The Association between Plasma Omega-6/Omega-3 Ratio and Anthropometric Traits Differs by Racial/Ethnic Groups and NFKB1 Genotypes in Healthy Young Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Anthropometric Traits
2.3. Dietary Assessment
2.4. Measurement of Plasma n-6 and n-3 Concentrations
2.5. Single Nucleotide Polymorphism Selection and Genotyping
2.6. Statistical Analyses
3. Results
3.1. General Characteristics
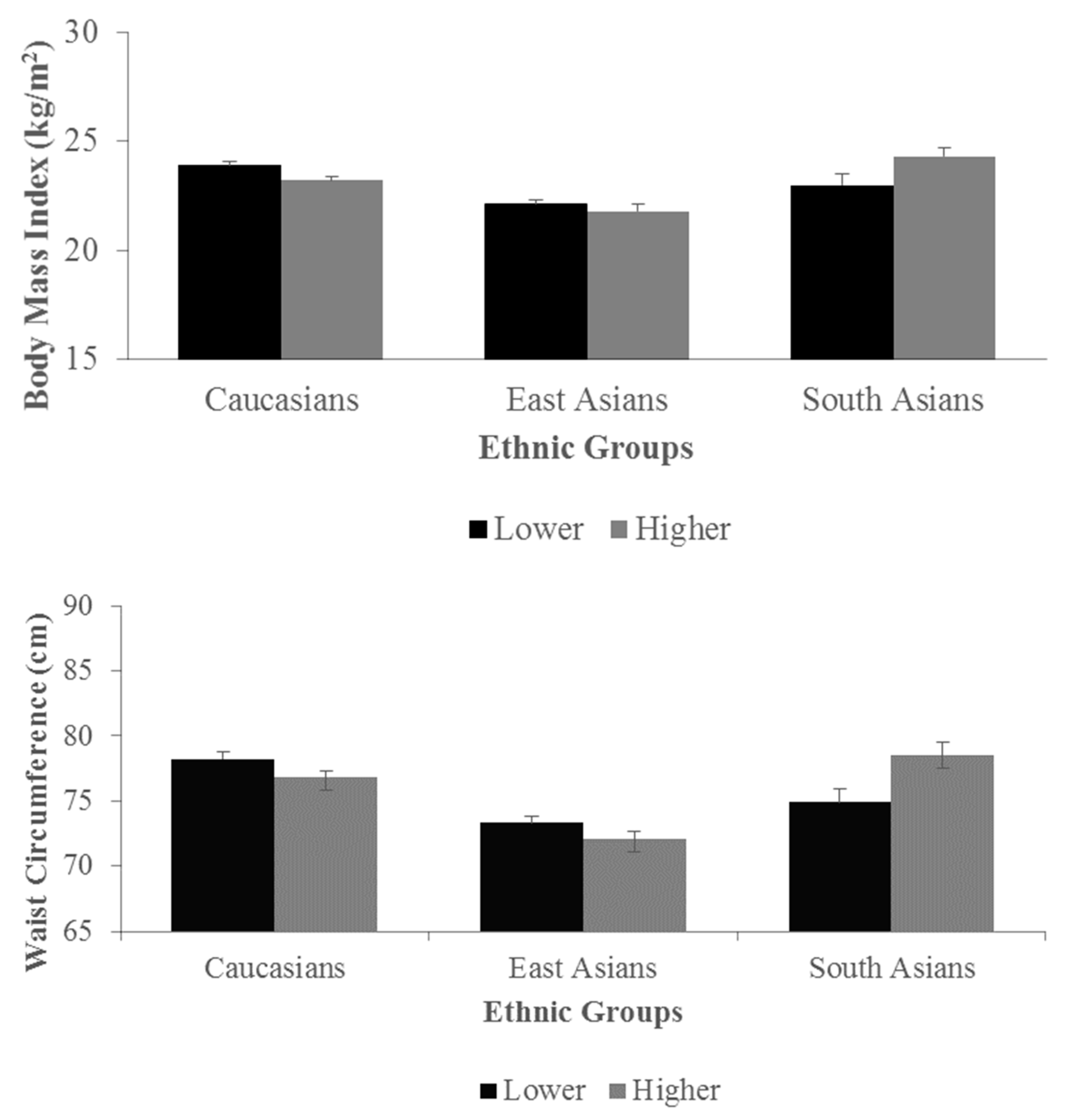
3.2. Associations between n-6/n-3 Ratios, NFKB1 Genotypes, and Anthropometric Traits
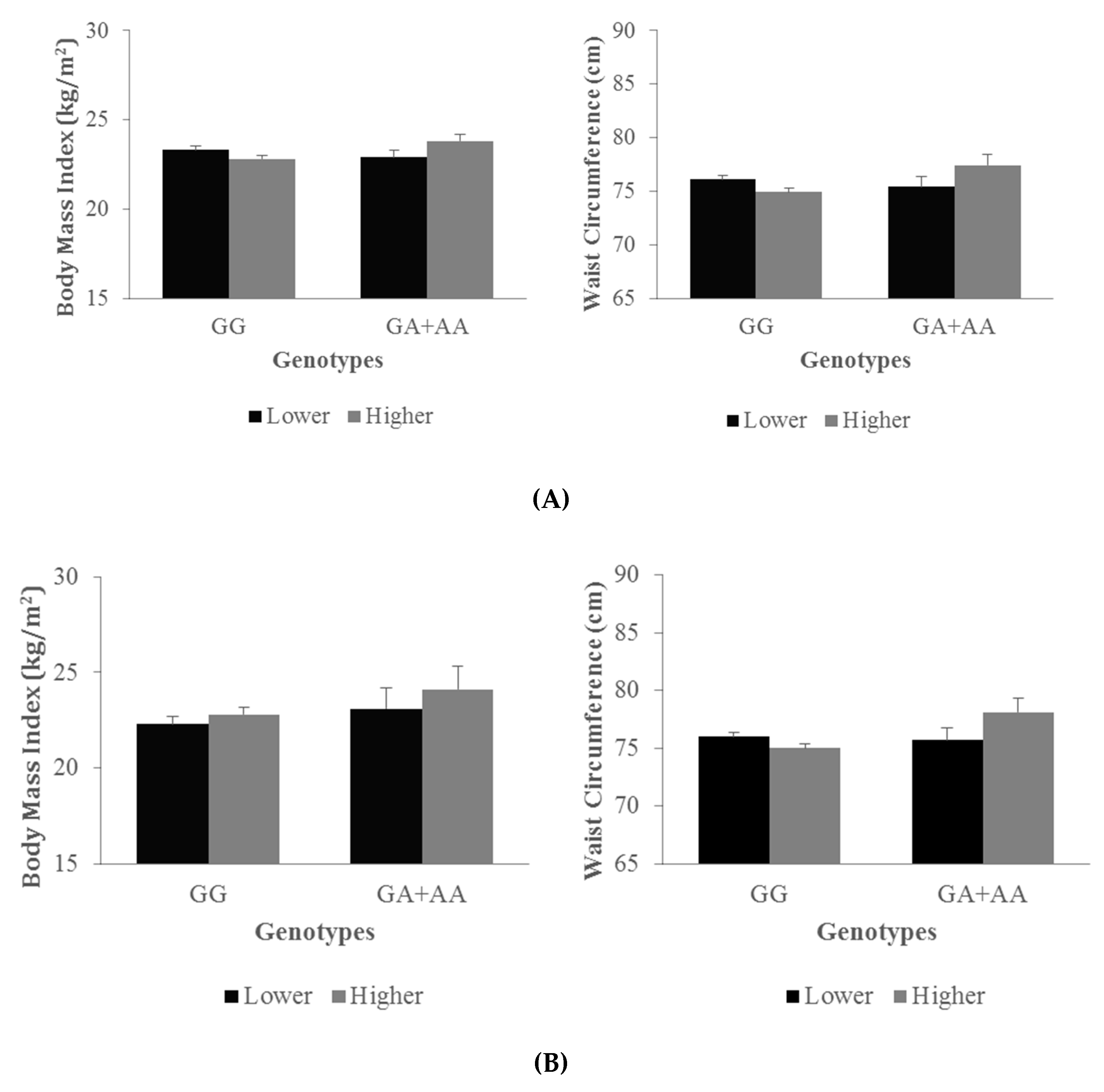
3.3. Interactions between Lower and Higher n-6/n-3 Ratio Groups and NFKB1 Genotypes on Anthropometric Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zarate, R.; El Jaber-Vazdekis, N.; Tejera, N.; Perez, J.A.; Rodriguez, C. Significance of long chain polyunsaturated fatty acids in human health. Clin. Transl. Med. 2017, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: Nutritional implications for chronic diseases. Biomed. Pharmacother. 2006, 60, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Buckley, J.D.; Howe, P.R.C. Anti-obesity effects of long-chain omega-3 polyunsaturated fatty acids. Obes. Rev. 2009, 10, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Naughton, S.S.; Mathai, M.L.; Hryciw, D.H.; McAinch, A.J. Linoleic acid and the pathogenesis of obesity. Prostaglandins Other Lipid Mediat. 2016, 125, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, M.U.; Madsen, L.; Skjoth, F.; Berentzen, T.L.; Halkjaer, J.; Tjonneland, A.; Schmidt, E.B.; Sorensen, T.I.; Kristiansen, K.; Overvad, K. Dietary intake and adipose tissue content of long-chain n-3 PUFAs and subsequent. Am. J. Clin. Nutr. 2017, 105, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-W.; Chien, Y.-S.; Chen, Y.-J.; Ajuwon, K.M.; Mersmann, H.M.; Ding, S.-T. Role of n-3 Polyunsaturated Fatty Acids in Ameliorating the Obesity-Induced Metabolic Syndrome in Animal Models and Humans. Int. J. Mol. Sci. 2016, 17, 1689. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Liu, W.; Zhao, T.Y.; Tian, H.M. Efficacy of Omega-3 Polyunsaturated Fatty Acids Supplementation in Managing Overweight and Obesity: A Meta-Analysis of Randomized Clinical Trials. J. Nutr. Health Aging 2017, 21, 187–192. [Google Scholar] [CrossRef]
