The Emerging Role of Checkpoint Inhibition in Microsatellite Stable Colorectal Cancer
Abstract
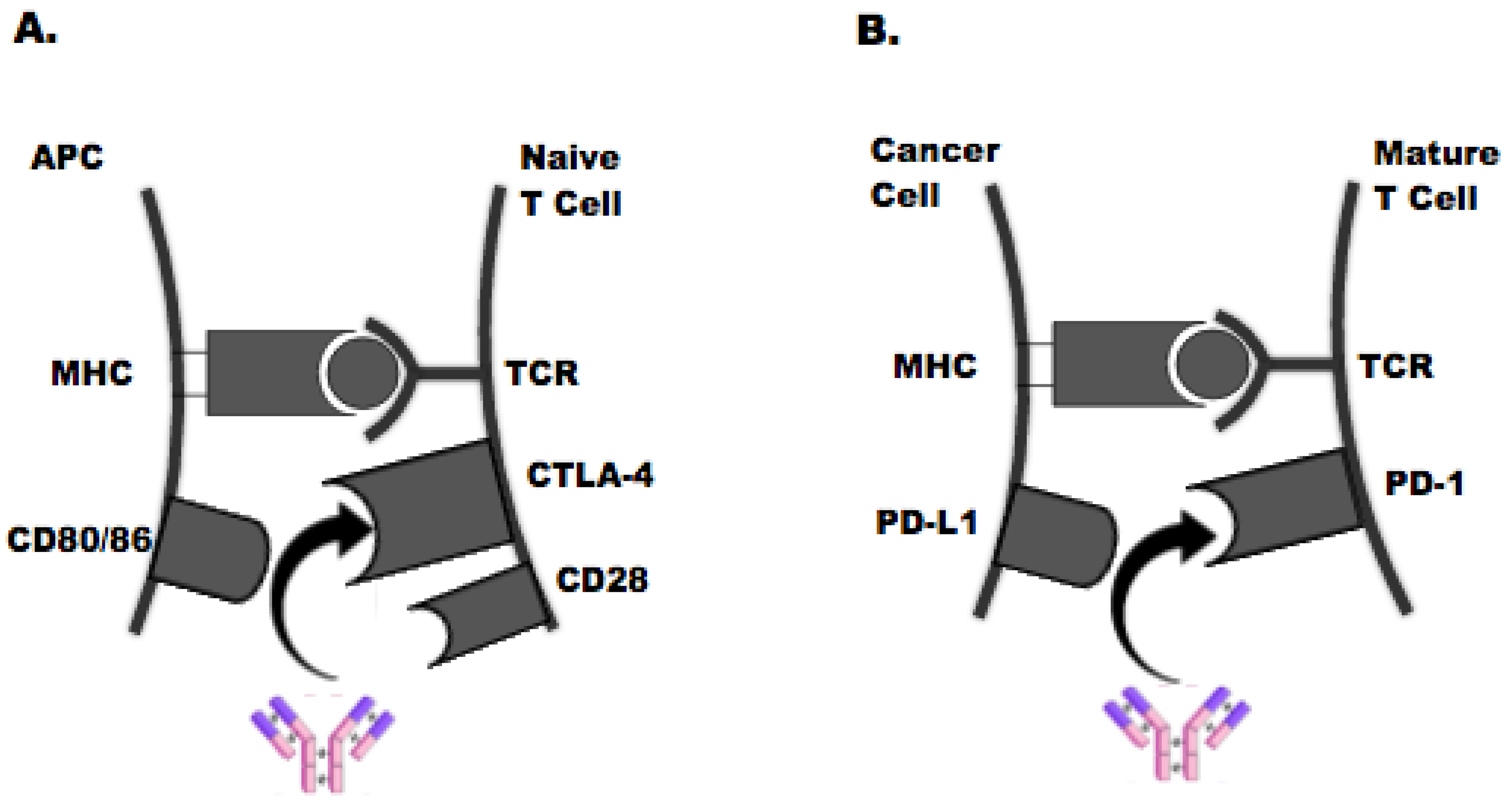
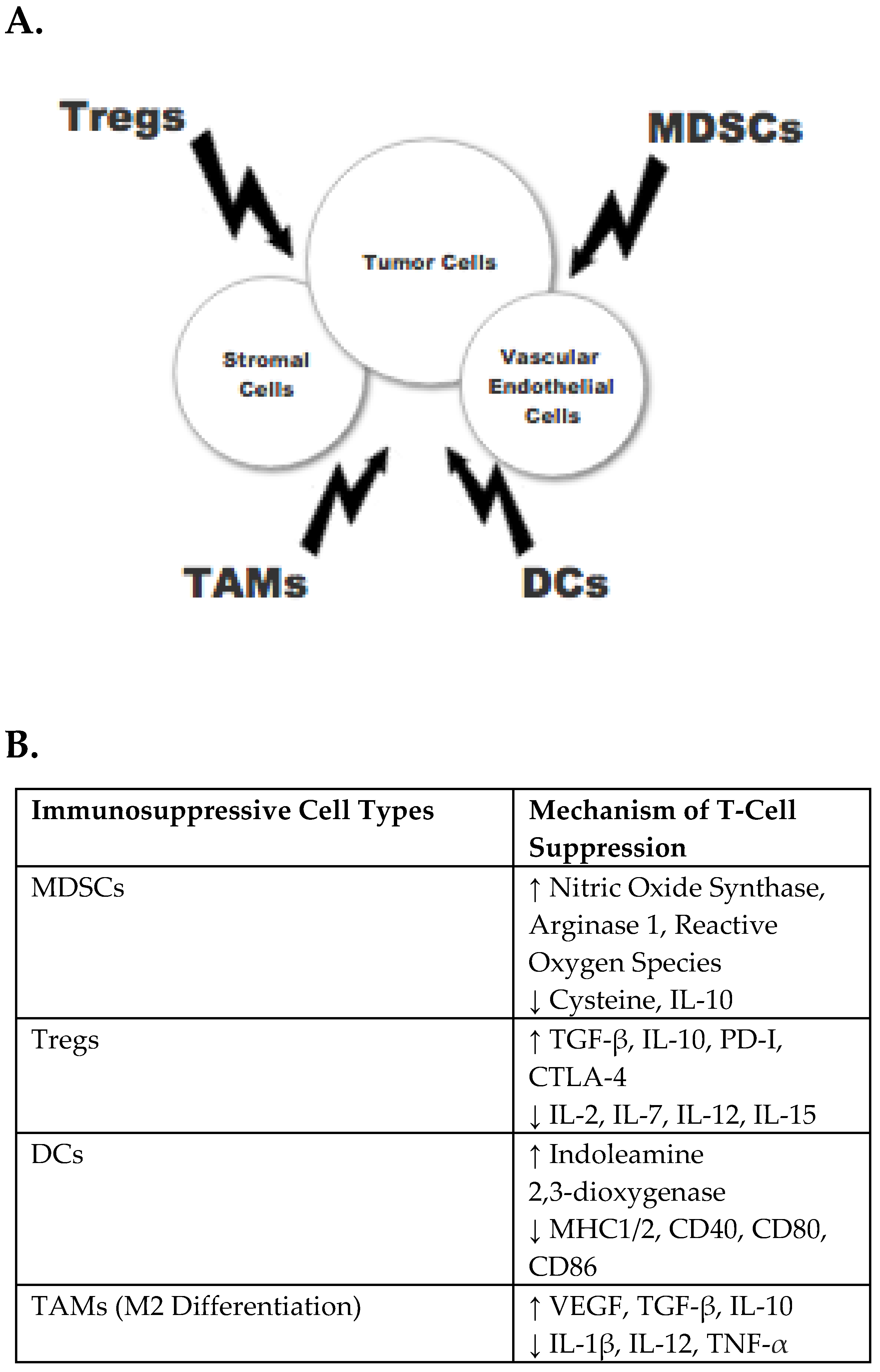
1. Introduction
2. Immune Checkpoint Inhibitors + Chemotherapy
3. Immune Checkpoint Inhibitors + VEGF/EGFR Inhibitors +/− Chemotherapy
4. Immune Checkpoint Inhibitors + Radiotherapy
5. Immune Checkpoint Inhibitors + MEK Inhibitors
6. Novel Combination Therapies
6.1. CSF1R Inhibitors
6.2. IDO1 Inhibitors
6.3. Autologous Tumor Vaccines and Oncolytic Viral Therapy
6.4. T-Cell Bispecific Antibodies
6.5. CD73 Inhibitors
6.6. Gut Microbiota
6.7. Poly-ICLC
6.8. Additional Investigative Agents
7. Conclusions
Funding
Conflicts of Interest
References
- Stidham, R.W.; Higgins, P.D.R. Colorectal Cancer in Inflammatory Bowel Disease. Clin. Colon. Rectal. Surg. 2018, 31, 168–178. [Google Scholar]
- Mei, Z.; Liu, Y.; Liu, C.; Cui, A.; Liang, Z.; Wang, G.; Peng, H.; Cui, L.; Li, C. Tumour-infiltrating inflammation and prognosis in colorectal cancer: Systematic review and meta-analysis. Br. J. Cancer 2014, 110, 1595–1605. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.M.; Kato, S.; Bazhenova, L.; Patel, S.P.; Frampton, G.M.; Miller, V.; Stephens, P.J.; Daniels, G.A.; Kurzrock, R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol. Cancer Ther. 2017, 16, 2598–2608. [Google Scholar] [CrossRef] [PubMed]
- Pagès, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet 2018, 391, 2128–2139. [Google Scholar] [CrossRef]
- Hermel, D.J.; Gruber, S.B. Hereditary Colorectal Cancer: Immunotherapy Approaches. In Hereditary Colorectal Cancer; Valle, L., Gruber, S., Capellá, G., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Llosa, N.J.; Cruise, M.; Tam, A.; Wicks, E.C.; Hechenbleikner, E.M.; Taube, J.M.; Blosser, R.L.; Fan, H.; Wang, H.; Luber, B.S.; et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015, 5, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Klempner, S.J.; Park, S.H.; Park, J.O.; Park, Y.S.; Lim, H.Y.; Kang, W.K.; Kim, K.M.; Lee, J. Correlating programmed death ligand 1 (PD-L1) expression, mismatch repair deficiency, and outcomes across tumor types: Implications for immunotherapy. Oncotarget 2017, 8, 77415–77423. [Google Scholar] [CrossRef]
