Impacts of Sex Differences in Pulse Pressure among Patients with Chronic Kidney Disease
Abstract
1. Introduction
2. Materials and Methods
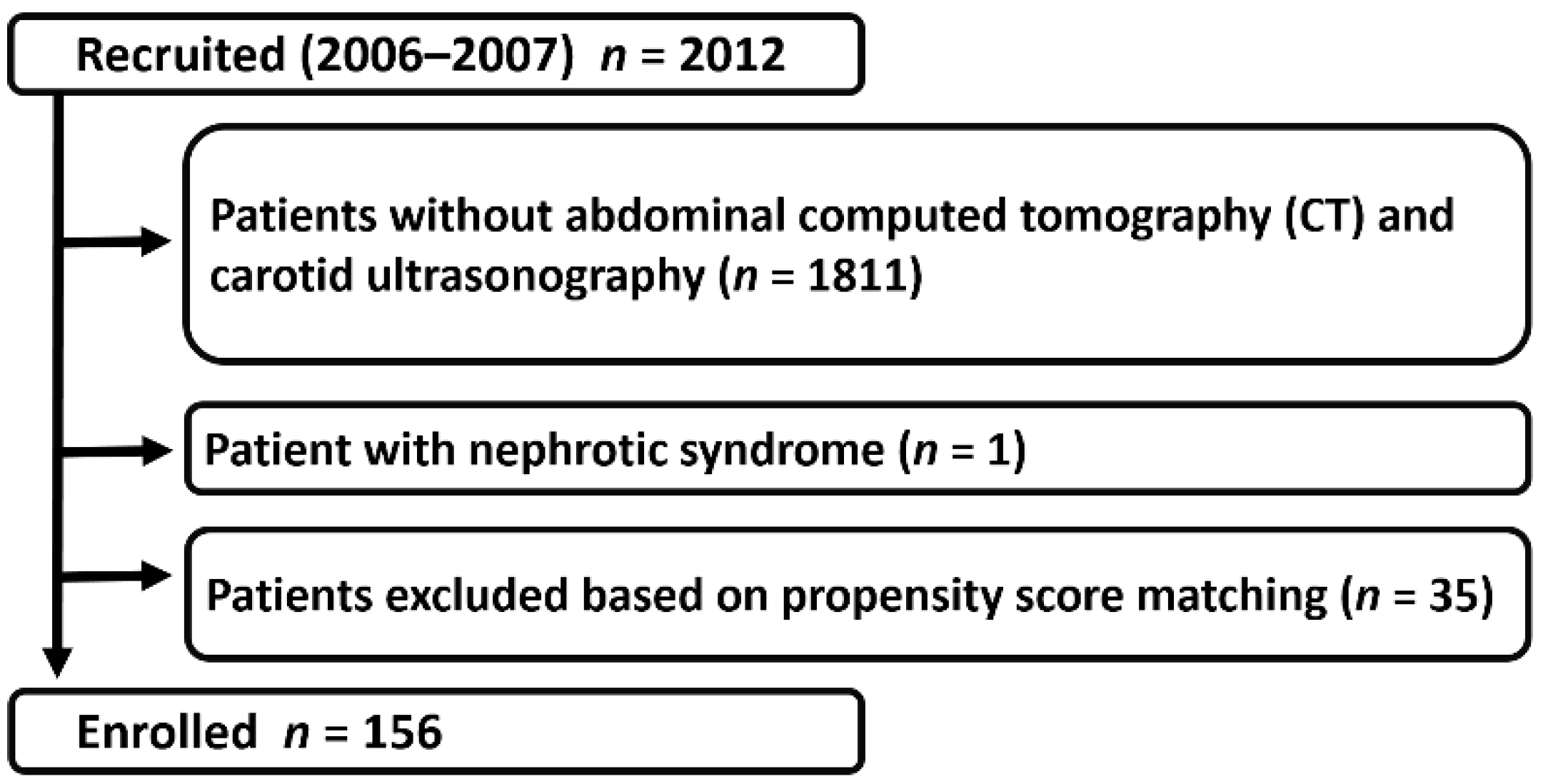
2.1. Study Population
2.2. Covariate Assessments
2.3. Definitions of the Comorbidities and Primary Causes of Chronic Kidney Disease
2.4. Study End Point
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Pulse Pressure as a Progression-Related Factor in Patients with Chronic Kidney Disease
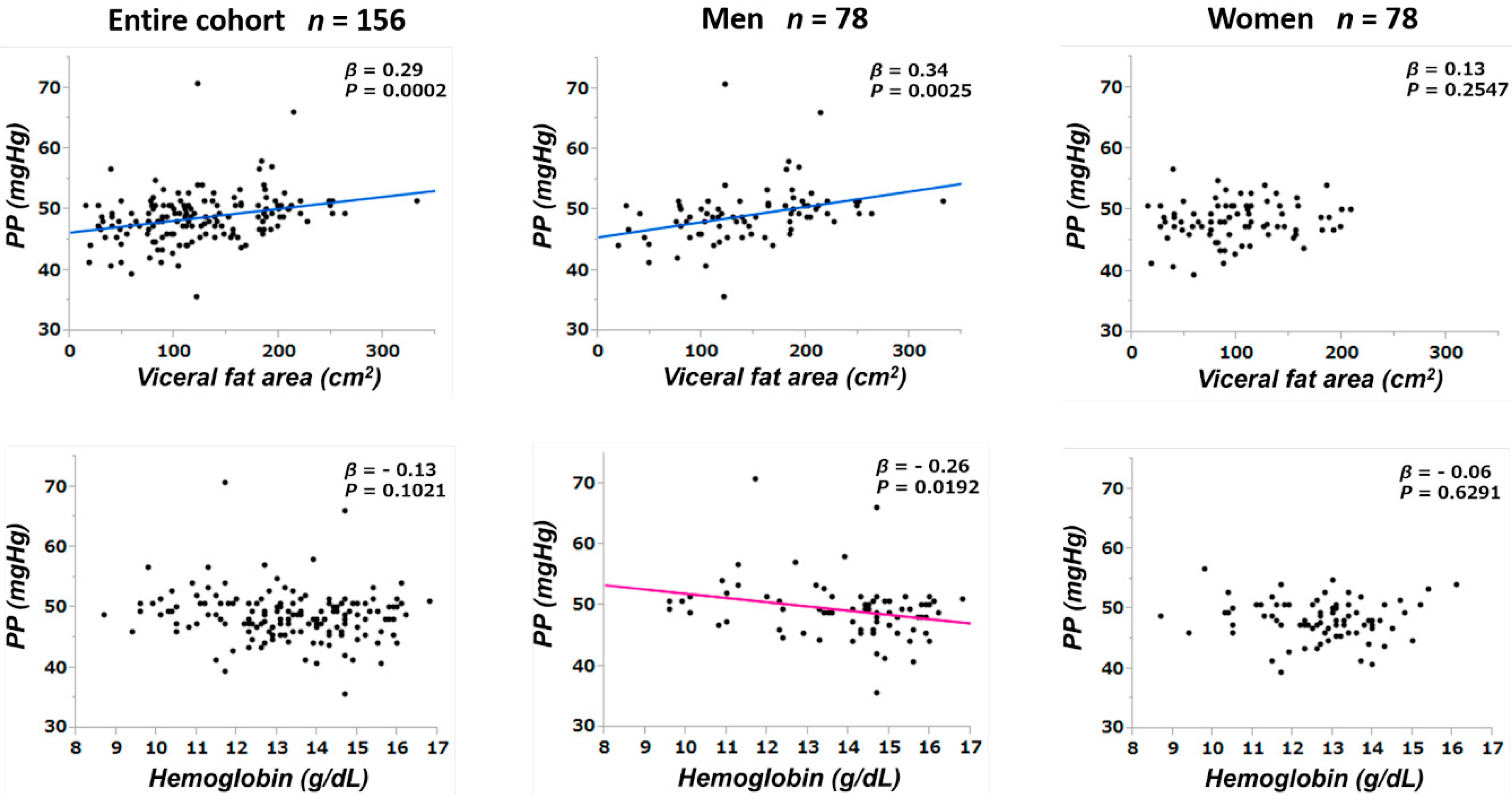
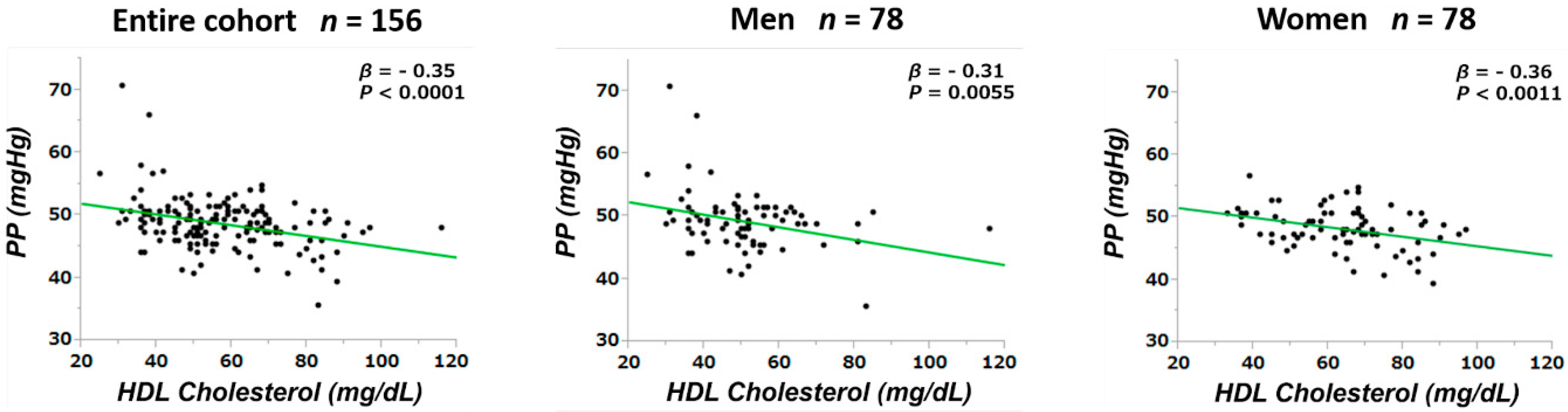
3.3. Correlations between Pulse Pressure and Other Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Statement of Ethics
References
- Klag, M.J.; Whelton, P.K.; Randall, B.L.; Neaton, J.D.; Brancati, F.L.; Ford, C.E.; Shulman, N.B.; Stamler, J. Blood pressure and end-stage renal disease in men. N. Engl. J. Med. 1996, 334, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Klahr, S.; Schreiner, G.; Ichikawa, I. The progression of renal disease. N. Engl. J. Med. 1988, 318, 1657–1666. [Google Scholar] [CrossRef]
- Parving, H.H.; Andersen, A.R.; Smidt, U.M.; Svendsen, P.A. Early aggressive antihypertensive treatment reduces rate of decline in kidney function in diabetic nephropathy. Lancet 1983, 1, 1175–1179. [Google Scholar] [CrossRef]
- Ruggenenti, P.; Perna, A.; Loriga, G.; Ganeva, M.; Ene-Iordache, B.; Turturro, M.; Lesti, M.; Perticucci, E.; Chakarski, I.N.; Leonardis, D.; et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (rein-2): Multicentre, randomised controlled trial. Lancet 2005, 365, 939–946. [Google Scholar] [CrossRef]
- Upadhyay, A.; Earley, A.; Haynes, S.M.; Uhlig, K. Systematic review: Blood pressure target in chronic kidney disease and proteinuria as an effect modifier. Ann. Intern. Med. 2011, 154, 541–548. [Google Scholar] [CrossRef]
- Appel, L.J.; Wright, J.T., Jr.; Greene, T.; Agodoa, L.Y.; Astor, B.C.; Bakris, G.L.; Cleveland, W.H.; Charleston, J.; Contreras, G.; Faulkner, M.L.; et al. Intensive blood-pressure control in hypertensive chronic kidney disease. N. Engl. J. Med. 2010, 363, 918–929. [Google Scholar] [CrossRef]
- Boutouyrie, P.; Bussy, C.; Lacolley, P.; Girerd, X.; Laloux, B.; Laurent, S. Association between local pulse pressure, mean blood pressure, and large-artery remodeling. Circulation 1999, 100, 1387–1393. [Google Scholar] [CrossRef]
- Madero, M.; Peralta, C.; Katz, R.; Canada, R.; Fried, L.; Najjar, S.; Shlipak, M.; Simonsick, E.; Lakatta, E.; Patel, K.; et al. Association of arterial rigidity with incident kidney disease and kidney function decline: The Health ABC study. Clin. J. Am. Soc. Nephrol. 2013, 8, 424–433. [Google Scholar] [CrossRef]
- Briet, M.; Boutouyrie, P.; Laurent, S.; London, G.M. Arterial stiffness and pulse pressure in CKD and ESRD. Kidney Int. 2012, 82, 388–400. [Google Scholar] [CrossRef]
- Banerjee, D.; Brincat, S.; Gregson, H.; Contreras, G.; Streather, C.; Oliveira, D.; Nelson, S. Pulse pressure and inhibition of renin-angiotensin system in chronic kidney disease. Nephrol. Dial. Transplant. 2006, 21, 975–978. [Google Scholar] [CrossRef][Green Version]