- Massiera, F.; Saint-Marc, P.; Seydoux, J.; Murata, T.; Kobayashi, T.; Narumiya, S.; Guesnet, P.; Amri, E.-Z.; Negrel, R.; Ailhaud, G. Arachidonic acid and prostacyclin signaling promote adipose tissue development: A human health concern? J. Lipid Res. 2003, 44, 271–279. [Google Scholar] [CrossRef]
- Hassanali, Z.; Ametaj, B.N.; Field, C.J.; Proctor, S.D.; Vine, D.F. Dietary supplementation of n-3 PUFA reduces weight gain and improves postprandial lipaemia and the associated inflammatory response in the obese JCR:LA-cp rat. Diabetes Obes. Metab. 2010, 12, 139–147. [Google Scholar] [CrossRef]
- Li, J.; Li, F.R.; Wei, D.; Jia, W.; Kang, J.X.; Stefanovic-Racic, M.; Dai, Y.; Zhao, A.Z. Endogenous omega-3 polyunsaturated fatty acid production confers resistance to obesity, dyslipidemia, and diabetes in mice. Mol. Endocrinol. 2014, 28, 1316–1328. [Google Scholar] [CrossRef] [PubMed]
- Ruzickova, J.; Rossmeisl, M.; Prazak, T.; Flachs, P.; Sponarova, J.; Veck, M.; Tvrzicka, E.; Bryhn, M.; Kopecky, J. Omega-3 PUFA of marine origin limit diet-induced obesity in mice by reducing cellularity of adipose tissue. Lipids 2004, 39, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Leeman, J.R.; Gilmore, T.D. Alternative splicing in the NF-kappaB signaling pathway. Gene 2008, 423, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Tortosa-Caparrós, E.; Navas-Carrillo, D.; Marín, F.; Orenes-Piñero, E. Anti-inflammatory effects of omega 3 and omega 6 polyunsaturated fatty acids in cardiovascular disease and metabolic syndrome. Crit. Rev. Food Sci. Nutr. 2017, 57, 3421–3429. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Long chain fatty acids and gene expression in inflammation and immunity. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol. Nutr. Food Res. 2008, 52, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Chaudhary, A.; Sethi, S. Oxidized omega-3 fatty acids inhibit NF-kappaB activation via a PPARalpha-dependent pathway. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Allam-Ndoul, B.; Guénard, F.; Barbier, O.; Vohl, M.-C. Effect of n-3 fatty acids on the expression of inflammatory genes in THP-1 macrophages. Lipids Health Dis. 2016, 15, 69. [Google Scholar] [CrossRef]
- Camandola, S.; Leonarduzzi, G.; Musso, T.; Varesio, L.; Carini, R.; Scavazza, A.; Chiarpotto, E.; Baeuerle, P.A.; Poli, G. Nuclear factor kB is activated by arachidonic acid but not by eicosapentaenoic acid. Biochem. Biophys. Res. Commun. 1996, 229, 643–647. [Google Scholar] [CrossRef]
- Schmitz, G.; Ecker, J. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef]
- NFKB1 gene; nuclear factor kappa B subunit 1. Available online: https://ghr.nlm.nih.gov/gene/NFKB1 (accessed on 14 November 2018).
- Soydas, T.; Karaman, O.; Arkan, H.; Yenmis, G.; Ilhan, M.M.; Tombulturk, K.; Tasan, E.; Kanigur Sultuybek, G. The Correlation of Increased CRP Levels with NFKB1 and TLR2 Polymorphisms in the Case of Morbid Obesity. Scand. J. Immunol. 2016, 84, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Stegger, J.G.; Schmidt, E.B.; Berentzen, T.L.; Tjonneland, A.; Vogel, U.; Rimm, E.; Sorensen, T.I.A.; Overvad, K.; Jensen, M.K. Interaction between obesity and the NFKB1 - 94ins/delATTG promoter polymorphism in relation to incident acute coronary syndrome: A follow up study in three independent cohorts. PLoS ONE 2013, 8, e63004. [Google Scholar] [CrossRef] [PubMed]
- Costantine, M.M. Physiologic and pharmacokinetic changes in pregnancy. Front. Pharm. 2014, 5, 65. [Google Scholar] [CrossRef] [PubMed]
- El-sohemy, A.; Cahill, L.E. Functional genetic variants of glutathione S-transferase protect against. Am. J. Clin. Nutr. 2009, 90, 1411–1417. [Google Scholar]