- Fabrizio, D.A.; George, T.J., Jr.; Dunne, R.F.; Frampton, G.; Sun, J.; Gowen, K.; Kennedy, M.; Greenbowe, J.; Schrock, A.B.; Hezel, A.F.; et al. Beyond microsatellite testing: Assessment of tumor mutational burden identifies subsets of colorectal cancer who may respond to immune checkpoint inhibition. J. Gastrointest. Oncol. 2018, 9, 610–617. [Google Scholar] [CrossRef]
- Dosset, M.; Vargas, T.R.; Lagrange, A.; Boidot, R.; Végran, F.; Roussey, A.; Chalmin, F.; Dondaine, L.; Paul, C.; Marie-Joseph, E.L.; et al. PD-1/PD-L1 pathway: An adaptive immune resistance mechanism to immunogenic chemotherapy in colorectal cancer. Oncoimmunology 2018, 7, e1433981. [Google Scholar] [CrossRef]
- Liu, W.M.; Fowler, D.W.; Smith, P.; Dalgleish, A.G. Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br. J. Cancer 2010, 102, 115–123. [Google Scholar] [CrossRef]
- Song, W.; Shen, L.; Wang, Y.; Liu, Q.; Goodwin, T.J.; Li, J.; Dorosheva, O.; Liu, T.; Liu, R.; Huang, L. Synergistic and low adverse effect cancer immunotherapy by immunogenic chemotherapy and locally expressed PD-L1 trap. Nat. Commun. 2018, 9, 2237. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Shahda, S.; Noonan, A.M.; Bekaii-Saab, T.S.; O’Neil, B.H.; Sehdev, A.; Shaib, W.L.; Helft, P.R.; Loehrer, P.J.; Tong, Y.; Liu, Z.; et al. A phase II study of pembrolizumab in combination with mFOLFOX6 for patients with advanced colorectal cancer. J. Clin. Oncol. 2017, 35, 3541. [Google Scholar] [CrossRef]
- MacDonald, F.; Zaiss, D.M.W. The Immune System’s Contribution to the Clinical Efficacy of EGFR Antagonist Treatment. Front. Pharmacol. 2017, 8, 575. [Google Scholar] [CrossRef]
- Yang, J.; Yan, J.; Liu, B. Targeting VEGF/VEGFR to Modulate Antitumor Immunity. Front. Immunol. 2018, 9, 978. [Google Scholar] [CrossRef]
- Boland, P.M.; Hutson, A.; Maguire, O.; Minderman, H.; Fountzilas, C.; Iyer, R.V. A phase Ib/II study of cetuximab and pembrolizumab in RAS-wt mCRC. J. Clin. Oncol. 2018, 36, 834. [Google Scholar] [CrossRef]
- Wallin, J.; Pishvaian, M.J.; Hernandez, G.; Yadav, M.; Jhunjhunwala, S.; Delamarre, L.; He, X.; Powderly, J.; Lieu, C.; Eckhardt, S.G.; et al. Clinical activity and immune correlates from a phase Ib study evaluating atezolizumab (anti-PDL1) in combination with FOLFOX and bevacizumab (anti-VEGF) in metastatic colorectal carcinoma. Cancer Res. 2016, 76, 2651. [Google Scholar] [CrossRef]
