Personalized Assessment of the Coronary Atherosclerotic Arteries by Intravascular Ultrasound Imaging: Hunting the Vulnerable Plaque
Abstract
:1. Introduction
2. Vulnerable Plaque
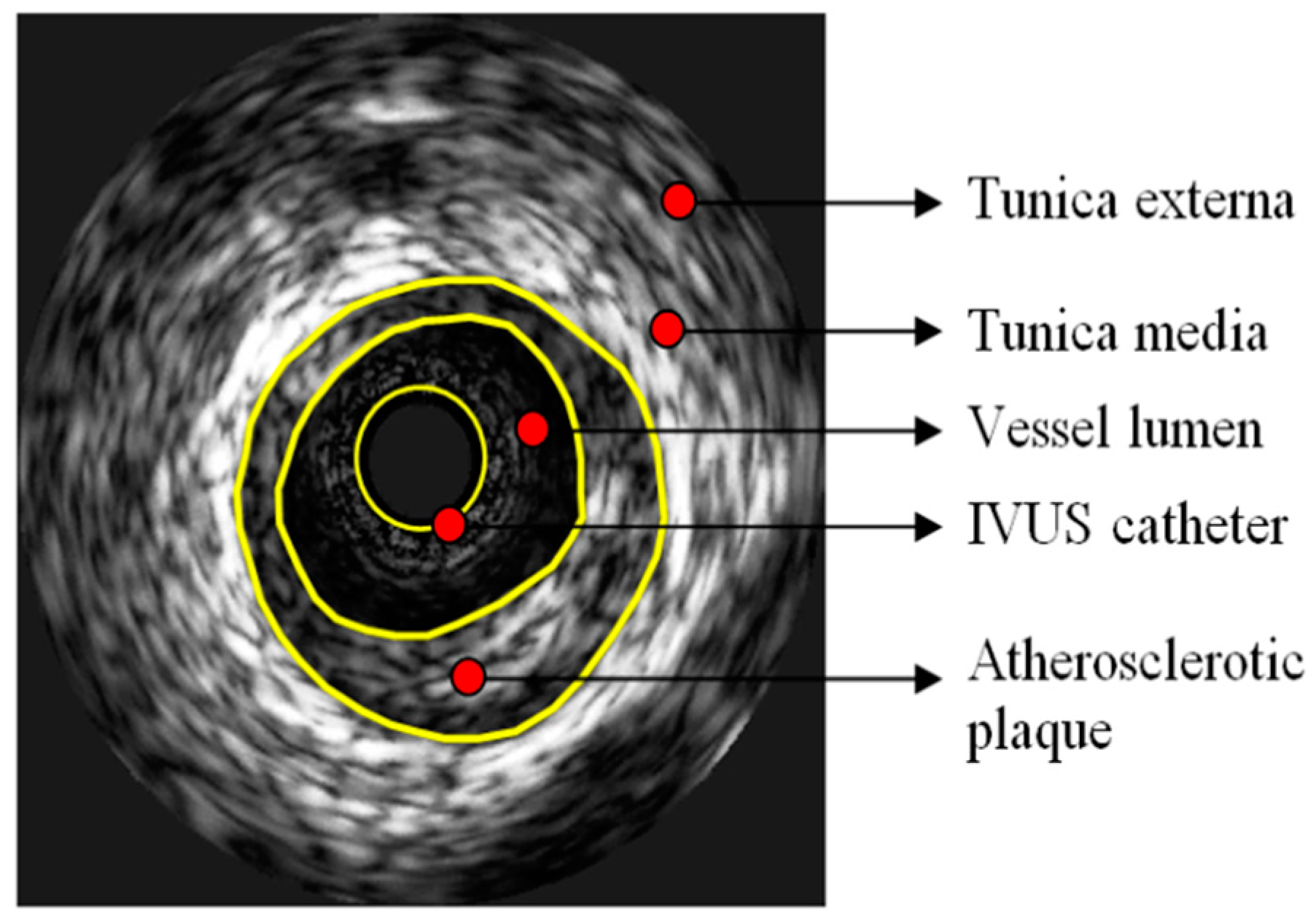
2.1. Intravascular Ultrasound: IVUS
2.2. Ruptured Plaque
2.3. Calcification
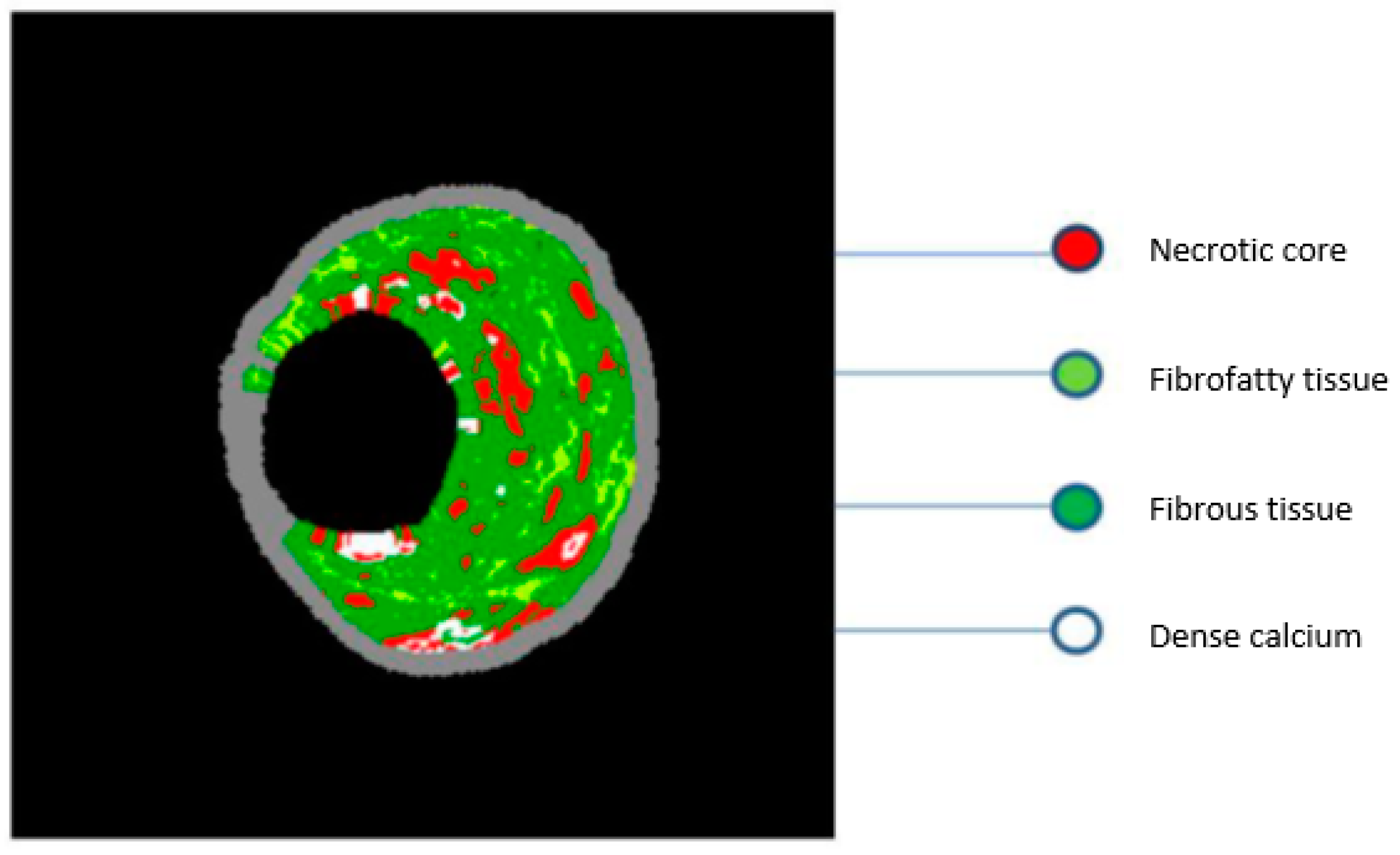
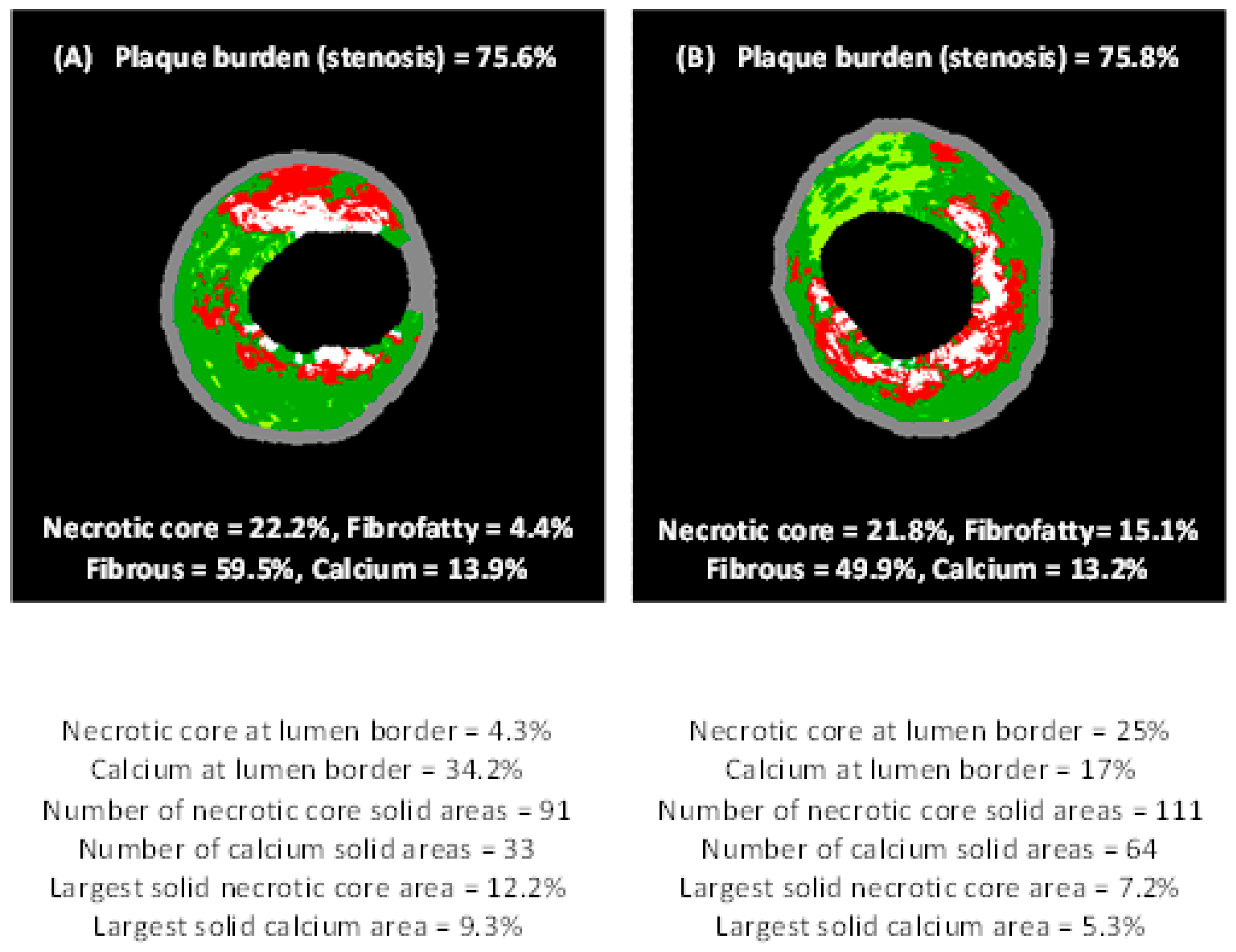
2.4. Virtual Histology–IVUS
3. Clinical Applications of IVUS
3.1. Assessment of Intermediate Coronary Lesions
3.2. Non-Left Main Lesions
3.3. Left Main Disease
3.4. IVUS-Guided Interventions
4. IVUS in Various Stages of Coronary Intervention
4.1. Prior to Intervention, Selection of Optimal Stent Sizing
4.2. Optimization after Stent Implantation
4.3. Assessment of the Mechanism of Stent Failure
4.4. New Developments in IVUS Imaging
5. Conclusions
Funding
Conflicts of Interest
References
- Falk, E. Plaque rupture with severe pre-existing stenosis precipitating coronary thrombosis. Characteristics of coronary atherosclerotic plaques underlying fatal occlusive thrombi. Br. Heart J. 1983, 50, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Van den Bovenkamp, G.J. Role of thrombus in plaque formation in the human diseased coronary artery. Br. J. Exp. Pathol. 1966, 47, 550–557. [Google Scholar] [PubMed]
- Davies, M.J. A macro and micro view of coronary vascular insult in ischemic heart disease. Circulation 1990, 82, 38–46. [Google Scholar]
- Libby, P.; Theroux, P. Pathophysiology of coronary artery disease. Circulation 2005, 111, 3481–3488. [Google Scholar] [CrossRef] [PubMed]
- Kalampogias, A.; Siasos, G.; Oikonomou, E.; Tsalamandris, S.; Mourouzis, K.; Tsigkou, V.; Vavuranakis, M.; Zografos, T.; Deftereos, S.; Stefanadis, C.; et al. Basic Mechanisms in Atherosclerosis: The Role of Calcium. Med. Chem. 2016, 12, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Stary, H.C.; Chandler, A.B.; Dinsmore, R.E.; Fuster, V.; Glagov, S.; Insull, W., Jr.; Rosenfeld, M.E.; Schwartz, C.J.; Wagner, W.D.; Wissler, R.W. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1995, 92, 1355–1374. [Google Scholar] [CrossRef] [PubMed]
- Tuzcu, E.M.; Kapadia, S.R.; Tutar, E.; Ziada, K.M.; Hobbs, R.E.; McCarthy, P.M.; Young, J.B.; Nissen, S.E. High prevalence of coronary atherosclerosis in asymptomatic teenagers and young adults: Evidence from intravascular ultrasound. Circulation 2001, 103, 2705–2710. [Google Scholar] [CrossRef] [PubMed]
- Stefanadis, C.; Antoniou, C.-K.; Tsiachris, D.; Pietri, P. Coronary Atherosclerotic Vulnerable Plaque: Current Perspectives. J. Am. Heart Assoc. 2017, 6, e005543. [Google Scholar] [CrossRef]
- Liang, M.; Puri, A.; Devlin, G. The Vulnerable Plaque: The Real Villain in Acute Coronary Syndromes. Open Cardiovasc. Med. J. 2011, 5, 123–129. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef]
