Evidence to Support Inclusion of Pharmacogenetic Biomarkers in Randomised Controlled Trials
Abstract
1. Introduction
2. Details of Included Trials
3. TARGET
4. EU-PACT
5. SHIVA
6. GGST Statin Trial
7. Precision Medicine Guided Treatment for Cancer Pain
8. Discussion
Recommendations
- -
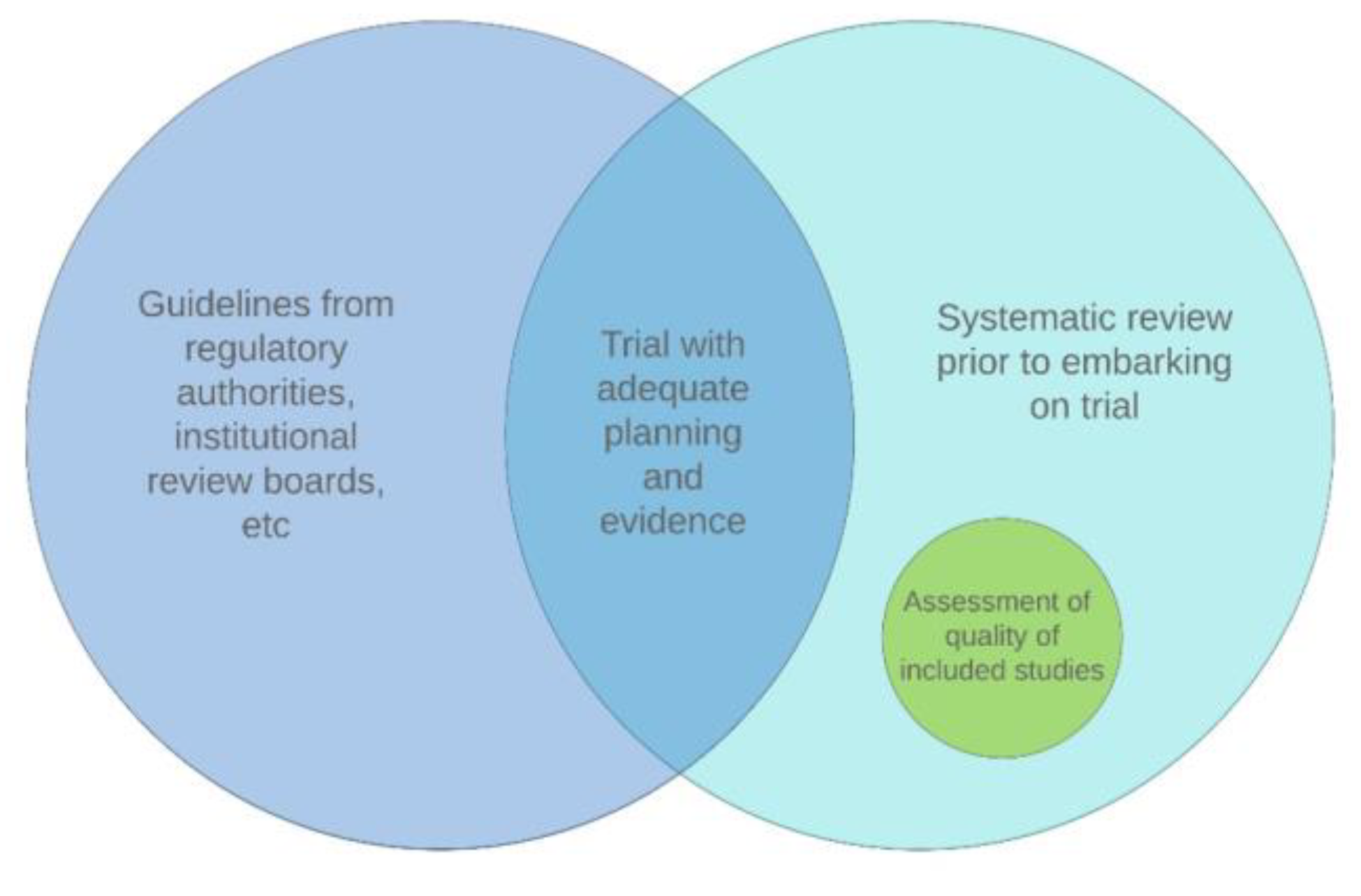
- Systematic review before embarking on a trial
- -
- Guidelines are required
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Teutsch, S.M.; Bradley, L.A.; Palomaki, G.E.; Haddow, J.E.; Piper, M.; Calonge, N.; Dotson, W.D.; Douglas, M.P.; Berg, A.O.; EGAPP Working Group. The Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Initiative: Methods of the EGAPP Working Group. Genet. Med. 2009, 11, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Williams-Jones, B.; Corrigan, O.P. Rhetoric and hype: where’s thee thics in pharmacogenomics? Am. J. Pharm. 2003, 3, 375–383. [Google Scholar]
- Buchanan, A.; Califano, A.; Kahn, J.; McPherson, E.; Robertson, J.; Brody, B. Pharmacogenetics: Ethical issues and policy options. Kennedy Inst. Ethics 2002, 12, 1–15. [Google Scholar] [CrossRef]
- Pirmohamed, M. Acceptance of biomarker-based tests for application in clinical practice: Criteria and obstacles. Clin. Pharmacol. Ther. 2010, 88, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Pirmohamed, M.; Park, B.K. Genetic susceptibility to adverse drug reactions. Trends Pharm. Sci 2001, 22, 298–305. [Google Scholar] [CrossRef]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.D.; Sigman, C.C.; Kelloff, G.J.; Hylton, N.M.; Berry, D.A.; Esserman, L.J. I-SPY 2: An adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin. Pharmacol. Ther. 2009, 86, 97–100. [Google Scholar] [CrossRef]
- Weinberg, D.S.; Myers, R.E.; Keenan, E.; Ruth, K.; Sifri, R.; Ziring, B.; Ross, E.; Manne, S.L. Genetic and environmental risk assessment and colorectal cancer screening in an average-risk population: A randomized trial. Ann. Intern. Med. 2014, 161, 537–545. [Google Scholar] [CrossRef]
- Pirmohamed, M.; Burnside, G.; Eriksson, N.; Jorgensen, A.L.; Toh, C.H.; Nicholson, T.; Kesteven, P.; Christersson, C.; Wahlstrom, B.; Stafberg, C.; et al. A randomized trial of genotype-guided dosing of warfarin. N. Engl. J. Med. 2013, 369, 2294–2303. [Google Scholar] [CrossRef]
- Newman, W.G.; Payne, K.; Tricker, K.; Roberts, S.A.; Fargher, E.; Pushpakom, S.; Alder, J.E.; Sidgwick, G.P.; Payne, D.; Elliott, R.A.; et al. A pragmatic randomized controlled trial of thiopurine methyltransferase genotyping prior to azathioprine treatment: The TARGET study. Pharmacogenomics 2011, 12, 815–826. [Google Scholar] [CrossRef]
- Siramshetty, V.B.; Nickel, J.; Omieczynski, C.; Gohlke, B.O.; Drwal, M.N.; Preissner, R. WITHDRAWN—A resource for withdrawn and discontinued drugs. Nucleic Acids Res. 2015, 44, D1080–D1086. [Google Scholar] [CrossRef]
- Onakpoya, I.J.; Heneghan, C.J.; Aronson, J.K. Worldwide withdrawal of medicinal products because of adverse drug reactions: A systematic review and analysis. Crit. Rev. Toxicol. 2016, 46, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Need, A.C.; Motulsky, A.G.; Goldstein, D.B. Priorities and standards in pharmacogenetic research. Nat. Genet. 2005, 37, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Pirmohamed, M.; James, S.; Meakin, S.; Green, C.; Scott, A.K.; Walley, T.J.; Farrar, K.; Park, B.K.; Breckenridge, A.M. Adverse drug reactions as cause of admission to hospital: Prospective analysis of 18 820 patients. BMJ 2004, 329, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Laatikainen, O.; Miettunen, J.; Sneck, S.; Lehtiniemi, H.; Tenhunen, O.; Turpeinen, M. The prevalence of medication-related adverse events in inpatients-a systematic review and meta-analysis. Eur. J. Clin. Pharm. 2017, 73, 1539–1549. [Google Scholar] [CrossRef]
- Verbelen, M.; Weale, M.E.; Lewis, C.M. Cost-effectiveness of pharmacogenetic-guided treatment: Are we there yet? Pharm. J. 2017, 17, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Plumpton, C.O.; Roberts, D.; Pirmohamed, M.; Hughes, D.A. A Systematic Review of Economic Evaluations of Pharmacogenetic Testing for Prevention of Adverse Drug Reactions. Pharmacoeconomics 2016, 34, 771–793. [Google Scholar] [CrossRef]
- Mallal, S.; Phillips, E.; Carosi, G.; Molina, J.M.; Workman, C.; Tomazic, J.; Jagel-Guedes, E.; Rugina, S.; Kozyrev, O.; Cid, J.F.; et al. HLA-B*5701 screening for hypersensitivity to abacavir. N. Engl. J. Med. 2008, 358, 568–579. [Google Scholar] [CrossRef]
- Hughes, D.A.; Vilar, F.J.; Ward, C.C.; Alfirevic, A.; Park, B.K.; Pirmohamed, M. Cost-effectiveness analysis of HLA B*5701 genotyping in preventing abacavir hypersensitivity. Pharmacogenetics 2004, 14, 335–342. [Google Scholar] [CrossRef]
- Hughes, D.A. Economics of Pharmacogenetic-Guided Treatments: Underwhelming or Overstated? Clin. Pharmacol. Ther. 2018, 103, 749–751. [Google Scholar] [CrossRef]
- Food and Drug Administration (US). Table of Pharmacogenomic Biomarkers in Drug Labeling. Available online: https://www.fda.gov/Drugs/ScienceResearch/ucm572698.htm (accessed on 25 July 2019).
