Knowledge, Attitudes and Referral Patterns of Lynch Syndrome: A Survey of Clinicians in Australia
Abstract
:1. Introduction
2. Experimental
2.1. Participants and Procedures
2.2. Instrumentation
2.3. Data Analyses
3. Results
| Total | GP | GYN | GE | ONC | SURG | Others b | |
|---|---|---|---|---|---|---|---|
| N (%) c | N (%) c | N (%) c | N (%) c | N (%) c | N (%) c | N (%) c | |
| Total | 144 (100) | 18 (100) | 24 (100) | 11 (100) | 59 (100) | 27 (100) | 5 (100) |
| Age (years) | |||||||
| <50 | 84 (58) | 10 (56) | 11 (46) | 6 (55) | 37 (63) | 17 (63) | 3 (60) |
| ≥50 | 60 (42) | 8 (44) | 13 (54) | 5 (46) | 22 (37) | 10 (37) | 2 (40) |
| Gender | |||||||
| Female | 67 (47) | 8 (44) | 8 (33) | 0 | 37 (63) | 9 (33) | 5 (100) |
| Male | 77 (53) | 10 (56) | 16 (67) | 11 (100) | 22 (37) | 18 (67) | 0 |
| Years of Practice in Specialty | |||||||
| <10 | 59 (41) | 5 (28) | 7 (29) | 4 (36) | 27 (46) | 12 (44) | 4 (80) |
| ≥10 | 85 (59) | 13 (72) | 17 (71) | 7 (64) | 32 (54) | 15 (56) | 1 (20) |
| State d | |||||||
| NSW/ACT | 44 (31) | 6 (33) | 9 (38) | 0 | 18 (31) | 9 (33) | 3 (60) |
| VIC/TAS | 44 (31) | 2 (11) | 5 (21) | 4 (36) | 20 (34) | 11 (41) | 2 (40) |
| QLD | 29 (20) | 5 (28) | 11 (46) | 2 (18) | 9 (15) | 3 (11) | 0 |
| SA | 11 (8) | 2 (11) | 0 | 3 (27) | 6 (10) | 0 | 0 |
| WA | 16 (11) | 3 (17) | 1 (4) | 2 (18) | 6 (10) | 4 (15) | 0 |
3.1. Referral to Genetics Services and Ordering Diagnostic Testing for Lynch Syndrome
| Total (N = 144) | GP (N = 18) | GYN (N = 24) | GE (N = 11) | ONC (N = 59) | SURG (N = 27) | Others c (N = 5) | |
|---|---|---|---|---|---|---|---|
| N(%; 95%CI) d | N(%;95%CI) d p-value | N(%;95%CI) d p-value | N(%;95%CI) d p-value | N(%;95%CI) d p-value | N(%;95%CI)d p-value | N(%;95%CI) d p-value | |
| Referred patients to genetic services | 112 (78; 70–84) | 9 (50; 29–71) 0.003 | 19 (79; 60–91) 0.969 | 10 (91; 62–98) 0.458 | 50 (85; 73–92) 0.095 | 22 (81; 63–92) 0.799 | 2 (50) 0.196 |
| Ordered tumour IHC testing | 77 (53; 45–61) | 4 (22; 9–45) 0.002 | 6 (25; 12–45) 0.003 | 8 (73; 43–90) 0.524 | 39 (66; 53–77) 0.030 | 20 (74; 55–87) 0.004 | 0 - |
| Ordered tumour MSI testing | 55 (38; 31–46) | 1 (6; 1–26) 0.002 | 3 (13; 4–31) 0.004 | 6 (55; 28–79) 0.527 | 29 (49; 37–62) 0.075 | 16 (59; 41–75) 0.005 | 0 - |
| Ordered DNA mutation testing | 67 (47; 39–55) | 6 (33; 16–56) 0.125 | 7 (29; 15–49) 0.034 | 3 (27; 10–57) 0.308 | 36 (61; 48–72) 0.007 | 15 (56; 37–72) 0.249 | 0 - |
3.2. Risk and Surveillance Strategies among Clinicians
3.3. Prevalence of Motivators and Barriers to Referral
| Have not Referred for Genetic Services (Total = 30) N (%) a | Referred for Genetic Services (Total = 112) N (%) a | p-value b | |||
|---|---|---|---|---|---|
| Motivators | |||||
| To provide genetic counselling for the patient | 20 | (66) | 103 | (92) | <0.001 |
| Patient interest or request | 20 | (66) | 92 | (82) | 0.065 |
| To provided appropriate screening and/or management for the patient’s family | 15 | (50) | 94 | (84) | <0.001 |
| To provide appropriate cancer risk assessments for the patient | 21 | (70) | 98 | (88) | 0.544 |
| To provide genetic testing for germline mutations | 14 | (47) | 83 | (74) | 0.004 |
| Reassurance for the patient and family | 16 | (53) | 73 | (65) | 0.234 |
| Ethical and legal responsibility | 14 | (47) | 70 | (63) | 0.117 |
| To provide appropriate screening and management for the patient | 20 | (66) | 81 | (72) | 0.544 |
| Others c | 0 | 5 | (4) | - | |
| Barriers | |||||
| Patient was not interested when referral was offered | 17 | (57) | 62 | (55) | 0.898 |
| Patient may be at risk for insurance discrimination | 5 | (17) | 19 | (17) | 0.969 |
| Recommendations and guidelines were not available to select patients for referral | 8 | (27) | 11 | (10) | 0.016 |