- Naghavi, M.; Libby, P.; Falk, E.; Casscells, S.W.; Litovsky, S.; Rumberger, J.; Badimon, J.J.; Stefanadis, C.; Moreno, P.; Pasterkamp, G.; et al. From vulnerable plaque to vulnerable patient: A call for new definitions and risk assessment strategies: Part, I. Circulation 2003, 108, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Arbustini, E.; Dal Bello, B.; Morbini, P.; Burke, A.P.; Bocciarelli, M.; Specchia, G.; Virmani, R. Plaque erosion is a major substrate for coronary thrombosis in acute myocardial infarction. Heart 1999, 82, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Zhou, B.; Hsiai, T.K.; Shung, K.K. A Review of Intravascular Ultrasound–Based Multimodal Intravascular Imaging: The Synergistic Approach to Characterizing Vulnerable Plaques. Ultrason Imaging 2016, 38, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Kolodgie, F.D.; Burke, A.P.; Farb, A.; Gold, H.K.; Yuan, J.; Narula, J.; Finn, A.V.; Virmani, R. The thin-cap fibroatheroma: A type of vulnerable plaque: The major precursor lesion to acute coronary syndromes. Curr. Opin. Cardiol. 2001, 16, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Burke, A.P.; Farb, A.; Kolodgie, F.D. Pathology of the vulnerable plaque. J. Am. Coll. Cardiol. 2006, 47, C13–C18. [Google Scholar] [CrossRef] [PubMed]
- Schaar, J.A.; Muller, J.E.; Falk, E.; Virmani, R.; Fuster, V.; Serruys, P.W.; Colombo, A.; Stefanadis, C.; Ward Casscells, S.; Moreno, P.R.; et al. Terminology for high-risk and vulnerable coronary artery plaques. Report of a meeting on the vulnerable plaque, June 17 and 18, 2003, Santorini, Greece. Eur. Heart J. 2004, 25, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Stefanadis, C.; Vavuranakis, M.; Toutouzas, P. Vulnerable plaque: The challenge to identify and treat it. J. Interv. Cardiol. 2003, 16, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Narula, J.; Nakano, M.; Virmani, R.; Kolodgie, F.D.; Petersen, R.; Newcomb, R.; Malik, S.; Fuster, V.; Finn, A.V. Histopathologic characteristics of atherosclerotic coronary disease and implications of the findings for the invasive and noninvasive detection of vulnerable plaques. J. Am. Coll. Cardiol. 2013, 61, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Toutouzas, K.; Synetos, A.; Nikolaou, C.; Tsiamis, E.; Tousoulis, D.; Stefanadis, C. Matrix metalloproteinases and vulnerable atheromatous plaque. Curr. Top. Med. Chem. 2012, 12, 1166–1180. [Google Scholar] [CrossRef]
- Rodriguez-Granillo, G.A.; Serruys, P.W.; Garcia-Garcia, H.M.; Aoki, J.; Valgimigli, M.; van Mieghem, C.A.G.; McFadden, E.; de Jaegere, P.P.T.; de Feyter, P. Coronary artery remodelling is related to plaque composition. Heart 2006, 92, 388–391. [Google Scholar] [CrossRef]
- Nakatani, S.; Proniewska, K.; Pociask, E.; Paoletti, G.; de Winter, S.; Muramatsu, T.; Bruining, N. How clinically effective is intravascular ultrasound in interventional cardiology? Present and future perspectives. Expert Rev. Med. Devices 2013, 10, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Batkoff, B.W.; Linker, D.T. Safety of intracoronary ultrasound: Data from a Multicenter European Registry. Cathet. Cardiovasc. Diagn. 1996, 38, 238–241. [Google Scholar] [CrossRef]
- Mintz, G.S.; Nissen, S.E.; Anderson, W.D.; Bailey, S.R.; Erbel, R.; Fitzgerald, P.J.; Pinto, F.J.; Rosenfield, K.; Siegel, R.J.; Tuzcu, E.M. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J. Am. Coll. Cardiol. 2001, 37, 1478–1492. [Google Scholar] [PubMed]
- Maehara, A.; Mintz, G.S.; Bui, A.B.; Walter, O.R.; Castagna, M.T.; Canos, D.; Pichard, A.D.; Satler, L.F.; Waksman, R.; Suddath, W.O.; et al. Morphologic and angiographic features of coronary plaque rupture detected by intravascular ultrasound. J. Am. Coll. Cardiol. 2002, 40, 904–910. [Google Scholar] [CrossRef]