- Bendell, J.C.; Powderly, J.D.; Lieu, C.H.; Eckhardt, S.G.; Hurwitz, H.; Hochster, H.S.; Murphy, J.E.; Funke, R.P.; Rossi, C.; Wallin, J.; et al. Safety and efficacy of MPDL3280A (anti-PDL1) in combination with bevacizumab (bev) and/or FOLFOX in patients (pts) with metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2015, 33, 15. [Google Scholar] [CrossRef]
- Walle, T.; Martinez Monge, R.; Cerwenka, A.; Ajona, D.; Melero, I.; Lecanda, F. Radiation effects on antitumor immune responses: Current perspectives and challenges. Ther. Adv. Med. Oncol. 2018, 10, 1758834017742575. [Google Scholar] [CrossRef]
- Segal, N.H.; Kemeny, N.E.; Cercek, A.; Reidy, D.L.; Raasch, P.J.; Warren, P.; Hrabovsky, A.E.; Campbell, N.; Shia, J.; Goodman, K.A.; et al. Non-randomized phase II study to assess the efficacy of pembrolizumab (Pem) plus radiotherapy (RT) or ablation in mismatch repair proficient (pMMR) metastatic colorectal cancer (mCRC) patients. J. Clin. Oncol. 2016, 34, 3539. [Google Scholar] [CrossRef]
- Neuzillet, C.; Tijeras-Raballand, A.; de Mestier, L.; Cros, J.; Faivre, S.; Raymond, E. MEK in cancer and cancer therapy. Pharmacol. Ther. 2014, 141, 160–171. [Google Scholar] [CrossRef]
- Oikonomou, E.; Koustas, E.; Goulielmaki, M.; Pintzas, A. BRAF vs RAS oncogenes: Are mutations of the same pathway equal? Differential signalling and therapeutic implications. Oncotarget 2014, 5, 11752–11777. [Google Scholar] [CrossRef] [PubMed]
- Hermel, D.J.; Ott, P.A. Combining forces: The promise and peril of synergistic immune checkpoint blockade and targeted therapy in metastatic melanoma. Cancer Metastasis Rev. 2017, 36, 43–50. [Google Scholar] [CrossRef]
- Ebert, P.J.R.; Cheung, J.; Yang, Y.; McNamara, E.; Hong, R.; Moskalenko, M.; Gould, S.E.; Maecker, H.; Irving, B.A.; Kim, J.M.; et al. MAP Kinase Inhibition Promotes T Cell and Anti-tumor Activity in Combination with PD-L1 Checkpoint Blockade. Immunity 2016, 44, 609–621. [Google Scholar] [CrossRef]