| Patient is unlikely to benefit from genetic counselling/testing | 1 | (3) | 16 | (14) | 0.123 |
| I do not feel familiar with hereditary cancer syndromes | 5 | (17) | 6 | (5) | 0.040 |
| Long waiting time for appointment at genetics clinic | 2 | (7) | 7 | (6) | 0.934 |
| I do not know how to make a referral to the local genetic health service | 7 | (23) | 1 | (1) | <0.001 |
| I do not have access to genetic health service | 6 | (20) | 2 | (2) | 0.001 |
| I do not feel it is my responsibility | 2 | (7) | 1 | (1) | 0.113 |
| Others d | 0 | 4 | (4) | - | |
3.4. Referral preferences, Perceived Role and Desired Support for the Delivery of Genetic Services among Clinicians
| Perceived Roles | Have not referred for genetic services (Total = 30) N (%) a | Referred for genetic services (Total = 112) N (%) a | p-value b |
|---|---|---|---|
| Providing emotional support after genetic testing | 21 (70) | 64 (57) | 0.202 |
| Identifying patients for referral to genetic services | 19 (63) | 104 (93) | <0.001 |
| Interpreting germline DNA-based genetic test results | 0 | 22 (20) | 0.004 |
| Collecting a three-generation family history information | 11 (37) | 54 (48) | 0.260 |
| Ordering pre-genetic testing of tumour tissue (e.g., MSI or IHC) | 5 (17) | 53 (47) | 0.002 |
| Counselling patients about their cancer risks after genetic testing | 8 (27) | 38 (34) | 0.450 |
| Counselling patients about their cancer risks before genetic testing | 7 (23) | 42 (38) | 0.147 |
| Calculating relative risk of cancer associated with family cancer history | 4 (13) | 25 (22) | 0.321 |
| Discussing the need for cancer surveillance or prophylaxis with patients when required | 17 (57) | 90 (80) | 0.011 |
| Providing regular clinical examination and care to patients with hereditary cancer syndromes | 15 (50) | 82 (73) | 0.015 |
| Other | 3 (10) c | 5 (5) | - |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lynch, H.T.; Watson, P.; Shaw, T.G.; Lynch, J.F.; Harty, A.E.; Franklin, B.A.; Kapler, C.R.; Tinley, S.T.; Liu, B.; Lerman, C. Clinical impact of molecular genetic diagnosis, genetic counseling, and management of hereditary cancer. Cancer 1999, 86, 2449–2456. [Google Scholar] [CrossRef]
- Dinh, T.A.; Rosner, B.I.; Atwood, J.C.; Boland, C.R.; Syngal, S.; Vasen, H.F.; Gruber, S.B.; Burt, R.W. Health benefits and cost-effectiveness of primary genetic screening for Lynch syndrome in the general population. Cancer Prev. Res. 2011, 4, 9–22. [Google Scholar] [CrossRef]
- Vasen, H.F.; Blanco, I.; Aktan-Collan, K.; Gopie, J.P.; Alonso, A.; Aretz, S.; Bernstein, I.; Bertario, L.; Burn, J.; Capella, G. Revised guidelines for the clinical management of Lynch syndrome (HNPCC): Recommendations by a group of European experts. Gut 2013, 62, 812–823. [Google Scholar] [CrossRef]
- Barrow, E.; Hill, J.; Evans, D.G. Cancer risk in Lynch Syndrome. Fam. Cancer 2013, 12, 229–240. [Google Scholar] [CrossRef]
- Win, A.K.; Lindor, N.M.; Winship, I.; Tucker, K.M.; Buchanan, D.D.; Young, J.P.; Rosty, C.; Leggett, B.; Giles, G.G.; Goldblatt, J. Risks of colorectal and other cancers after endometrial cancer for women with Lynch syndrome. J. Natl. Cancer Inst. 2013, 105, 274–279. [Google Scholar] [CrossRef]
- Wong, C.; Gibbs, P.; Johns, J.; Jones, I.; Faragher, I.; Lynch, E.; Macrae, F.; Lipton, L. Value of database linkage: Are patients at risk of familial colorectal cancer being referred for genetic counselling and testing? Intern. Med. J. 2008, 38, 328–333. [Google Scholar] [CrossRef]
- Singh, H.; Schiesser, R.; Anand, G.; Richardson, P.A.; El-Serag, H.B. Underdiagnosis of Lynch syndrome involves more than family history criteria. Clin. Gastroenterol. Hepatol. 2010, 8, 523–529. [Google Scholar] [CrossRef]