- Shuldiner, A.R.; Relling, M.V.; Peterson, J.F.; Hicks, J.K.; Freimuth, R.R.; Sadee, W.; Pereira, N.L.; Roden, D.M.; Johnson, J.A.; Klein, T.E.; et al. The Pharmacogenomics Research Network Translational Pharmacogenetics Program: Overcoming challenges of real-world implementation. Clin. Pharmacol. Ther. 2013, 94, 207–210. [Google Scholar] [CrossRef] [PubMed]
- García-González, X.; Cabaleiro, T.; Herrero María, J.; McLeod, H.; López-Fernández Luis, A. Clinical implementation of pharmacogenetics. Drug Metab. Pers. Ther. 2016, 31, 9. [Google Scholar] [CrossRef] [PubMed]
- Slob, E.M.A.; Vijverberg, S.J.H.; Pijnenburg, M.W.; Koppelman, G.H.; Maitland-van der Zee, A.H. What do we need to transfer pharmacogenetics findings into the clinic? Pharmacogenomics 2018, 19, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Pirmohamed, M.; Hughes, D.A. Pharmacogenetic tests: The need for a level playing field. Nat. Rev. Drug Discov. 2013, 12, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Najafzadeh, M.; Davis, J.C.; Joshi, P.; Marra, C. Barriers for integrating personalized medicine into clinical practice: A qualitative analysis. Am. J. Med. Genet. Part A 2013, 16, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Chin, L.; Devine, B.; Baradaran, S.; Keyloun, K.; Canestaro, W.; Pham, J. Characterizing the Strength of Evidence in FDA Labels for Pharmacogenomic Biomarker-Guided Medication Use. Amia Summits Transl. Sci. Proc. 2017, 2017, 30–39. [Google Scholar] [PubMed]
- Vivot, A.; Boutron, I.; Ravaud, P.; Porcher, R. Guidance for pharmacogenomic biomarker testing in labels of FDA-approved drugs. Genet. Med. 2015, 17, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Whirl-Carrillo, M.; McDonagh, E.M.; Hebert, J.M.; Gong, L.; Sangkuhl, K.; Thorn, C.F.; Altman, R.B.; Klein, T.E. Pharmacogenomics knowledge for personalized medicine. Clin. Pharmacol. Ther. 2012, 92, 414–417. [Google Scholar] [CrossRef] [PubMed]
- van der Wouden, C.H.; Cambon-Thomsen, A.; Cecchin, E.; Cheung, K.C.; Davila-Fajardo, C.L.; Deneer, V.H.; Dolzan, V.; Ingelman-Sundberg, M.; Jonsson, S.; Karlsson, M.O.; et al. Implementing Pharmacogenomics in Europe: Design and Implementation Strategy of the Ubiquitous Pharmacogenomics Consortium. Clin. Pharmacol. Ther. 2017, 101, 341–358. [Google Scholar] [CrossRef]
- Lesko, L.J.; Atkinson, A.J., Jr. Use of biomarkers and surrogate endpoints in drug development and regulatory decision making: Criteria, validation, strategies. Annu. Rev. Pharm. Toxicol. 2001, 41, 347–366. [Google Scholar] [CrossRef]
- Rolan, P. The contribution of clinical pharmacology surrogates and models to drug development--A critical appraisal. Br. J. Clin. Pharmacol. 1997, 44, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Torgerson, D.; Torgerson, C. Designing Randomised Trials in Health, Education and the Social Sciences: An Introduction; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Barton, S. Which clinical studies provide the best evidence? The best RCT still trumps the best observational study. BMJ 2000, 321, 255–256. [Google Scholar] [CrossRef] [PubMed]
- Hyde, P. Fool’s Gold: Examining the Use of Gold Standards in the Production of Research Evidence. Br. J. Occup. Ther. 2016, 67, 89–94. [Google Scholar] [CrossRef]
- Vivot, A.; Boutron, I.; Beraud-Chaulet, G.; Zeitoun, J.D.; Ravaud, P.; Porcher, R. Evidence for Treatment-by-Biomarker interaction for FDA-approved Oncology Drugs with Required Pharmacogenomic Biomarker Testing. Sci. Rep. 2017, 7, 6882. [Google Scholar] [CrossRef] [PubMed]
- Poste, G. Bring on the biomarkers. Nature 2011, 469, 156–157. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, M.; Jorgensen, A.L.; Kolamunnage-Dona, R. Biomarker-Guided Adaptive Trial Designs in Phase II and Phase III: A Methodological Review. PLoS ONE 2016, 11, e0149803. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, M.; Jorgensen, A.L.; Kolamunnage-Dona, R. Biomarker-guided trial designs (BiGTeD): An online tool to help develop personalised medicine. Available online: http://www.bigted.org/ (accessed on 18 May 2018).
- Antoniou, M.; Kolamunnage-Dona, R.; Jorgensen, A.L. Biomarker-Guided Non-Adaptive Trial Designs in Phase II and Phase III: A Methodological Review. J. Pers Med. 2017, 7, 1. [Google Scholar] [CrossRef]
- Yee, L.M.; Lively, T.G.; McShane, L.M. Biomarkers in early-phase trials: Fundamental issues. Bioanalysis 2018, 10, 933–944. [Google Scholar] [CrossRef]
- Hayes, D.F.; Allen, J.; Compton, C.; Gustavsen, G.; Leonard, D.G.; McCormack, R.; Newcomer, L.; Pothier, K.; Ransohoff, D.; Schilsky, R.L.; et al. Breaking a vicious cycle. Sci. Transl. Med. 2013, 5, 196cm6. [Google Scholar] [CrossRef]
- Le Tourneau, C.; Delord, J.P.; Goncalves, A.; Gavoille, C.; Dubot, C.; Isambert, N.; Campone, M.; Tredan, O.; Massiani, M.A.; Mauborgne, C.; et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): A multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015, 16, 1324–1334. [Google Scholar] [CrossRef]
- Singh, K.; Peyser, B.; Trujillo, G.; Milazzo, N.; Savard, D.; Haga, S.B.; Musty, M.; Voora, D. Rationale and design of the SLCO1B1 genotype guided statin therapy trial. Pharmacogenomics 2016, 17, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Peyser, B.; Perry, E.P.; Singh, K.; Gill, R.D.; Mehan, M.R.; Haga, S.B.; Musty, M.D.; Milazzo, N.A.; Savard, D.; Li, Y.J.; et al. Effects of Delivering SLCO1B1 Pharmacogenetic Information in Randomized Trial and Observational Settings. Circ. Genom. Precis. Med. 2018, 11, e002228. [Google Scholar] [CrossRef] [PubMed]
- Mosley, S.A.; Hicks, J.K.; Portman, D.G.; Donovan, K.A.; Gopalan, P.; Schmit, J.; Starr, J.; Silver, N.; Gong, Y.; Langaee, T.; et al. Design and rational for the precision medicine guided treatment for cancer pain pragmatic clinical trial. Contemp. Clin. Trials 2018, 68, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Ollier, B.; Newman, B.; Payne, K.; Poulton, K.; Andrews, J.; Elliott, R.; Ray, D.; Elles, R.; Houston, B.; Bruce, I.; et al.; (University of Manchester, Manchester, UK) Personal Communication, 2015.