- Higuma, T.; Soeda, T.; Abe, N.; Yamada, M.; Yokoyama, H.; Shibutani, S.; Vergallo, R.; Minami, Y.; Ong, D.S.; Lee, H.; et al. A Combined Optical Coherence Tomography and Intravascular Ultrasound Study on Plaque Rupture, Plaque Erosion, and Calcified Nodule in Patients with ST-Segment Elevation Myocardial Infarction: Incidence, Morphologic Characteristics, and Outcomes After Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2015, 8, 1166–1176. [Google Scholar] [PubMed]
- Kusama, I.; Hibi, K.; Kosuge, M.; Nozawa, N.; Ozaki, H.; Yano, H.; Sumita, S.; Tsukahara, K.; Okuda, J.; Ebina, T.; et al. Impact of plaque rupture on infarct size in ST-segment elevation anterior acute myocardial infarction. J. Am. Coll. Cardiol. 2007, 50, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Soeda, T.; Higuma, T.; Abe, N.; Yamada, M.; Yokoyama, H.; Shibutani, S.; Ong, D.S.; Vergallo, R.; Minami, Y.; Lee, H.; et al. Morphological predictors for no reflow phenomenon after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction caused by plaque rupture. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 103–110. [Google Scholar] [CrossRef]
- Abdul Jabbar, A.; Houston, J.; Burket, M.; Il’Giovine, Z.J.; Srivastava, B.K.; Agarwal, A. Screening for subclinical subclavian artery stenosis before coronary artery bypass grafting: Should we do it? Echocardiography 2017, 34, 928–933. [Google Scholar] [CrossRef]
- Burke, A.P.; Kolodgie, F.D.; Farb, A.; Weber, D.K.; Malcom, G.T.; Smialek, J.; Virmani, R. Healed plaque ruptures and sudden coronary death: Evidence that subclinical rupture has a role in plaque progression. Circulation 2001, 103, 934–940. [Google Scholar] [CrossRef]
- Mintz, G.S. Intravascular imaging of coronary calcification and its clinical implications. JACC Cardiovasc. Imaging 2015, 8, 461–471. [Google Scholar] [CrossRef]
- Kataoka, Y.; Wolski, K.; Uno, K.; Puri, R.; Tuzcu, E.M.; Nissen, S.E.; Nicholls, S.J. Spotty calcification as a marker of accelerated progression of coronary atherosclerosis: Insights from serial intravascular ultrasound. J. Am. Coll. Cardiol. 2012, 59, 1592–1597. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Mintz, G.S.; Biro, S.; Lee, J.B.; Sum, S.T.; Madden, S.P.; Burke, A.P.; Zhang, P.; He, B.; Goldstein, J.A.; et al. Insights into echo-attenuated plaques, echolucent plaques, and plaques with spotty calcification: Novel findings from comparisons among intravascular ultrasound, near-infrared spectroscopy, and pathological histology in 2294 human coronary artery segments. J. Am. Coll. Cardiol. 2014, 63, 2220–2233. [Google Scholar] [CrossRef] [PubMed]
- Koneru, S.; Collier, P.; Goldberg, A.; Sanghi, V.; Grimm, R.; Rodriguez, L.; Griffin, B.; Budd, T.; James, K.; Popovic, Z.B.; et al. Temporal Variability of Global Longitudinal Strain in Stable Patients Undergoing Chemotherapy with Trastuzumab. Am. J. Cardiol. 2016, 118, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Genereux, P.; Madhavan, M.V.; Mintz, G.S.; Maehara, A.; Palmerini, T.; Lasalle, L.; Xu, K.; McAndrew, T.; Kirtane, A.; Lansky, A.J.; et al. Ischemic outcomes after coronary intervention of calcified vessels in acute coronary syndromes. Pooled analysis from the HORIZONS-AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) and ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) TRIALS. J. Am. Coll. Cardiol. 2014, 63, 1845–1854. [Google Scholar] [PubMed]