- Tan, Y.Y.; McGaughran, J.; Ferguson, K.; Walsh, M.D.; Buchanan, D.D.; Young, J.P.; Webb, P.M.; Obermair, A.; Spurdle, A.B. Improving identification of lynch syndrome patients: A comparison of research data with clinical records. Int. J. Cancer 2013, 132, 2876–2883. [Google Scholar] [CrossRef]
- Pujol, P.; Lyonnet, D.S.; Frebourg, T.; Blin, J.; Picot, M.C.; Lasset, C.; Dugast, C.; Berthet, P.; de Paillerets, B.B.; Sobol, H.; et al. Lack of referral for genetic counseling and testing in BRCA1/2 and Lynch syndromes: A nationwide study based on 240,134 consultations and 134,652 genetic tests. Breast Cancer Res. Treat. 2013, 141, 135–144. [Google Scholar] [CrossRef]
- Grover, S.; Stoffel, E.M.; Bussone, L.; Tschoegl, E.; Syngal, S. Physician assessment of family cancer history and referral for genetic evaluation in colorectal cancer patients. Clin. Gastroenterol. Hepatol. 2004, 2, 813–819. [Google Scholar] [CrossRef]
- Suther, S.; Goodson, P. Barriers to the provision of genetic services by primary care physicians: A systematic review of the literature. Genet. Med. 2003, 5, 70–76. [Google Scholar] [CrossRef]
- Tan, Y.Y.; Noon, L.L.; McGaughran, J.M.; Spurdle, A.B.; Obermair, A. Referral of patients with suspected hereditary breast-ovarian cancer or Lynch syndrome for genetic services: A systematic review. J. Community Med. Health Educ. 2013. [Google Scholar] [CrossRef]
- Prochniak, C.F.; Martin, L.J.; Miller, E.M.; Knapke, S.C. Barriers to and motivations for physician referral of patients to cancer genetics clinics. J. Genet. Couns. 2011, 21, 305–325. [Google Scholar]
- Domanska, K.; Carlsson, C.; Bendahl, P.O.; Nilbert, M. Knowledge about hereditary nonpolyposis colorectal cancer; mutation carriers and physicians at equal levels. BMC Med. Genet. 2009. [Google Scholar] [CrossRef]
- Frey, M.K.; Biewald, M.A.; Worley, M.J., Jr.; Taylor, J.S.; Lin, S.N.; Holcomb, K. Lynch Syndrome: Awareness among Medical Students at a United States Medical School. Curr. Womens Health Rev. 2012, 8, 242–247. [Google Scholar] [CrossRef]
- Tan, Y.Y.; Fitzgerald, L.J. Barriers and Motivators for Referral of Patients with Suspected Lynch Syndrome to Cancer Genetic Services: A Qualitative Study. J. Pers. Med. 2014, 4, 20–34. [Google Scholar] [CrossRef]
- Vasen, H.; Watson, P.; Mecklin, J.; Lynch, H. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 1999, 116, 1453–1456. [Google Scholar] [CrossRef]
- Umar, A.; Boland, C.R.; Terdiman, J.P.; Syngal, S.; Chapelle, A.; Rüschoff, J.; Fishel, R.; Lindor, N.M.; Burgart, L.J.; Hamelin, R.; et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J. Natl. Cancer Inst. 2004, 96, 261–268. [Google Scholar] [CrossRef]
- Lancaster, J.M.; Powell, C.B.; Kauff, N.D.; Cass, I.; Chen, L.M.; Lu, K.H.; Mutch, D.G.; Berchuck, A.; Karlan, B.Y.; Herzog, T.J.; et al. Society of Gynecologic Oncologists Education Committee statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol. Oncol. 2007, 107, 159–162. [Google Scholar] [CrossRef]
- Gaff, C.L.; Aitken, M.; Flouris, A.; Metcalfe, S.A. A model for the development of genetics education programs for health professionals. Genet. Med. 2007, 9, 451–457. [Google Scholar] [CrossRef]
- Buchanan, D.D.; Tan, Y.Y.; Walsh, M.D.; Clendenning, M.; Metcalf, A.M.; Ferguson, K.; Arnold, S.T.; Thompson, B.A.; Lose, F.A.; Parsons, M.T.; et al. Tumor mismatch repair immunohistochemistry and DNA MLH1 methylation testing of patients with endometrial cancer diagnosed at age younger than 60 years optimizes triage for population-level germline mismatch repair gene mutation testing. J. Clin. Oncol. 2014, 32, 90–100. [Google Scholar] [CrossRef]
- CAP. Technology Assessment Committee. POET Report: Perspectives on Emerging Technology Prognostic Uses of MSI Testing 2011. Available online: http://www.cap.org/apps/docs/committees/technology/microsatellite_testing.pdf (accessed on 4 April 2014).