- Voora, D.; Singh, K.; Trujillo, G.; Milazzo, N.; Savard, D.; Haga, S.; Musty, M.D.; Li, Y.J.; Peyser, B. SLCO1B1 Genotype-Guided Statin Therapy Lowers LDL Cholesterol in Patients With Statin-Intolerance-A Randomized Controlled Trial. Circulation 2016, 134, A12183. [Google Scholar]
- van Schie, R.M.; Wadelius, M.I.; Kamali, F.; Daly, A.K.; Manolopoulos, V.G.; de Boer, A.; Barallon, R.; Verhoef, T.I.; Kirchheiner, J.; Haschke-Becher, E.; et al. Genotype-guided dosing of coumarin derivatives: The European pharmacogenetics of anticoagulant therapy (EU-PACT) trial design. Pharmacogenomics 2009, 10, 1687–1695. [Google Scholar] [CrossRef]
- R Studio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2016. [Google Scholar]
- Ren, K.; Russell, K. Package ‘formattable’. Available online: https://cran.r-project.org/web/packages/formattable/formattable.pdf (accessed on 25 July 2019).
- Lucid Software Inc. Online Diagram Software: Lucidchart; Lucid Software Inc.: South Jordan, UT, USA, 2013. [Google Scholar]
- Thompson, A.J.; Newman, W.G.; Elliott, R.A.; Roberts, S.A.; Tricker, K.; Payne, K. The cost-effectiveness of a pharmacogenetic test: A trial-based evaluation of TPMT genotyping for azathioprine. Value Health J. Int. Soc. Pharm. Outcomes Res. 2014, 17, 22–33. [Google Scholar] [CrossRef]
- Weinshilboum, R.M.; Sladek, S.L. Mercaptopurine pharmacogenetics: Monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am. J. Hum. Genet. 1980, 32, 651–662. [Google Scholar]
- Lennard, L.; Van Loon, J.A.; Weinshilboum, R.M. Pharmacogenetics of acute azathioprine toxicity: Relationship to thiopurine methyltransferase genetic polymorphism. Clin. Pharmacol. Ther. 1989, 46, 149–154. [Google Scholar] [CrossRef]
- Dubinsky, M.C.; Lamothe, S.; Yang, H.Y.; Targan, S.R.; Sinnett, D.; Theoret, Y.; Seidman, E.G. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology 2000, 118, 705–713. [Google Scholar] [CrossRef]
- McLeod, H.L.; Lin, J.S.; Scott, E.P.; Pui, C.H.; Evans, W.E. Thiopurine methyltransferase activity in American white subjects and black subjects. Clin. Pharmacol. Ther. 1994, 55, 15–20. [Google Scholar] [CrossRef]
- Yates, C.R.; Krynetski, E.Y.; Loennechen, T.; Fessing, M.Y.; Tai, H.L.; Pui, C.H.; Relling, M.V.; Evans, W.E. Molecular diagnosis of thiopurine S-methyltransferase deficiency: Genetic basis for azathioprine and mercaptopurine intolerance. Ann. Intern. Med. 1997, 126, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Bloomfeld, R.S.; Onken, J.E. Mercaptopurine metabolite results in clinical gastroenterology practice. Aliment. Pharmacol. Ther. 2003, 17, 69–73. [Google Scholar] [CrossRef] [PubMed]
- McLeod, H.L.; Coulthard, S.; Thomas, A.E.; Pritchard, S.C.; King, D.J.; Richards, S.M.; Eden, O.B.; Hall, A.G.; Gibson, B.E. Analysis of thiopurine methyltransferase variant alleles in childhood acute lymphoblastic leukaemia. Br. J. Haematol. 1999, 105, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Black, A.J.; McLeod, H.L.; Capell, H.A.; Powrie, R.H.; Matowe, L.K.; Pritchard, S.C.; Collie-Duguid, E.S.; Reid, D.M. Thiopurine methyltransferase genotype predicts therapy-limiting severe toxicity from azathioprine. Ann. Intern. Med. 1998, 129, 716–718. [Google Scholar] [CrossRef] [PubMed]
- Pandya, B.; Thomson, W.; Poulton, K.; Bruce, I.; Payne, D.; Qasim, F. Azathioprine toxicity and thiopurine methyltransferase genotype in renal transplant patients. Transpl. Proc. 2002, 34, 1642–1645. [Google Scholar] [CrossRef]
- Murphy, L.A.; Atherton, D. A retrospective evaluation of azathioprine in severe childhood atopic eczema, using thiopurine methyltransferase levels to exclude patients at high risk of myelosuppression. Br. J. Dermatol. 2002, 147, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Holme, S.A.; Duley, J.A.; Sanderson, J.; Routledge, P.A.; Anstey, A.V. Erythrocyte thiopurine methyl transferase assessment prior to azathioprine use in the UK. Qjm Mon. J. Assoc. Physicians 2002, 95, 439–444. [Google Scholar] [CrossRef]
- Seidman, E.G. Clinical use and practical application of TPMT enzyme and 6-mercaptopurine metabolite monitoring in IBD. Rev. Gastroenterol. Disord. 2003, 3, S30–S38. [Google Scholar]
- Marra, C.A.; Esdaile, J.M.; Anis, A.H. Practical pharmacogenetics: The cost effectiveness of screening for thiopurine s-methyltransferase polymorphisms in patients with rheumatological conditions treated with azathioprine. J. Rheumatol. 2002, 29, 2507–2512. [Google Scholar]
- Tavadia, S.M.; Mydlarski, P.R.; Reis, M.D.; Mittmann, N.; Pinkerton, P.H.; Shear, N.; Sauder, D.N. Screening for azathioprine toxicity: A pharmacoeconomic analysis based on a target case. J. Am. Acad. Dermatol. 2000, 42, 628–632. [Google Scholar] [CrossRef]
- Tan, B.B.; Lear, J.T.; Gawkrodger, D.J.; English, J.S. Azathioprine in dermatology: A survey of current practice in the U.K. Br. J. Dermatol. 1997, 136, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Pirmohamed, M.; Stoddern, J.; Prince, C.; Toh, C.H.; Nicholson, T.; Kesteven, P.; Jorgensen, A.; Daly, A.; Maitland-van der Zee, A.H.; Williamson, P.; et al. A Randomized Trial Comparing Genotype-Guided Dosing of Warfarin to Standard Dosing: The EU Pharmacogenetics of Anticoagulant Therapy (EU-PACT) Warfarin Study. N. Engl. J. Med. 2013, 369, 2294–2303. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, T.I.; Ragia, G.; de Boer, A.; Barallon, R.; Kolovou, G.; Kolovou, V.; Konstantinides, S.; Le Cessie, S.; Maltezos, E.; van der Meer, F.J.; et al. A randomized trial of genotype-guided dosing of acenocoumarol and phenprocoumon. N. Engl. J. Med. 2013, 369, 2304–2312. [Google Scholar] [CrossRef] [PubMed]
- Manolopoulos, V.G.; Ragia, G.; Tavridou, A.; Kolovou, V.; Kolovou, G.; Maltezos, E.; Tziakas, D.; Konstantinides, S. Effectiveness of Acenocoumarol Genetic and Clinical Dosing Algorithms in Predicting Stable Dose in the Greek Cohort of the Eu-Pact Trial. Clin. Ther. 2015, 37, E6. [Google Scholar] [CrossRef]
- James, A.H.; Britt, R.P.; Raskino, C.L.; Thompson, S.G. Factors affecting the maintenance dose of warfarin. J. Clin. Pathol. 1992, 45, 704–706. [Google Scholar] [CrossRef] [PubMed]
- Wadelius, M.; Chen, L.Y.; Lindh, J.D.; Eriksson, N.; Ghori, M.J.; Bumpstead, S.; Holm, L.; McGinnis, R.; Rane, A.; Deloukas, P. The largest prospective warfarin-treated cohort supports genetic forecasting. Blood 2009, 113, 784–792. [Google Scholar] [CrossRef]
- Takeuchi, F.; McGinnis, R.; Bourgeois, S.; Barnes, C.; Eriksson, N.; Soranzo, N.; Whittaker, P.; Ranganath, V.; Kumanduri, V.; McLaren, W.; et al. A genome-wide association study confirms VKORC1, CYP2C9, and CYP4F2 as principal genetic determinants of warfarin dose. PLoS Genet. 2009, 5, e1000433. [Google Scholar] [CrossRef] [PubMed]
- International Warfarin Pharmacogenetics Consortium; Klein, T.E.; Altman, R.B.; Eriksson, N.; Gage, B.F.; Kimmel, S.E.; Lee, M.T.; Limdi, N.A.; Page, D.; Roden, D.M.; et al. Estimation of the warfarin dose with clinical and pharmacogenetic data. N. Engl. J. Med. 2009, 360, 753–764. [Google Scholar]
- Rosendaal, F.R. The Scylla and Charybdis of oral anticoagulant treatment. N. Engl. J. Med. 1996, 335, 587–589. [Google Scholar] [CrossRef]
- Pirmohamed, M. Warfarin: Almost 60 years old and still causing problems. Br. J. Clin. Pharmacol. 2006, 62, 509–511. [Google Scholar] [CrossRef]