- Sedaghat, S.; Mattace-Raso, F.U.; Hoorn, E.J.; Uitterlinden, A.G.; Hofman, A.; Ikram, M.A.; Franco, O.H.; Dehghan, A. Arterial stiffness and decline in kidney function. Clin. J. Am. Soc. Nephrol. 2015, 10, 2190–2197. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, M.F.; Safar, M.E. Relationship between aortic stiffening and microvascular disease in brain and kidney: Cause and logic of therapy. Hypertension 2005, 46, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Peralta, C.A.; Jacobs, D.R., Jr.; Katz, R.; Ix, J.H.; Madero, M.; Duprez, D.A.; Sarnak, M.J.; Criqui, M.H.; Kramer, H.J.; Palmas, W.; et al. Association of pulse pressure, arterial elasticity, and endothelial function with kidney function decline among adults with estimated GFR >60 ml/min/1.73 m(2): The multi-ethnic study of atherosclerosis (mesa). Am. J. Kidney Dis. 2012, 59, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J. Gender differences in chronic kidney disease: Underpinnings and therapeutic implications. Kidney Blood Press. Res. 2010, 33, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Coggins, C.H.; Breyer Lewis, J.; Caggiula, A.W.; Castaldo, L.S.; Klahr, S.; Wang, S.R. Differences between women and men with chronic renal disease. Nephrol. Dial. Transplant. 1998, 13, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.C.; Wu, H.Y.; Peng, Y.S.; Ko, M.J.; Wu, M.S.; Hung, K.Y.; Wu, K.D.; Chu, T.S.; Chien, K.L. Risk factors for development and progression of chronic kidney disease: A systematic review and exploratory meta-analysis. Medicine 2016, 95, e3013. [Google Scholar] [CrossRef] [PubMed]
- de Hauteclocque, A.; Ragot, S.; Slaoui, Y.; Gand, E.; Miot, A.; Sosner, P.; Halimi, J.M.; Zaoui, P.; Rigalleau, V.; Roussel, R.; et al. The influence of sex on renal function decline in people with type 2 diabetes. Diabet. Med. 2014, 31, 1121–1128. [Google Scholar] [CrossRef]
- Fernandez-Prado, R.; Fernandez-Fernandez, B.; Ortiz, A. Women and renal replacement therapy in Europe: Lower incidence, equal access to transplantation, longer survival than men. Clin. Kidney J. 2018, 11, 1–6. [Google Scholar] [CrossRef]
- Iseki, K.; Nakai, S.; Shinzato, T.; Nagura, Y.; Akiba, T. Increasing gender difference in the incidence of chronic dialysis therapy in Japan. Ther. Apher. Dial. 2005, 9, 407–411. [Google Scholar] [CrossRef]
- Mitchell, G.F.; Gudnason, V.; Launer, L.J.; Aspelund, T.; Harris, T.B. Hemodynamics of increased pulse pressure in older women in the community-based age, gene/environment susceptibility-reykjavik study. Hypertension 2008, 51, 1123–1128. [Google Scholar] [CrossRef]
