The Impact of Potassium Channel Gene Polymorphisms on Antiepileptic Drug Responsiveness in Arab Patients with Epilepsy
Abstract
1. Introduction
2. Material and Methods
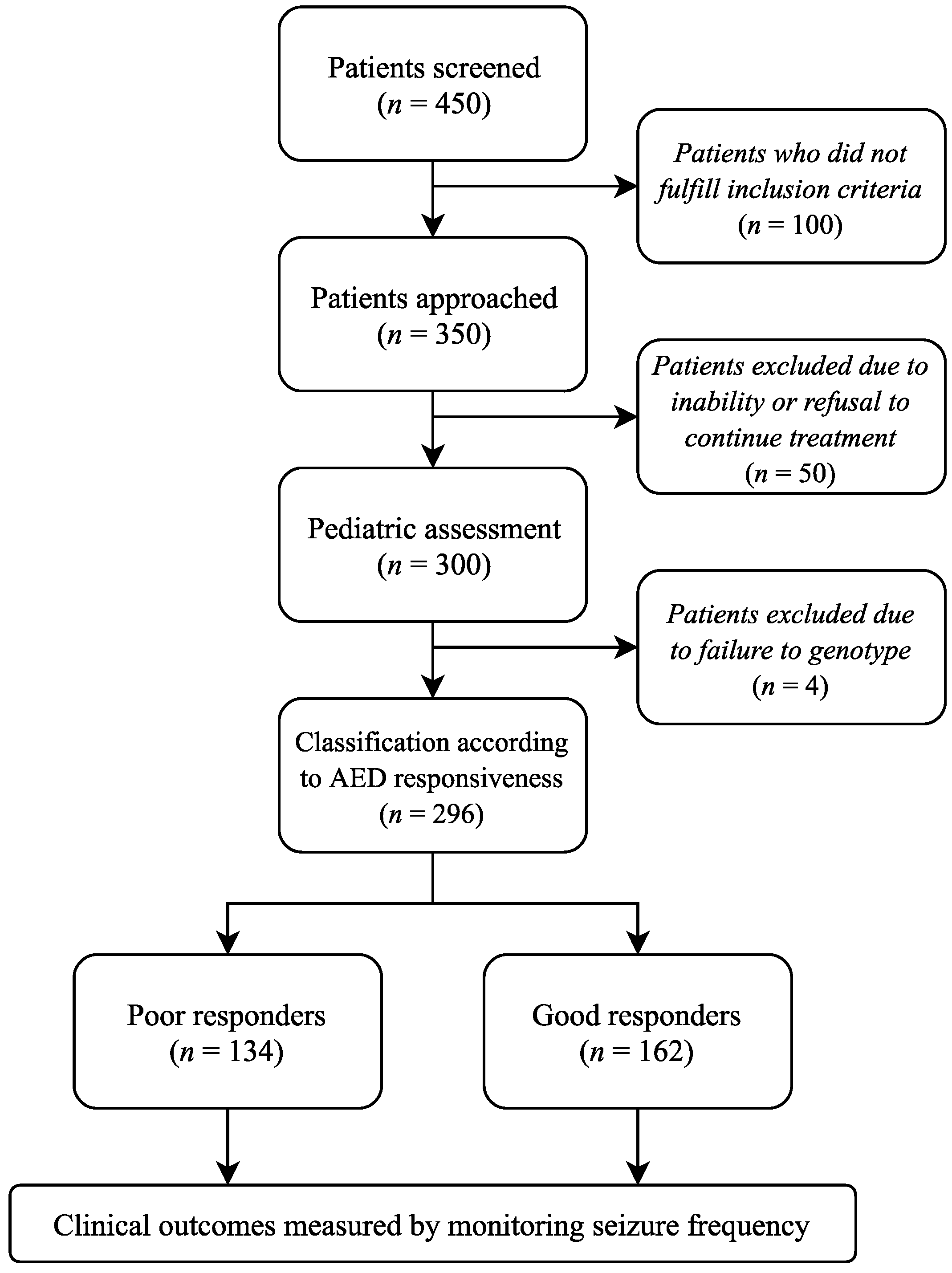
2.1. Study Population and Design
2.2. Study Design
2.3. Treatment Approach
2.4. Outcome Measure
2.5. SNP Selection and Genotyping
2.6. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Hardy-Weinberg Equilibrium Test
3.3. Quality Control
3.4. Allelic and Genotypic Distribution in Epileptic Patients
3.5. Association of KCNA1, KCNA2, and KCNV2 SNPs with Susceptibility to Generalized Epilepsy
3.6. Association of SNPs KCNA1, KCNA2 and KCNV2 Genotypes with Partial Epilepsy Susceptibility
3.7. Association of SNPs KCNA1, KCNA2, and KCNV2 Genotypes with Epilepsy Patients Responsiveness
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Epilepsy: Aetiology, Epidemiology and Prognosis. Fact Sheet No. 165. 2001. Available online: http://www.who.int/news-room/fact-sheets/detail/epilepsy (accessed on 20 July 2005).
