427 °C. The critical point of water is 374 °C and 218 atmospheres, and hence liquid water at the average Venus surface temperature is out of the question. Furthermore, at temperatures well below 427 °C enzymes are speedily inactivated, proteins denatured, and most biological organic molecules pyrolized. All in all, it seems quite safe to conjecture that the mean surface temperature of Venus excludes terrestrial forms of life.
Contrariwise, it has long been speculated that the clouds of Venus offer a favorable habitat for life, but regulated to be domiciled at an essentially fixed altitude [3]. The archetype living thing being the spherical hydrogen gasbag isopyenic organism (sHgio). The average sHgio size can be obtained by demanding that its mass must be equal to the displaced mass of the atmosphere, viz.,
where and are, respectively, the atmospheric and hydrogen densities at 0.5 atm pressure level, and is the density of the skin modelled as a membrane of outer radius and inner radius . For a typical skin thickness of roughly , i.e.,
Equation (1) implies that sHgios would have an average diameter of .
Millimeter-waveband spectra of Venus from both the James Clerk Maxwell Telescope (JCMT) and the Atacama Large Millimeter/submillimeter Array (ALMA) telescopes show conclusive evidence (signal-to-noise ratio ∼) of a phosphine (chemical formula PH3) absorption-line profile against the thermal background from deeper, hotter layers of the atmosphere [4]. Data reanalyses to address some critiques questioning the bandpass calibration [5], statistics on flase positives [6], and SO2 contamination [7,8] were presented in [9,10,11].
The PH3 signal has also been claimed to be present in historical data collected by the Pioneer Venus Large Probe Neutral Mass Spectrometer [12]. However, this is a very doubtful detection. The mass resolution of this instrument together with the limited data transmitted clearly does not allow to identify PH3 on 34.1 This could very well be 34S or even H2S. Especially 34S as a fragment of all the sulfur bearing species in the atmosphere of Venus is probably more abundant than PH3.
The punch line of a potential PH3 detection is that phosphine is a biosignature gas associated with anaerobic ecosystems [13]. Thus, the JCMT and ALMA intriguing observations have reinvigorated investigations looking into the possibility of life in the atmosphere of Venus [14,15,16,17]. In particular, it was proposed in [15] that microbial life could reside inside liquid droplets/aerosols, which could protect the microbes from a fatal net loss of liquid to the atmosphere, an unavoidable problem for any free-floating living thing. However, the aerosol habitat could only have a limited lifetime because it would inexorably grow into droplets of a large enough size that are forced by gravity to settle downward to hotter, uninhabitable layers of the atmosphere. In this paper, we generalize a study of airborne coronavirus transmission [18] to constrain the maximum size of the droplets that could be floating in the Venusian atmosphere by demanding that their Stokes fallout times to reach moderately high temperatures are pronouncedly larger than the microbe’s replication time.
The layout of the paper is as follows. In Section 2, we provide a concise summary of the aerial life cycle put forward in [15]. In Section 3, we compare the Stokes fallout time of droplets with typical microbe’s replication times on Earth (under optimum conditions) to determine the maximum allowed radius for the life cycle to persist indefinitely in the atmosphere of Venus. In Section 4, we comment on the effect of cosmic ray showers on the evolution of aerial microbial life. In Section 5, we explore whether some kind of organic chemistry could develop in the concentrated sulfuric acid of Venus’ clouds. The paper wraps up in Section 6 with conclusions.
2. Life Cycle for Venusian Aerial Microbes
Venus’ clouds encircle the entire planet, resulting in a high planetary albedo ∼0.8 [19]. The base of this thick cloud cover is situated at roughly 47 km above the surface (the temperature at this position is around 100 °C) and extends up to over 70 km in altitude. In equatorial and mid-latitudes, the cloud top is located at 74 km, but decreases towards the poles to roughly 65 km [20]. The size distribution of aerosol particles drifting inside the clouds enables a subdivision into three layers: upper (56.5 to 70 km altitude), middle (50.5 to 56.5 km), and lower (47.5 to 50.5 km); the smallest type-1 droplets (with a radius of ) and type-2 droplets (with a radius of 1 to 2 ) are present in all three cloud layers, whereas the largest type-3 droplets (with a radius of ) are only present in the middle and lower cloud layers [21].
It has long been suspected that the cloud decks of Venus offer an aqueous habitat where microorganisms can grow and flourish [22]. Carbon dioxide, sulfuric acid compounds, and ultraviolet (UV) light could give microbes food and energy. It is generally accepted that the existence of life is most likely at an altitude of 50 km, where the temperature is between 60 and 90 degrees Celsius (140 and 194 degrees Fahrenheit), and the pressure is about 1 atm. An optimist might even imagine that the microbial life actually arose in a good-natured surface habitat, perhaps in a primitive ocean, before the planet suffered a runaway greenhouse and the microbes lofted into the clouds [23]. On the other hand, a recent study seems to indicate that the planet has never been liquid-water habitable [24].
Even though the Earth’s atmosphere does not provide long-lived aerosols with temperatures or acidities comparable to Venus, the presence of life at high altitudes has been well documented: bacteria, pollen, and algae have been observed in the Earth’s atmosphere as high as 15 km [25]. Furthermore, evidence has been found for the growth of bacteria in droplets sampled from a super-cooled cloud near a meteorological station on a mountain top in the Alps [26]. It is commonly understood that these microorganisms would have likely reached such heights through evaporation, storms, eruptions, or meteor impacts. Of course, all of these processes could also have occurred in the early history of Venus. In contrast to the Earth, Venusian clouds are not transient entities but represent a global, uninterrupted phenomenon, with the potential for aerosol particles to be sustained for a long period of time, rather than just a few days as in the terrestrial atmosphere. Thereby, the Venusian clouds could provide a stable niche if microbes remain lofted in this aerial.
