Anti-Amyloid Monoclonal Antibodies for Alzheimer’s Disease: Evidence, ARIA Risk, and Precision Patient Selection
Abstract
1. Introduction
2. Method
3. The Amyloid Hypothesis
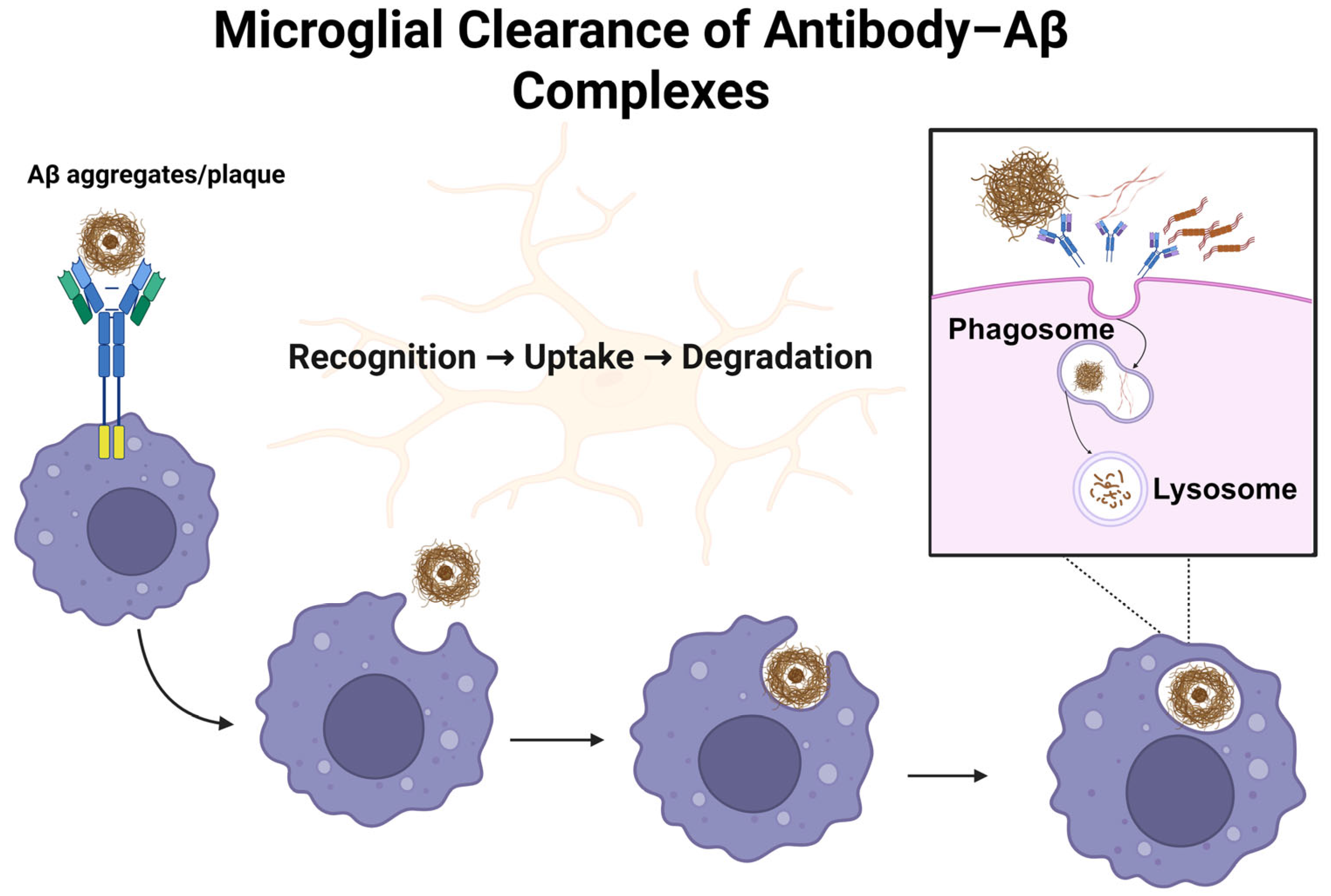
4. Monoclonal Antibodies in Alzheimer’s Disease
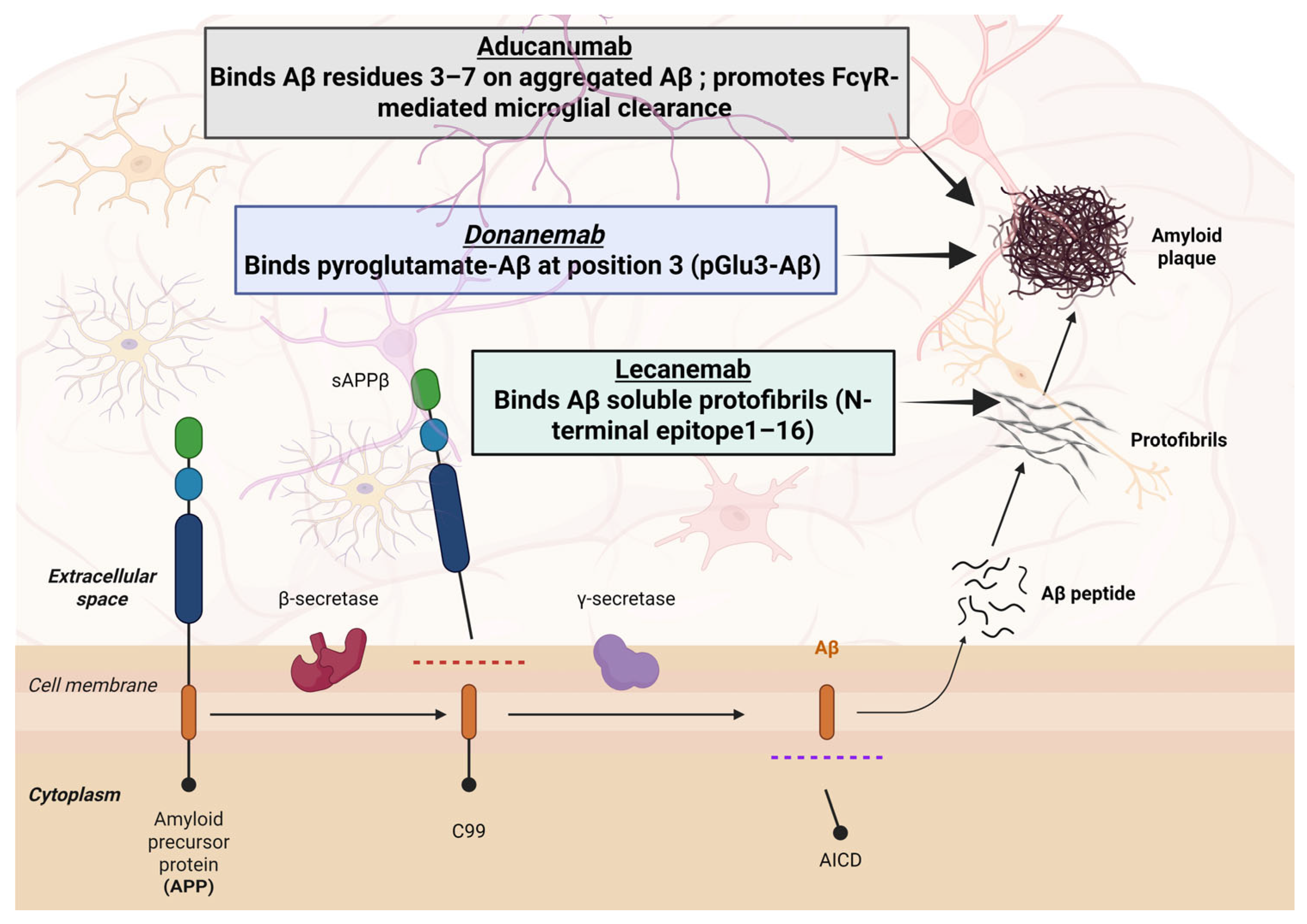
4.1. Aducanumab (Aduhelm®)
Clinical Development and Efficacy
4.2. Lecanemab (Leqembi®)
Clinical Development and Efficacy
4.3. Donanemab (Kisunla®)
Clinical Development and Efficacy
| NCT Number | Phase | Design Details | Results and References |
| Aducanumab | |||
| NCT05108922 | Phase 3 | Open-Label, Parallel-Group, 2-Arm Study. | At 6 months, 37.9% of patients achieved amyloid clearance compared with 1.6% in the aducanumab group. The median time to clearance was shorter with donanemab (359 days) than with aducanumab (568 days). In addition, donanemab produced a greater reduction in phospho-tau at 6 months, although this difference diminished with continued treatment [92]. |