- Bendell, J.; Ciardiello, F.; Tabernero, J.; Tebbutt, N.; Eng, C.; Di Bartolomeo, M.; Falcone, A.; Fakih, M.; Kozloff, M.; Segal, N.; et al. Efficacy and safety results from IMblaze370, a randomised Phase III study comparing atezolizumab+cobimetinib and atezolizumab monotherapy vs regorafenib in chemotherapy-refractory metastatic colorectal cancer. Ann. Oncol. 2018, 29, 123. [Google Scholar] [CrossRef]
- Bendell, J.; Bang, Y.-J.; Chee, C.E.; Ryan, D.P.; McRee, A.J.; Chow, L.Q.; Desai, J.; Wongchenko, M.; Yan, Y.; Pitcher, B.; et al. A phase Ib study of safety and clinical activity of atezolizumab (A) and cobimetinib (C) in patients (pts) with metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2018, 36, 560. [Google Scholar] [CrossRef]
- Wiehagen, K.R.; Girgis, N.M.; Yamada, D.H.; Smith, A.A.; Chan, S.R.; Grewal, I.S.; Quigley, M.; Verona, R.I. Combination of CD40 Agonism and CSF-1R Blockade Reconditions Tumor-Associated Macrophages and Drives Potent Antitumor Immunity. Cancer Immunol. Res. 2017, 5, 1109–1121. [Google Scholar] [CrossRef] [PubMed]
- Wainberg, Z.; Piha-Paul, S.; Luke, J. First in-human phase 1 dose escalation and expansion of a novel combination, anti-CSF-1 receptor (cabiralizumab) plus anti–PD-1 (nivolumab), in patients with advanced solid tumors. Presented at: 32th Society for Immunotherapy of Cancer Annual Meeting, National Harbor, MD, USA, 8–12 November 2017. [Google Scholar]
- Gyori, D.; Lim, E.L.; Grant, F.M.; Spensberger, D.; Roychoudhuri, R.; Shuttleworth, S.J.; Okkenhaug, K.; Stephens, L.R.; Hawkins, P.T. Compensation between CSF1R+ macrophages and Foxp3+ Treg cells drives resistance to tumor immunotherapy. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Ladomersky, E.; Lenzen, A.; Nguyen, B.; Patel, R.; Lauing, K.L.; Wu, M.; Wainwright, D.A. IDO1 in cancer: A Gemini of immune checkpoints. Cell Mol. Immunol. 2018, 15, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Andtbacka, R.H.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients with Advanced Melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Chaurasiya, S.; Warner, S. Viroimmunotherapy for Colorectal Cancer: Clinical Studies. Biomedicines 2017, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- LaRocca, C.J.; Warner, S.G. Oncolytic viruses and checkpoint inhibitors: Combination therapy in clinical trials. Clin. Transl. Med. 2018, 7, 35. [Google Scholar] [CrossRef]