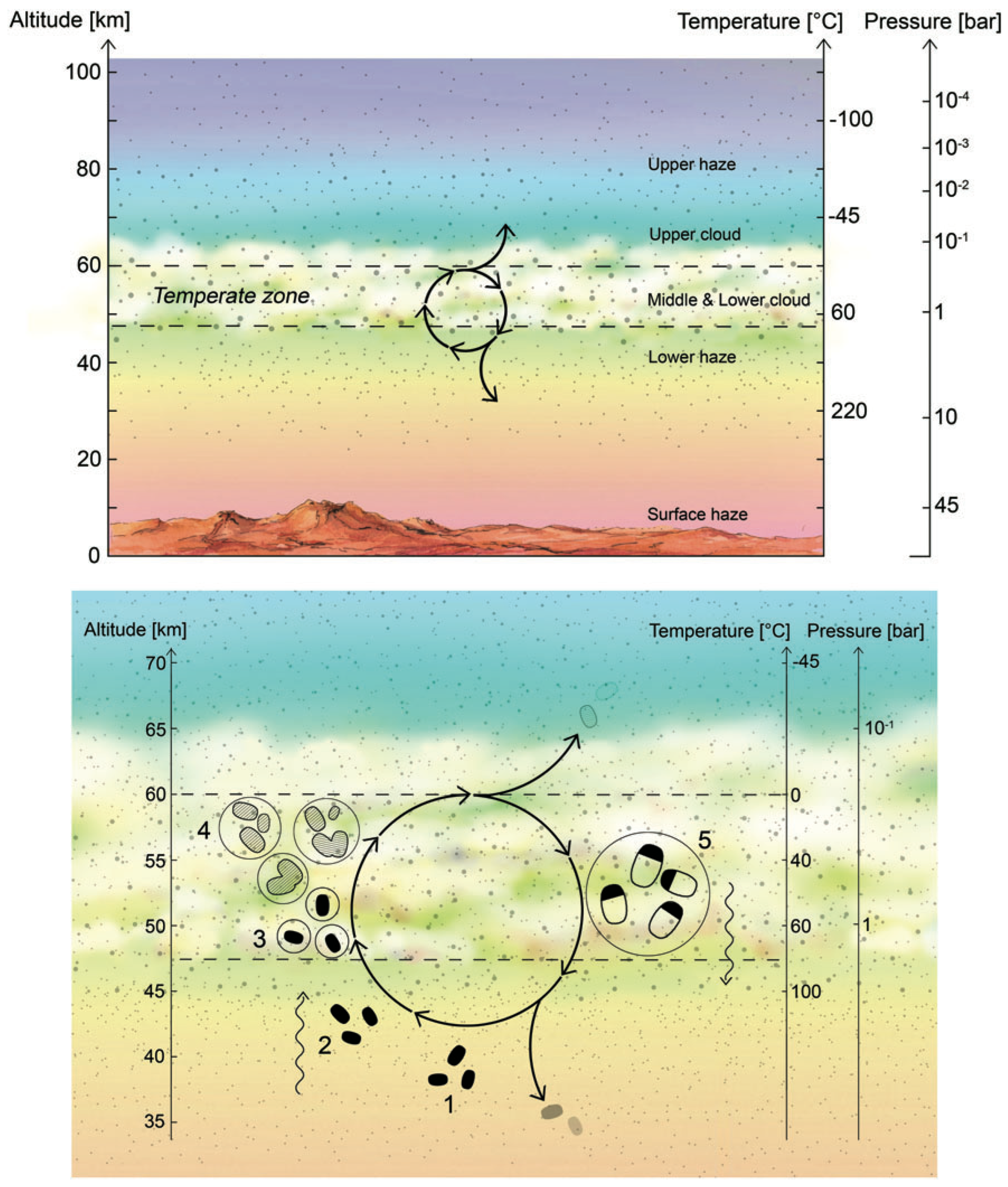
A cycle for Venusian aerial microbial life was developed in [15]. The general idea, which is shown in Figure 1, follows five steps that can be summarized as follows:
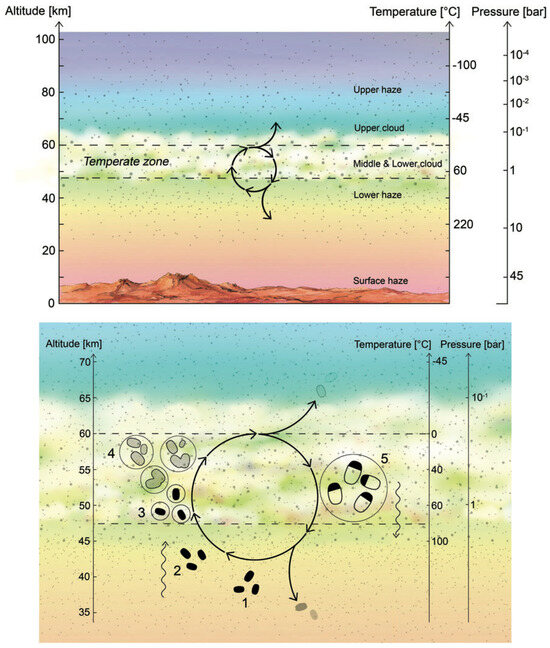
Figure 1.
Hypothetical life cycle of the Venusian microorganisms. (Top panel) Cloud cover on Venus is permanent and continuous, with the middle and lower cloud layers at temperatures that are suitable for life. (Bottom panel) Cycle for Venusian aerial microbial life; see text for details. Taken from Ref. [15].
- The updraft of spores transports them up to the habitable layer. For example, the spores could travel up to the clouds via the effect of gravity waves. Despite the fact that gravity waves can only lead to the net transport of energy and momentum and not matter, they can compress atmosphere layers as they travel, producing vertical winds (which have been measured directly by the Venera landing probes 9 and 10 at the atmospheric lower haze layers [27]).
- Shortly after reaching the (middle and lower cloud) habitable layer, the spores act as cloud condensation nuclei, and once surrounded by liquid (with necessary chemicals dissolved) germinate and become metabolically active.
- Metabolically active microbes (dashed blobs in Figure 1) grow and divide within liquid droplets (shown as solid circles in the figure). The liquid droplets grow by coagulation.