- Abdelmagid, S.A.; Clarke, S.E.; Nielsen, D.E.; Badawi, A.; El-Sohemy, A.; Mutch, D.M.; Ma, D.W.L. Comprehensive profiling of plasma fatty acid concentrations in young healthy canadian adults. PLoS ONE 2015, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Douketis, J.B.; Paradis, G.; Keller, H.; Martineau, C. Canadian guidelines for body weight classification in adults: Application in clinical practice to screen for overweight and obesity and to assess disease risk. Can. Med. Assoc. J. 2005, 172, 995–998. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Otto, M.C.; Wu, J.H.Y.; Baylin, A.; Vaidya, D.; Rich, S.S.; Tsai, M.Y.; Jacobs, D.R.; Mozaffarian, D. Circulating and dietary omega-3 and omega-6 polyunsaturated fatty acids and incidence of CVD in the Multi-Ethnic Study of Atherosclerosis. J. Am. Heart Assoc. 2013, 2, e000506. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Peterson, C.M.; Thomas, D.M.; Heo, M.; Schuna, J.M. Why are there race/ethnic differences in adult body mass index-adiposity relationships? A quantitative critical review: BMI and race/ethnicity. Obes. Rev. 2016, 17, 262–275. [Google Scholar] [CrossRef]
- WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [CrossRef]
- Mani, I.; Kurpad, A.V. Fats & fatty acids in Indian diets: Time for serious introspection. Indian J. Med. Res. 2016, 144, 507–514. [Google Scholar]
- Chilton, F.H.; Murphy, R.C.; Wilson, B.A.; Sergeant, S.; Ainsworth, H.; Seeds, M.C.; Mathias, R.A. Diet-gene interactions and PUFA metabolism: A potential contributor to health disparities and human diseases. Nutrients 2014, 6, 1993–2022. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Mallik, S.; Majumder, P.P. A two-step genetic study on quantitative precursors of coronary artery disease in a homogeneous Indian population: Case-control association discovery and validation by transmission-disequilibrium test. J. Biosci. 2011, 36, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Seufert, B.L.; Poole, E.M.; Whitton, J.; Xiao, L.; Makar, K.W.; Campbell, P.T.; Kulmacz, R.J.; Baron, J.A.; Newcomb, P.A.; Slattery, M.L.; et al. IκBKβ and NFκB1, NSAID use and risk of colorectal cancer in the Colon Cancer Family Registry. Carcinogenesis 2013, 34, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Curtin, K.; Wolff, R.K.; Herrick, J.S.; Abo, R.; Slattery, M.L. Exploring multilocus associations of inflammation genes and colorectal cancer risk using hapConstructor. BMC Med. Genet. 