- Steinlein, O.K. Genetic mechanisms that underlie epilepsy. Nat. Rev. Neurosci. 2004, 5, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; van Emde Boas, W.; Blume, W.; Elger, C.; Genton, P.; Lee, P.; Engel, J., Jr. Epileptic seizures and epilepsy: Definitions proposed by the International League against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005, 46, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Nordli, D.R., Jr. Idiopathic generalized epilepsies recognized by the International League against Epilepsy. Epilepsia 2005, 46 (Suppl. 9), 48–56. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.N.; Prasad, C.; Stafstrom, C.E. Recent advances in the genetics of epilepsy: Insights from human and animal studies. Epilepsia 1999, 40, 1329–1359. [Google Scholar] [CrossRef] [PubMed]
- D’Adamo, M.C.; Catacuzzeno, L.; Di Giovanni, G.; Franciolini, F.; Pessia, M. K+ channelepsy: Progress in the neurobiology of potassium channels and epilepsy. Front. Cell. Neurosci. 2013, 7, 134. [Google Scholar] [CrossRef] [PubMed]
- Camerino, D.C.; Tricarico, D.; Desaphy, J.F. Ion channel pharmacology. Neurotherapeutics 2007, 4, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.H.; Aizenman, E. Voltage-gated potassium channels at the crossroads of neuronal function, ischemic tolerance, and neurodegeneration. Transl. Stroke Res. 2014, 5, 38–58. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Lu, S.; Lu, Z.; Xu, P.; Xiang, D.; Qu, Q. Pharmacogenetic and case–control study on potassium channel related gene variants and genetic generalized epilepsy. Medicine 2017, 96, 7321. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.; Combi, R. Potassium channels and human epileptic phenotypes: An updated overview. Front. Cell. Neurosci. 2016, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Kohling, R.; Wolfart, J. Potassium channels in epilepsy. Cold Spring Harb. Perspect. Med. 2016, 6, a022871. [Google Scholar] [CrossRef] [PubMed]
- Zuberi, S.M.; Eunson, L.H.; Spauschus, A.; De Silva, R.; Tolmie, J.; Wood, N.W.; McWilliam, R.C.; Stephenson, J.B.; Kullmann, D.M.; Hanna, M.G. A novel mutation in the human voltage-gated potassium channel gene (Kv1.1) associates with episodic ataxia type 1 and sometimes with partial epilepsy. Brain 1999, 122, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.M.; Conroy, J.; Shahwan, A.; Lynch, B.; Correa, R.G.; Pena, S.D.; McCreary, D.; Magalhães, T.R.; Ennis, S.; Lynch, S.A.; et al. Unexplained early onset epileptic encephalopathy: Exome screening and phenotype expansion. Epilepsia 2016, 57, e12–e17. [Google Scholar] [CrossRef] [PubMed]