- The droplets reach a size large enough to gravitationally settle down out of the atmosphere; higher temperatures and droplet evaporation trigger cell division and sporulation. The spores are small enough to withstand further downward sedimentation, remaining suspended in the lower haze layer (a depot of hibernating microbial life) to restart the cycle.
One of the key assumptions of the aerial life cycle put forward in [15] is the timescale on which droplets would persist in the habitable layer to empower replication. It is this that we now turn to study.
3. Replication Rates and Fallout Times
Self-replication is a faculty inherent to every species of living thing, and standard intuition imposes that such a physical process must invariably be fueled by the production of entropy [28]. It is of interest then to estimate a lower bound for the amount of heat that is produced during a process of self-replication in a system coupled to a thermal bath. The minimum value for the physically allowed rate of heat production is determined by the growth rate, internal entropy, and durability of the replicator. Impressively, bacteria replicate close (within a factor of two or three) to the physical efficiency [29].
Bacteria replicate by binary fission, a process by which one bacterium splits into two. Therefore, bacteria increase their numbers by geometric progression whereby their population doubles every generation time. Generation time is the time it takes for a population of bacteria to double in number. For many common bacteria, the generation time is quite short, 20 to 60 min under optimum conditions. The typical example is Escherichia coli (or E. coli), which can divide every 20 min under aerobic, nutrient-rich conditions (but of course the generation time for bacteria in the wild are substantially greater than those in the laboratory) [30]. Actually, Vibrio natriegens (previously known as Pseudomonas natriegens and Beneckea natriegens) is a free-living marine bacterium with the fastest generation time known, between 7 and 10 min [31,32,33]. In our calculations, we adopt as benchmark the E. coli generation time under optimum conditions. The duplication times of other types of bacterium are listed in Table 1.

Table 1.
Bacteria generation times.
The mean division time for bacteria population is then 20 min. If the observation begins with one bacterium, we can estimate how many bacteria are present after six hours. The E. coli divides every 20 min, and so this bacterium divide (60/20 = 3) three times every hour. If the bacteria grow for twelve hours, each bacterium divides three times per hour × 12 h = 36 times. Every time the bacteria reproduce, the number doubles. Then, the number of bacteria at the end of the growth period is found to be
where is number of bacteria at the beginning of the growth period and n is the number of divisions. For and , we have bacteria. We conclude that to maintain the colony of microbes alive, we require Stokes fallout times longer than half an Earth day.
Assuming that the droplets are spherical, the mass can be simply estimated as
where is the droplet’s density; in our calculations, we consider the E. coli dry mass density [43] and the density of water .
Under the action of gravity, droplets of mass and size acquire a downward terminal speed that follows from Stokes law and is given by
where is the acceleration due to gravity in Venus,
is the organism mobility in the fluid, and where is the dynamic viscosity of the Venusian atmosphere [44]. For the dynamic viscosity, we adopt the estimate of Ref. [45], .
The acceleration of gravity at the surface of Venus can be derived from measurements of Mariner V [46]
and is found to be , where is Newton’s gravitational constant and where we take the radius of Venus to be to accommodate existing observations [47,48,49].
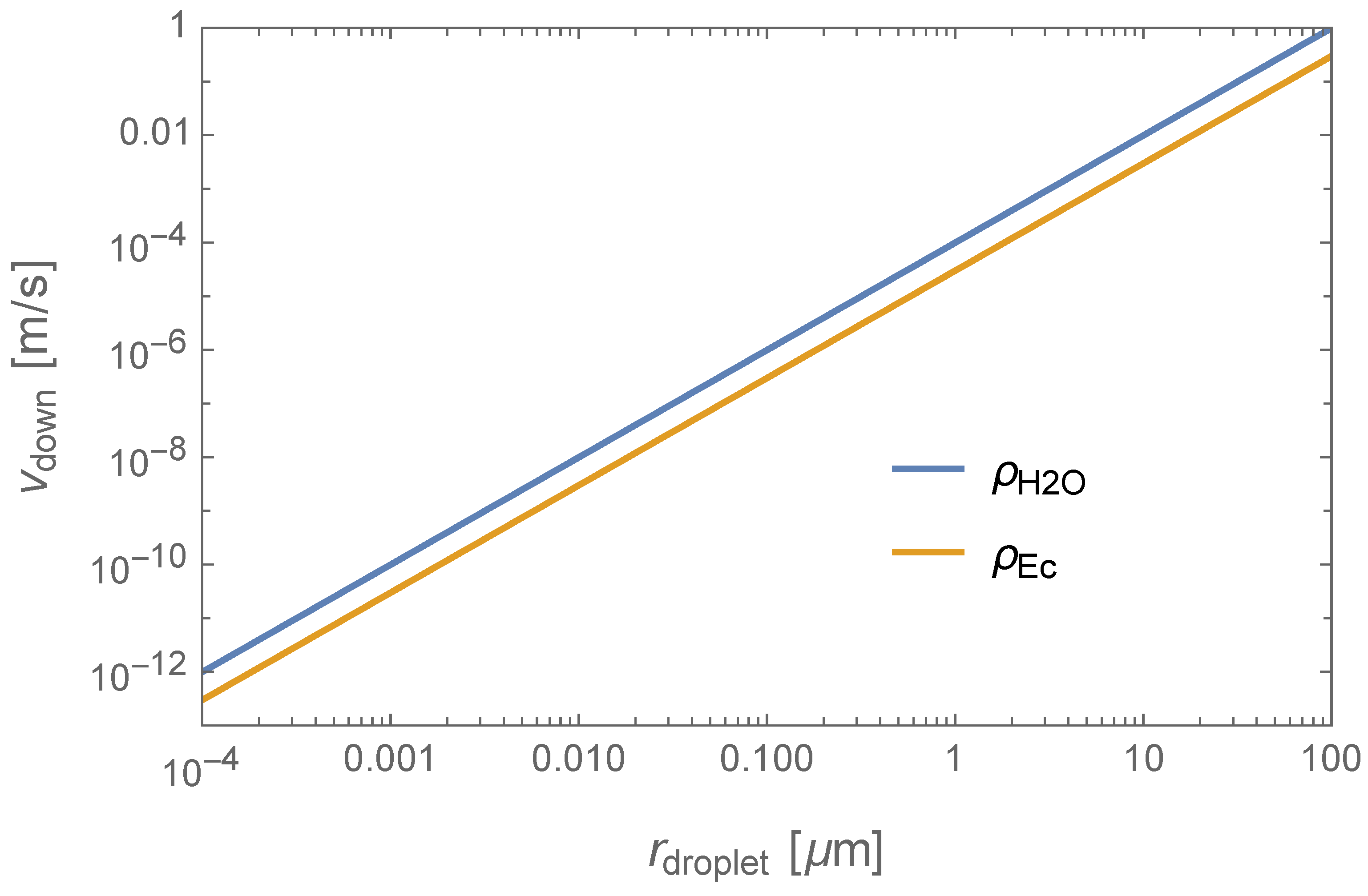
Putting all this together, we can now combine Equations (5) and (6) to estimate the downward terminal speed of the droplets. In Figure 2, we show the terminal velocity as a function of the droplet’s radius. As one can check by inspection, the terminal speed of aerosols (viz., droplets with ) is negligible, and so we can conclude that gravity would play no role in the motion of the microbes through the atmosphere. As the droplet size approaches , the droplets would start sinking to the lower haze layers. For example, a droplet with density and a radius would attain a terminal speed, of and would thus fall about 4 km in half an Earth day.