- Jonker, D.J.; Tang, P.A.; Kennecke, H.; Welch, S.A.; Cripps, M.C.; Asmis, T.; Chalchal, H.; Tomiak, A.; Lim, H.; Ko, Y.J.; et al. A Randomized Phase II Study of FOLFOX6/Bevacizumab With or Without Pelareorep in Patients With Metastatic Colorectal Cancer: IND.210, a Canadian Cancer Trials Group Trial. Clin. Colorectal Cancer 2018, 17. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.J.; Peng, K.W.; Bell, J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012, 30, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Workenhe, S.T.; Mossman, K.L. Oncolytic virotherapy and immunogenic cancer cell death: Sharpening the sword for improved cancer treatment strategies. Mol. Ther. 2014, 22, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Argiles, G.; Saro, J.; Segal, N.H.; Melero, I.; Ros, W.; Marabelle, A.; Rodriguez, M.E.; Albanell, J.; Calvo, E.; Moreno, V.; et al. Novel carcinoembryonic antigen T-cell bispecific (CEA-TCB) antibody: Preliminary clinical data as a single agent and in combination with atezolizumab in patients with metastatic colorectal cancer (mCRC). Ann. Oncol. 2017, 28. [Google Scholar] [CrossRef]
- Allard, D.; Allard, B.; Gaudreau, P.O.; Chrobak, P.; Stagg, J. CD73-adenosine: A next-generation target in immuno-oncology. Immunotherapy 2016, 8, 145–163. [Google Scholar] [CrossRef]
- Ma, Y.L.; Peng, J.Y.; Zhang, P.; Liu, W.J.; Huang, L.; Qin, H.L. Immunohistochemical analysis revealed CD34 and Ki67 protein expression as significant prognostic factors in colorectal cancer. Med. Oncol. 2010, 27, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Humphries, A.; Daud, A. The gut microbiota and immune checkpoint inhibitors. Hum. Vacc. Immunother. 2018, 14, 2178–2182. [Google Scholar] [CrossRef]
- Song, W.; Tiruthani, K.; Wang, Y.; Shen, L.; Hu, M.; Dorosheva, O.; Qiu, K.; Kinghorn, K.A.; Liu, R.; Huang, L. Trapping of Lipopolysaccharide to Promote Immunotherapy against Colorectal Cancer and Attenuate Liver Metastasis. Adv. Mater. 2018. [CrossRef]
- Kyi, C.; Roudko, V.; Sabado, R.; Saenger, Y.; Loging, W.; Mandeli, J.; Thin, T.H.; Lehrer, D.; Donovan, M.; Posner, M.; et al. Therapeutic Immune Modulation against Solid Cancers with Intratumoral Poly-ICLC: A Pilot Trial. Clin. Cancer Res. 2018, 24, 4937–4948. [Google Scholar] [CrossRef]