- Schmeler, K.M.; Lynch, H.T.; Chen, L.M.; Munsell, M.F.; Soliman, P.T.; Clark, M.B.; Daniels, M.S.; White, K.G.; Boyd-Rogers, S.G.; Conrad, P.G.; et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N. Engl. J. Med. 2006, 354, 261–269. [Google Scholar] [CrossRef]
- Lu, K.H.; Loose, D.S.; Yates, M.S.; Nogueras-Gonzalez, G.M.; Munsell, M.F.; Chen, L.M.; Lynch, H.; Cornelison, T.; Boyd-Rogers, S.; Rubin, M.; et al. Prospective, multi-center randomized intermediate biomarker study of oral contraceptive vs. depo-provera for prevention of endometrial cancer in women with Lynch Syndrome. Cancer Prev. Res. 2013, 6, 774–781. [Google Scholar] [CrossRef]
- Jarvinen, H.J.; Aarnio, M.; Mustonen, H.; Aktan-Collan, K.; Aaltonen, L.A.; Peltomaki, P.; de la Chapelle, A.; Mecklin, J.P. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology 2000, 118, 829–834. [Google Scholar] [CrossRef]
- Jarvinen, H.J.; Renkonen-Sinisalo, L.; Aktan-Collan, K.; Peltomaki, P.; Aaltonen, L.A.; Mecklin, J.P. Ten years after mutation testing for Lynch syndrome: Cancer incidence and outcome in mutation-positive and mutation-negative family members. J. Clin. Oncol. 2009, 27, 4793–4797. [Google Scholar] [CrossRef]
- De Jong, A.E.; Hendriks, Y.M.; Kleibeuker, J.H.; de Boer, S.Y.; Cats, A.; Griffioen, G.; Nagengast, F.M.; Nelis, F.G.; Rookus, M.A.; Vasen, H.F. Decrease in mortality in Lynch syndrome families because of surveillance. Gastroenterology 2006, 130, 665–671. [Google Scholar] [CrossRef]
- Batra, S.; Valdimarsdottir, H.; McGovern, M.; Itzkowitz, S.; Brown, K. Awareness of genetic testing for colorectal cancer predisposition among specialists in gastroenterology. Am. J. Gastroenterol. 2002, 97, 729–733. [Google Scholar] [CrossRef]
- Kelly, K.M.; Love, M.M.; Pearce, K.A.; Porter, K.; Barron, M.A.; Andrykowski, M. Cancer risk assessment by rural and Appalachian family medicine physicians. J. Rural Health 2009, 25, 372–377. [Google Scholar] [CrossRef]
- Carroll, J.C.; Cappelli, M.; Miller, F.; Wilson, B.J.; Grunfeld, E.; Peeters, C.; Hunter, A.G.; Gilpin, C.; Prakash, P. Genetic services for hereditary breast/ovarian and colorectal cancers—Physicians’ awareness, use and satisfaction. Community Genet. 2008, 11, 43–51. [Google Scholar] [CrossRef]
- Freedman, A.N.; Wideroff, L.; Olson, L.; Davis, W.; Klabunde, C.; Srinath, K.P.; Reeve, B.B.; Croyle, R.T.; Ballard-Barbash, R. US physicians’ attitudes toward genetic testing for cancer susceptibility. Am. J. Med. Genet. A 2003, 120, 63–71. [Google Scholar]
- Cox, S.L.; Zlot, A.I.; Silvey, K.; Elliott, D.; Horn, T.; Johnson, A.; Leman, R.F. Patterns of cancer genetic testing: A randomized survey of Oregon clinicians. J. Cancer Epidemiol. 2012. [Google Scholar] [CrossRef]
- Linstone, H.; Turoff, M. The Delphi Method: Techniques and Applications; Addison-Wesley Pub. Co.: Newark, NJ, USA, 1975. [Google Scholar]
- Cancer Australia. Report to the Nation—Uterine Cancer 2012. Available online: http://canceraustralia.gov.au/publications-resources/cancer-australia-publications/report-nation-uterine-cancer-2012 ( accessed on 4 April 2014).
- Cancer Australia. Diagnostic guide for general practitioners and gynaecologists. Available online: http://www.canceraustralia.gov.au/sites/default/files/publications/ncgc-vaginal-bleeding-flowcharts-march-20111_504af02038614.pdf (accessed on 17 April 2014).
- Wakefield, C.; Kasparian, N.; Meiser, B.; Homewood, J.; Kirk, J.; Tucker, K. Attitudes toward genetic testing for cancer risk after genetic counseling and decision support: A qualitative comparison between hereditary cancer types. Genet. Test. 2007, 11, 401–412. [Google Scholar] [CrossRef]
- Australian Law Reform Commission. Essentially yours: The protection of human genetic information in Australia (ALRC Report 96). Genetic counselling and medical education: Access to medical genetic testing and counselling. Available online: http://www.alrc.gov.au/publications/23-genetic-counselling-and-medical-education/access-medical-genetic-testing-and-counsel/ (accessed on 8 October 2013).