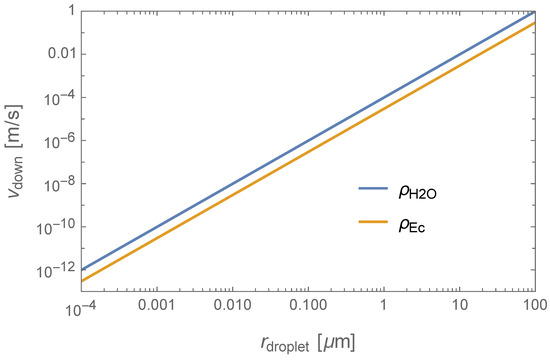
Figure 2.
Terminal speed as a function of the microbe’s radius.
4. Cosmic Ray Effects on Microbial Life
We have long been suspecting that cosmic radiation plays a pivotal role in the evolution of life on Earth [50]; for a more recent perspective, see [51]. In this section, we briefly comment on whether energetic particle radiation could provide a threat for any type of microbe populating the clouds of Venus.
Pioneer Venus measurements are consistent with the absence of an intrinsic magnetic field that could provide a shield against low- and high-energy charged particles reaching the atmosphere [52,53]. If taken at face value, the lack of field seems to indicate that the solar wind would interact directly with the upper atmosphere. However, the upper atmosphere is ionized by UV radiation, yielding the so-called “ionosphere”. Currents arising from the interaction between the solar wind and the electrically conductive venusian ionosphere induce a magnetic field, and so the incoming solar wind particles are slowed down and diverted around the planet [54]. However, the induced magnetic field is weak [55] and can only deflect charged energetic particles with energies up to several hundreds of keV. Thus, most of the cosmic radiation have unrestricted access to the Venusian atmosphere.
Cosmic rays with energies above about 1 GeV induce particle cascades via inelastic scattering with atmospheric nuclei, producing fluxes of secondary, tertiary, and subsequent generations of particles. All these particles together create a cascade (or shower). As the cascade develops longitudinally, the particles become less and less energetic since the energy of the incoming cosmic ray is redistributed among more and more participants. The transverse momenta acquired by the secondaries cause the particles to spread laterally as they propagate through the atmospheric target [56].
As can be seen in Figure 1, the top region of the temperate zone is at 62 km altitude, and thus it receives less than shielding depth against cosmic ray showers. This is far less than the biosphere on the surface of the Earth, which is beneath . The atmospheric grammage at the middle cloud layer is, however, , suggesting the cosmic ray-induced effects would be comparable to those on the Earth’s surface. Indeed, numerical simulations show that cosmic radiation would not have had any hazardous effect on putative microorganisms within the potentially temperate zone (51 to ) [57,58,59].
5. Life Outside the Habitable Zone
A habitable planet, in the context of exoplanets, is a planet that has the potential to support life, typically defined by its location within a star’s habitable zone, where temperatures are suitable for liquid water (a key ingredient for life as we know it) to exist on the surface. These are the so-called “Goldilocks planets”. A broader and certainly more nontypical definition of a habitable planet is a frontier to be explored and requires pushing the boundaries of our terracentric viewpoint for what we deem to be a habitable environment [60,61,62,63]. In this section, we relax our assumption about Earthly life to briefly discuss recent findings which suggest life may be supported in the extreme solvent conditions of the Venusian acid clouds [64,65].
The study of sulfuric acid hydrolysis of proteins dates back to 1820 [66]. Subsequently, studies continued to explore the reactivity of biological matter in concentrated sulfuric acid (and other acids). This pioneer work searched for the chemical composition of biological material and eventually attempted to decipher the amino acid sequence in protein polymers. Such studies on the reactivity of proteins in concentrated sulfuric acid aimed to chemically split peptide bonds at specific amino acid positions in polypeptides [67,68]. Today, we know that a rich organic chemistry can evolve from simple precursor molecules seeded into concentrated sulfuric acid.
The sulfuric acid concentration in the clouds of Venus has not been directly measured, but instead inferred from Pioneer Venus measurements of gases by the mass spectrometer [69]. The gases evolved from cloud particles that clogged the inlet and are consistent with cloud droplets composed of 15% w/w H2O and 85% w/w H2SO4.3 Based on models, it is likely that the sulfuric acid concentration of the cloud particles varies with altitude, reaching 79% w/w in the cloud tops and 98% w/w [70] in the lower clouds.