- Syrbe, S.; Hedrich, U.B.; Riesch, E.; Djémié, T.; Müller, S.; Møller, R.S.; Maher, B.; Hernandez-Hernandez, L.; Synofzik, M.; Caglayan, H.S.; et al. De novo loss- or gain-of-function mutations in KCNA2 cause epileptic encephalopathy. Nat. Genet. 2015, 47, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Brew, H.M.; Gittelman, J.X.; Silverstein, R.S.; Hanks, T.D.; Demas, V.P.; Robinson, L.C.; Robbins, C.A.; McKee-Johnson, J.; Chiu, S.Y.; Messing, A.; et al. Seizures and reduced life span in mice lacking the potassium channel subunit Kv1.2, but hypoexcitability and enlarged Kv1 currents in auditory neurons. J. Neurophysiol. 2007, 98, 1501–1525. [Google Scholar] [CrossRef] [PubMed]
- Jorge, B.S.; Campbell, C.M.; Miller, A.R.; Rutter, E.D.; Gurnett, C.A.; Vanoye, C.G.; George, A.L., Jr.; Kearney, J.A. Voltage-gated potassium channel KCNV2 (Kv8.2) contributes to epilepsy susceptibility. Proc. Natl. Acad. Sci. USA 2011, 108, 5443–5448. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Saito, Y.; Shiseki, K.; Fukushima-Uesaka, H.; Hasegawa, R.; Ozawa, S.; Sugai, K.; Katoh, M.; Saitoh, O.; Ohnuma, T.; et al. Haplotype structures of EPHX1 and their effects on the metabolism of carbamazepine-10,11-epoxide in Japanese epileptic patients. Eur. J. Clin. Pharmacol. 2005, 61, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.R.; Tan, N.C.; Kwan, P.; Brodie, M.J. Newly diagnosed epilepsy and pharmacogenomics research: A step in the right direction. Epilepsy Behav. 2011, 22, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Kasperaviciute, D.; Sisodiya, SM. Epilepsy pharmacogenetics. Pharmacogenomics 2009, 10, 817–836. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhou, B.T.; Yin, J.Y.; Xu, X.J.; Zhao, Y.C.; Lei, G.H.; Tang, Q.; Zhou, H.H.; Liu, Z.Q. ABCC2 polymorphisms and haplotype are associated with drug resistance in Chinese epileptic patients. CNS Neurosci. Ther. 2012, 18, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.T.; Zhou, Q.H.; Yin, J.Y.; Li, G.L.; Qu, J.; Xu, X.J.; Liu, D.; Zhou, H.H.; Liu, Z.Q. Effects of SCN1A and GABA receptor genetic polymorphisms on carbamazepine tolerability and efficacy in Chinese patients with partial seizures: 2-year longitudinal clinical