- Eckman, M.H.; Rosand, J.; Greenberg, S.M.; Gage, B.F. Cost-effectiveness of using pharmacogenetic information in warfarin dosing for patients with nonvalvular atrial fibrillation. Ann. Intern. Med. 2009, 150, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Schalekamp, T.; Boink, G.J.; Visser, L.E.; Stricker, B.H.; de Boer, A.; Klungel, O.H. CYP2C9 genotyping in acenocoumarol treatment: Is it a cost-effective addition to international normalized ratio monitoring? Clin. Pharmacol. Ther. 2006, 79, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.A.; Pirmohamed, M. Warfarin pharmacogenetics: Economic considerations. Pharmacoeconomics 2007, 25, 899–902. [Google Scholar] [CrossRef] [PubMed]
- Tarcic, G.; Kamal, M.; Edelheit, O.; Barbash, Z.; Vidne, M.; Miron, B.; Callens, C.; Servant, N.; Bieche, I.; Le Tourneau, C. Functional mutational analysis to assess the oncogenic activity of variant of uncertain significance (VUS) detected in patients included in the SHIVA trial. Eur. J. Cancer 2016, 69, S6–S7. [Google Scholar] [CrossRef]
- Kamal, M.; Servant, N.; Pierron, G.; Callens, C.; Gentien, D.; Lermine, A.; Lucotte, G.; Bernard, V.; Vincent-Salomon, A.; Bièche, I.; et al. Abstract 1524: Mutations and gene copy number variations landscape of metastases of various cancer types from patients enrolled in the SHIVA trial. Cancer Res. 2016, 76, 1524. [Google Scholar]
- Thatcher, N.; Chang, A.; Parikh, P.; Rodrigues Pereira, J.; Ciuleanu, T.; von Pawel, J.; Thongprasert, S.; Tan, E.H.; Pemberton, K.; Archer, V.; et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: Results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 2005, 366, 1527–1537. [Google Scholar] [CrossRef]
- Mok, T.S.; Wu, Y.L.; Thongprasert, S.; Yang, C.H.; Chu, D.T.; Saijo, N.; Sunpaweravong, P.; Han, B.; Margono, B.; Ichinose, Y.; et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009, 361, 947–957. [Google Scholar] [CrossRef]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef]
- Lievre, A.; Bachet, J.B.; Boige, V.; Cayre, A.; Le Corre, D.; Buc, E.; Ychou, M.; Bouche, O.; Landi, B.; Louvet, C.; et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 374–379. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Stephenson, J.J., Jr.; Rosen, P.; Loesch, D.M.; Borad, M.J.; Anthony, S.; Jameson, G.; Brown, S.; Cantafio, N.; Richards, D.A.; et al. Pilot study using molecular profiling of patients’ tumors to find potential targets and select treatments for their refractory cancers. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 4877–4883. [Google Scholar] [CrossRef]
- Doroshow, J.H. Selecting systemic cancer therapy one patient at a time: Is there a role for molecular profiling of individual patients with advanced solid tumors? J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 4869–4871. [Google Scholar] [CrossRef] [PubMed]
- Sosman, J.A.; Kim, K.B.; Schuchter, L.; Gonzalez, R.; Pavlick, A.C.; Weber, J.S.; McArthur, G.A.; Hutson, T.E.; Moschos, S.J.; Flaherty, K.T.; et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N. Engl. J. Med. 2012, 366, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Druker, B.J.; Talpaz, M.; Resta, D.J.; Peng, B.; Buchdunger, E.; Ford, J.M.; Lydon, N.B.; Kantarjian, H.; Capdeville, R.; Ohno-Jones, S.; et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 2001, 344, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Sekulic, A.; Migden, M.R.; Oro, A.E.; Dirix, L.; Lewis, K.D.; Hainsworth, J.D.; Solomon, J.A.; Yoo, S.; Arron, S.T.; Friedlander, P.A.; et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N. Engl. J. Med. 2012, 366, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Tsimberidou, A.M.; Iskander, N.G.; Hong, D.S.; Wheler, J.J.; Falchook, G.S.; Fu, S.; Piha-Paul, S.; Naing, A.; Janku, F.; Luthra, R.; et al. Personalized medicine in a phase I clinical trials program: The MD Anderson Cancer Center initiative. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 6373–6383. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Kim, D.W.; Nakagawa, K.; Seto, T.; Crino, L.; Ahn, M.J.; De Pas, T.; Besse, B.; Solomon, B.J.; Blackhall, F.; et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N. Engl. J. Med. 2013, 368, 2385–2394. [Google Scholar] [CrossRef]
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Press, M.; Mackey, J.; Glaspy, J.; Chan, A.; Pawlicki, M.; et al. Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med. 2011, 365, 1273–1283. [Google Scholar] [CrossRef]
- Maemondo, M.; Inoue, A.; Kobayashi, K.; Sugawara, S.; Oizumi, S.; Isobe, H.; Gemma, A.; Harada, M.; Yoshizawa, H.; Kinoshita, I.; et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 2010, 362, 2380–2388. [Google Scholar] [CrossRef]
- Ramsey, L.B.; Johnson, S.G.; Caudle, K.E.; Haidar, C.E.; Voora, D.; Wilke, R.A.; Maxwell, W.D.; McLeod, H.L.; Krauss, R.M.; Roden, D.M.; et al. The clinical pharmacogenetics implementation consortium guideline for SLCO1B1 and simvastatin-induced myopathy: 2014 update. Clin. Pharmacol. Ther. 2014, 96, 423–428. [Google Scholar] [CrossRef]
- Wilke, R.A.; Ramsey, L.B.; Johnson, S.G.; Maxwell, W.D.; McLeod, H.L.; Voora, D.; Krauss, R.M.; Roden, D.M.; Feng, Q.; Cooper-Dehoff, R.M.; et al. The clinical pharmacogenomics implementation consortium: CPIC guideline for SLCO1B1 and simvastatin-induced myopathy. Clin. Pharmacol. Ther. 2012, 92, 112–117. [Google Scholar] [CrossRef]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Bairey Merz, C.N.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults. J. Am. Coll. Cardiol. 2014, 63, 2889–2934. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, R.C.; Smith, S.C.; Bairey-Merz, C.N.; Grundy, S.M.; Cleeman, J.I.; Lenfant, C. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J. Am. Coll. Cardiol. 2002, 40, 567–572. [Google Scholar] [CrossRef]
- Stroes, E.S.; Thompson, P.D.; Corsini, A.; Vladutiu, G.D.; Raal, F.J.; Ray, K.K.; Roden, M.; Stein, E.; Tokgozoglu, L.; Nordestgaard, B.G.; et al. Statin-associated muscle symptoms: Impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur. Heart J. 2015, 36, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Després, J.-P.; Fullerton, H.J.; Howard, V.J.; et al. Executive Summary: Heart Disease and Stroke Statistics—2015 Update. Circulation 2015, 131, 434–441. [Google Scholar] [CrossRef]
- Alfirevic, A.; Neely, D.; Armitage, J.; Chinoy, H.; Cooper, R.G.; Laaksonen, R.; Carr, D.F.; Bloch, K.M.; Fahy, J.; Hanson, A.; et al. Phenotype standardization for statin-induced myotoxicity. Clin. Pharmacol. Ther. 2014, 96, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Ong, F.S.; Deignan, J.L.; Kuo, J.Z.; Bernstein, K.E.; Rotter, J.I.; Grody, W.W.; Das, K. Clinical utility of pharmacogenetic biomarkers in cardiovascular therapeutics: A challenge for clinical implementation. Pharmacogenomics 2012, 13, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Voora, D.; Ginsburg, G.S. Clinical application of cardiovascular pharmacogenetics. J. Am. Coll. Cardiol. 2012, 60, 9–20. [Google Scholar] [CrossRef]
- Patel, J.; Superko, H.R.; Martin, S.S.; Blumenthal, R.S.; Christopher-Stine, L. Genetic and immunologic susceptibility to statin-related myopathy. Atherosclerosis 2015, 240, 260–271. [Google Scholar] [CrossRef]
- Hirsh, B.J.; Smilowitz, N.R.; Rosenson, R.S.; Fuster, V.; Sperling, L.S. Utilization of and Adherence to Guideline-Recommended Lipid-Lowering Therapy After Acute Coronary Syndrome: Opportunities for Improvement. J. Am. Coll. Cardiol. 2015, 66, 184–192. [Google Scholar] [CrossRef]
- Osterberg, L.; Blaschke, T. Adherence to medication. N. Engl. J. Med. 2005, 353, 487–497. [Google Scholar] [CrossRef]
- Niemi, M.; Pasanen, M.K.; Neuvonen, P.J. Organic anion transporting polypeptide 1B1: A genetically polymorphic transporter of major importance for hepatic drug uptake. Pharm. Rev. 2011, 63, 157–181. [Google Scholar] [CrossRef] [PubMed]