It has been shown that the majority of amino acids are stable in the range of Venus’ cloud sulfuric acid concentrations (81% and 98% w/w, the rest being water) [71,72,73]. Another step towards true biochemistry in this aggressive solvent is the observation of stability of lipid membranes [74]. Finally, some dipeptides, which are precursors to larger peptides and proteins, could also be stable at both sulfuric acid concentrations for many months, if not longer [75,76]. The stability of nucleic acid bases and lipids in concentrated sulfuric acid advances the idea that chemistry to support life may exist in the Venus cloud particle environment.
In closing, we note that our estimate of the Stokes fall-out times required for droplets of sulfuric acid to reach the lower haze layers of the atmosphere remains well grounded, because the density of sulfuric acid, , is only a factor of two larger than that of water. However, whether organic chemistry in concentrated sulfuric acid would allow the bacterial replication time to be shorter than the microbe’s lifetime remains an open question.
6. Conclusions
The potential detection of of PH3 absorption lines in Venus’ atmosphere, a gas often associated with biological processes on Earth, has sparked excitement and renewed interest in the possibility of life beyond Earth. At present, the scientific community is divided into those who support the detection of phosphine [4,9,10,11] and those who question the measurements and the presence of PH3 [5,6,7,8]. Certainly, more data are needed to shed light on this conundrum.
From the perspective of terrestrial chemistry, phosphine is considered a biogenic molecule, typically produced by living organisms. Possible pathways for PH3 production in a Venusian environment were investigated, with the conclusion that the observed abundance of PH3 cannot be explained by conventional gas-phase reactions, surface and subsurface geochemical reactions, photochemistry, and other non-equilibrium processes [14,17]. The observed PH3 absorption lines must then originate in some unknown geochemistry/photochemistry process. An even more extreme possibility is that a strictly aerial microbial biosphere is responsible for the observed PH3 signal.
Even though the detection of a PH3 signal is yet under debate, it is interesting to entertain the possibility that it could be the footprint of aerial microbial life. A proposed Venusian life cycle suggests that microbial life could exist within the lower cloud layers, cycling between a dormant, spore-like state in the haze and an active, metabolically active state within cloud droplets [15]. We re-examined whether one can actually envisage life inside the clouds of Venus, operating entirely on known terrestrial principles. We showed that for aerosols, the Stokes fallout times to reach the lower haze atmospheric layers is pronouncedly larger than the typical bacterium replication time on Earth. Bearing this in mind, we can conclude that if updraughts exist, a stable population of microorganisms that in the early history of Venus emigrated from the surface to the atmospheric clouds and now remain confined to aerosols may be possible. However, as noted in [24], caution must be taken. If Venus was once habitable, we could forecast a water-rich interior of the planet, which can be probed inspecting its volcano activity. Now, the gases that Venus’ volcanoes are releasing about 6% water, whereas the expectation from Earth-like volcanism in Venus would lead to about 96% of water. Thus, the dryness we observe today in the interior of Venus seems to be inconsistent with the planet ever having oceans. Leaving aside our working hypothesis, we can argue that if Venus has always been a hellish hot planet, then organic chemistry developing in concentrated sulfuric acid may be an alternative. Whether it is Earth-like or not, an aerial microbial life may be awaiting for the Venus Life Finder (VLF) Missions to arrive [77].
Author Contributions
All authors contributed equally to the conceptualization, investigation, and writing of this paper. All authors have read and agreed to the published version of the manuscript.
Funding
The research of L.A.A. is supported by the U.S. National Science Foundation (NSF grant PHY-2412679).
Data Availability Statement
No new data were generated for the paper.
Acknowledgments
We thank William Bains and Janusz Petkowski for permission to reproduce Figure 1.
Conflicts of Interest
The authors declare no conflicts of interest.
Notes
| 1 | In mass spectrometry, represents the ratio of an ion’s mass (m) to its charge (z). |
| 2 | In the spirit of [15], throughout we use the term “spore” to indicate a cell in a dormant state of long-term metabolic inactivity, which is further resistant to (and protected from) environmental stresses. |
| 3 | The weight concentration of a solution is expressed as w/w, which stands for weight-per-weight. The volume of each chemical is disregarded and only the weight counts. |
References
- Sagan, C. The planet Venus. Science 1961, 133, 849. [Google Scholar] [CrossRef]