- McCann, S.; MacAuley, D.; Barnett, Y. Genetic consultations in primary care: GPs’ responses to three scenarios. Scand. J. Prim. Health Care 2005, 23, 109–114. [Google Scholar] [CrossRef]
- Sifri, R.; Myers, R.; Hyslop, T.; Turner, B.; Cocroft, J.; Rothermel, T.; Grana, J.; Schlackman, N. Use of cancer susceptibility testing among primary care physicians. Clin. Genet. 2003, 64, 355–360. [Google Scholar] [CrossRef]
- Fry, A.; Campbell, H.; Gudmundsdottir, H.; Rush, R.; Porteous, M.; Gorman, D.; Cull, A. GPs’ views on their role in cancer genetics services and current practice. Fam. Pract. 1999, 16, 468–474. [Google Scholar] [CrossRef]
- Renaud, M.C.; Le, T.; Le, T.; Bentley, J.; Farrell, S.; Fortier, M.P.; Giede, C.; Kupets, R.; Plante, M.; Power, P.; et al. Epidemiology and investigations for suspected endometrial cancer. J. Obstet. Gynaecol. Can. 2013, 35, 380–383. [Google Scholar]
- Palomaki, G.E.; McClain, M.R.; Melillo, S.; Hampel, H.L.; Thibodeau, S.N. EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genet. Med. 2009, 11, 42–65. [Google Scholar] [CrossRef]
- Acheson, L.S.; Stange, K.C.; Zyzanski, S. Clinical genetics issues encountered by family physicians. Genet. Med. 2005, 7, 501–508. [Google Scholar] [CrossRef]
- Taylor, M.R.; Edwards, J.G.; Ku, L. Lost in transition: Challenges in the expanding field of adult genetics. Am. J. Med. Genet. C Semin Med. Genet. 2006, 142C, 294–303. [Google Scholar] [CrossRef]
- Qureshi, N.; Wilson, B.; Santaguida, P.; Carroll, J.; Allanson, J.; Culebro, C.R.; Brouwers, M.; Raina, P. Collection and use of cancer family history in primary care. Evid. Rep. Technol. Assess. 2007, 159, 1–84. [Google Scholar]
- Watson, E.K.; Shickle, D.; Qureshi, N.; Emery, J.; Austoker, J. The “new genetics” and primary care: GPs’ views on their role and their educational needs. Fam. Pract. 1999, 16, 420–425. [Google Scholar] [CrossRef]
- Lanceley, A.; Eagle, Z.; Ogden, G.; Gessler, S.; Razvi, K.; Ledermann, J.A.; Side, L. Family history and women with ovarian cancer: Is it asked and does it matter?: An observational study. Int. J. Gynecol. Cancer 2012, 22, 254–259. [Google Scholar] [CrossRef]
- Trano, G.; Wasmuth, H.H.; Sjursen, W.; Hofsli, E.; Vatten, L.J. Awareness of heredity in colorectal cancer patients is insufficient among clinicians: A Norwegian population-based study. Colorectal Dis. 2009, 11, 456–461. [Google Scholar] [CrossRef]
- Van Altena, A.M.; van Aarle, S.; Kiemeney, L.A.; Hoogerbrugge, N.; Massuger, L.F.; de Hullu, J.A. Adequacy of family history taking in ovarian cancer patients: A population-based study. Fam. Cancer 2012, 11, 343–349. [Google Scholar] [CrossRef]
- Suthers, G.K.; Armstrong, J.; McCormack, J.; Trott, D. Letting the family know: Balancing ethics and effectiveness when notifying relatives about genetic testing for a familial disorder. J. Med. Genet. 2006, 43, 665–670. [Google Scholar] [CrossRef]
- Keogh, L.A.; Southey, M.C.; Maskiell, J.; Young, M.A.; Gaff, C.L.; Kirk, J.; Tucker, K.M.; Rosenthal, D.; McCredie, M.R.; Giles, G.G. Uptake of offer to receive genetic information about BRCA1 and BRCA2 mutations in an Australian population-based study. Cancer Epidemiol. Biomark. Prev. 2004, 13, 2258–2263. [Google Scholar]
- Wakefield, C.E.; Ratnayake, P.; Meiser, B.; Suthers, G.; Price, M.A.; Duffy, J.; Tucker, K. Kathleen Cuningham National Consortium for Research into Familial Breast Cancer (kConFab). “For all my family’s sake, I should go and find out”: An Australian report on genetic counseling and testing uptake in individuals at high risk of breast and/or ovarian cancer. Genet. Test. Mol. Biomark. 2011, 15, 379–385. [Google Scholar] [CrossRef]
- Qureshi, N.; Carroll, J.C.; Wilson, B.; Santaguida, P.; Allanson, J.; Brouwers, M.; Raina, P. The current state of cancer family history collection tools in primary care: A systematic review. Genet. Med. 2009, 11, 495–506. [Google Scholar] [CrossRef]
- Emery, J.; Morris, H.; Goodchild, R.; Fanshawe, T.; Prevost, A.T.; Bobrow, M.; Kinmonth, A.L. The GRAIDS Trial: A cluster randomised controlled trial of computer decision support for the management of familial cancer risk in primary care. Br. J. Cancer 2007, 97, 486–493. [Google Scholar] [CrossRef]
- Scheuner, M.T.; Hamilton, A.B.; Peredo, J.; Sale, T.J.; Austin, C.; Gilman, S.C.; Bowen, M.S.; Goldzueig, C.L.; Lee, M.; Mittman, B.S.; et al. A cancer genetics toolkit improves access to genetic services through documentation and use of the family history by primary-care clinicians. Genet. Med. 2014, 16, 60–69. [Google Scholar]
- Braithwaite, D.; Emery, J.; de Lusignan, S.; Sutton, S. Using the Internet to conduct surveys of health professionals: A valid alternative? Fam. Pract. 2003, 20, 545–551. [Google Scholar] [CrossRef]
- Scott, A.; Jeon, S.H.; Joyce, C.M.; Humphreys, J.S.; Kalb, G.; Witt, J.; Leahy, A. A randomised trial and economic evaluation of the effect of response mode on response rate, response bias, and item non-response in a survey of doctors. BMC Med. Res. Methodol. 2011. [CrossRef]
- Chen, L.-S.; Kwok, O.-M.; Goodson, P. US health educators’ likelihood of adopting genomic competencies into health promotion. Am. J. Public Health 2008, 98, 1651–1657. [Google Scholar] [CrossRef]
- McMahon, S.R.; Iwamoto, M.; Massoudi, M.S.; Yusuf, H.R.; Stevenson, J.M.; David, F.; Chu, S.Y.; Pickering, L.K. Comparison of e-mail, fax, and postal surveys of pediatricians. Pediatrics 2003, 111, e299–e303. [Google Scholar] [CrossRef]
Appendix 1: Questionnaire
Health Professionals’ Knowledge and Attitudes towards Genetic Services for Patients with or at High-Risk of Lynch syndrome
Demographic of Respondents
- What is your gender?