2010, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Gibby, C.C.; Wang, J.; Silvas, M.R.T.; Yu, R.; Yeung, S.-C.J.; Shete, S. MAPK1/ERK2 as novel target genes for pain in head and neck cancer patients. BMC Genet. 2016, 17, 40. [Google Scholar] [CrossRef]
- Cerhan, J.R.; Liu-Mares, W.; Fredericksen, Z.S.; Novak, A.J.; Cunningham, J.M.; Kay, N.E.; Dogan, A.; Liebow, M.; Wang, A.H.; Call, T.G.; et al. Genetic variation in tumor necrosis factor and the nuclear factor-kappaB canonical pathway and risk of non-Hodgkin’s lymphoma. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 3161–3169. [Google Scholar] [CrossRef]
- Slattery, M.L.; John, E.M.; Torres-Mejia, G.; Lundgreen, A.; Herrick, J.S.; Baumgartner, K.B.; Hines, L.M.; Stern, M.C.; Wolff, R.K. Genetic variation in genes involved in hormones, inflammation and energetic factors and breast cancer risk in an admixed population. Carcinogenesis 2012, 33, 1512–1521. [Google Scholar] [CrossRef]
- Slattery, M.L.; Lundgreen, A.; Bondurant, K.L.; Wolff, R.K. Interferon-signaling pathway: associations with colon and rectal cancer risk and subsequent survival. Carcinogenesis 2011, 32, 1660–1667. [Google Scholar] [CrossRef]
| Total | Lower n-6/n-3 | Higher n-6/n-3 | P | |
|---|---|---|---|---|
| Sample size, n (men/women) | 898 (260/638) | 449 (122/327) | 449 (138/311) | 0.24 |
| Age (years) | 22.7 ± 0.1 | 22.7 ± 0.1 | 22.7 ± 0.1 | 0.93 |
| Smokers, n (%) | 1 (0.1) | 0 (0) | 1 (0.2) | 1.0 |
| Physical activity level (MET) | 7.5 ± 0.1 | 7.4 ± 0.1 | 7.7 ± 0.1 | 0.07 |
| BMI (kg/m2) | 22.7 ± 0.1 | 22.7 ± 0.2 | 22.7 ± 0.2 | 0.95 |
| WC (cm) | 73.7 ± 0.3 | 73.6 ± 0.4 | 73.9 ± 0.4 | 0.65 |
| Racial/ethnic groups, n (%) | <0.0001 | |||
| Caucasians | 455 (51) | 195 (44) | 260 (58) | |
| East Asians | 338 (37) | 203 (45) | 135 (30) | |