follow-up. CNS Neurosci. Ther. 2012, 18, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.L.; Wu, X.Y.; Zheng, J.; Wu, Z.Y.; Hong, Z.; Zhong, M.K. Association of SCN1A, SCN2A and ABCC2 gene polymorphisms with the response to antiepileptic drugs in Chinese Han patients with epilepsy. Pharmacogenomics 2014, 15, 1323–1336. [Google Scholar] [CrossRef] [PubMed]
- EPICURE Consortium; EMINet Consortium; Steffens, M.; Leu, C.; Ruppert, A.K.; Zara, F.; Striano, P.; Robbiano, A.; Capovilla, G.; Tinuper, P.; et al. Genome-wide association analysis of genetic generalized epilepsies implicates susceptibility loci at 1q43, 2p16.1, 2q22.3 and 17q21.32. Hum. Mol. Genet. 2012, 21, 5359–5372. [Google Scholar] [PubMed]
- Kimchi-Sarfaty, C.; Oh, J.M.; Kim, I.W.; Sauna, Z.E.; Calcagno, A.M.; Ambudkar, S.V.; Gottesman, M.M. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science 2007, 315, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.L.; Pan, J.; Ohnuma, S.; Lund, P.E.; Pixley, J.N.; Kimchi-Sarfaty, C.; Ambudkar, S.V.; Gottesman, M.M. MDR1 synonymous polymorphisms alter transporter specificity and protein stability in a stable epithelial monolayer. Cancer Res. 2014, 74, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Eunson, L.; Rea, R.; Zuberi, S.; Youroukos, S.; Panayiotopoulos, C.; Liguori, R.; Avoni, P.; McWilliam, R.; Stephenson, J.; Hanna, M.; et al. Clinical, genetic, and expression studies of mutations in the potassium channel gene KCNA1 reveal new phenotypic variability. Ann. Neurol. 2000, 48, 647–656. [Google Scholar] [CrossRef]
| Gene | SNP ID | Model | Cases (%) | Controls (%) | OR (95% CI) | p-Value * |
|---|---|---|---|---|---|---|
| KCNA1 | rs2227910 | CC/GC/GG | 41.1/40.4/18.5 | 38.6/45.0/16.4 | _ | 0.52 |
| CC/(GC + GG) | 41.1/58.9 | 38.6/61.4 | 0.90 (0.65−1.25) | 0.53 | ||
| (CC + GC)/GG | 81.5/18.5 | 83.6/16.4 | 1.15 (0.75−1.76) | 0.51 | ||
| rs112561866 | GA | 0.0 | 0.3 | _ | 0.24 | |
| rs7974459 | CC/TC/TT | 40.5/43.7/15.8 | 39.6/41.0/19.4 | 1.04 (0.72−1.50) | 0.54 | |
| CC/(TC + TT) | 40.5/59.5 | 39.6/60.4 | 0.97 (0.69−1.35) | 0.84 | ||
| (CC + TC)/TT | 84.2/15.8 | 80.7/19.3 | 0.79 (0.51−1.21) | 0.28 | ||
| KCNA2 | rs3887820 | CC/CA/AA | 79.8/18.5/1.7 | 82.9/15.4/1.7 | _ | 0.6 |
| CC/(CA + AA) | 79.8/20.2 | 82.9/17.1 | 1.23 (0.81−1.87) | 0.33 | ||
| (CC + CA)/AA | 98.3/1.7 | 98.3/1.7 | 1.00 (0.29−3.50) | 1 | ||
| KCNV2 | rs7029012 | GG/GC/CC | 37.7/47.4/14.9 | 37/47.5/15.5 | 0.94 (0.66−1.35) | 0.98 |
