Personalized Medicine’s Bottleneck: Diagnostic Test Evidence and Reimbursement
Abstract
:1. Introduction
2. Methods
3. Results
| Brand-Name (generic)/Indication | Test(s)/Biomarker(s) | Co-Developed | Medicare Part B or Part D |
|---|---|---|---|
| Herceptin (trastuzumab)—breast cancer | HER-2/neu receptor | Yes | Part B |
| Gleevec (imatinib)—chronic myeloid leukemia | Philadelphia chromosome/BCR-ABL | No | Part D |
| Erbitux (cetuximab)—colorectal cancer | EGFR expression/K-RAS mutation | No | Part B |
| Tarceva (erlotinib)—non-small cell lung cancer | Cobas EGFR mutation | No | Part D |
| Sprycel (dasatinib)—chronic myeloid leukemia | Philadelphia chromosome/BCR-ABL | No | Part D |
| Vectibix (panitumumab)—colorectal cancer | EGFR expression/K-RAS mutation | No | Part B |
| Tykerb (lapatinib)—breast cancer | HER-2/neu receptor | No | Part D |
| Selzentry (maraviroc)—HIV | CCR5 receptor | No | Part D |
| Zelboraf (vemurafenib)—non-small cell lung cancer | Cobas BRAF V600E | Yes | Part D |
| Xalkori (crizotinib)—melanoma | ALK | Yes | Part D |
| Drug Name (Generic) | Companion Diagnostic/Biomarker | U.S. Approval Date | 2013 Global Forecast (in millions of U.S. $) | 2012 Global Sales (in millions of U.S. $) | 2011 Global Sales (in millions of U.S. $) | 2012 Annual Cost per U.S. Patient |
|---|---|---|---|---|---|---|
| Herceptin (trastuzumab) | HER-2/neu receptor | 9/25/1998 | 6,589 | 6,282 | 5,944 | $47,000 |
| Gleevec (imatinib) | Philadelphiachromosome/BCR-ABL | 5/10/2001 | 4,618 | 4,675 | 4,659 | $76,000 |