- Gogas, B.D.; Farooq, V.; Serruys, P.W.; Hector, M. Garcìa-Garcìa. Assessment of coronary atherosclerosis by IVUS and IVUS-based imaging modalities: Progression and regression studies, tissue composition and beyond. Int. J. Cardiovasc. Imaging 2011, 27, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Maehara, A.; Lansky, A.J.; de Bruyne, B.; Cristea, E.; Mintz, G.S.; Mehran, R.; McPherson, J.; Farhat, N.; Marso, S.P.; et al. for the PROSPECT Investigators. A Prospective Natural-History Study of Coronary Atherosclerosis. N. Engl. J. Med. 2011, 364, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Thim, T.; Kallestrup Hagensen, M.; Wallace-Bradley, D.; Granada, J.F.; Kaluza, G.L.; Drouet, L.; Paaske, W.P.; Bøtker, H.E.; Falk, E. Unreliable Assessment of Necrotic Core by Virtual Histology Intravascular Ultrasound in Porcine Coronary Artery Disease. Circ. Cardiovasc. Imaging 2010, 3, 384–391. [Google Scholar] [CrossRef]
- Jensen, L.O.; Thayssen, P.; Mintz, G.S.; Egede, R.; Maeng, M.; Junker, A.; Galloee, A.; Christiansen, E.H.; Pedersen, K.E.; Hansen, H.S.; et al. Comparison of intravascular ultrasound and angiographic assessment of coronary reference segment size in patients with type 2 diabetes mellitus. Am. J. Cardiol. 2008, 101, 590–595. [Google Scholar] [CrossRef]
- Kern, M.J.; Samady, H. Current concepts of integrated coronary physiology in the catheterization laboratory. J. Am. Coll. Cardiol. 2010, 55, 173–185. [Google Scholar] [CrossRef]
- Abizaid, A.; Mintz, G.S.; Pichard, A.D.; Kent, K.M.; Satler, L.F.; Walsh, C.L.; Popma, J.J.; Leon, M.B. Clinical, intravascular ultrasound, and quantitative angiographic determinants of the coronary flow reserve before and after percutaneous transluminal coronary angioplasty. Am. J. Cardiol. 1998, 82, 423–428. [Google Scholar] [CrossRef]
- Briguori, C.; Anzuini, A.; Airoldi, F.; Gimelli, G.; Nishida, T.; Adamian, M.; Corvaja, N.; Di Mario, C.; Colombo, A. Intravascular ultrasound criteria for the assessment of the functional significance of intermediate coronary artery stenoses and comparison with fractional flow reserve. Am. J. Cardiol. 2001, 87, 136–141. [Google Scholar] [CrossRef]
- Takagi, A.; Tsurumi, Y.; Ishii, Y.; Suzuki, K.; Kawana, M.; Kasanuki, H. Clinical potential of intravascular ultrasound for physiological assess- ment of coronary stenosis: Relationship between quantitative ultrasound tomography and pressure-derived fractional flow reserve. Circulation 1999, 100, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Tai, B.C.; Soon, C.Y.; Low, A.F.; Poh, K.K.; Yeo, T.C.; Lim, G.H.; Yip, J.; Omar, A.R.; Teo, S.G.; et al. New set of intravascular ultrasound-derived anatomic criteria for defining functionally significant stenoses in small coronary arteries (results from Intravascular Ultrasound Diag- nostic Evaluation of Atherosclerosis in Singapore [IDEAS] study). Am. J. Cardiol. 2010, 105, 1378–1384. [Google Scholar] [CrossRef]