| GG/(GC + CC) | 37.7/62.3 | 37/63 | 1.02(0.73−1.43) | 0.91 | ||
| (GG + GC)/CC | 85.1/14.9 | 84.5/15.5 | 1.04(0.66−1.63) | 0.87 | ||
| rs10967705 | GG/CG/CC | 34.4/50.3/15.3 | 35.5/49.1/15.4 | _ | 0.95 | |
| GG/(CG + CC) | 34.3/65.7 | 35.5/64.5 | 1.05 (0.75−1.47) | 0.78 | ||
| (GG + CG)/CC | 84.7/15.3 | 84.6/15.4 | 0.99 (0.64−1.55) | 0.98 | ||
| rs10967728 | GG/GC/CC | 31.0/48.3/20.7 | 27.8/49.6/22.6 | _ | 0.67 | |
| GG/(GC + CC) | 31.0/69.0 | 27.8/72.2 | 0.85 (0.60−1.22) | 0.39 | ||
| (GG + GC)/CC | 79.3/20.7 | 77.4/22.6 | 0.89 (0.60−1.33) | 0.58 |
| Gene | SNP ID | Model | Cases (%) | Controls (%) | OR (95% CI) | p-Value * |
|---|---|---|---|---|---|---|
| KCNA1 | rs2227910 | CC/GC/GG | 40.0/42.9/17.1 | 38.6/45.0/16.4 | 0.92 (0.61−1.39) | 0.91 |
| CC/(GC + GG) | 40.0/60.0 | 38.6/61.4 | 0.94 (0.64−1.39) | 0.76 | ||
| (CC + GC)/GG | 82.9/19.1 | 83.6/16.4 | 1.05 (0.63−1.73) | 0.86 | ||
| rs112561866 | GA | 0.0 (0.0) | 1.0 (0.3) | 0.00 (0.00-NA) | 0.34 | |
| rs7974459 | CC/TC/TT | 38.3/43.7/18.0 | 39.6/41.1/19.3 | 1.10 (0.72−1.68) | 0.85 | |
| CC/(TC + TT) | 38.3/61.7 | 39.6/60.4 | 1.06 (0.71−1.56) | 0.78 | ||
| (CC + TC)/TT | 82.0/18.0 | 80.7/19.3 | 0.92 (0.56−1.50) | 0.73 | ||
| KCNA2 | rs3887820 | CC/CA/AA | 50.6/47.0/2.4 | 82.9/15.4/1.7 | 5.02 (3.23−7.80) | <0.0001 |
| CC/(CA + AA) | 50.6/49.4 | 82.9/17.1 | 4.75 (3.09−7.28) | <0.0001 | ||
| (CC + CA)/AA | 97.7/2.3 | 98.3/1.7 | 1.39 (0.37−5.24) | 0.63 | ||
| KCNV2 | rs7029012 | GG/GC/CC | 39.3/47.6/13.1 | 34.8/49.3/15.9 | 0.86 (0.57−1.29) | 0.54 |
| GG/(GC + CC) | 39.3/60.7 | 34.8/65.2 | 0.82 (0.56−1.22) | 0.34 | ||
| (GC + GG)/CC | 86.9/13.1 | 84.1/15.9 | 0.80 (0.46−1.38) | 0.41 | ||
| rs10967705 | GG/CG/CC | 34.5/50.9/14.6 | 35.5/49.2/15.3 | 1.06 (0.70−1.61) | 0.94 | |
| GG/(CG + CC) | 34.5/65.5 | 35.5/64.5 | 1.04 (0.70−1.55) | 0.84 | ||
| (GG + CG)/CC | 85.4/14.6 | 84.6/15.4 | 0.94 (0.56−1.60) | 0.82 | ||
| rs10967728 | GG/GC/CC | 30.8/49.7/19.5 | 27.8/49.6/22.6 | 0.90 (0.58−1.40) | 0.67 | |
| GG/(GC + CC) | 30.8/69.2 | 27.8/72.2 | 0.87 (0.57−1.31) | 0.5 | ||
| (GG + GC)/CC | 80.5/19.5 | 77.4/22.6 | 0.83 (0.52−1.33) | 0.44 |
| Epilepsy Subtype | SNP ID | Allele/Genotype | Cases N (%) | Controls N (%) | OR (95% CI) | p-Value * |
|---|---|---|---|---|---|---|
| GM | rs7029012 | C | 41 (36) | 240 (41) | 1.00 | 0.15 |
| G | 73 (64) | 352 (59) | ||||
| GG | 20 (35) | 103 (35) | 0.99 (0.54−1.79) | 0.97 | ||