| Erbitux (cetuximab) | EGFR expression/ K-RAS mutation | 2/12/2004 | 1,839 | 1,843 | 1,881 | $80,000 |
| Tarceva (erlotinib) | Cobas EGFR mutation | 11/18/2004 | 1,439 | 1,402 | 1,415 | $73,000 |
| Sprycel(dasatinib) | Philadelphiachromosome/BCR-ABL | 6/28/2006 | 1,257 | 1,019 | 803 | $123,000 |
| Vectibix (panitumumab) | EGFR expression/ K-RAS mutation | 9/27/2006 | 589 | 954 | 539 | $52,000 |
| Tykerb (lapatinib) | HER-2/neu receptor | 3/13/2007 | 335 | 379 | 102 | $67,000 |
| Selzentry (maraviroc) | CCR5 receptor | 8/6/2007 | 231 | 203 | 176 | $15,000 |
| Zelboraf (vemurafenib) | BRAF V600E | 9/17/2011 | 389 | 250 | 35 | $78,000 |
| Xalkori (crizotinib) | ALK | 8/26/2011 | 282 | 123 | 16 | $149,000 |
3.1. Drug Reimbursement
3.2. Diagnostic Reimbursement
| Diagnostic/Biomarker (drug) | U.S. Price per Test | FDA-Approved | Analyte-Specific Coding |
|---|---|---|---|
| HER-2/neu receptor (trastuzumab, lapatinib) | $300 | Yes | Yes |
| EGFR expression (cetuximab, panitumumab) | $300 | No | Yes |
| K-RAS mutation (cetuximab, panitumumab) | $450 | Yes | Yes |
| CCR5 receptor (maraviroc) | $2000 | No | Yes |
| Philadelphia Chromosome/BCR-ABL (imatinib, dasatinib) | $200 | Yes | No |
| ALK (crizotinib) | $1500 | Yes | Yes |
| BRAF V600E mutation (vemurafenib) | $500 | Yes | Yes |
| Cobas EGFR mutation (erlotinib) | $1500 | Yes | Yes |
| Drug, Documentation on Testing (Number of LCDs) | Drug Approved for Reimbursement | Diagnostic/Biomarker(s) Associated with Drug |
|---|---|---|
| Trastuzumab (2x) | Yes (on-label) | HER-2 |
| Cetuximab (3x) | Yes (on- and off-label) | K-RAS |