- Greenland, P.; Lauer, M.S. Cholesterol Lowering in 2015: Still Answering Questions About How and in Whom. JAMA 2015, 314, 127–128. [Google Scholar] [CrossRef]
- Thompson, P.D.; Clarkson, P.M.; Rosenson, R.S. National Lipid Association Statin Safety Task Force Muscle Safety Expert, P. An assessment of statin safety by muscle experts. Am. J. Cardiol. 2006, 97, 69C–76C. [Google Scholar] [CrossRef] [PubMed]
- Search Collaborative Group; Link, E.; Parish, S.; Armitage, J.; Bowman, L.; Heath, S.; Matsuda, F.; Gut, I.; Lathrop, M.; Collins, R. SLCO1B1 variants and statin-induced myopathy--a genomewide study. N. Engl. J. Med. 2008, 359, 789–799. [Google Scholar] [PubMed]
- Pencina, M.J.; Navar-Boggan, A.M.; D’Agostino, R.B., Sr.; Williams, K.; Neely, B.; Sniderman, A.D.; Peterson, E.D. Application of new cholesterol guidelines to a population-based sample. N. Engl. J. Med. 2014, 370, 1422–1431. [Google Scholar] [CrossRef] [PubMed]
- Bermingham, M.; Hayden, J.; Dawkins, I.; Miwa, S.; Gibson, D.; McDonald, K.; Ledwidge, M. Prospective analysis of LDL-C goal achievement and self-reported medication adherence among statin users in primary care. Clin. Ther. 2011, 33, 1180–1189. [Google Scholar] [CrossRef]
- Ho, P.M.; Magid, D.J.; Shetterly, S.M.; Olson, K.L.; Maddox, T.M.; Peterson, P.N.; Masoudi, F.A.; Rumsfeld, J.S. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am. Heart J. 2008, 155, 772–779. [Google Scholar] [CrossRef]
- Vodonos, A.; Ostapenko, I.; Toledano, R.; Henkin, Y.; Zahger, D.; Wolak, T.; Sherf, M.; Novack, V. Statin adherence and LDL cholesterol levels. Should we assess adherence prior to statin upgrade? Eur. J. Intern. Med. 2015, 26, 268–272. [Google Scholar] [CrossRef]
- Franklin, J.M.; Krumme, A.A.; Tong, A.Y.; Shrank, W.H.; Matlin, O.S.; Brennan, T.A.; Choudhry, N.K. Association between trajectories of statin adherence and subsequent cardiovascular events. Pharm. Drug Saf. 2015, 24, 1105–1113. [Google Scholar] [CrossRef]
- Pasanen, M.K.; Backman, J.T.; Neuvonen, P.J.; Niemi, M. Frequencies of single nucleotide polymorphisms and haplotypes of organic anion transporting polypeptide 1B1 SLCO1B1 gene in a Finnish population. Eur. J. Clin. Pharm. 2006, 62, 409–415. [Google Scholar] [CrossRef]
- Li, J.H.; Joy, S.V.; Haga, S.B.; Orlando, L.A.; Kraus, W.E.; Ginsburg, G.S.; Voora, D. Genetically guided statin therapy on statin perceptions, adherence, and cholesterol lowering: A pilot implementation study in primary care patients. J. Pers. Med. 2014, 4, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, L.A.; Doney, A.S.; Tavendale, R.; Lang, C.C.; Pearson, E.R.; Colhoun, H.M.; McCarthy, M.I.; Hattersley, A.T.; Morris, A.D.; Palmer, C.N. Common nonsynonymous substitutions in SLCO1B1 predispose to statin intolerance in routinely treated individuals with type 2 diabetes: A go-DARTS study. Clin. Pharmacol. Ther. 2011, 89, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Birmingham, B.K.; Bujac, S.R.; Elsby, R.; Azumaya, C.T.; Wei, C.; Chen, Y.; Mosqueda-Garcia, R.; Ambrose, H.J. Impact of ABCG2 and SLCO1B1 polymorphisms on pharmacokinetics of rosuvastatin, atorvastatin and simvastatin acid in Caucasian and Asian subjects: A class effect? Eur. J. Clin. Pharm. 2015, 71, 341–355. [Google Scholar] [CrossRef] [PubMed]
- de Keyser, C.E.; Peters, B.J.; Becker, M.L.; Visser, L.E.; Uitterlinden, A.G.; Klungel, O.H.; Verstuyft, C.; Hofman, A.; Maitland-van der Zee, A.H.; Stricker, B.H. The SLCO1B1 c. 521T> C polymorphism is associated with dose decrease or switching during statin therapy in the Rotterdam Study. Pharm. Genom. 2014, 24, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Voora, D.; Shah, S.H.; Spasojevic, I.; Ali, S.; Reed, C.R.; Salisbury, B.A.; Ginsburg, G.S. The SLCO1B1*5 genetic variant is associated with statin-induced side effects. J. Am. Coll. Cardiol. 2009, 54, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Danik, J.S.; Chasman, D.I.; MacFadyen, J.G.; Nyberg, F.; Barratt, B.J.; Ridker, P.M. Lack of association between SLCO1B1 polymorphisms and clinical myalgia following rosuvastatin therapy. Am. Heart J. 2013, 165, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.G.; Li, K.W.; Murray, H.; Putt, W.; Packard, C.J.; Humphries, S.E. The effects of a single nucleotide polymorphism in SLCO1B1 on the pharmacodynamics of pravastatin. Br. J. Clin. Pharmacol. 2012, 73, 303–306. [Google Scholar] [CrossRef][Green Version]
- Taylor, F.; Huffman, M.D.; Macedo, A.F.; Moore, T.H.; Burke, M.; Davey Smith, G.; Ward, K.; Ebrahim, S. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2013, 31, CD004816. [Google Scholar] [CrossRef]
- De Vera, M.A.; Bhole, V.; Burns, L.C.; Lacaille, D. Impact of statin adherence on cardiovascular disease and mortality outcomes: A systematic review. Br. J. Clin. Pharmacol. 2014, 78, 684–698. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists Collaborators. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: Meta-analysis of individual data from 27 randomised trials. Lancet 2012, 380, 581–590. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists Collaborators. Efficacy and safety of LDL-lowering therapy among men and women: Meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet 2015, 385, 1397–1405. [Google Scholar] [CrossRef]
- Hou, Q.; Li, S.; Li, L.; Li, Y.; Sun, X.; Tian, H. Association Between SLCO1B1 Gene T521C Polymorphism and Statin-Related Myopathy Risk: A Meta-Analysis of Case-Control Studies. Medicine 2015, 94, e1268. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.M.; Nau, D.P.; Cramer, J.A.; Benner, J.; Gwadry-Sridhar, F.; Nichol, M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health J. Int. Soc. Pharm. Outcomes Res. 2007, 10, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Cholesterol Treatment Trialists Collaborators. CTT Collaboration. Available online: https://www.cttcollaboration.org/ (accessed on 26 February 2019).
- Eckhardt, K.; Li, S.; Ammon, S.; Schanzle, G.; Mikus, G.; Eichelbaum, M. Same incidence of adverse drug events after codeine administration irrespective of the genetically determined differences in morphine formation. Pain 1998, 76, 27–33. [Google Scholar] [CrossRef]
- Temel, J.S.; Greer, J.A.; Muzikansky, A.; Gallagher, E.R.; Admane, S.; Jackson, V.A.; Dahlin, C.M.; Blinderman, C.D.; Jacobsen, J.; Pirl, W.F.; et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N. Engl. J. Med. 2010, 363, 733–742. [Google Scholar] [CrossRef]
- Angst, M.S.; Phillips, N.G.; Drover, D.R.; Tingle, M.; Ray, A.; Swan, G.E.; Lazzeroni, L.C.; Clark, J.D. Pain sensitivity and opioid analgesia: A pharmacogenomic twin study. Pain 2012, 153, 1397–1409. [Google Scholar] [CrossRef]
- Lotsch, J.; Rohrbacher, M.; Schmidt, H.; Doehring, A.; Brockmoller, J.; Geisslinger, G. Can extremely low or high morphine formation from codeine be predicted prior to therapy initiation? Pain 2009, 144, 119–124. [Google Scholar] [CrossRef]
- Samer, C.F.; Daali, Y.; Wagner, M.; Hopfgartner, G.; Eap, C.B.; Rebsamen, M.C.; Rossier, M.F.; Hochstrasser, D.; Dayer, P.; Desmeules, J.A. The effects of CYP2D6 and CYP3A activities on the pharmacokinetics of immediate release oxycodone. Br. J. Pharm. 2010, 160, 907–918. [Google Scholar] [CrossRef]
- Zwisler, S.T.; Enggaard, T.P.; Noehr-Jensen, L.; Pedersen, R.S.; Mikkelsen, S.; Nielsen, F.; Brosen, K.; Sindrup, S.H. The hypoalgesic effect of oxycodone in human experimental pain models in relation to the CYP2D6 oxidation polymorphism. Basic Clin. Pharm. Toxicol. 2009, 104, 335–344. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Adult Cancer Pain. Version 2. 2016. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx (accessed on 21 August 2019).