| CG + CC | 37 (65) | 193 (65) | ||||
| GM | rs10967705 | C | 44 (39) | 239 (40) | 1.00 | 0.51 |
| G | 70 (61) | 359 (60) | ||||
| GG | 19 (33) | 106 (35) | 1.10 (0.60−2.00) | 0.76 | ||
| CG + CC | 38 (67) | 193 (65) | ||||
| GM | rs3887820 | C | 55 (48) | 531 (91) | 1.00 | <0.0001 |
| A | 59 (52) | 55 (9) | ||||
| AA | 2 (4) | 10 (1.7) | 1.00 | <0.0001 | ||
| CC + CA | 55 (96) | 288 (98.3) | ||||
| GTC | rs10967728 | G | 125 (56) | 303 (53) | 1.00 | 0.72 |
| C | 99 (44) | 273 (47) | ||||
| GG | 35 (31) | 80 (28) | 0.85 (0.53−1.36) | 0.49 | ||
| GC + CC | 77 (69) | 208 (72) | ||||
| GTC | rs10967705 | G | 135 (59) | 359 (60) | 1.00 | 0.95 |
| C | 93 (41) | 239 (40) | ||||
| GG | 40 (35) | 106 (35) | 1.00 | 0.94 | ||
| GC + CC | 74 (65) | 193 (65) |
| Gene | SNP ID | Model | Cases (%) | Controls (%) | OR (95% CI) | p-Value * |
|---|---|---|---|---|---|---|
| KCNA1 | rs2227910 | CC/GC/GG | 42.6/36.9/20.5 | 38.6/45.0/16.4 | 0.74 (0.46−1.19) | 0.29 |
| CC/(GC + GG) | 42.6/57.4 | 38.6/61.4 | 0.85 (0.55−1.30) | 0.44 | ||
| (CC + GC)/GG | 79.5/20.5 | 83.6/16.4 | 1.31 (0.77−2.24) | 0.33 | ||
| rs112561866 | GA | 0.0 (0.0) | 1.0 (0.3) | 0.00 (0.00–NA) | 0.4 | |
| rs7974459 | CC/TC/TT | 43.6/43.6/12.8 | 39.6/41.1/19.3 | 0.97 (0.61−1.54) | 0.28 | |
| CC/(TC + TT) | 43.6/56.4 | 39.6/60.4 | 0.85 (0.55−1.31) | 0.47 | ||
| (CC + TC)/TT | 87.2/12.8 | 80.7/19.3 | 0.61 (0.33−1.14) | 0.11 | ||
| KCNV2 | rs7029012 | GG/GC/CC | 41.0/42.6/16.4 | 34.8/49.3/15.9 | 0.73 (0.46−1.17) | 0.42 |
| GG/(GC + CC) | 41.0/59.0 | 34.8/65.2 | 0.77 (0.50−1.18) | 0.23 | ||
| (GC + GG)/CC | 83.6/16.4 | 84.1/15.9 | 1.04 (0.59−1.84) | 0.9 | ||
| rs10967705 | GG/CG/CC | 34.1/49.6/16.3 | 35.5/49.1/15.4 | 1.05 (0.66−1.67) | 0.96 | |
| GG/(CG + CC) | 34.2/65.8 | 35.5/64.5 | 1.06 (0.68−1.65) | 0.8 | ||
| (GG + CG)/CC | 83.7/16.3 | 84.6/15.4 | 1.07 (0.60−1.89) | 0.82 | ||
| rs10967728 | GG/GC/CC | 31.4/46.3/22.3 | 27.8/49.6/22.6 | 0.82 (0.50−1.35) | 0.75 | |
| GG/(GC + CC) | 31.4/68.6 | 27.8/72.2 | 0.84 (0.53−1.33) | 0.46 | ||
| (GG + GC)/CC | 77.7/22.3 | 77.4/22.6 | 0.99 (0.59−1.64) | 0.96 |
| Gene | SNP ID | Model | Poor Responders (%) | Good Responders (%) | OR (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| KCNA1 | rs2227910 | CC/GC/GG | 40.2/46.2/13.6 | 41.9/35.6/22.5 | 1.35 (0.81−2.25) | 0.07 |
| CC/(GC + GG) | 40.1/59.9 | 41.9/58.1 | 0.54 (0.29−1.01) | 0.77 | ||