- Skurnick, J.H.; Aladjem, M.; Aviv, A. Sex differences in pulse pressure trends with age are cross-cultural. Hypertension 2010, 55, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.S.; Gustin, W.T.; Wong, N.D.; Larson, M.G.; Weber, M.A.; Kannel, W.B.; Levy, D. Hemodynamic patterns of age-related changes in blood pressure. The framingham heart study. Circulation 1997, 96, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; de Jong, P.E.; Coresh, J.; El Nahas, M.; Astor, B.C.; Matsushita, K.; Gansevoort, R.T.; Kasiske, B.L.; Eckardt, K.U. The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int. 2011, 80, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Spooner, R.J.; Weir, R.J.; Frier, B.M. Detection of microalbuminuria in diabetic patients using a simple latex agglutination test. Clin. Chim. Acta 1987, 166, 247–253. [Google Scholar] [CrossRef]
- Matsushita, K.; Chen, J.; Sang, Y.; Ballew, S.H.; Shimazaki, R.; Fukagawa, M.; Imai, E.; Coresh, J.; Hishida, A. Risk of end-stage renal disease in Japanese patients with chronic kidney disease increases proportionately to decline in estimated glomerular filtration rate. Kidney Int. 2016, 90, 1109–1114. [Google Scholar] [CrossRef]
- van Dijk, P.C.; Zwinderman, A.H.; Dekker, F.W.; Schon, S.; Stel, V.S.; Finne, P.; Jager, K.J. Effect of general population mortality on the north-south mortality gradient in patients on replacement therapy in Europe. Kidney Int. 2007, 71, 53–59. [Google Scholar] [CrossRef][Green Version]
- Duru, O.K.; Li, S.; Jurkovitz, C.; Bakris, G.; Brown, W.; Chen, S.C.; Collins, A.; Klag, M.; McCullough, P.A.; McGill, J.; et al. Race and sex differences in hypertension control in CKD: Results from the kidney early evaluation program (KEEP). Am. J. Kidney Dis. 2008, 51, 192–198. [Google Scholar] [CrossRef]
- Muiesan, M.L.; Ambrosioni, E.; Costa, F.V.; Leonetti, G.; Pessina, A.C.; Salvetti, M.; Trimarco, B.; Volpe, M.; Pontremoli, R.; Deferrari, G.; et al. Sex differences in hypertension-related renal and cardiovascular diseases in italy: The i-demand study. J. Hypertens. 2012, 30, 2378–2386. [Google Scholar] [CrossRef]
- Gill, J.S.; Hendren, E.; Dong, J.; Johnston, O.; Gill, J. Differential association of body mass index with access to kidney transplantation in men and women. Clin. J. Am. Soc. Nephrol. 2014, 9, 951–959. [Google Scholar] [CrossRef]
- Ogawa, T.; Nitta, K. Pathogenesis and management of vascular calcification in patients with end-stage renal disease. Contrib. Nephrol. 2018, 196, 71–77. [Google Scholar] [PubMed]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-related mechanisms in chronic kidney disease prediction, progression, and outcome. J. Immunol. Res. 2018, 2018, 2180373. [Google Scholar] [CrossRef] [PubMed]
- Viazzi, F.; Leoncini, G.; Derchi, L.E.; Pontremoli, R. Ultrasound doppler renal resistive index: A useful tool for the management of the hypertensive patient. J. Hypertens. 2014, 32, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Doublier, S.; Lupia, E.; Catanuto, P.; Elliot, S.J. Estrogens and progression of diabetic kidney damage. Curr. Diabetes Rev. 2011, 7, 28–34. [Google Scholar] [CrossRef]
- Mule, G.; Nardi, E.; Cottone, S.; Cusimano, P.; Incalcaterra, F.; Palermo, A.; Giandalia, M.E.; Mezzatesta, G.; Andronico, G.; Cerasola, G. Relationship of metabolic syndrome with pulse pressure in patients with essential hypertension. Am. J. Hypertens. 2007, 20, 197–203. [Google Scholar] [CrossRef]