| South Asians | 105 (12) | 51 (11) | 54 (12) | |
| n-6 concentrations (µg/mL) 1 | 800 ± 253 | 798 ± 257 | 807 ± 255 | 0.22 |
| n-3 concentrations (µg/mL) 1 | 62.6 ± 35.1 | 77.3 ± 34.5 | 51.6 ± 19.5 | <0.0001 |
| Circulating n-6/n-3 ratio | 13.0 ± 4.7 | 10.7 ± 2.4 | 15.4 ± 3.2 | <0.0001 |
| Caloric intake (kcal) | 1947 ± 21 | 1936 ± 30 | 1958 ± 30 | 0.61 |
| Dietary n-6 intake (g) 1 | 10.7 ± 6.3 | 10.4 ± 6.3 | 10.7 ± 6.4 | 0.04 |
| Dietary n-3 intake (g) 1 | 1.21 ± 0.82 | 1.28 ± 0.91 | 1.15 ± 0.68 | 0.001 |
| Dietary n-6/n-3 ratio 1 | 8.7 ± 3.0 | 8.0 ± 3.0 | 9.3 ± 0.4 | <0.0001 |
| BMI (kg/m2) | WC (cm) | |||||||
|---|---|---|---|---|---|---|---|---|
| Genotypes (n) | Caucasians (n = 455) | East Asians (n = 338) | South Asians (n = 105) | Pint. | Caucasians (n = 455) | East Asians (n = 338) | South Asians (n = 105) | Pint. |
| rs11722146 | 0.02 | 0.04 | ||||||
| GG (392) | 23.3 ± 0.3 | 21.8 ± 0.3 | 24.2 ± 0.5 | 77.0 ± 0.6 | 72.2 ± 0.6 | 77.8 ± 1.2 | ||
| GA (394) | 23.5 ± 0.3 | 22.3 ± 0.2 | 23.8 ± 0.6 | 77.5 ± 0.6 | 73.0 ± 0.5 | 77.3 ± 1.6 | ||
| AA (112) | 23.6 ± 0.5 | 22.3 ± 0.4 | 20.6 ± 1.2 | 78.0 ± 1.2 | 73.6 ±0.8 | 69.8 ± 3.1 | ||
| P | 0.75 | 0.26 | 0.02 | 0.61 | 0.33 | 0.06 | ||
| rs1609798 | 0.003 | 0.02 | ||||||
| CC (402) | 23.4 ± 0.3 | 21.9 ± 0.2 | 24.5 ± 0.5 | 77.2 ± 0.6 | 72.3 ± 0.6 | 78.2 ± 1.3 | ||
| CT (377) | 23.4 ± 0.3 | 22.3 ± 0.2 | 23.5 ± 0.6 | 77.2 ± 0.6 | 73.0 ± 0.5 | 76.6 ± 1.6 | ||
| TT (119) | 23.7 ± 0.5 | 22.2 ± 0.4 | 21.0 ± 1.0 | 78.3 ± 1.1 | 73.4 ±0.8 | 71.7 ± 2.7 | ||
| P | 0.89 | 0.44 | 0.01 | 0.66 | 0.42 | 0.09 | ||
| rs230511 | 0.01 | 0.03 | ||||||
| GG (379) | 23.3 ± 0.3 | 21.8 ± 0.3 | 24.4 ± 0.5 | 76.9 ± 0.6 | 72.4 ± 0.6 | 78.1 ± 1.2 | ||
| GA (396) | 23.6 ± 0.3 | 22.2 ± 0.2 | 23.5 ± 0.6 | 77.6 ± 0.6 | 72.9 ± 0.5 | 76.7 ± 1.6 | ||
| AA (123) | 23.5 ± 0.5 | 22.2 ± 0.3 | 20.6 ± 1.3 | 77.8 ± 1.1 | 73.4 ±0.8 | 69.6 ± 3.3 | ||
| P | 0.67 | 0.40 | 0.02 | 0.64 | 0.54 | 0.06 | ||
| MAF | BMI (kg/m2) | WC (cm) | |||||
|---|---|---|---|---|---|---|---|
| Genotype | n-6/n-3 | Interaction | Genotype | n-6/n-3 | Interaction | ||