| NCT02484547 | Phase 3 | Randomized, Parallel assessment, Double-Blind. | High-dose treatment produced a statistically significant reduction in decline across CDR-SB, ADAS-Cog13, ADCS-ADL-MCI, and MMSE, whereas the low-dose group showed no significant difference from placebo [93]. |
| NCT02477800 | Phase 3 | Randomized, Parallel assessment, Double-Blind. | Neither high- nor low-dose treatment groups showed significant cognitive benefit over placebo across assessed endpoints [93]. |
| NCT05310071 | Phase 3 | Randomized, Parallel Assignment, quadruple masking (patient, care provider, PI, outcome assessing researcher). | Specific statistical analysis results not published [94]. |
| Donanemab | |||
| NCT05108922 | Phase 3 | Open-Label, Parallel-Group, 2-Arm Study. | At 6 months, 37.9% of patients achieved amyloid clearance versus 1.6% with aducanumab. Median clearance time was shorter with donanemab (359 days) than with aducanumab (568 days). Donanemab also showed greater phospho-tau reduction at 6 months, though this effect decreased with continued treatment [89]. |
| NCT04640077 | Phase 2 | Non-randomized, sequential. | The donanemab reduction of tau was more pronounced in patients with complete amyloid clearance [95]. |
| NCT04437511 | Phase 3 | Randomized, parallel, double masking (patient, investigator). | Cognitive decline was slow compared to the placebo group. Amyloid clearance significantly increased, with no change in tau pathology [87]. |
| NCT03367403 | Phase 2 | Randomized, parallel, double masking (patient, investigator). | Significant slowing of clinical decline (in iADRS and ADAS, no significant difference in CDR-SB and MMSE), increase in amyloid clearance, mixed tau pathology outcome [86]. |
| Lecanemab | |||