- Zhao, J.; Ou, B.; Feng, H.; Wang, P.; Yin, S.; Zhu, C.; Wang, S.; Chen, C.; Zheng, M.; Zong, Y.; et al. Overexpression of CXCR2 predicts poor prognosis in patients with colorectal cancer. Oncotarget 2017, 8, 28442–28454. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jiao, C.; Zhu, Y.; Liang, D.; Zao, M.; Meng, X.; Gao, J.; He, Y.; Liu, W.; Hou, J.; et al. Activation of CXCL12/CXCR4 renders colorectal cancer cells less sensitive to radiotherapy via up-regulating the expression of survivin. Exp. Biol. Med. 2017, 242, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Akce, M.; Alese, O.B.; Shaib, W.L.; Wu, C.S.-Y.; Lesinski, G.B.; El-Rayes, B.F. Phase Ib trial of pembrolizumab and XL888 in patients with advanced gastrointestinal malignancies. J. Clin. Oncol. 2018, 36. [Google Scholar] [CrossRef]
- Masso-Valles, D.; Jauset, T.; Soucek, L. Ibrutinib repurposing: From B-cell malignancies to solid tumors. Oncoscience 2016, 3, 147–148. [Google Scholar]
- Grasso, C.S.; Giannakis, M.; Wells, D.K.; Hamada, T.; Mu, X.J.; Quist, M.; Nowak, J.A.; Nishihara, R.; Qian, Z.R.; Inamura, K.; et al. Genetic Mechanisms of Immune Evasion in Colorectal Cancer. Cancer Discov. 2018, 8, 730–749. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Lan, C.; Li, L.; Yang, D.; Xia, X.; Liao, Q.; Fu, W.; Chen, X.; An, S.; Wang, W.E.; et al. A novel porcupine inhibitor blocks WNT pathways and attenuates cardiac hypertrophy. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3459–3467. [Google Scholar] [CrossRef]
- Kuboki, Y.; Kawazoe, A.; Komatsu, Y.; Nishina, T.; Shinozaki, E.; Hara, H.; Yuki, S.; Fukutani, M.; Tsukahara, N.; Hasegawa, H.; et al. Multicenter phase I/II trial of BBI608 and pembrolizumab combination in patients with metastatic colorectal cancer (SCOOP Study): EPOC1503. J. Clin. Oncol. 2017, 35. [Google Scholar] [CrossRef]
| Combination Treatment | ICI | Study Population | Trial ID | Phase | Status |
|---|---|---|---|---|---|
| Trifluridine + Tipiracil Hychloride | Nivolumab | Refractory, Metastatic MSS CRC | NCT02860546 | II | Completed |
| Romidepsin +/− 5-Azacitidine | Pembrolizumab | Refractory, Metastatic MSS CRC | NCT02512172 | I | Recruiting |