- Sagan, C. Life on the surface of Venus? Nature 1967, 216, 1198. [Google Scholar] [CrossRef]
- Morowitz, H.; Sagan, C. Life in the clouds of Venus? Nature 1967, 215, 1259. [Google Scholar] [CrossRef]
- Greaves, J.S.; Richards, A.M.S.; Bains, W.; Rimmer, P.B.; Sagawa, H.; Clements, D.L.; Seager, S.; Petkowski, J.J.; Sousa-Silva, C.; Ranjan, S.; et al. Phosphine gas in the cloud decks of Venus. Nat. Astron. 2020, 5, 665. [Google Scholar] [CrossRef]
- Villanueva, G.L.; Cordiner, M.; Irwin, P.; de Pater, I.; Butler, B.; Gurwell, M.; Milam, S.; Nixon, C.; Luszcz-Cook, S.; Wilson, C.; et al. No evidence of phosphine in the atmosphere of Venus from independent analyses. Nat. Astron. 2021, 5, 631–635. [Google Scholar] [CrossRef]
- Thompson, M.A. The statistical reliability of 267-GHz JCMT observations of Venus: No significant evidence for phosphine abibitebsorption. Mon. Not. R. Astron. Soc. 2021, 501, L18. [Google Scholar] [CrossRef]
- Akins, A.B.; Lincowski, A.P.; Meadows, V.S.; Steffes, P.G. Complications in the ALMA detection of phosphine at Venus. As-trophys. J. Lett. 2021, 907, L27. [Google Scholar] [CrossRef]
- Lincowski, A.P.; Meadows, V.S.; Crisp, D.; Akins, A.B.; Schwieterman, E.W.; Arney, G.N.; Wong, M.L.; Steffes, P.G.; Parenteau, M.N.; Domagal-Goldman, S. Claimed detection of PH3 in the clouds of Venus is consistent with mesospheric SO2. Astrophys. J. Lett. 2021, 908, L44. [Google Scholar] [CrossRef]
- Greaves, J.S.; Richards, A.M.; Bains, W.; Rimmer, P.B.; Sagawa, H.; Clements, D.L.; Seager, S.; Petkowski, J.J.; Sousa-Silva, C.; Ranjan, S.; et al. Adendum: Phosphine gas in the cloud decks of Venus. Nat. Astron. 2021, 5, 726. [Google Scholar] [CrossRef]
- Greaves, J.S.; Richards, A.M.; Bains, W.; Rimmer, P.B.; Clements, D.L.; Seager, S.; Petkowski, J.J.; Sousa-Silva, C.; Ranjan, S.; Fraser, H.J. Reply to: No evidence of phosphine in the atmosphere of Venus from independent analyses. Nat. Astron. 2021, 5, 636–639. [Google Scholar] [CrossRef]
- Greaves, J.S.; Rimmer, P.B.; Richards, A.M.; Petkowski, J.J.; Bains, W.; Ranjan, S.; Seager, S.; Clements, D.L.; Silva, C.S.; Fraser, H.J. Low levels of sulphur dioxide contamination of venusian phosphine spectra. Mon. Not. R. Astron. Soc. 2022, 514, 2994. [Google Scholar] [CrossRef]
- Mogul, R.; Limaye, S.S.; Way, M.J.; Cordova, J.A. Venus’ mass spectra show signs of disequilibria in the middle clouds. Geophys. Res. Lett. 2021, 48, e91327. [Google Scholar] [CrossRef]
- Sousa-Silva, C.; Seager, S.; Ranjan, S.; Petkowski, J.J.; Zhan, Z.; Hu, R.; Bains, W. Phosphine as a Biosignature Gas in Exoplanet Atmospheres. Astrobiology 2020, 20, 235. [Google Scholar] [CrossRef]
- Bains, W.; Petkowski, J.J.; Seager, S.; Ranjan, S.; Sousa-Silva, C.; Rimmer, P.B.; Zhan, Z.; Greaves, J.S.; Richards, A.M.S. Phosphine on Venus cannot be explained by conventional processes. Astrobilogy 2021, 21, 1277. [Google Scholar] [CrossRef]
- Seager, S.; Petkowski, J.J.; Gao, P.; Bains, W.; Bryan, N.C.; Ranjan, S.; Greaves, J. The venusian lower atmosphere haze as a depot for desiccated microbial life: A propose cycle persistence of the venusian aerial biosphere. Astrobiology 2021, 21, 1206. [Google Scholar] [CrossRef]
- Clements, D.L. Venus, Phosphine and the Possibility of Life. Contemp. Phys. 2023, 63, 180. [Google Scholar] [CrossRef]
- Bains, W.; Seager, S.; Clements, D.L.; Greaves, J.S.; Rimmer, P.B.; Petkowski, J.J. Source of phosphine on Venus—An unsolved problem. Front. Astron. Space Sci. 2024, 11, 1372057. [Google Scholar] [CrossRef]
- Anchordoqui, L.A.; Chudnovsky, E.M. A physicist view of COVID-19 airborne infection through convective airflow in indoor spaces. SciMed. J. 2020, 2, 68. [Google Scholar] [CrossRef]
- Marov, M.; Grinspoon, D. The Planet Venus; Yale University Press: New Haven, CT, USA, 1998. [Google Scholar]
- Ignatiev, N.; Titov, D.V.; Piccioni, G.; Drossart, P.; Markiewicz, W.J.; Cottini, V.; Roatsch, T.; Almeida, M.; Manoel, N. Altimetry of the Venus cloud tops from the Venus Express observations. J. Geophys. Res. 2009, 114, E00B43. [Google Scholar] [CrossRef]
- Knollenberg, R.; Hunten, D. The microphysics of the clouds of Venus: Results of the Pioneer Venus particle size spectrometer experiment. J. Geophys. Res. 1980, 85, 8039. [Google Scholar] [CrossRef]
- Grinspoon, D.H. Venus Revealed: A New Look Below the Clouds of Our Mysterious Twin Planet; Perseus Publishing: Cambridge, MA, USA, 1987. [Google Scholar]
- Schulze-Makuch, D.; Irwin, L.N.; Faire’n, A.G. Drastic environmental change and its effects on a planetary biosphere. Icarus 2013, 225, 775. [Google Scholar] [CrossRef]
- Constantinou, T.; Shorttle, O.; Rimmer, P.B. A dry Venusian interior constrained by atmospheric chemistry. Nat. Astron. 2025, 9, 189. [Google Scholar] [CrossRef]
- Womack, A.; Bohannan, B.; Green, J. Biodiversity and biogeography of the atmosphere. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3645. [Google Scholar] [CrossRef]
- Sattler, B.; Puxbaum, H.; Psenner, R. Bacterial growth in supercooled cloud droplets. Geophys. Res. Lett. 2001, 28, 239. [Google Scholar] [CrossRef]