- Susce, M.T.; Murray-Carmichael, E.; de Leon, J. Response to hydrocodone, codeine and oxycodone in a CYP2D6 poor metabolizer. Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 30, 1356–1358. [Google Scholar] [CrossRef] [PubMed]
- Ciszkowski, C.; Madadi, P.; Phillips, M.S.; Lauwers, A.E.; Koren, G. Codeine, ultrarapid-metabolism genotype, and postoperative death. N. Engl. J. Med. 2009, 361, 827–828. [Google Scholar] [CrossRef] [PubMed]
- Gasche, Y.; Daali, Y.; Fathi, M.; Chiappe, A.; Cottini, S.; Dayer, P.; Desmeules, J. Codeine intoxication associated with ultrarapid CYP2D6 metabolism. N. Engl. J. Med. 2004, 351, 2827–2831. [Google Scholar] [CrossRef] [PubMed]
- Rawlins, M. De Testimonio: On the evidence for decisions about the use of therapeutic interventions. Clin. Med. (Lond. Engl.) 2008, 8, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Murad, M.H.; Asi, N.; Alsawas, M.; Alahdab, F. New evidence pyramid. Evid. Based Med. 2016, 21, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Amur, S.; LaVange, L.; Zineh, I.; Buckman-Garner, S.; Woodcock, J. Biomarker Qualification: Toward a Multiple Stakeholder Framework for Biomarker Development, Regulatory Acceptance, and Utilization. Clin. Pharmacol. Ther. 2015, 98, 34–46. [Google Scholar] [CrossRef]
- Gammie, T.; Lu, C.Y.; Babar, Z.U. Access to Orphan Drugs: A Comprehensive Review of Legislations, Regulations and Policies in 35 Countries. PLoS ONE 2015, 10, e0140002. [Google Scholar] [CrossRef]
- Hughes, D.A.; Plumpton, C.O. Rare disease prevention and treatment: The need for a level playing field. Pharmacogenomics 2018, 19, 243–247. [Google Scholar] [CrossRef]
- Green, M.J.; Botkin, J.R. “Genetic exceptionalism” in medicine: Clarifying the differences between genetic and nongenetic tests. Ann. Intern. Med. 2003, 138, 571–575. [Google Scholar] [CrossRef]
- The Lancet. Information for Authors. Available online: https://els-jbs-prod-cdn.literatumonline.com/pb/assets/raw/Lancet/authors/tl-info-for-authors-1530878364923.pdf (accessed on 18 October 2018).
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schunemann, H.J.; Group, G.W. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Altar, C.A.; Amakye, D.; Bounos, D.; Bloom, J.; Clack, G.; Dean, R.; Devanarayan, V.; Fu, D.; Furlong, S.; Hinman, L.; et al. A prototypical process for creating evidentiary standards for biomarkers and diagnostics. Clin. Pharmacol. Ther. 2008, 83, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernan, M.A.; Reeves, B.C.; Savovic, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zhang, Y.; Kwong, J.S.; Zhang, C.; Li, S.; Sun, F.; Niu, Y.; Du, L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: A systematic review. J. Evid. Based Med. 2015, 8, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, A.L.; Williamson, P.R. Methodological quality of pharmacogenetic studies: Issues of concern. Stat. Med. 2008, 27, 6547–6569. [Google Scholar] [CrossRef] [PubMed]
- Geller, S.E.; Koch, A.; Pellettieri, B.; Carnes, M. Inclusion, analysis, and reporting of sex and race/ethnicity in clinical trials: Have we made progress? J. Womens Health 2011, 20, 315–320. [Google Scholar] [CrossRef]
- Ghafoor, A.; Jemal, A.; Ward, E.; Cokkinides, V.; Smith, R.; Thun, M. Trends in breast cancer by race and ethnicity. CA Cancer J. Clin. 2003, 53, 342–355. [Google Scholar] [CrossRef]
- Ward, E.; Jemal, A.; Cokkinides, V.; Singh, G.K.; Cardinez, C.; Ghafoor, A.; Thun, M. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J. Clin. 2004, 54, 78–93. [Google Scholar] [CrossRef]
- Popejoy, A.B.; Fullerton, S.M. Genomics is failing on diversity. Nature 2016, 538, 161–164. [Google Scholar] [CrossRef]
- Murthy, V.H.; Krumholz, H.M.; Gross, C.P. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA 2004, 291, 2720–2726. [Google Scholar] [CrossRef] [PubMed]
- Suther, S.; Kiros, G.E. Barriers to the use of genetic testing: A study of racial and ethnic disparities. Genet. Med. 2009, 11, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Forman, A.D.; Hall, M.J. Influence of race/ethnicity on genetic counseling and testing for hereditary breast and ovarian cancer. Breast J. 2009, 15, S56–S62. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, J.; Oliver, S.; Lorenc, T. Reflections on developing and using PROGRESS-Plus. Equity Update 2008, 2, 1–3. [Google Scholar]
- Sterne, J.A.; Sutton, A.J.; Ioannidis, J.P.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rucker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef] [PubMed]
- Relling, M.V.; Klein, T.E. CPIC: Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clin. Pharmacol. Ther. 2011, 89, 464–467. [Google Scholar] [CrossRef] [PubMed]
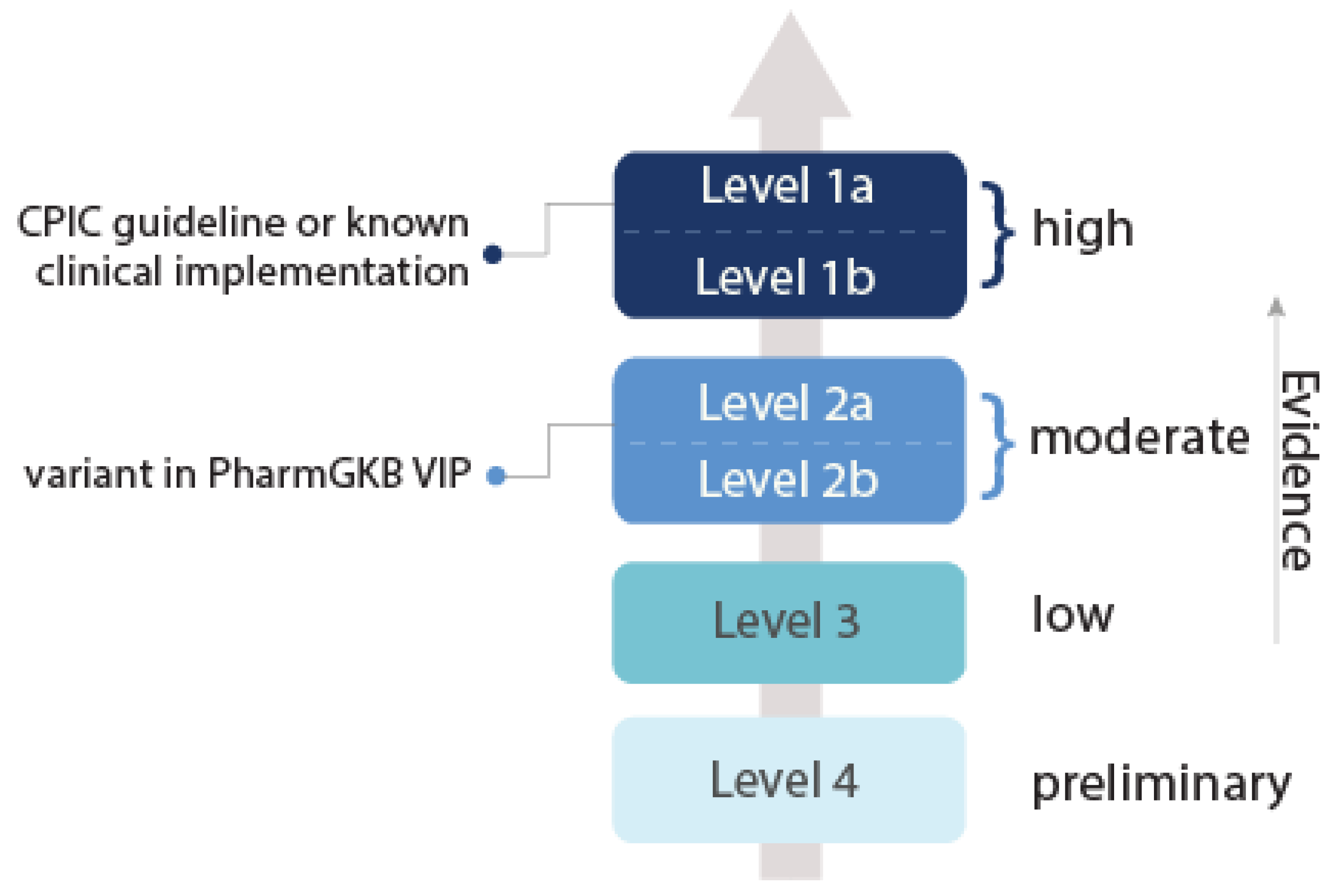
- Clinical Pharmacogenetics Implementation Consortium. Levels of Evidence. Available online: https://cpicpgx.org/levels-of-evidence/ (accessed on 25 March 2019).