| Vemurafenib (2x) | Yes (on-label only) | K-RAS |
| Diagnostic testing (1x) | Yes, all tests for biomarkers listed in right-hand column | K-RAS, BRAF, ALK, HER-2 |
| Biomarkers for oncology (1x) | Yes, all tests for biomarkers listed in right-hand column | K-RAS, BRAF, ALK, HER-2 |
| Brand (generic) | Indication | Number of cost-effectiveness studies | Test included in cost-effectiveness study | Considered cost-effectiveness |
|---|---|---|---|---|
| Herceptin (trastuzumab) | Breast cancer | 8 | Yes, 2 of 8 | 6 of 8 |
| Gleevec (imatinib) | Chronic myeloid leukemia | 4 | No | 2 of 4 |
| Erbitux (cetuximab) | Colorectal cancer | 1 | Yes | Yes |
| Tarceva (erlotinib) | Non-small cell lung cancer | 1 | Yes | Inconclusive |
| Sprycel (dasatinib) | Chronic myeloid leukemia | 1 | No | Yes |
| Vectibix (panitumumab) | Colorectal cancer | 0 | No | N.A. |
| Tykerb (lapatinib) | Breast cancer | 1 | No | No |
| Selzentry (maraviroc) | HIV | 0 | No | N.A. |
| Xalkori (crizotinib) | Non-small cell lung cancer | 0 | No | N.A. |
| Zelboraf (vemurafenib) | Melanoma | 0 | No | N.A. |
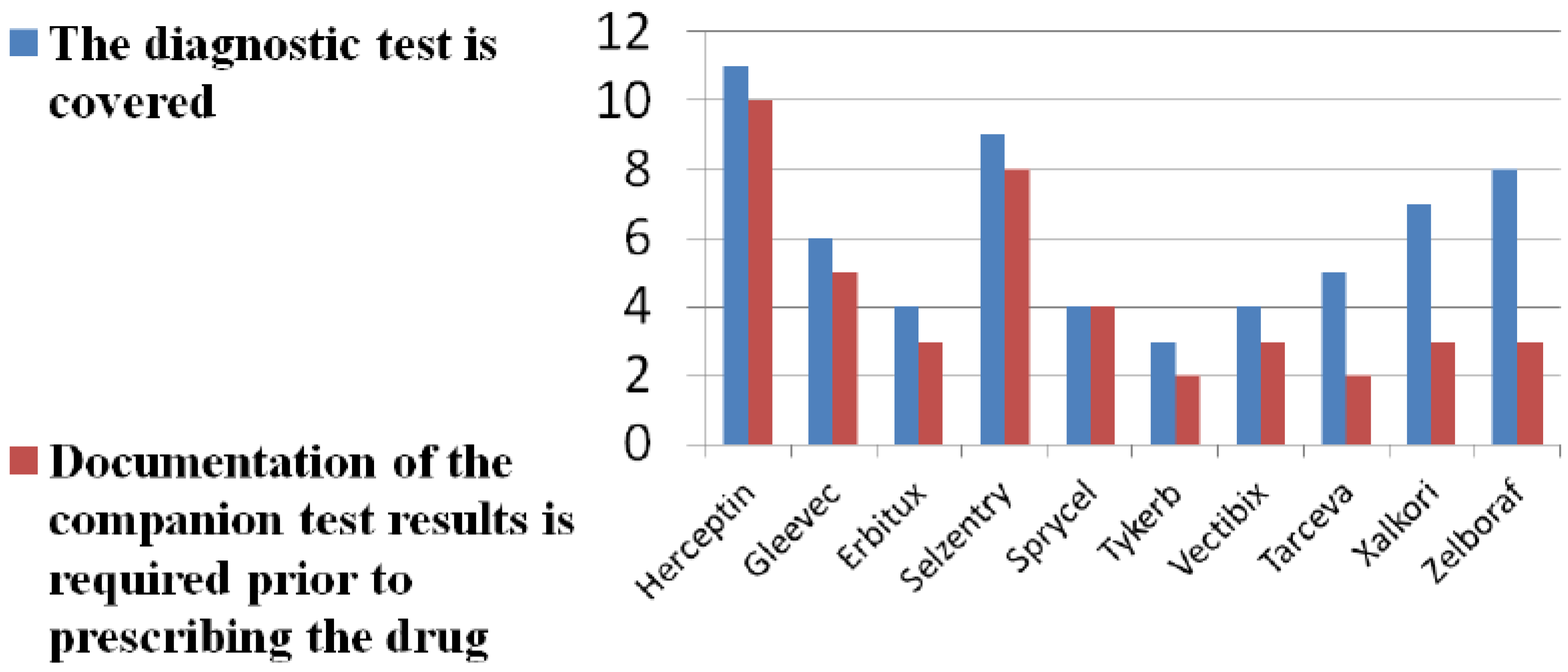
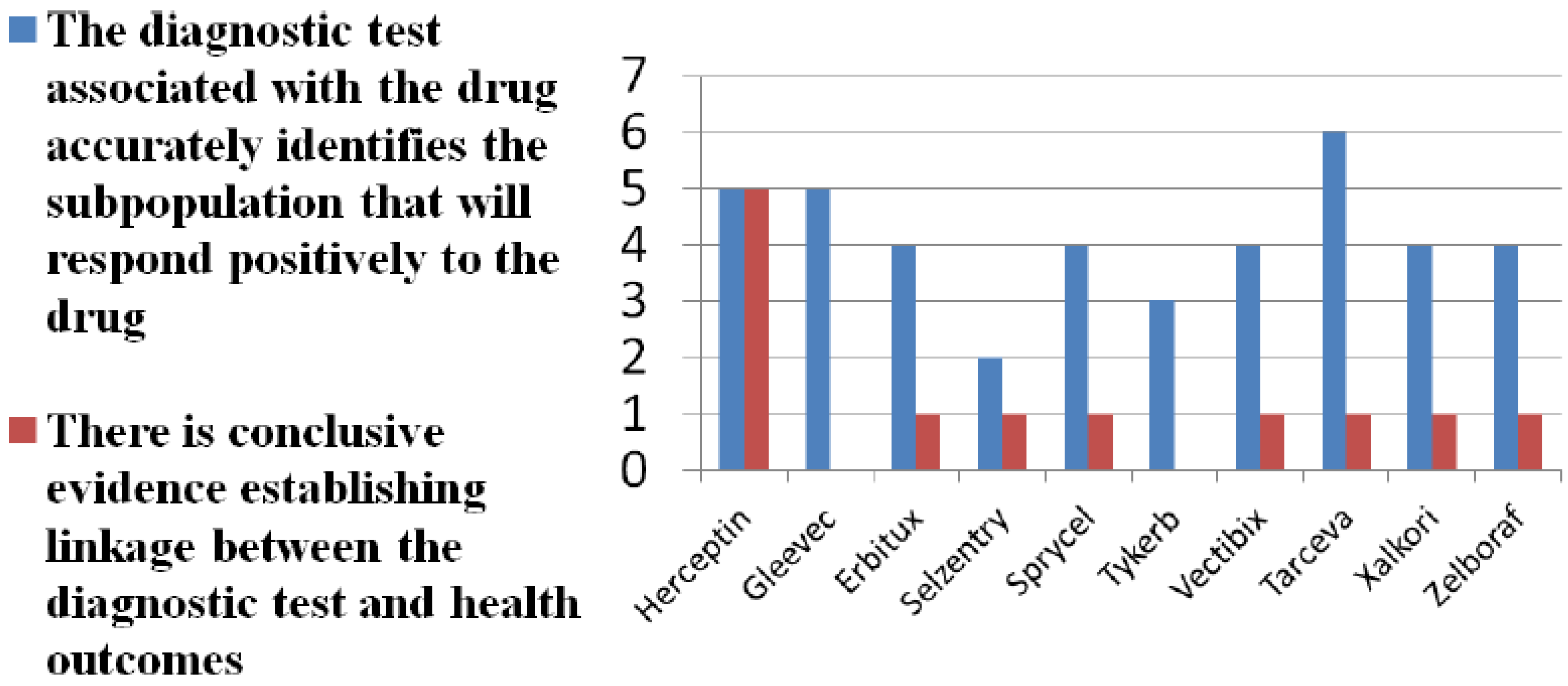
4. Discussion and Conclusions
Supplementary Files
Acknowledgement
Author Contributions
Conflicts of Interest
References
- Simoncelli, T. Paving the Way for Personalized Medicine: FDA’s Role in a New Era of Medical Product Development. October 2013. Available online: http://www.fda.gov/downloads/ScienceResearch/SpecialTopics/PersonalizedMedicine/UCM372421.pdf (accessed on 13 November 2013).