- 〇
- Male
- 〇
- Female
- What is your age?
- 〇
- 18–29
- 〇
- 30–39
- 〇
- 40–49
- 〇
- 50–59
- 〇
- 60 +
- What is your current specialty?
- 〇
- General practic
- 〇
- Gynaecology
- 〇
- Gastroenterology
- 〇
- Gynaecology oncology
- 〇
- Medical oncology
- 〇
- Radiation oncology
- 〇
- General surgery
- 〇
- Colorectal surgery
- 〇
- Other (please specify__________________)
- How long have you been practicing in your current field?
- 〇
- 0–5 year
- 〇
- 6–10 year
- 〇
- 11–20 year
- 〇
- More than 20 years
- What is the postcode of your primary location of practice?
- Please specify _____________________
You and Your Practice
- 6.
- In the past 12 months, have you referred patients for:
Yes No Not sure a. Genetic services? ❐1 ❐2 b. DNA mutation testing? ❐1 ❐2 ❐3 c. Tumour immunohistochemistry (IHC) testing ❐1 ❐2 ❐3 d. Tumour microsatellite instability (MSI) testing ❐1 ❐2 ❐3 - 7.
- Do you consider the following testing a germline test?
Yes No Not sure a. IHC testing? ❐1 ❐2 ❐3 b. MSI testing? ❐1 ❐2 ❐3
Case Scenario
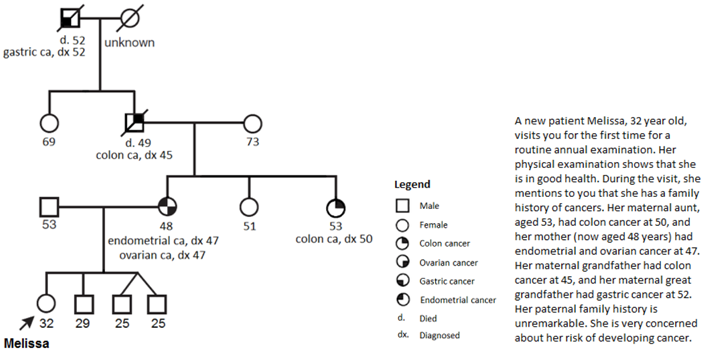
- 8.
- In your medical opinion, what is Melissa’s risk for developing the following cancers as compared to the general population? (Please answer all items).
Much higher Somewhat higher Same Somewhat lower Not sure a. Breast cancer ❐5 ❐4 ❐3 ❐2 ❐1 b. Gastric cancer ❐5 ❐4 ❐3 ❐2 ❐1 c. Ovarian cancer ❐5 ❐4 ❐3 ❐2 ❐1 d. Thyroid cancer ❐5 ❐4 ❐3 ❐2 ❐1 e. Colorectal cancer ❐5 ❐4 ❐3 ❐2 ❐1 f. Endometrial cancer ❐5 ❐4 ❐3 ❐2 ❐1 - 9.
- About a year later, Melissa was diagnosed with endometrial cancer. How would you proceed?
Very likely Somewhat likely Not likely Very unlikely Not sure a. Offer surveillance ❐5 ❐4 ❐3 ❐2 ❐1 b. Referral to a geneticist ❐5 ❐4 ❐3 ❐2 ❐1 c. Family history assessment ❐5 ❐4 ❐3 ❐2 ❐1 d. Discussion of risk-reducing surgery ❐5 ❐4 ❐3 ❐2 ❐1 e. Referral to a non-genetics specialist ❐5 ❐4 ❐3 ❐2 ❐1 f. Order genetic testing for germline mutations ❐5 ❐4 ❐3 ❐2 ❐1 g. Order pre-genetic testing of tumour tissue (e.g., MSI or IHC) ❐5 ❐4 ❐3 ❐2 ❐1 h. Discussion about Lynch syndrome cancers with patient ❐5 ❐4 ❐3 ❐2 ❐1 i. No action ❐5 ❐4 ❐3 ❐2 ❐1 - 10
- If you have chosen to offer surveillance, what would you offer? (check all that apply)
- ❐
- Colonoscopy
- ❐
- Gastroscopy
- ❐
- CA125
- ❐
- Mammography
- ❐
- Other, please specify _____________________
- 11.
- If you have chosen to discuss risk-reducing surgery with patients, what kind of risk-reducing surgery would you discuss with the patient?
- Please specify _____________________
General Attitudes about Genetic Services
- 12.
- In your opinion, which of the following factors play a role in your decision to refer a patient for genetic services? (Check all that apply)
- ❐1
- Patient interest or request
- ❐2
- Ethical and legal responsibility
- ❐3
- Reassurance for the patient and family
- ❐4
- To provide genetic counselling for the patient
- ❐5
- Genetic testing for germline mutations
- ❐6
- To provide appropriate cancer risk assessment for the patient
- ❐7
- To provide appropriate screening and management for the patient
- ❐8
- To provide appropriate screening and/or management for the patients’ family
- ❐9
- Other, please specify: ________________________________
- 13.