| rs11722146 | 0.34 | 0.60 | 0.42 | 0.82 | 0.33 | 0.38 | 0.72 |
| rs13117745 | 0.12 | 0.69 | 0.12 | 0.41 | 0.73 | 0.14 | 0.80 |
| rs1609798 | 0.25 | 0.79 | 0.35 | 0.99 | 0.74 | 0.21 | 0.84 |
| rs4648090 1 | 0.07 | 0.31 | 0.48 | 0.02 | 0.23 | 0.57 | 0.03 |
| rs4648022 1 | 0.05 | 0.11 | 0.43 | 0.06 | 0.12 | 0.43 | 0.04 |
| rs1599961 | 0.38 | 0.34 | 0.11 | 0.35 | 0.19 | 0.06 | 0.27 |
| rs230511 | 0.36 | 0.78 | 0.27 | 0.81 | 0.51 | 0.24 | 0.92 |
| rs7674640 | 0.49 | 0.63 | 0.91 | 0.44 | 0.53 | 0.68 | 0.55 |
| rs3774932 | 0.50 | 0.52 | 0.58 | 0.10 | 0.18 | 0.62 | 0.12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauman-Fortin, J.; Ma, D.W.L.; Mutch, D.M.; Abdelmagid, S.A.; Badawi, A.; El-Sohemy, A.; Fontaine-Bisson, B. The Association between Plasma Omega-6/Omega-3 Ratio and Anthropometric Traits Differs by Racial/Ethnic Groups and NFKB1 Genotypes in Healthy Young Adults. J. Pers. Med. 2019, 9, 13. https://doi.org/10.3390/jpm9010013
Bauman-Fortin J, Ma DWL, Mutch DM, Abdelmagid SA, Badawi A, El-Sohemy A, Fontaine-Bisson B. The Association between Plasma Omega-6/Omega-3 Ratio and Anthropometric Traits Differs by Racial/Ethnic Groups and NFKB1 Genotypes in Healthy Young Adults. Journal of Personalized Medicine. 2019; 9(1):13. https://doi.org/10.3390/jpm9010013
Chicago/Turabian StyleBauman-Fortin, Jeremy, David W.L. Ma, David M. Mutch, Salma A. Abdelmagid, Alaa Badawi, Ahmed El-Sohemy, and Bénédicte Fontaine-Bisson. 2019. "The Association between Plasma Omega-6/Omega-3 Ratio and Anthropometric Traits Differs by Racial/Ethnic Groups and NFKB1 Genotypes in Healthy Young Adults" Journal of Personalized Medicine 9, no. 1: 13. https://doi.org/10.3390/jpm9010013
APA StyleBauman-Fortin, J., Ma, D. W. L., Mutch, D. M., Abdelmagid, S. A., Badawi, A., El-Sohemy, A., & Fontaine-Bisson, B. (2019). The Association between Plasma Omega-6/Omega-3 Ratio and Anthropometric Traits Differs by Racial/Ethnic Groups and NFKB1 Genotypes in Healthy Young Adults. Journal of Personalized Medicine, 9(1), 13. https://doi.org/10.3390/jpm9010013