- PharmGKB. Clinical Annotation Levels of Evidence. Available online: https://www.pharmgkb.org/page/clinAnnLevels (accessed on 25 March 2018).
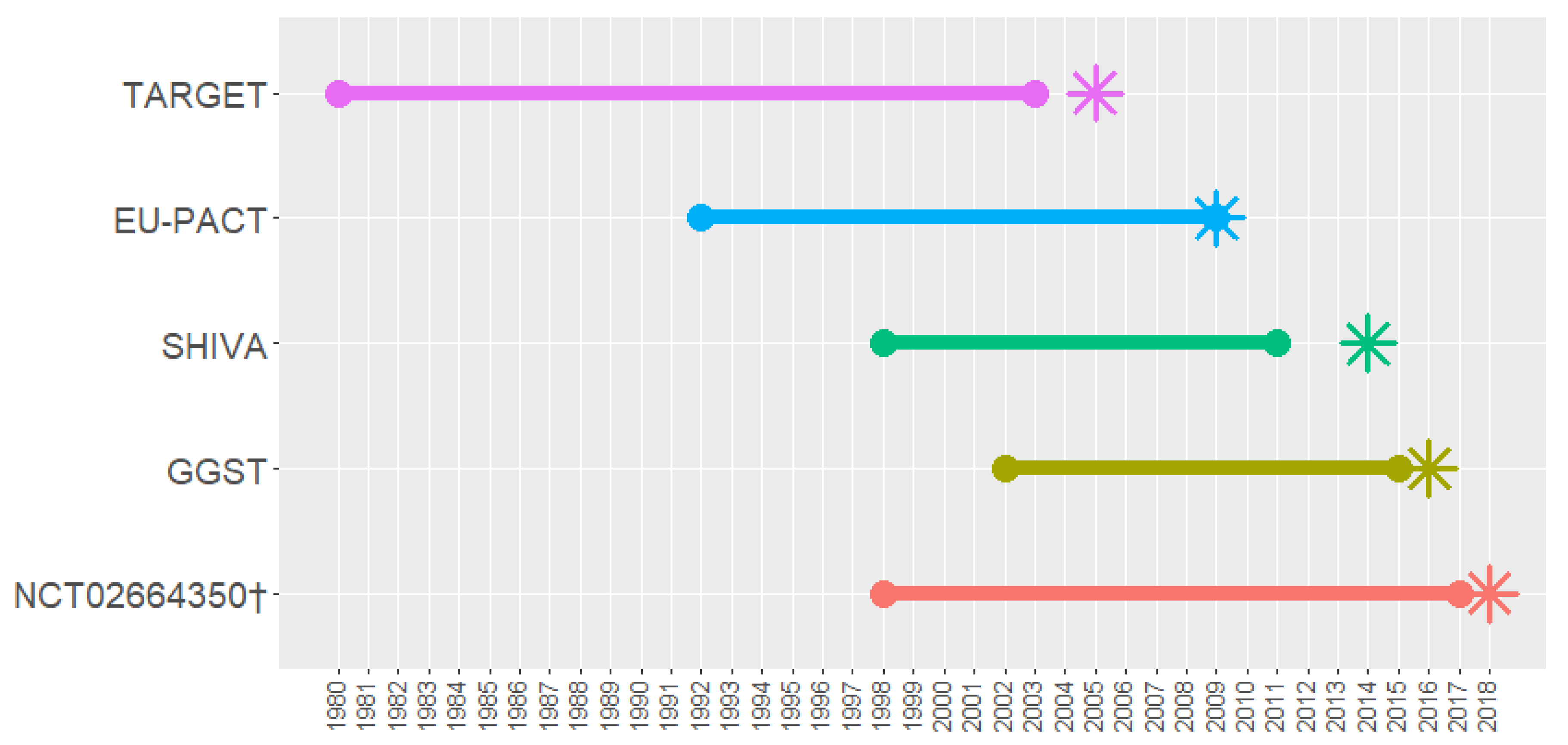
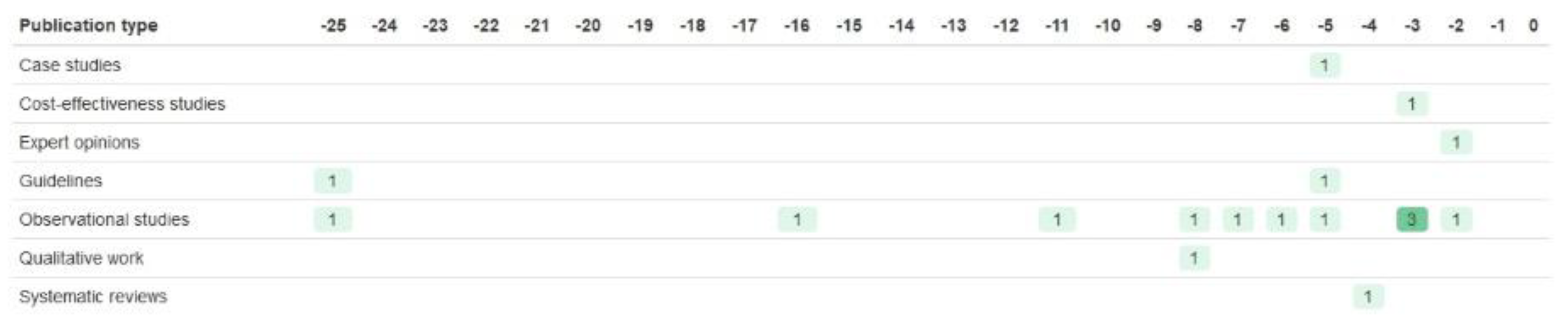
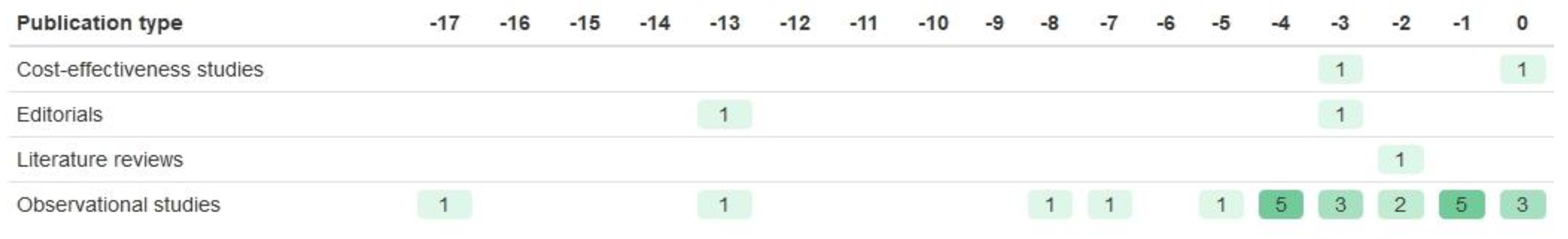
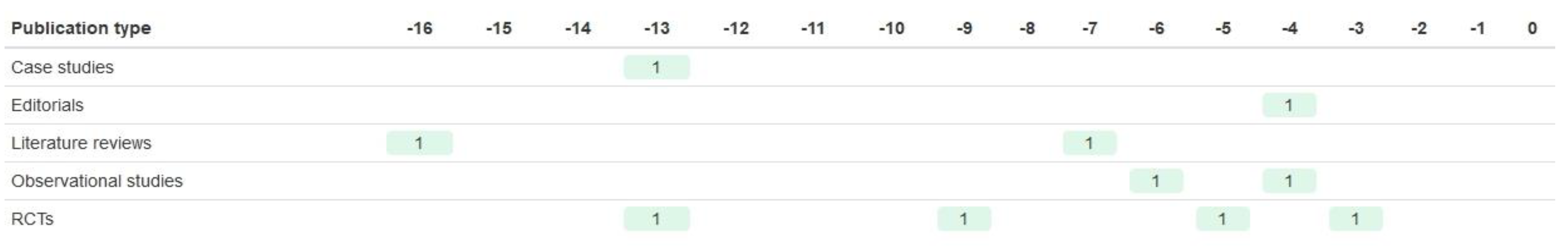
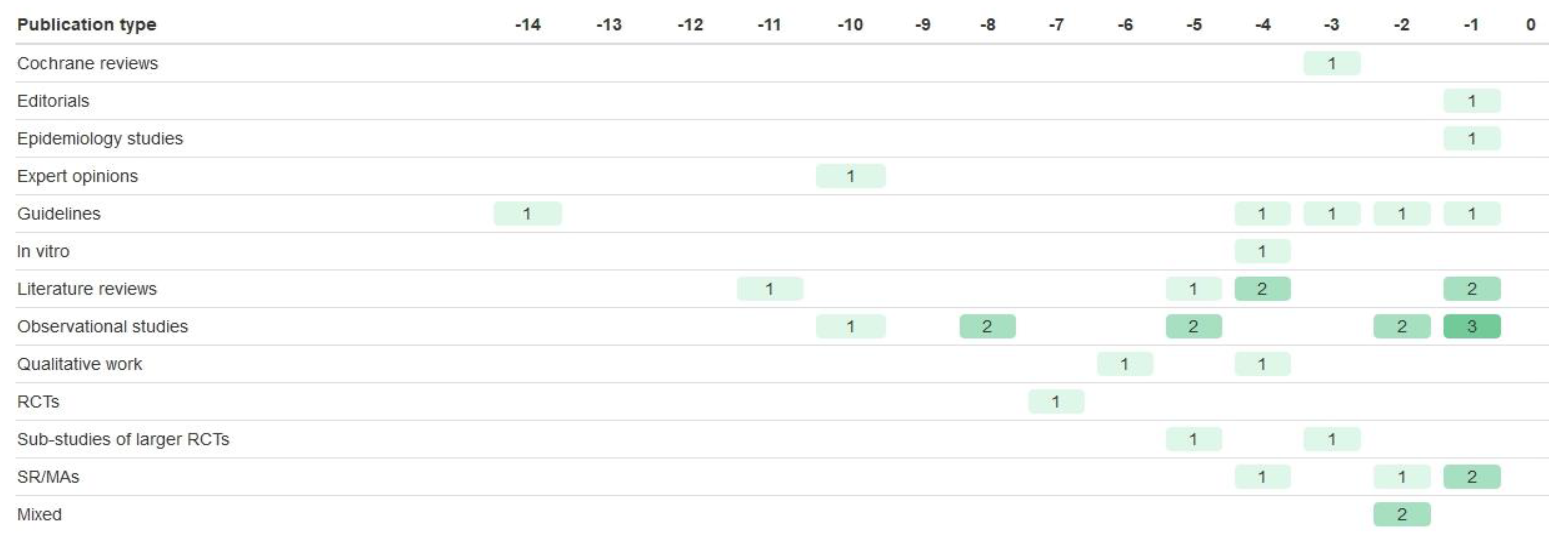
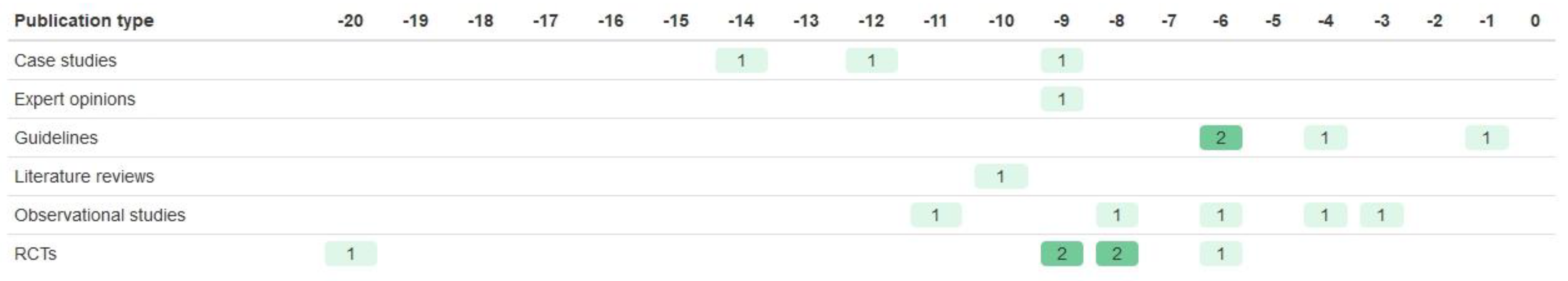
| Registration Number | Trial Name | Start Year | Year of Results Publication | Paper References Taken from | BM Trial Design | Biomarker | Drug of Interest | Sample Size (n Randomised) | Age of Participants | Sex of Participants | Ethnicity of Participants | Study Location |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ISRCTN30748308 | TARGET (protocol) [10,47] | 2005 | 2011 | 2005 protocol obtained from authors | Biomarker strategy design (without biomarker assessment in control arm) | TPMT | Azathioprine | 333 | Mean 43.2 (control) | 50.6%/49.4% F/M (control) | 92.2% white, 4.8% South Asian, 0.6% Black, 2.4% mixed/other (control) | UK |
| Mean 41.0 (genotyped) | 50.3%/49.7% F/M (genotyped) | 89.8% white, 7.2% South Asian, 3.0% Black, 0% mixed/other (genotyped) | ||||||||||
| NCT01119300 | EU-PACT [49] | 2011 | 2013 | 2009 paper 10.2217/pgs.09.125 | Biomarker strategy design (without biomarker assessment in control arm) | CYP2C9*2 | Warfarin | 455 | Mean 66.9 (control) | 42.1%/57.9% F/M (control) | 98.7% white, 0.9% Black, 0.4% Asian (control) | UK, Sweden |
| CYP2C9*3 | Mean 67.8 (genotyped) | 35.8%/64.2% F/M (genotyped) | 98.2% white, 1.3% Black, 0.4% Asian (genotyped) | |||||||||
| VKORC1 | ||||||||||||
| NCT01771458 | SHIVA [43] (protocol) | 2012 | 2015 | 2014 protocol obtained from authors | Enrichment design | Hormone receptors pathway | Targeted chemotherapy agent, based on genotyping | 195 | Median 63 (control) | 72%/28% F/M (control) | Not reported | France |