- Kerzhanovich, V.V.; Marov, M.I. The Atmospheric Dynamics of Venus According to Doppler Measurements by the Venera Entry Probes Venus; University of Arizona Press: Tucson, AZ, USA, 1983; p. 766. [Google Scholar]
- Chudnovsky, E.M. Thermodynamics of natural selection. J. Stat. Phys. 1985, 41, 877. [Google Scholar] [CrossRef]
- England, J.L. Statistical physics of self-replication. J. Chem. Phys. 2013, 139, 121923. [Google Scholar] [CrossRef]
- Gibson, B.; Wilson, D.J.; Fell, E.; Eyre-Walker, A. The distribution of bacterial doubling time in the wild. Proc. R. Soc. B 2018, 285, 20180789. [Google Scholar] [CrossRef]
- Eagon, R.G. Pseudomonas natriegens, a marine bacterium with a generation time of less than 10 minutes. J. Bacteriol. 1962, 83, 736. [Google Scholar] [CrossRef]
- Maida, I.; Bosi, E.; Perrin, E.; Papaleo, M.C.; Orlandini, V.; Fondi, M.; Fani, R.; Wiegel, J.; Bianconi, G.; Canganella, F. Draft genome sequence of the fast-growing Bacterium Vibrio Natriegens Strain DSMZ 759. Genome Announc. 2013, 1, e00648-13. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Ostrov, N.; Wong, B.G.; Gold, M.A.; Khalil, A.S.; Church, G.M. Functional genomics of the rapidly replicating bacte-rium Vibrio natriegens by CRISPRi. Nat. Microbiol. 2019, 4, 1105. [Google Scholar] [CrossRef] [PubMed]
- Errington, J.; Aart, L.T.V. Microbe Profile: Bacillus subtilis: Model organism for cellular development, and industrial workhorse. Microbiology 2020, 166, 425. [Google Scholar] [CrossRef]
- LaBauve, A.E.; Wargo, M.J. Growth and laboratory maintenance of Pseudomonas aeruginosa. Curr. Protoc. Microbiol. 2010, 6. [Google Scholar] [CrossRef]
- Das, S.; Chatterjee, A.; Datta, P.P. Knockdown Experiment Reveals an Essential GTPase CgtA’s Involvement in Growth, Viability, Motility, Morphology, and Persister Phenotypes in Vibrio cholerae. Microbiol. Spectr. 2023, 11, e03181-22. [Google Scholar] [CrossRef]
- Choi, H.J.; Kang, S.J.; Hong, K.W. Comparison of NheA toxin production and doubling time between Bacillus cereus and Bacillus thuringiensis. Appl. Biol. Chem. 2017, 60, 545. [Google Scholar] [CrossRef]
- Jennison, A.V.; Verma, N.K. Shigella flexneri infection: Pathogenesis and vaccine development. FEMS Microbiol. Rev. 2004, 28, 43. [Google Scholar] [CrossRef]
- Gera, K.; McIver, K.S. Laboratory growth and maintenance of Streptococcus pyogenes (the Group A Streptococcus, GAS). Curr. Protoc. Microbiol. 2013, 30, 9D.2.1–9D.2.13. [Google Scholar] [CrossRef] [PubMed]
- Lowrie, D.B.; Aber, V.R.; Carrol, M.E. Division and death rates of Salmonella typhimurium inside macrophages: Use of penicillin as a probe. J. Gen. Microbiol. 1979, 110, 409–419. [Google Scholar] [CrossRef]
- Li, J.; Paredes-Sabja, D.; Sarker, M.R.; Mc-Clane, B.A. Clostridium perfringens Sporulation and Sporulation-Associated Toxin Production. Microbiol. Spectr. 2016, 4, TBS-0022-2015. [Google Scholar] [CrossRef]
- Caldwell, D.E.; Lawrence, J.R. Growth Kinetics of Pseudomonas Fluorescens Microcolonies within the Hydrodynamic Boundary Layers of Surface Microenvironments. Microb. Ecol. 1986, 12, 299. [Google Scholar] [CrossRef]
- Pang, T.Y.; Lercher, M.J. Optimal density of bacterial cells. PLoS Comput. Biol. 2023, 19, e1011177. [Google Scholar] [CrossRef]
- Einarsson, J.; Mehlig, B. Spherical particle sedimenting in weakly viscoelastic shear flow. Phys. Rev. Fluids 2017, 2, 063301. [Google Scholar] [CrossRef]
- Beck, A.J.; Schiffer, R.A. Models of Venus Atmosphere; NASA Technical Report, Document ID: 19690011554; Report/Patent: NASA-SP-8011, Accession Number: 69N20916; NASA: Washington, DC, USA, 1968. [Google Scholar]
- Anderson, J.D.; Pease, G.E.; Efron, L.; Tausworthe, R.C. Celestial Mechanics Experiment. Science 1967, 158, 1689. [Google Scholar] [CrossRef]
- Ash, A.; Shapiro, I.; Smith, W. Astronomical Constants and Planetary Ephemerides Deduced from Radar and Optical Observa-tions. Astron. J. 1967, 72, 338. [Google Scholar] [CrossRef]
- Ash, M.E.; Campbell, D.B.; Dyce, R.B.; Ingalls, R.P.; Jurgens, R.; Pettengill, G.H.; Shapiro, I.I.; Slade, M.A.; Thompson, T.W. The Case for the Radar Radius of Venus. Science 1968, 160, 985. [Google Scholar] [CrossRef]
- Anderson, J.D.; Cain, D.L.; Efron, L.; Goldstein, R.M.; Melbourne, W.G.; O’Handley, D.A.; Pease, G.E.; Tausworth, R.C. The Radius of Venus as Determined by Planetary Radar and Mariner V Radio Tracking Data. J. Atmos. Sci. 1968, 25, 1171. [Google Scholar] [CrossRef]
- Joly, J. Cosmic radiation and evolution. Nature 1929, 123, 981. [Google Scholar] [CrossRef]
- Dartnell, L.R. Ionizing radiation and life. Astrobiology 2011, 11, 551. [Google Scholar] [CrossRef]
- Phillips, J.L.; Russell, C.T. Upper limit on the intrinsic magnetic field of Venus. J. Geophys. Res. 1987, 92, 2253. [Google Scholar] [CrossRef]
- Russell, C.T.; Elphic, R.C.; Slavin, J.A. Limits on the possible intrinsic magnetic field of Venus. J. Geophys. Res. 1980, 85, 8319. [Google Scholar] [CrossRef]
- Zhang, T.L.; Delva, M.; Baumjohann, W.; Auster, H.-U.; Carr, C.; Russell, C.T.; Barabash, S.; Balikhin, M.; Kudela, K.; Berghofer, G.; et al. Little or no solar wind enters Venus’ atmosphere at solar minimum. Nature 2007, 450, 654. [Google Scholar] [CrossRef]