- McDaniel, M.; Eshtehardi, P.; Sawaya, F.; Douglas, J.; Samady, H. Contemporary clinical applications of coronary intravascular ultrasound. JACC Cardiovasc. Interv. 2011. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.A.; Deumite, N.J.; Chaitman, B.R.; Davis, K.B.; Killip, T.; Rogers, W.J. Asymptomatic left main coronary artery disease in the Coronary Artery Surgery Study (CASS) registry. Circulation 1989, 79, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- De la Torre Hernandez, J.M.; Hernández Hernandez, F.; Alfonso, F.; Rumoroso, J.R.; Lopez-Palop, R.; Sadaba, M.; Carrillo, P.; Rondan, J.; Lozano, I.; Nodar, J.M.R.; et al. Prospective application of pre-defined intravascular ultrasound criteria for assessment of intermediate left main coronary artery lesions: Results from the multicenter LITRO study. J. Am. Coll. Cardiol. 2011, 58, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Räber, L.; Mintz, G.S.; Koskinas, K.C.; Johnson, T.W.; Holm, N.R.; Onuma, Y.; Radu, M.D.; Joner, M.; Yu, B.; Jia, H.; et al. Clinical use of intracoronary imaging. Part 1: Guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur. Heart J. 2018, 39, 3281–3300. [Google Scholar] [CrossRef]
- Cheneau, E.; Leborgne, L.; Mintz, G.S.; Kotani, J.I.; Pichard, A.D.; Satler, L.F.; Canos, D.; Castagna, M.; Weissman, N.J.; Waksman, R. Predictors of subacute stent thrombosis: Results of a systematic intravascular ultrasound study. Circulation 2003, 108, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Cutlip, D.E.; Baim, D.S.; Ho, K.K.; Popma, J.J.; Lansky, A.J.; Cohen, D.J.; Carrozza, J.P., Jr.; Chauhan, M.S.; Rodriguez, O.; Kuntz, R.E. Stent thrombosis in the modern era: A pooled analysis of multicenter coronary stent clinical trials. Circulation 2001, 103, 1967–1971. [Google Scholar] [CrossRef]
- Uren, N.G.; Schwarzacher, S.P.; Metz, J.A.; Lee, D.P.; Honda, Y.; Yeung, A.C.; Fitzgerald, P.J.; Yock, P.G. Predictors and outcomes of stent thrombosis: An intra- vascular ultrasound registry. Eur. Heart J. 2002, 23, 124–132. [Google Scholar] [CrossRef]
- Fitzgerald, P.J.; Oshima, A.; Hayase, M.; Metz, J.A.; Bailey, S.R.; Baim, D.S.; Cleman, M.W.; Deutsch, E.; Diver, D.J.; Leon, M.B.; et al. Final results of the Can Routine Ultrasound Influence Stent Expansion (CRUISE) study. Circulation 2000, 102, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Parise, H.; Maehara, A.; Stone, G.W.; Leon, M.B.; Mintz, G.S. Meta- analysis of randomized studies comparing intravascular ultrasound versus angiographic guidance of percutaneous coronary intervention in pre-drug-eluting stent era. Am. J. Cardiol. 2011, 107, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Baz, J.A.; Alfonso, F.M.; Garcia, T.C.; de Carlos Gimeno, F.; Roura, G.F.; Recalde, A.S.; Martínez-Luengas, I.L.; Gomez, J.L.; Hernandez, F.H.; Pérez-Vizcayno, M.J.; et al. Clinical impact of intravascular ultrasound guidance in drug-eluting stent implantation for unprotected left main coronary disease: Pooled analysis at the patient-level of 4 registries. JACC Cardiovasc. Interv. 2014, 7, 244–254. [Google Scholar]