| PI3K/AKT/mTOR pathway | Median 61 (genotyped) | 61%/39% F/M (genotyped) | ||||||||||
| RAF/MEK pathway | ||||||||||||
| NCT01894230 | GGST statin trial [44] | 2013 | 2018 | 2016 paper 10.2217/pgs-2016-0065 | Biomarker strategy design (with biomarker assessment in control arm) | SLCO1B1*5 | Any statin | 159 | Mean 62.5 (control) | 65.8%/34.2% F/M (control) | 80.3% white, 14.5% Black, 5.3% other (control) | USA |
| Mean 62.7 (genotyped) | 49.4%/50.6% F/M (genotyped) | 79.5% white, 16.9% Black, 3.6% other (genotyped) | ||||||||||
| NCT02664350 | n/a [46] | 2016 | Results not yet published | 2018 paper 10.1016/j.cct.2018.03.001 | Biomarker strategy design (without biomarker assessment in control arm) | CYP2D6 | Opioids | 200 (forecast) | Not available | Not available | Not available | USA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, D.; Hughes, D.; Pirmohamed, M.; Jorgensen, A. Evidence to Support Inclusion of Pharmacogenetic Biomarkers in Randomised Controlled Trials. J. Pers. Med. 2019, 9, 42. https://doi.org/10.3390/jpm9030042
Johnson D, Hughes D, Pirmohamed M, Jorgensen A. Evidence to Support Inclusion of Pharmacogenetic Biomarkers in Randomised Controlled Trials. Journal of Personalized Medicine. 2019; 9(3):42. https://doi.org/10.3390/jpm9030042
Chicago/Turabian StyleJohnson, Danielle, Dyfrig Hughes, Munir Pirmohamed, and Andrea Jorgensen. 2019. "Evidence to Support Inclusion of Pharmacogenetic Biomarkers in Randomised Controlled Trials" Journal of Personalized Medicine 9, no. 3: 42. https://doi.org/10.3390/jpm9030042
APA StyleJohnson, D., Hughes, D., Pirmohamed, M., & Jorgensen, A. (2019). Evidence to Support Inclusion of Pharmacogenetic Biomarkers in Randomised Controlled Trials. Journal of Personalized Medicine, 9(3), 42. https://doi.org/10.3390/jpm9030042