- In your opinion, which of the following factors play a role in your decision to NOT refer a patient for genetic services? (Check all that apply)
- ❐1
- I do not feel it is my responsibility
- ❐2
- I do not have access to a genetic health service
- ❐3
- Patients may be at risk for insurance discrimination
- ❐4
- Long waiting time for appointment at a genetic clinic
- ❐5
- Patient was not interested when referral was offered
- ❐6
- I do not feel familiar with hereditary cancer syndromes
- ❐7
- Patient is unlikely to benefit from genetic counselling and/or testing
- ❐8
- I do not know how to make a referral to the local genetic health service
- ❐9
- Recommendations and guidelines are not available to select patients for referral
- ❐10
- Other, please specify: ________________________________________
- 14.
- Who do you think is best to refer patients for genetic services (i.e., to make the initial offer)? (Please choose only one of the following, and provide the reason(s) for your selection)
- 〇
- General practitioners
- 〇
- Gynaecologists
- 〇
- Gastroenterologists
- 〇
- Gynaecology oncologists
- 〇
- Medical oncologists
- 〇
- Radiation oncologists
- 〇
- Colorectal surgeons
- 〇
- General surgeons
- 〇
- Any of the above
- 〇
- Make a comment on your choice here: ______________________________
- 15.
- When is the best time for the initial referral for genetic services to be made to the patient? (Please choose only one of the following, and provide the reason(s) for your selection)
- 〇
- When family history is collected
- 〇
- At diagnosis of cancer
- 〇
- After surgery and before commencement of adjuvant therapy
- 〇
- During adjuvant therapy
- 〇
- After treatment is finished
- 〇
- At any time
- 〇
- Make a comment on your choice here: ______________________________
- 16.
- I feel my role as a physician includes: (Check all that apply)
- ❐1
- Providing emotional support after genetic testing
- ❐2
- Identifying patients for referral to genetic services
- ❐3
- Interpreting germline DNA-based genetic test results
- ❐4
- Collecting a three-generation family history information
- ❐5
- Ordering pre-genetic testing of tumour tissue (e.g. MSI or IHC)
- ❐6
- Counselling patients about their cancer risks after genetic testing
- ❐7
- Counselling patients about their cancer risks before genetic testing
- ❐8
- Calculating relative risk of cancer associated with family cancer history