| Pemetrexed +/− Oxaliplatin | Pembrolizumab | Refractory, Metastatic MSS CRC | NCT03626922 | I | Not yet Recruiting |
| Nordic FLOX Regimen | Nivolumab | Untreated, Metastatic MSS CRC | NCT03388190 | II | Recruiting |
| Azacitidine | Durvalumab | Refractory, Metastatic MSS CRC | NCT02811497 | II | Recruiting |
| Guadecitabine | Nivolumab | Refractory, Metastatic MSS CRC | NCT03576963 | Ib/II | Not yet Recruiting |
| FOLFOX | Tremelimumab + Durvalumab | First-line, KRAS-mt CRC | NCT03202758 | Ib/II | Recruiting |
| TATE | Nivolumab or Pembrolizumab | Metastatic CRC to liver | NCT03259867 | II | Recruiting |
| Combination Regimen | ICI | Study Population | Trial ID | Phase | Status |
|---|---|---|---|---|---|
| Capecitabine + Bevacizumab | Atezolizumab | Refractory, Metastatic CRC | NCT02873195 | II | Not Recruiting |
| SOC Chemotherapy + Bevacizumab | Nivolumab | Metastatic CRC; No Prior Chemotherapy | NCT03414983 | II/III | Recruiting |
| Trifluridine/Tipiracil + Oxaliplatin +/− bevacizumab | Nivolumab | Refractory, Metastatic CRC | NCT02848443 | I | Recruiting |
| Regorafenib | PDR001 | Refractory, Metastatic MSS CRC | NCT03081494 | I | Recruiting |
| Capecitabine + Bevacizumab | Pembrolizumab | Refractory, MSS CRC | NCT03396926 | II | Recruiting |
| Cetuximab + Irinotecan | Avelumab | Refractory, BRAF V600E-WT, MSS CRC | NCT03608046 | II | Not yet Recruiting |
| Bevacizumab + mFOLFOX6 | PDR001 | Treatment naïve, MSS CRC | NCT03176264 | Ib | Completed |
| Panitumumab | Nivolumab + Ipilimumab | Refractory, KRAS/NRAS/BRAF-WT, MSS CRC | NCT03442569 | II | Recruiting |
| Radiation Regimen | ICI | Study Population | Study ID | Phase | Status |
|---|---|---|---|---|---|
| Standard Radiation Therapy | Nivolumab+ Ipilimumab | MSS and MSI-H CRC | NCT03104439 | II | Recruiting |
| Hypofractionated palliative radiation | Durvalumab and Tremelimumab | Metastatic MSS CRC | NCT03007407 | II | Recruiting |
| Chemo-radiation | Durvalumab | Stage II-IV, MSS Rectal Cancer | NCT03102047 | II | Recruiting |
| SBRT to Liver | Pembrolizumab | Metastatic CRC to Liver | NCT02837263 | I | Recruiting |
| Radioembolization | Durvalumab and Tremelimumab | Metastatic MSS CRC to Liver | NCT03005002 | I | Active, not recruiting |