- Russell, C.T.; Luhmann, J.G.; Strangeway, R.J. The solar wind interaction with Venus through the eyes of the Pioneer Venus Orbiter. Planet. Space Sci. 2006, 54, 1482. [Google Scholar] [CrossRef]
- Anchordoqui, L.A. Ultra-high-energy cosmic rays. Phys. Rep. 2019, 801, 1. [Google Scholar] [CrossRef]
- Dartnell, L.R.; Nordheim, T.A.; Patel, M.R.; Mason, J.P.; Coates, A.J.; Jones, G.H. Constraints on a potential aerial biosphere on Venus I: Cosmic rays. Icarus 2015, 257, 396. [Google Scholar] [CrossRef]
- Herbst, K.; Banjac, S.; Atri, D.; Nordheim, T.A. Revisiting the cosmic-ray induced Venusian radiation dose in the context of habitability. Astron. Astrophys. 2020, 633, A15. [Google Scholar] [CrossRef]
- Anchordoqui, L.A.; Garcia Canal, C.A.; Sciutto, S.J. The Venusian Chronicles. In Proceedings of the 7th International Symposium on Ultra High Energy Cosmic Rays (UHECR2024), Malargüe, Argentina, 17–21 November 2024. [Google Scholar] [CrossRef]
- Lingam, M.; Loeb, A. Subsurface exolife. Int. J. Astrobiol. 2019, 18, 112. [Google Scholar] [CrossRef]
- Anchordoqui, L.A.; Weber, S.M. Upper limit on the fraction of alien civilizations that develop communication technology. arXiv 2019, arXiv:1908.01335. [Google Scholar]
- Hoehler, T.M.; Bains, W.; Davila, A.; Parenteau, M.N.; Pohorille, A. Life’s requirements, habitability, and biological potential. In Planetary Astrobiology; Meadows, V.S., Arney, G.N., Schmidt, B.E., Marais, D.J.D., Eds.; Space Science Series; University of Arizona Press: Tucson, AZ, USA, 2020; pp. 37–69. ISBN 978-0-8265-4006-8. [Google Scholar] [CrossRef]
- Anchordoqui, L.A.; Chudnovsky, E.M. Can self-replicating species flourish in the interior of a star? LHEP 2020, 2020, 166. [Google Scholar] [CrossRef]
- Bains, W.; Petkowski, J.J.; Seager, S. A data resource for sulfuric acid reactivity of organic chemicals. Data 2021, 6, 24. [Google Scholar] [CrossRef]
- Bains, W.; Petkowski, J.J.; Zhan, Z.; Seager, S. Evaluating alternatives to water as solvents for life: The example of sulfuric acid. Life 2021, 11, 400. [Google Scholar] [CrossRef]
- Braconnot, H.M. Sur la conversion des matie’res animales ennouvelles substances par le moyen de l’acide sulfurique. Ann. Chim. Phys. Ser. 1820, 2, 113. [Google Scholar]
- Reitz, H.C.; Ferrel, R.E.; Fraenkel-Conrat, H.; Olcott, H.S. Action of sulfating agents on proteins and model substances I: Con-centrated sulfuric acid. J. Am. Chem. Soc. 1946, 68, 1024. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, L.K.; McConnell, W.B. The Action of Sulphuric Acid on Gliadin: With special reference to the N-peptidyl → O-peptidyl Bond Rearrangement. Can. J. Chem. 1955, 33, 1638. [Google Scholar] [CrossRef]
- Hoffman, J.H.; Oyama, V.I.; Von Zahn, U. Measurements of the Venus lower atmosphere composition: A comparison of results. J. Geophys. Res. Space Phys. 1980, 85, 7871. [Google Scholar] [CrossRef]
- Krasnopolsky, V.A. Vertical profiles of H2O, H2SO4, and sulfuric acid concentration at 45–75 km on Venus. Icarus 2015, 252, 327. [Google Scholar] [CrossRef]
- Seager, S.; Petkowski, J.J.; Seager, M.D.; Grimes, J.H., Jr.; Zinsli, Z.; Vollmer-Snarr, H.R.; El-Rahman, M.K.A.; Wishart, D.S.; Lee, B.L.; Gautam, V.; et al. Stability of nucleic acid bases in concentrated sulfuric acid: Implications for the habitability of Venus’ clouds. Proc. Natl. Acad. Sci. USA 2023, 120, e2220007120. [Google Scholar] [CrossRef]
- Seager, M.D.; Seager, S.; Bains, W.; Petkowski, J.J. Stability of 20 biogenic amino acids in concentrated sulfuric acid: Implications for the habitability of Venus’ clouds. Astrobiology 2024, 24, 386–396. [Google Scholar] [CrossRef]
- Seager, S.; Petkowski, J.J.; Seager, M.D.; Grimes, J.H., Jr.; Zinsli, Z.; Vollmer-Snarr, H.R.; El-Rahman, M.K.A.; Wishart, D.S.; Lee, B.L.; Gautam, V.; et al. Year-Long Stability of Nucleic Acid Bases in Concentrated Sulfuric Acid: Implications for the Persistence of Organic Chemistry in Venus’ Clouds. Life 2024, 14, 538. [Google Scholar] [CrossRef]
- Duzdevich, D.; Nisler, C.; Petkowski, J.J.; Bains, W.; Kaminsky, C.K.; Szostak, J.W.; Seager, S. Simple lipids form stable high-er-order structures in concentrated sulfuric acid. Astrobiology 2025, 25, 270–283. [Google Scholar] [CrossRef]
- Petkowski, J.J.; Seager, M.D.; Bains, W.; Seager, S. General instability of dipeptides in concentrated sulfuric acid as relevant for the Venus cloud habitability. Sci. Rep. 2024, 14, 17083. [Google Scholar] [CrossRef]
- Petkowski, J.J.; Seager, M.D.; Bains, W.; Grimes, J.H., Jr.; Seager, S. Mechanism for Peptide Bond Solvolysis in 98% w/w Con-centrated Sulfuric Acid. Am. Chem. Soc. Omega 2025, 10, 9623. [Google Scholar] [CrossRef]
- Seager, S.; Petkowski, J.J.; Carr, C.E.; Grinspoon, D.; Ehlmann, B.; Saikia, S.J.; Agrawal, R.; Buchanan, W.; Weber, M.U.; French, R.; et al. Venus Life Finder Mission Study, A suit of mission concepts to explore Venus to study habitability and potentially find life. arXiv 2021, arXiv:2112.05153. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).