- Food and Drug Administration. Guidance for Industry and Food and Drug Administration Staff— In Vitro Companion Diagnostic Devices. 24 April 2013. Available online: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm262292.htm (accessed on 11 November 2013).
- Food and Drug Administration. Pharmacogenomic Biomarkers in Drug Labels. 22 January 2014. Available online: http://www.fda.gov/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/ucm083378.htm (accessed on 1 November 2013).
- Lester, D. Will personalized medicine help in “ransforming” the business of healthcare. Pers. Med. 2009, 6, 555–565. [Google Scholar] [CrossRef]
- Ray, T. Clovis Pulls Plug on Personalized Cancer Drug after Trial Shows No Benefit for Drug or Biomarker. Pharmacogenomics Reporter. 14 November 2012. Available online: http://www.genomeweb.com/clinical-genomics/clovis-pulls-plug-personalized-cancer-drug-after-trial-shows-no-benefit-drug-or (accessed on 1 November 2013).
- Thomson Reuters Cortellis (Competitive Intelligence). Available online: http://thomsonreuters.com/cortellis-competitive-intelligence/ (accessed on 10 November 2013).
- DataRx. Drug Price/Pharmacy Finder. Available online: http://data-rx.com/index.php?id=84 (accessed on 10 November 2013).
- Centers for Medicare and Medicare Services (CMS). Medicare Part B Drug Average Sales Price (ASP) data. Available online: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/index.html (accessed on 1 November 2013).
- The Official U.S. Government Site for Medicare. Medicare Part D Formulary Plan Finder. Available online: https://www.medicare.gov/find-a-plan/questions/home.aspx?AspxAutoDetectCookieSupport=1 (accessed on 2 November 2013).
- GE Healthcare. Clarient Diagnostic Test Menu. Available online: http://www.clarientinc.com/ (accessed on 1 November 2013).
- Dako. Dako Product List: Reagents and Kits. Available online: http://www.dako.com/us (accessed on 2 November 2013).
- Integrated Oncology: LabCorp Specialty Testing Group. Oncology Testing. Available online: https://www.labcorp.com/wps/portal/integratedoncology?WCM_GLOBAL_CONTEXT=&bypass=true (accessed on 1 November 2013).
- Food and Drug Administration. Companion Diagnostic Devices: In vitro and Imaging Tools. 4 March 2014. Available online: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm301431.htm (accessed on 2 November 2013). [Google Scholar]
- Centers for Medicare and Medicaid Services. Local Coverage Determinations. Available online: http://www.cms.gov/Medicare/Coverage/DeterminationProcess/LCDs.html (accessed on 10 November 2013).
- Tufts Medical Center Cost-Effectiveness Analysis Registry (CEA). Available online: https://research.tufts-nemc.org/cear4/Default.aspx (accessed on 12 November 2013).
- Gustavsen, G.; Phillips, K.; Pothier, K. Reimbursement Landscape for Novel Diagnostics. Health Advances. 2010. Available online: http://www.healthadvances.com/pdf/novel_diag_reimbursement.pdf (accessed on 10 November 2013).
- Malone, B. Molecular Diagnostics Reimbursement in Flux: What Will New Codes Mean for Labs? Clinical Laboratory News. January 2013. Available online: http://www.aacc.org/publications/cln/2013/january/Pages/Molecular.aspx# (accessed on 10 November 2013).