- Kim, B.-K.; Shin, D.-H.; Hong, M.-K.; Park, H.S.; Rha, S.-W.; Mintz, G.S.; Kim, J.-S.; Kim, J.S.; Lee, S.-J.; Kim, H.-Y.; et al. Clinical impact of intravascular ultrasound-guided chronic total occlusion intervention with zotarolimus-eluting versus biolimus-eluting stent implantation: Randomized study. Circ. Cardiovasc. Interv. 2015, 8, e002592. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Kim, B.K.; Shin, D.H.; Nam, C.M.; Kim, J.S.; Ko, Y.G.; Choi, D.; Kang, T.S.; Kang, W.C.; Her, A.Y.; et al. Effect of intravascular ultrasound-guided vs angiography-guided everolimus-eluting stent implantation: The IVUS-XPL Randomized Clinical Trial. JAMA 2015, 314, 2155–2163. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, I.Y.; Mahmoud, A.N.; Elgendy, A.Y.; Bavry, A.A. Outcomes with intravascular ultrasound-guided stent implantation: A meta-analysis of randomized trials in the era of drug-eluting stents. Circ. Cardiovasc. Interv. 2016, 9, e003700. [Google Scholar] [CrossRef] [PubMed]
- Witzenbichler, B.; Maehara, A.; Weisz, G.; Neumann, F.-J.; Rinaldi, M.J.; Metzger, D.C.; Henry, T.D.; Cox, D.A.; Duffy, P.L.; Brodie, B.R.; et al. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents: The Assessment of Dual Antiplatelet Therapy With Drug-Eluting Stents (ADAPT- DES) Study. Circulation 2014, 129, 463–470. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Pang, S.; Chen, X.-Y.; Bourantas, C.V.; Pan, D.-R.; Dong, S.-J.; Wu, W.; Ren, X.-M.; Zhu, H.; Shi, S.-Y.; et al. Comparison of intra- vascular ultrasound guided versus angiography guided drug eluting stent im- plantation: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2015, 15, 153. [Google Scholar] [CrossRef]
- Buccheri, S.; Franchina, G.; Romano, S.; Puglisi, S.; Venuti, G.; D’Arrigo, P.; Francaviglia, B.; Scalia, M.; Condorelli, A.; Barbanti, M.; et al. Clinical outcomes following intravascular imaging-guided versus coronary angiography-guided percutaneous coronary intervention with stent implantation: A systematic review and Bayesian network meta-analysis of 31 studies and 17,882 patients. JACC Cardiovasc. Interv. 2017, 10, 2488–2498. [Google Scholar] [CrossRef]
- Mariani, J.; Guedes, C.; Soares, P.; Zalc, S.; Campos, C.; Lopes, A.; Spadaro, A.; Perin, M.; Esteves Filho, A.; Takimura, C.; et al. Intravascular Ultrasound Guidance to Minimize the Use of Iodine Contrast in Percutaneous Coronary Intervention, The MOZART (Minimizing cOntrast utilization With IVUS Guidance in coronary angioplasTy) Randomized Controlled Trial. JACC Cardiovasc. Interv. 2014, 7, 1287–1293. [Google Scholar] [CrossRef]
- Song, H.G.; Kang, S.J.; Ahn, J.M.; Kim, W.J.; Lee, J.Y.; Park, D.W.; Lee, S.W.; Kim, Y.H.; Lee, C.W.; Park, S.W.; et al. Intravascular ultrasound assessment of optimal stent area to prevent in-stent restenosis after zotarolimus-, everolimus-, and sirolimus-eluting stent implantation. Catheter. Cardiovasc. Interv. 2014, 83, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-Y.; Witzenbichler, B.; Maehara, A.; Lansky, A.J.; Guagliumi, G.; Brodie, B.; Kellett, M.A.; Dressler, O.; Parise, H.; Mehran, R.; et al. Intravascular ultrasound findings of early stent thrombosis after primary percutaneous intervention in acute myocardial infarction: A Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS- AMI) substudy. Circ. Cardiovasc. Interv. 2011, 4, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Cho, Y.R.; Park, G.M.; Ahn, J.M.; Kim, W.J.; Lee, J.Y.; Park, D.W.; Lee, S.W.; Kim, Y.H.; Lee, C.W.; et al. Intravascular ultrasound pre- dictors for edge restenosis after newer generation drug-eluting stent implant- ation. Am. J. Cardiol. 