- Wykretowicz, A.; Rutkowska, A.; Krauze, T.; Przymuszala, D.; Guzik, P.; Marciniak, R.; Wysocki, H. Pulse pressure amplification in relation to body fatness. Br. J. Clin. Pharmacol. 2012, 73, 546–552. [Google Scholar] [CrossRef][Green Version]
- Protogerou, A.D.; Blacher, J.; Mavrikakis, M.; Lekakis, J.; Safar, M.E. Increased pulse pressure amplification in treated hypertensive subjects with metabolic syndrome. Am. J. Hypertens. 2007, 20, 127–133. [Google Scholar] [CrossRef][Green Version]
- Pond, C.M. An evolutionary and functional view of mammalian adipose tissue. Proc. Nutr. Soc. 1992, 51, 367–377. [Google Scholar] [CrossRef]
- Fox, C.S.; Massaro, J.M.; Hoffmann, U.; Pou, K.M.; Maurovich-Horvat, P.; Liu, C.Y.; Vasan, R.S.; Murabito, J.M.; Meigs, J.B.; Cupples, L.A.; et al. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the framingham heart study. Circulation 2007, 116, 39–48. [Google Scholar] [CrossRef]
- Liu, J.; Fox, C.S.; Hickson, D.A.; May, W.D.; Hairston, K.G.; Carr, J.J.; Taylor, H.A. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: The jackson heart study. J. Clin. Endocrinol. Metab. 2010, 95, 5419–5426. [Google Scholar] [CrossRef]
- Kvist, H.; Chowdhury, B.; Grangard, U.; Tylen, U.; Sjostrom, L. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: Predictive equations. Am. J. Clin. Nutr. 1988, 48, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, J.C.; Champagne, C.M.; de Jonge, L.; Xie, H.; Smith, S.R. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int. J. Obes. 2008, 32, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.C. The emergence of the metabolic syndrome with menopause. J. Clin. Endocrinol. Metab. 2003, 88, 2404–2411. [Google Scholar] [CrossRef] [PubMed]
- Ozbey, N.; Sencer, E.; Molvalilar, S.; Orhan, Y. Body fat distribution and cardiovascular disease risk factors in pre-and postmenopausal obese women with similar bmi. Endocr. J. 2002, 49, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, P.; Michos, E.D.; Karas, R.H. Hormone replacement therapy and the cardiovascular system lessons learned and unanswered questions. J. Am. Coll. Cardiol. 2006, 47, 1741–1753. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Papacosta, O.; Lawlor, D.A.; Whincup, P.H.; Lowe, G.D.; Ebrahim, S.; Sattar, N. Do women exhibit greater differences in established and novel risk factors between diabetes and non-diabetes than men? The British regional heart study and British women’s heart health study. Diabetologia 2012, 55, 80–87. [Google Scholar] [CrossRef]
- Peters, S.A.; Huxley, R.R.; Woodward, M. Diabetes as a risk factor for stroke in women compared with men: A systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet 2014, 383, 1973–1980. [Google Scholar] [CrossRef]
- Shaikh, W.A.; Patel, M.; Singh, S. Association of adiposity with pulse pressure amongst Gujarati Indian adolescents. Indian J. Community Med. 2010, 35, 406–408. [Google Scholar] [CrossRef]
- Stevenson, J.C.; Crook, D.; Godsland, I.F. Influence of age and menopause on serum lipids and lipoproteins in healthy women. Atherosclerosis 1993, 98, 83–90. [Google Scholar] [CrossRef]