- Cohen, J. Overcoming regulatory and economic challenges facing pharmacogenomics. Nat. Biotechnol. 2012, 29, 751–756. [Google Scholar]
- Ofili, E.; Sproles, D. Conference Scene: The healthcare reform act, comparative effectiveness research and personalized medicine. Pers. Med. 2011, 8, 133–135. [Google Scholar] [CrossRef]
- Trosman, J.; van Bebber, S.; Phillips, K. Health Technology Assessment and Private Payer’s Coverage of Personalized Medicine. J. Oncol. Pract. 2011, 7, 18s–24s. [Google Scholar] [CrossRef]
- Weldon, C.; Trosman, J.; Gradishar, W.; Benson, A.; Schink, J. Barriers to the Use of Personalized Medicine in Breast Cancer. J. Oncol. Pract. 2012, 8, e24–e31. [Google Scholar] [CrossRef]
- Ansari, M. The Regulation of Companion Diagnostics: A Global Perspective. Ther. Innov. Regul. Sci. 2013, 47, 405–415. [Google Scholar] [CrossRef]
- McCaughan, M. The Companion Diagnostics Dilemma. The RPM Report, 13 March 2013. [Google Scholar]
- Reinke, T. Targeted Medications: New Focus on Companion Tests. Manag. Care 2012, 21, 35–38. [Google Scholar]
- Quinn, B.; Hoag, F. Current Issues and Options: Coverage and Reimbursement for Molecular Diagnostics. Available online: http://aspe.hhs.gov/health/reports/2010/CovReimCMD/index.shtml (accessed on 10 November 2013).
- Palmetto’s MolDx program. Available online: http://www.palmettogba.com/palmetto/MolDX.nsf/DocsCat/MolDx (accessed on 10 November 2013).
- Usdin, S. Coding for utility. BioCentury, 15 July 2013. [Google Scholar]
- Eisenberg, A. Variations on a Gene, and Tools to Find Them. New York Times, 27 April 2013. [Google Scholar]
- Twachtman, G. With Personalized Medicine, Payers Want a Role in Early Development. The Pink Sheet, 3 June 2013. [Google Scholar]
- Epstein, R.; Russell Teagarden, J. Comparative Effectiveness Research and Personalized Medicine: Catalyzing or Colliding? Pharmacoeconomics 2010, 28, 905–913. [Google Scholar] [CrossRef]
- Patrick, A.; Avorn, J.; Choudhry, N. Cost-effectiveness of genotype-guided warfarin dosing for patients with atrial fibrillation. Circ. Cardiovasc. Qual. Outcomes 2009, 2, 429–436. [Google Scholar] [CrossRef]
- National Coverage Determination (NCD) for Pharmacogenomic Testing for Warfarin. Available online: http://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=333&ncdver=1&bc=BAAAgAAAAAAA& (accessed on 1 March 2014).
- Grosse, S. Economic analyses of genetic tests in personalized medicine: Clinical utility first, then cost utility. Genet. Med. 2014, 16, 225–227. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cohen, J.P.; Felix, A.E. Personalized Medicine’s Bottleneck: Diagnostic Test Evidence and Reimbursement. J. Pers. Med. 2014, 4, 163-175. https://doi.org/10.3390/jpm4020163
Cohen JP, Felix AE. Personalized Medicine’s Bottleneck: Diagnostic Test Evidence and Reimbursement. Journal of Personalized Medicine. 2014; 4(2):163-175. https://doi.org/10.3390/jpm4020163
Chicago/Turabian StyleCohen, Joshua P., and Abigail E. Felix. 2014. "Personalized Medicine’s Bottleneck: Diagnostic Test Evidence and Reimbursement" Journal of Personalized Medicine 4, no. 2: 163-175. https://doi.org/10.3390/jpm4020163
APA StyleCohen, J. P., & Felix, A. E. (2014). Personalized Medicine’s Bottleneck: Diagnostic Test Evidence and Reimbursement. Journal of Personalized Medicine, 4(2), 163-175. https://doi.org/10.3390/jpm4020163