| (CC + GC)/GG | 86.4/13.6 | 77.5/22.5 | 1.07 (0.67−1.72) | 0.05 | ||
| rs7974459 | CC/TC/TT | 40.6/45.3/14.1 | 40.4/42.3/17.3 | 1.06 (0.64−1.77) | 0.73 | |
| CC/(TC + TT) | 40.6/59.4 | 40.4/59.6 | 0.99 (0.62−1.59) | 0.97 | ||
| (CC + TC)/TT | 85.9/14.1 | 82.7/17.3 | 0.78 (0.41−1.50) | 0.45 | ||
| KCNA2 | rs3887820 | CC/CA/AA | 79.4/17.6/3.0 | 80.2/19.2/0.6 | 0.92 (0.51−1.67) | 0.26 |
| CC/(CA + AA) | 79.4/20.6 | 80.1/19.9 | 1.05 (0.59−1.86) | 0.88 | ||
| (CC + CA)/AA | 97.0/3.0 | 99.4/0.6 | 5.04 (0.56−45.60) | 0.1 | ||
| KCNV2 | rs7029012 | GG/GC/CC | 40.5/43.5/16.0 | 39.6/47.2/13.2 | 0.90 (0.55−1.49) | 0.73 |
| GG/(GC + CC) | 40.5/59.5 | 39.6/60.4 | 0.97 (0.60−1.55) | 0.89 | ||
| (GG + GC)/CC | 84.0/16.0 | 86.8/13.2 | 1.25 (0.65−2.41) | 0.5 | ||
| rs10967705 | GG/CG/CC | 37.3/45.5/17.2 | 31.9/54.4/13.7 | 0.72 (0.43−1.19) | 0.31 | |
| GG/(CG + CC) | 37.3/62.7 | 31.9/68.1 | 0.79 (0.49−1.27) | 0.33 | ||
| (GG + CG)/CC | 82.8/17.2 | 86.2/13.8 | 1.30 (0.69−2.45) | 0.42 | ||
| rs10967728 | GG/GC/CC | 30.5/45.8/23.7 | 31.4/50.3/18.3 | 0.94 (0.55−1.60) | 0.51 | |
| GG/(GC + CC) | 30.5/69.5 | 31.5/68.5 | 1.04 (0.63−1.72) | 0.87 | ||
| (GG + GC)/CC | 76.3/23.7 | 81.8/18.2 | 1.39 (0.79−2.46) | 0.26 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
AL-Eitan, L.N.; Al-Dalalah, I.M.; Elshammari, A.K.; Khreisat, W.H.; Almasri, A.Y. The Impact of Potassium Channel Gene Polymorphisms on Antiepileptic Drug Responsiveness in Arab Patients with Epilepsy. J. Pers. Med. 2018, 8, 37. https://doi.org/10.3390/jpm8040037
AL-Eitan LN, Al-Dalalah IM, Elshammari AK, Khreisat WH, Almasri AY. The Impact of Potassium Channel Gene Polymorphisms on Antiepileptic Drug Responsiveness in Arab Patients with Epilepsy. Journal of Personalized Medicine. 2018; 8(4):37. https://doi.org/10.3390/jpm8040037
Chicago/Turabian StyleAL-Eitan, Laith N., Islam M. Al-Dalalah, Afrah K. Elshammari, Wael H. Khreisat, and Ayah Y. Almasri. 2018. "The Impact of Potassium Channel Gene Polymorphisms on Antiepileptic Drug Responsiveness in Arab Patients with Epilepsy" Journal of Personalized Medicine 8, no. 4: 37. https://doi.org/10.3390/jpm8040037
APA StyleAL-Eitan, L. N., Al-Dalalah, I. M., Elshammari, A. K., Khreisat, W. H., & Almasri, A. Y. (2018). The Impact of Potassium Channel Gene Polymorphisms on Antiepileptic Drug Responsiveness in Arab Patients with Epilepsy. Journal of Personalized Medicine, 8(4), 37. https://doi.org/10.3390/jpm8040037