2013, 111, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.M.; Kang, S.J.; Yoon, S.H.; Park, H.W.; Kang, S.M.; Lee, J.Y.; Lee, S.W.; Kim, Y.H.; Lee, C.W.; Park, S.W.; et al. Meta-analysis of outcomes after intravascular ultrasound-guided versus angiography-guided drug-eluting stent implantation in 26,503 patients enrolled in three randomized trials and 14observational studies. Am. J. Cardiol. 2014, 113, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Morino, Y.; Honda, Y.; Okura, H.; Oshima, A.; Hayase, M.; Bonneau, H.N.; Kuntz, R.E.; Yock, P.G.; Fitzgerald, P.J. An optimal diagnostic threshold for minimal stent area to predict target lesion revascularization following stent implantation in native coronary lesions. Am. J. Cardiol. 2001, 88, 301. [Google Scholar] [CrossRef]
- Doi, H.; Maehara, A.; Mintz, G.S.; Yu, A.; Wang, H.; Mandinov, L.; Popma, J.J.; Ellis, S.G.; Grube, E.; Dawkins, K.D.; et al. Impact of post-intervention minimal stent area on 9-month follow-up patency of paclitaxel-eluting stents: An integrated intravascular ultrasound analysis from the TAXUS IV, V, and VI and TAXUS ATLAS Workhorse, Long Lesion, and Direct Stent Trials. J. Am. Coll. Cardiol. Cardiovasc. Interv. 2009, 2, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, E.; Gatto, L.; La Manna, A.; Burzotta, F.; Taglieri, N.; Saia, F.; Amico, F.; Marco, V.; Ramazzotti, V.; Di Giorgio, A.; et al. Role of residual acute stent malapposition in percutaneous coronary interventions. Catheter. Cardiovasc. Interv. 2017, 90, 566–575. [Google Scholar] [CrossRef]
- Adriaenssens, T.; Joner, M.; Godschalk, T.; Malik, N.; Alfonso, F.; Xhepa, E.; De Cock, D.; Komukai, K.; Tada, T.; Cuesta, J.; et al. Prevention of Late Stent Thrombosis by an Interdisciplinary Global European Effort (PRESTIGE) Investigators. Optical coherence tomography findings in patients with coronary stent thrombosis: A report of the PREvention of Late Stent Thrombosis by an Interdisciplinary Global European Effort (PRESTIGE) Consortium. Circulation 2017, 136, 1007–1021. [Google Scholar]
- Maehara, A.; Mintz, G.S.; Bui, A.B.; Castagna, M.T.; Walter, O.R.; Pappas, C.; Pinnow, E.E.; Pichard, A.D.; Satler, L.F.; Waksman, R.; et al. Incidence, morphology, angiographic findings, and outcomes of intramural hematomas after percutaneous coronary interventions: an intravascular ultrasound study. Circulation 2002, 105, 2037–2042. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2018. [Google Scholar] [CrossRef]
- Goto, K.; Zhao, Z.; Matsumura, M.; Dohi, T.; Kobayashi, N.; Kirtane, A.J.; Rabbani, L.E.; Collins, M.B.; Parikh, M.A.; Kodali, S.K.; et al. Mechanisms and patterns of intravascular ultrasound in-stent restenosis among bare metal stents and first- and second-generation drug-eluting stents. Am. J. Cardiol. 2015, 116, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Ahn, J.M.; Song, H.; Kim, W.J.; Lee, J.Y.; Park, D.W.; Yun, S.C.; Lee, S.W.; Kim, Y.H.; Lee, C.W.; et al. Comprehensive intravascular ultrasound assessment of stent area and its impact on restenosis and adverse cardiac events in 403 patients with unprotected left main disease. Circ. Cardiovasc. Interv. 