- Kuk, J.L.; Ardern, C.I. Age and sex differences in the clustering of metabolic syndrome factors: Association with mortality risk. Diabetes Care 2010, 33, 2457–2461. [Google Scholar] [CrossRef]
- Yoon, H.; Lee, J.H.; Kim, G.S.; Kim, Y.J.; Hwang, E.Y.; Park, C.E.; Park, J. The relationship between anemia and pulse pressure and hypertension: The Korea national health and nutrition examination survey 2010–2012. Clin. Exp. Hypertens. 2018, 40, 650–655. [Google Scholar] [CrossRef]
- Tanimura, M.; Dohi, K.; Fujimoto, N.; Moriwaki, K.; Omori, T.; Sato, Y.; Sugiura, E.; Kumagai, N.; Nakamori, S.; Kurita, T.; et al. Effect of anemia on cardiovascular hemodynamics, therapeutic strategy and clinical outcomes in patients with heart failure and hemodynamic congestion. Circ. J. 2017, 81, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Drueke, T.B.; Parfrey, P.S. Summary of the KDIGO guideline on anemia and comment: Reading between the (guide)line(s). Kidney Int. 2012, 82, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.G. The sex difference in haemoglobin levels in adults—Mechanisms, causes, and consequences. Blood Rev. 2014, 28, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Akhondzadeh, S. Personalized medicine: A tailor made medicine. Avicenna J. Med. Biotechnol. 2014, 6, 191. [Google Scholar]
- Sacristan, J.A. Patient-centered medicine and patient-oriented research: Improving health outcomes for individual patients. BMC Med. Inform. Decis. Mak. 2013, 13, 6. [Google Scholar] [CrossRef]
- Bardes, C.L. Defining “patient-centered medicine”. N. Engl. J. Med. 2012, 366, 782–783. [Google Scholar] [CrossRef]
| Variables | Entire Cohort | Men | Female | p-Value |
|---|---|---|---|---|
| n = 156 | n = 78 | n = 78 | ||
| Clinical Findings | ||||
| Age (years) | 58.9 ± 13.1 | 59.2 ± 13.5 | 58.5 ± 12.8 | 0.7523 |
| Gender (Men; %) | 50.0 | 100.0 | 0.0 | <0.0001 |
| SBP (mmHg) | 125.3 ± 7.9 | 126.8 ± 7.9 | 123.9 ± 7.8 | 0.0226 |
| DBP (mmHg) | 76.7 ± 6.3 | 77.6 ± 6.5 | 75.7 ± 6.0 | 0.0643 |
| MBP (mmHg) | 92.9 ± 6.6 | 94.0 ± 6.6 | 91.8 ± 6.5 | 0.0368 |
| PP (mmHg) | 48.7 ± 4.1 | 49.2 ± 4.7 | 48.2 ± 3.3 | 0.1187 |
| BMI (kg/m2) | 24.1 ± 4.1 | 24.6 ± 3.7 | 23.5 ± 4.4 | 0.0891 |
| Visceral fat area (cm2) | 123.5 ± 60.9 | 146.7 ± 63.3 | 100.4 ± 48.7 | <0.0001 |
| IMT (mm) | 1.47 ± 0.77 | 1.83 ± 0.89 | 1.11 ± 0.38 | <0.0001 |
| Laboratory Findings | ||||
| Serum Albumin (g/dL) | 4.20 ± 0.33 | 4.17 ± 0.37 | 4.22 ± 0.28 | 0.3515 |
| Hemoglobin (g/dL) | 13.3 ± 1.7 | 13.9 ± 1.8 | 12.7 ± 1.4 | <0.0001 |
| Serum Creatinine (mg/dL) | 1.18 ± 0.84 | 1.33 ± 0.89 | 1.02 ± 0.76 | 0.0247 |
| eGFR (mL/min/1.73m2) | 56.4 ± 22.2 | 56.4 ± 22.3 | 56.4 ± 22.2 | 0.9991 |
| Uric Acid (mg/dL) | 5.84 ± 1.48 | 6.40 ± 1.31 | 5.28 ± 1.42 | <0.0001 |
| Triglyceride (mg/dL) | 142.4 ± 69.9 | 160.9 ± 73.2 | 123.9 ± 61.5 | 0.0008 |
| LDL Cholesterol (mg/dL) | 115.4 ± 35.3 | 109.4 ± 32.0 | 121.5 ± 37.7 | 0.0327 |
| HDL Cholesterol (mg/dL) | 57.3 ± 16.5 | 50.8 ± 14.7 | 63.9 ± 15.7 | <0.0001 |