- ❐9
- Discussing the need for cancer surveillance or prophylaxis with patients when required
- ❐10
- Providing regular clinical examination and care to patients with hereditary cancer syndromes
- ❐11
- Other, please specify: ______________________________
- 17.
- In your opinion, which of the following would support your practice? (Please choose the appropriate response for each item)
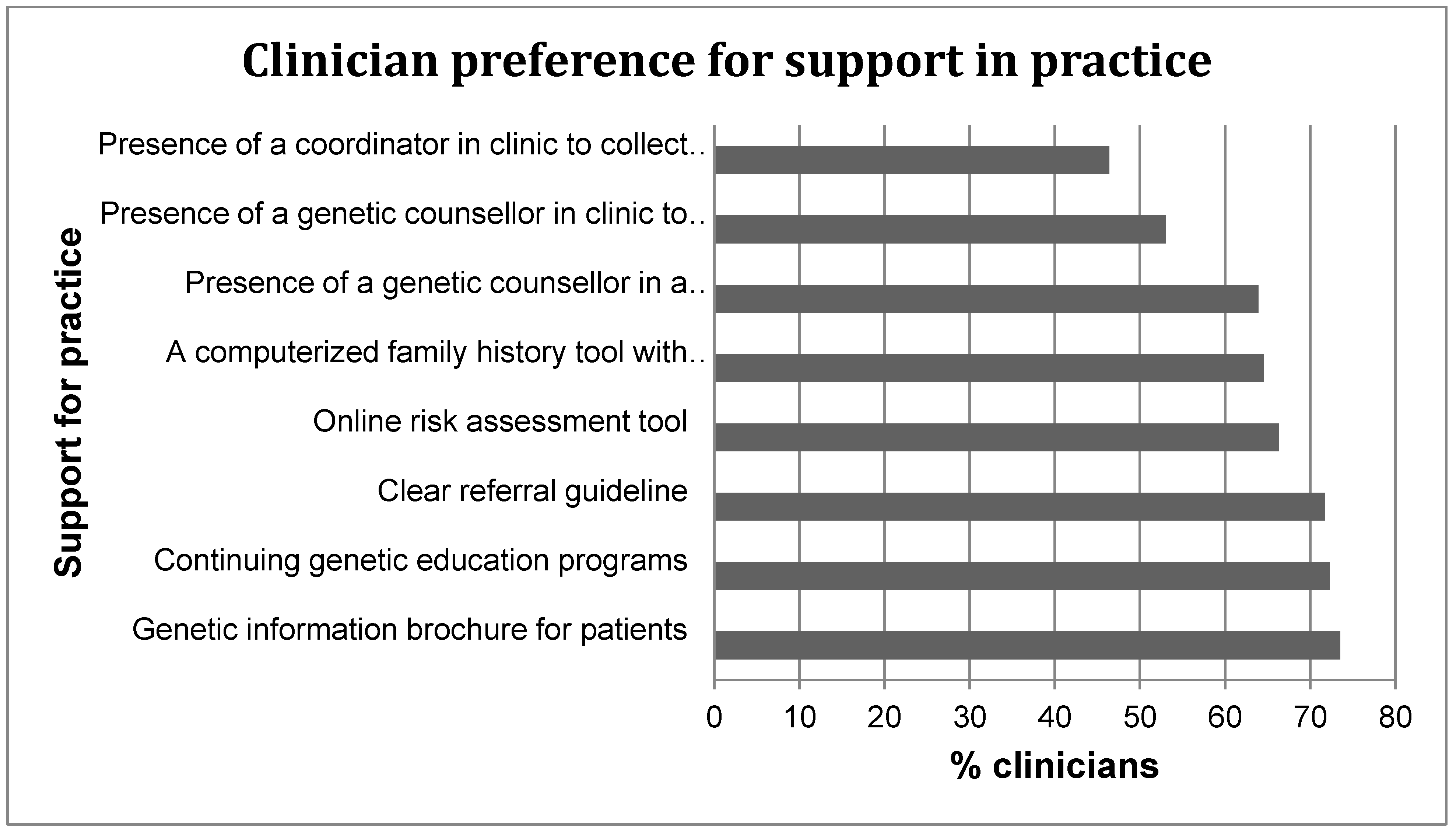
Very likely Somewhat likely Not likely Very unlikely Not sure a. Clear referral guideline ❐5 ❐4 ❐3 ❐2 ❐1 b. Online risk assessment tool ❐5 ❐4 ❐3 ❐2 ❐1 c. Continuing genetic education programmes ❐5 ❐4 ❐3 ❐2 ❐1 d. Genetic information brochure for patients ❐5 ❐4 ❐3 ❐2 ❐1 e. A coordinator in clinic to collect patient family history ❐5 ❐4 ❐3 ❐2 ❐1 f. A genetic counsellor in clinic to assess risk and to facilitate referral ❐5 ❐4 ❐3 ❐2 ❐1 g. A computerized family history tool with decision support for referral ❐5 ❐4 ❐3 ❐2 ❐1 h. The presence of a genetic counsellor in a multidisciplinary tumour board meeting ❐5 ❐4 ❐3 ❐2 ❐1 - 18.
- Is there anything else you would like to tell us about?
- 19.
- If you would like to receive a copy of the research summary report, please enter your email address here: _______________________________________________
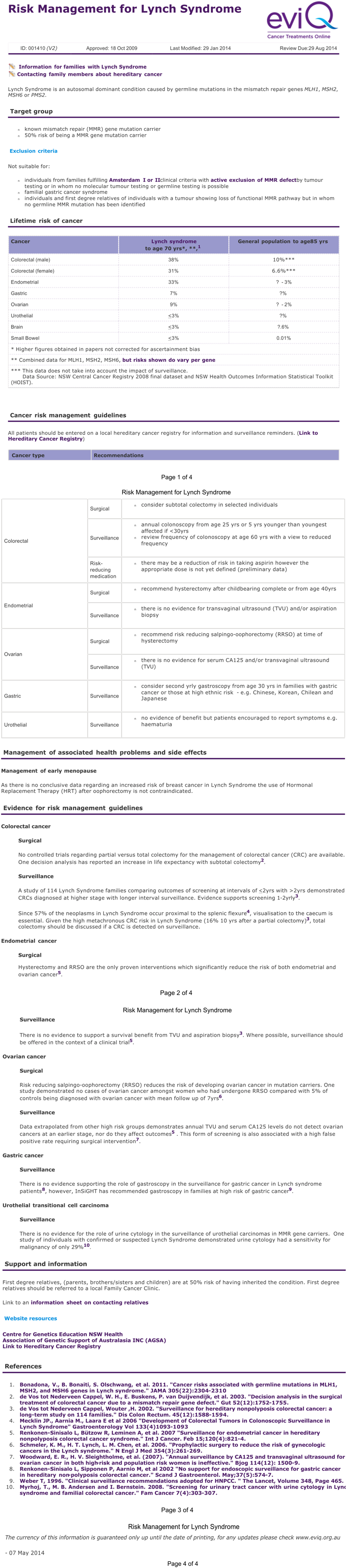
Appendix 2. Risk Management for Lynch Syndrome (eviQ version 2)
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tan, Y.Y.; Spurdle, A.B.; Obermair, A. Knowledge, Attitudes and Referral Patterns of Lynch Syndrome: A Survey of Clinicians in Australia. J. Pers. Med. 2014, 4, 218-244. https://doi.org/10.3390/jpm4020218
Tan YY, Spurdle AB, Obermair A. Knowledge, Attitudes and Referral Patterns of Lynch Syndrome: A Survey of Clinicians in Australia. Journal of Personalized Medicine. 2014; 4(2):218-244. https://doi.org/10.3390/jpm4020218
Chicago/Turabian StyleTan, Yen Y., Amanda B. Spurdle, and Andreas Obermair. 2014. "Knowledge, Attitudes and Referral Patterns of Lynch Syndrome: A Survey of Clinicians in Australia" Journal of Personalized Medicine 4, no. 2: 218-244. https://doi.org/10.3390/jpm4020218
APA StyleTan, Y. Y., Spurdle, A. B., & Obermair, A. (2014). Knowledge, Attitudes and Referral Patterns of Lynch Syndrome: A Survey of Clinicians in Australia. Journal of Personalized Medicine, 4(2), 218-244. https://doi.org/10.3390/jpm4020218