2011, 4, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Souteyrand, G.; Amabile, N.; Mangin, L.; Chabin, X.; Meneveau, N.; Cayla, G.; Vanzetto, G.; Barnay, P.; Trouillet, C.; Rioufol, G.; et al. Mechanisms of stent thrombosis analysed by optical coherence tomography: Insights from the national PESTO French registry. Eur. Heart J. 2016, 37, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Vavuranakis, M.; Kakadiaris, I.A.; O’Malley, S.M.; Papaioannou, T.G.; Sanidas, E.A.; Naghavi, M.; Carlier, S.; Tousoulis, D.; Stefanadis, C. A new method for assessment of plaque vulnerability based on vasa vasorum imaging, by using contrast-enhanced intravascular ultrasound and differential image analysis. Int. J. Cardiol. 2008, 130, 23–29. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, S.M.; Vavuranakis, M.; Naghavi, M.; Kakadiaris, I.A. Intravascular ultrasound-based imaging of vasa vasorum for the detection of vulnerable atherosclerotic plaque. Med. Image Comput. Comput. Assist. Interv. 2005, 8, 343–351. [Google Scholar] [PubMed]
- Ruiz, E.M.; Papaioannou, T.G.; Vavuranakis, M.; Stefanadis, C.; Naghavi, M.; Kakadiaris, I.A. Analysis of contrast-enhanced intravascular ultrasound images for the assessment of coronary plaque neoangiogenesis: Another step closer to the identification of the vulnerable plaque. Curr. Pharm. Des. 2012, 18, 2207–2213. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, T.G.; Schizas, D.; Vavuranakis, M.; Katsarou, O.; Soulis, D.; Stefanadis, C. Quantification of new structural features of coronary plaques by computational post-hoc analysis of virtual histology-intravascular ultrasound images. Comput. Methods Biomech. Biomed. Eng. 2014, 17, 643–651. [Google Scholar] [CrossRef]
- Vavuranakis, M.; Papaioannou, T.G.; Katsarou, O.A.; Vrachatis, D.A.; Sanidas, E.A.; Siasos, G.; Kalogeras, K.I.; Schizas, D.; Stefanadis, C.I.; Tousoulis, D. Impact of atherosclerotic plaque components and their distribution on stent deployment: An intravascular-ultrasound virtual histology observational study. Minerva Cardioangiol. 2016, 64, 507–516. [Google Scholar] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papaioannou, T.G.; Kalantzis, C.; Katsianos, E.; Sanoudou, D.; Vavuranakis, M.; Tousoulis, D. Personalized Assessment of the Coronary Atherosclerotic Arteries by Intravascular Ultrasound Imaging: Hunting the Vulnerable Plaque. J. Pers. Med. 2019, 9, 8. https://doi.org/10.3390/jpm9010008
Papaioannou TG, Kalantzis C, Katsianos E, Sanoudou D, Vavuranakis M, Tousoulis D. Personalized Assessment of the Coronary Atherosclerotic Arteries by Intravascular Ultrasound Imaging: Hunting the Vulnerable Plaque. Journal of Personalized Medicine. 2019; 9(1):8. https://doi.org/10.3390/jpm9010008
Chicago/Turabian StylePapaioannou, Theodore G., Charalampos Kalantzis, Efstratios Katsianos, Despina Sanoudou, Manolis Vavuranakis, and Dimitrios Tousoulis. 2019. "Personalized Assessment of the Coronary Atherosclerotic Arteries by Intravascular Ultrasound Imaging: Hunting the Vulnerable Plaque" Journal of Personalized Medicine 9, no. 1: 8. https://doi.org/10.3390/jpm9010008
APA StylePapaioannou, T. G., Kalantzis, C., Katsianos, E., Sanoudou, D., Vavuranakis, M., & Tousoulis, D. (2019). Personalized Assessment of the Coronary Atherosclerotic Arteries by Intravascular Ultrasound Imaging: Hunting the Vulnerable Plaque. Journal of Personalized Medicine, 9(1), 8. https://doi.org/10.3390/jpm9010008