| Glucose (mg/dL) | 106.5 ± 35.4 | 114.1 ± 46.7 | 98.9 ± 15.5 | 0.0072 |
| Hemoglobin A1c (NGSP) (%) | 6.05 ± 0.92 | 6.05 ± 0.85 | 6.05 ± 1.01 | 0.9804 |
| Hs-CRP (ng/mL) | 777.8 ± 1052.3 | 964.7 ± 1245.9 | 590.8 ± 778.5 | 0.0270 |
| UACR (mg/g Cre) | 303.8 ± 614.3 | 451.2 ± 779.7 | 156.4 ± 328.2 | 0.0025 |
| U-Prot (g/day) | 0.65 ± 1.23 | 0.93 ± 1.51 | 0.36 ± 0.76 | 0.0035 |
| Primary cause of CKD | ||||
| Diabetic nephropathy (%) | 6.4 | 7.7 | 5.1 | 0.7456 |
| Chronic glomerulonephritis (%) | 52.6 | 44.9 | 60.3 | 0.0543 |
| Nephrosclerosis (%) | 21.8 | 34.6 | 9.0 | 0.0001 |
| Others (%) | 19.2 | 12.8 | 25.6 | 0.0422 |
| Concomitant drugs | ||||
| Antihypertensive agents (%) | 68.6 | 76.9 | 60.3 | 0.0249 |
| ARB and or ACEI | 55.1 | 66.7 | 43.6 | 0.0038 |
| Ca blockade | 30.8 | 28.2 | 33.3 | 0.4878 |
| Antihyperuricemic agents (%) | 38.5 | 53.9 | 23.1 | <0.0001 |
| Antidiabetic agents (%) | 13.5 | 15.4 | 11.5 | 0.4816 |
| Corticosteroids (%) | 15.4 | 16.7 | 14.1 | 0.6572 |
| Immunosuppressants (%) | 6.4 | 6.4 | 6.4 | 1.0000 |
| Diuretics (%) | 23.1 | 16.7 | 29.5 | 0.0574 |
| Comorbidities | ||||
| Hypertension (%) | 68.0 | 75.6 | 60.3 | 0.0395 |
| Hyperuricemia (%) | 48.7 | 66.7 | 30.8 | <0.0001 |
| Hypertriglyceridemia (%) | 58.3 | 64.1 | 52.6 | 0.1439 |
| Hypercholesterolemia (%) | 58.3 | 55.1 | 61.5 | 0.4168 |
| Low HDL cholesterol (%) | 50.0 | 51.3 | 48.7 | 0.7488 |
| Diabetes mellitus (%) | 19.2 | 20.5 | 18.0 | 0.6845 |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Age (1 year increase) | 1.00 (0.98–1.03) | 0.7421 | 0.92 (0.88–0.96) | 0.0004 |
| Men (vs. women) | 1.69 (0.86–3.43) | 0.1266 | 2.08 (0.54–8.40) | 0.1391 |
| SBP (10 mmHg increase) | 1.80 (1.12–2.96) | 0.0149 | - | - |
| DBP (10 mmHg increase) | 1.43 (0.81–2.58) | 0.2220 | - | - |
| MBP (10 mmHg increase) | 1.64 (0.94–2.89) | 0.0814 | - | - |
| PP (10 mmHg increase) | 2.56 (1.25–4.60) | 0.0117 | 3.94 (1.32–10.03) | 0.0070 |
| Visceral fat area (10 cm2 increase) | 1.09 (1.03–1.14) | 0.0013 | 1.08 (1.02–1.15) | 0.0088 |
| IMT (1 mm increase) | 1.42 (0.97–1.96) | 0.0722 | 1.57 (0.85–2.72) | 0.1125 |
| eGFR (10 mL/min/1.73 m2 increase) | 0.52 (0.41–0.63) | <0.0001 | 0.49 (0.36–0.65) | <0.0001 |
| Hemoglobin (1 g/dL increase) | 0.62 (0.52–0.75) | <0.0001 | 0.88 (0.67–1.15) | 0.2966 |
| Serum albumin (1 g/dL increase) | 0.14 (0.07–0.31) | <0.0001 | 0.10 (0.03–0.32) | <0.0001 |
| Hs-CRP (10 ng/mL increase) | 1.00 (1.00–1.00) | 0.0761 | 1.00 (1.00–1.00) | 0.2528 |
| U-Prot (g/day) | 1.60 (1.38–1.81) | <0.0001 | - | - |
| Hypertriglyceridemia (vs. no) | 1.43 (0.71–3.04) | 0.3234 | - | - |
| Hypercholesterolemia (vs. no) | 1.25 (0.63–2.61) | 0.5274 | - | - |
| Low HDL cholesterol (vs. no) | 2.53 (1.25–5.55) | 0.0096 | 1.30 (0.53–3.30) | 0.5487 |
| Hypertension (vs. no) | 4.10 (1.62–13.81) | 0.0017 | 1.05 (0.38–3.75) | 0.9257 |
| Hyperuricemia (vs. no) | 6.44 (2.85–17.22) | <0.0001 | 1.04 (0.35–3.31) | 0.9495 |
| Diabetes mellitus (vs. no) | 2.29 (1.07–4.60) | 0.0332 | 2.05 (0.81–5.06) | 0.1237 |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Age (1 year increase) | 1.01 (0.97–1.05) | 0.6684 | 0.87 (0.81–0.94) | <0.0001 |