| High or low-dose radiation therapy | Durvalumab and Tremelimumab | Refractory Metastatic MSS CRC to Liver | NCT02888743 | II | Recruiting |
| Combination Regimen | ICI | Study Population | Study ID | Phase | Status |
|---|---|---|---|---|---|
| Trametinib | Nivolumab +/− Ipilimumab | RAS-mt; previously treated, metastatic MSS CRC | NCT03377361 | I/II | Recruiting |
| Binimetinib | Nivolumab +/− Ipilimumab | RAS-mt; previously treated, metastatic MSS CRC | NCT03271047 | I/II | Not Recruiting |
| Dabrafenib + Trametinib | PDR001 | BRAFV600E-mt; metastatic CRC | NCT03668431 | II | Recruiting |
| Trametinib | Durvalumab | Refractory, metastatic MSS CRC | NCT03428126 | II | By Invitation |
| Novel Agents | Therapeutic Targets | ICI | Study ID |
|---|---|---|---|
| Navarixin | CXCR2 | Pembrolizumab | NCT03473925 |
| Olaptesed Pegol | CXCL12 | Pembrolizumab | NCT03168139 |
| eFT508 | MNK 1/2 | Avelumab | NCT03258398 |
| Ibrutinib | BTK | Pembrolizumab | NCT03332498 |
| XL888 | HSP | Pembrolizumab | NCT03095781 |
| CGX1321 | PORCN | Pembrolizumab | NCT02675946 |
| BBI608 | STAT3/WNT | Pembrolizumab | NCT02851004 NCT03647839 |
| Vicriviroc | CCR5 | Pembrolizumab | NCT03631407, NCT03274804 |
| Grapiprant | EP4 | Pembrolizumab | NCT03658772 |
| Relatlimab | LAG-3 | Nivolumab | NCT03642067 |
| Copanlisib | PI3K | Nivolumab | NCT03711058 |
| MK-8353 | ERK1/2 | Pembrolizumab | NCT02972034 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hermel, D.J.; Sigal, D. The Emerging Role of Checkpoint Inhibition in Microsatellite Stable Colorectal Cancer. J. Pers. Med. 2019, 9, 5. https://doi.org/10.3390/jpm9010005
Hermel DJ, Sigal D. The Emerging Role of Checkpoint Inhibition in Microsatellite Stable Colorectal Cancer. Journal of Personalized Medicine. 2019; 9(1):5. https://doi.org/10.3390/jpm9010005
Chicago/Turabian StyleHermel, David J., and Darren Sigal. 2019. "The Emerging Role of Checkpoint Inhibition in Microsatellite Stable Colorectal Cancer" Journal of Personalized Medicine 9, no. 1: 5. https://doi.org/10.3390/jpm9010005
APA StyleHermel, D. J., & Sigal, D. (2019). The Emerging Role of Checkpoint Inhibition in Microsatellite Stable Colorectal Cancer. Journal of Personalized Medicine, 9(1), 5. https://doi.org/10.3390/jpm9010005