| SBP (10 mmHg increase) | 2.64 (1.28–5.80) | 0.0080 | - | - |
| DBP (10 mmHg increase) | 1.36 (0.62–3.13) | 0.4486 | - | - |
| MBP (10 mmHg increase) | 1.89 (0.84–4.45) | 0.1233 | - | - |
| PP (10 mmHg increase) | 2.61 (1.27–4.69) | 0.0035 | 8.35 (2.4–24.53) | 0.0004 |
| Visceral fat area (10 cm2 increase) | 1.06 (0.99–1.13) | 0.1127 | - | - |
| IMT (1 mm increase) | 1.30 (0.81–1.93) | 0.2609 | - | - |
| eGFR (10 mL/min/1.73 m2 increase) | 0.52 (0.39–0.67) | <0.0001 | 0.28 (0.15–0.51) | <0.0001 |
| Hemoglobin (1 g/dL increase) | 0.56 (0.44–0.71) | <0.0001 | 1.00 (0.67–1.48) | 0.9933 |
| Serum albumin (1 g/dL increase) | 0.14 (0.06–0.32) | <0.0001 | 0.02 (0.00–0.15) | <0.0001 |
| Hs-CRP (10 ng/mL increase) | 1.00 (1.00–1.00) | 0.0761 | 1.00 (1.00–1.00) | 0.8530 |
| U-Prot (g/day) | 1.52 (1.29–1.76) | <0.0001 | - | - |
| Hypertriglyceridemia (vs. no) | 1.36 (0.53–4.18) | 0.5449 | - | - |
| Hypercholesterolemia (vs. no) | 1.85 (0.74–5.22) | 0.1931 | - | - |
| Low HDL cholesterol (vs. no) | 2.04 (0.82–5.76) | 0.1286 | - | - |
| Hypertension (vs. no) | 7.05 (1.46–126.65) | 0.0098 | 1.95 (0.23–16.22) | 0.5041 |
| Hyperuricemia (vs. no) | 6.08 (1.75–38.27) | 0.0025 | 0.37 (0.06–2.36) | 0.3139 |
| Diabetes mellitus (vs. no) | 1.75 (0.62–4.37) | 0.2704 | - | - |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Age (1 year increase) | 1.00 (0.96–1.04) | 0.8692 | 0.98 (0.91–1.04) | 0.4647 |
| SBP (10 mmHg increase) | 1.21 (0.62–2.40) | 0.5731 | - | - |
| DBP (10 mmHg increase) | 1.39 (0.58–3.31) | 0.4568 | - | - |
| MBP (10 mmHg increase) | 1.33 (0.59–2.98) | 0.4917 | - | - |
| PP (10 mmHg increase) | 0.99 (0.21–4.99) | 0.9930 | - | - |
| Visceral fat area (10 cm2 increase) | 1.16 (1.05–1.29) | 0.0047 | 1.04 (0.93–1.17) | 0.4602 |
| IMT (1 mm increase) | 1.34 (0.34–3.52) | 0.6251 | - | - |
| eGFR (10 mL/min/1.73 m2 increase) | 0.50 (0.34–0.70) | <0.0001 | 0.93 (0.58–1.45) | 0.0027 |
| Hemoglobin (1 g/dL increase) | 0.59 (0.43–0.82) | 0.0019 | 0.93 (0.58–1.45) | 0.7560 |
| Serum albumin (1 g/dL increase) | 0.32 (0.03–2.37) | 0.2430 | - | - |
| Hs-CRP (10 ng/mL increase) | 1.00 (1.00–1.00) | 0.8702 | - | - |
| U-Prot (g/day) | 1.54 (0.98–2.12) | 0.0573 | - | - |
| Hypertriglyceridemia (vs. no) | 1.29 (0.45–3.93) | 0.6322 | - | |
| Hypercholesterolemia (vs. no) | 0.78 (0.27–2.36) | 0.6419 | - | - |
| Low HDL cholesterol (vs. no) | 3.23 (1.08–11.80) | 0.0356 | 1.93 (0.48–9.09) | 0.3595 |
| Hypertension (vs. no) | 2.71 (0.85–12.00) | 0.0965 | 1.83 (0.51–8.66) | 0.3708 |
| Hyperuricemia (vs. no) | 6.50 (2.17–23.71) | 0.0007 | 1.17 (0.21–6.47) | 0.8519 |
| Diabetes mellitus (vs. no) | 3.13 (0.96–9.08) | 0.0574 | 4.49 (1.03–21.24) | 0.0445 |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| β | p-Value | β | p-Value | |
| Age (years) | 0.25 | 0.0018 | 0.13 | 0.1405 |
| 0.62 | <0.0001 | - | - | |
| DBP (mmHg) | 0.13 | 0.1068 | - | - |
| MBP (mmHg) | 0.33 | <0.0001 | - | - |
| Visceral fat area (cm2) | 0.29 | 0.0002 | 0.14 | 0.1132 |
| IMT(mm) | 0.17 | 0.0314 | 0.01 | 0.9095 |
| eGFR (mL/min/1.73 m2) | −0.07 | 0.3995 | 0.13 | 0.1531 |
| Hemoglobin (g/dL) | −0.13 | 0.1021 | −0.21 | 0.0304 |
| Serum albumin (g/dL) | −0.06 | 0.4773 | 0.09 | 0.2961 |
| Uric acid (mg/dL) | 0.02 | 0.7743 | - | - |
| Triglyceride (mg/dL) | 0.06 | 0.4350 | - | - |
| LDL cholesterol (mg/dL) | −0.10 | 0.2108 | - | - |
| HDL cholesterol (mg/dL) | −0.35 | <0.0001 | −0.27 | 0.0018 |
| Glucose (mg/dL) | 0.06 | 0.4685 | - | - |
| Hemoglobin A1c (NGSP) (%) | 0.04 | 0.7030 | - | - |
| Hs-CRP (ng/mL) | 0.22 | 0.0057 | 0.08 | 0.0807 |
| U-Prot (g/day) | 0.31 | <0.0001 | - | - |
| Variables | Men (n = 78) | Women (n = 78) | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| β | p-Value | β | p-Value | β | p-Value | β | p-Value | |
| Age (years) | 0.25 | 0.0251 | 0.11 | 0.3789 | 0.24 | 0.0307 | 0.18 | 0.0914 |
| SBP (mmHg) | 0.57 | <0.0001 | - | - | 0.69 | <0.0001 | - | - |
| DBP (mmHg) | −0.04 | 0.7587 | - | - | 0.34 | 0.0020 | - | - |
| MBP (mmHg) | 0.20 | 0.0725 | - | - | 0.49 | <0.0001 | - | - |
| Visceral fat area (cm2) | 0.34 | 0.0025 | 0.24 | 0.0428 | 0.13 | 0.2547 | - | - |
| IMT (mm) | 0.13 | 0.2626 | - | - | 0.15 | 0.2002 | - | - |
| eGFR (mL/min/1.73 m2) | −0.12 | 0.3155 | 0.19 | 0.1629 | −0.00 | 0.9722 | - | - |
| Hemoglobin (g/dL) | −0.26 | 0.0192 | −0.30 | 0.0395 | −0.06 | 0.6291 | - | - |
| Serum albumin (g/dL) | −0.09 | 0.4331 | - | - | 0.03 | 0.7855 | - | - |
| Uric acid (mg/dL) | 0.01 | 0.9144 | - | - | −0.08 | 0.4957 | - | - |
| Triglyceride (mg/dL) | 0.01 | 0.9024 | - | - | 0.06 | 0.6016 | - | - |
| LDL cholesterol (mg/dL) | −0.21 | 0.0660 | - | - | 0.07 | 0.5549 | - | - |
| HDL cholesterol (mg/dL) | −0.31 | 0.0055 | −0.15 | 0.1886 | −0.36 | 0.0011 | −0.33 | 0.0030 |
| Glucose (mg/dL) | 0.01 | 0.8989 | - | - | 0.11 | 0.3476 | - | - |
| Hemoglobin A1c (NGSP) (%) | −0.00 | 0.9966 | - | - | 0.09 | 0.5053 | - | - |
| Hs-CRP (ng/mL) | 0.26 | 0.0199 | 0.18 | 0.1007 | 0.06 | 0.5808 | - | - |
| U-Prot (g/day) | 0.38 | 0.0006 | - | - | 0.05 | 0.6895 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kataoka, H.; Sawara, Y.; Kawachi, K.; Manabe, S.; Mochizuki, T.; Nitta, K. Impacts of Sex Differences in Pulse Pressure among Patients with Chronic Kidney Disease. J. Pers. Med. 2019, 9, 52. https://doi.org/10.3390/jpm9040052
Kataoka H, Sawara Y, Kawachi K, Manabe S, Mochizuki T, Nitta K. Impacts of Sex Differences in Pulse Pressure among Patients with Chronic Kidney Disease. Journal of Personalized Medicine. 2019; 9(4):52. https://doi.org/10.3390/jpm9040052
Chicago/Turabian StyleKataoka, Hiroshi, Yukako Sawara, Keiko Kawachi, Shun Manabe, Toshio Mochizuki, and Kosaku Nitta. 2019. "Impacts of Sex Differences in Pulse Pressure among Patients with Chronic Kidney Disease" Journal of Personalized Medicine 9, no. 4: 52. https://doi.org/10.3390/jpm9040052
APA StyleKataoka, H., Sawara, Y., Kawachi, K., Manabe, S., Mochizuki, T., & Nitta, K. (2019). Impacts of Sex Differences in Pulse Pressure among Patients with Chronic Kidney Disease. Journal of Personalized Medicine, 9(4), 52. https://doi.org/10.3390/jpm9040052