| NCT03887455 | Phase 3 | Randomized, parallel, quadruple masking (patient, care provider, PI, outcome assessing researcher). | At 18 mo, slowed decline (CDR-SB −27%, ADAS-Cog 14–26%, ADCOMS −24%, ADCS-ADL-MCI −37% vs. placebo); reduced brain amyloid by 59 CL; ARIA-E 12.6% (symptomatic 2.8%), ARIA-H 17.3% (symptomatic 0.7%); led to FDA traditional approval [96]. |
| NCT01767311 | Phase 2 | Randomized, parallel, triple masking (patient, care provider, PI). | Did not meet 12 mo primary endpoint; at 18 mo slowed decline (ADCOMS −30%, ADAS-Cog 14–47%, CDR-SB −26% vs. placebo); reduced brain Aβ and CSF p-tau; ARIA-E 9.9% (14.3% in APOE ε4) [97]. |
| NCT01230853 | Phase 1 | Randomized, quadruple masking (patient, care provider, PI, outcome assessing researcher). | This study showed that lecanemab was safe and well-tolerated across all treatment groups, even at progressively increasing doses [98]. |
4.4. Cognitive Outcome Measures and Clinical Meaningfulness
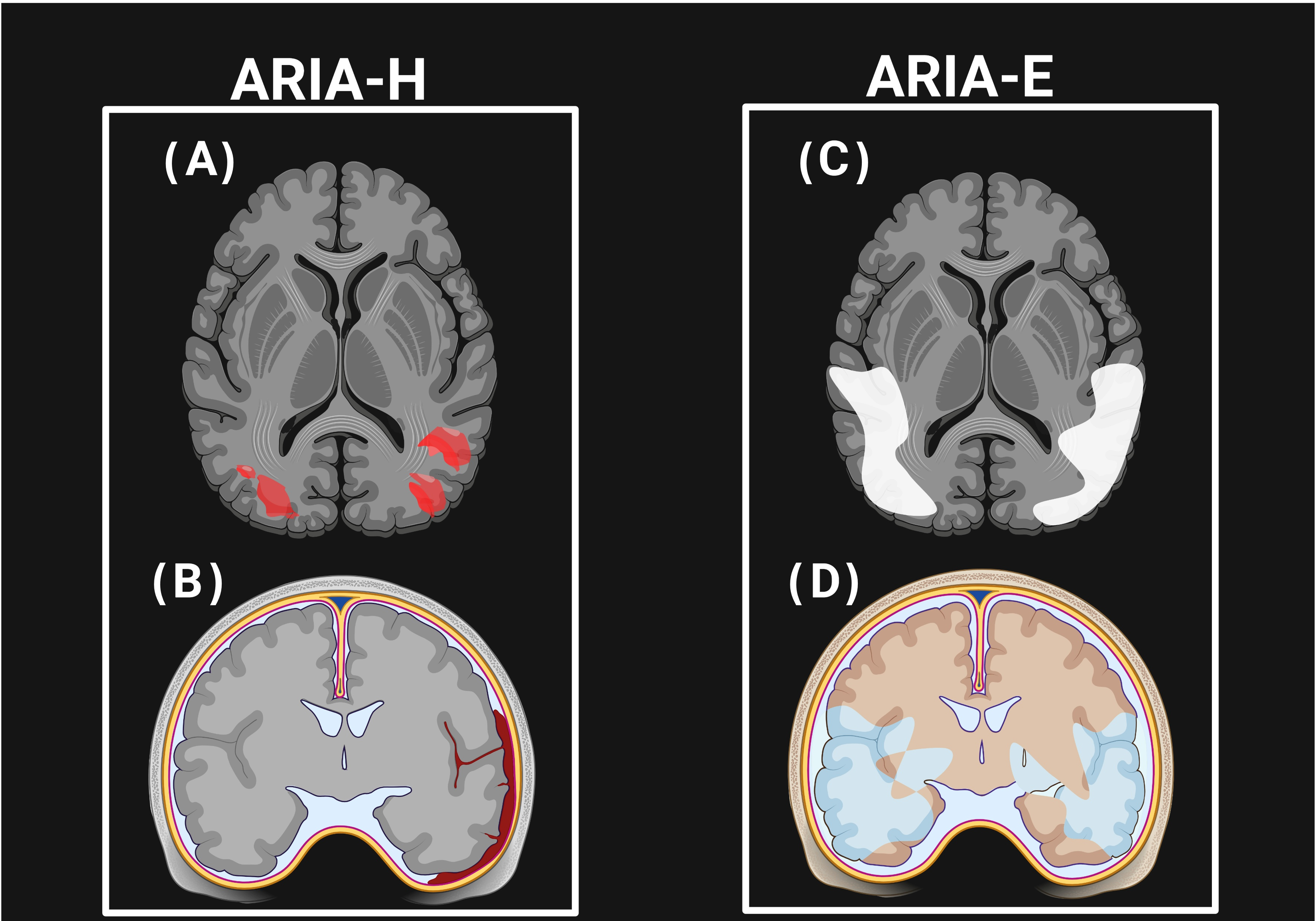
5. Amyloid-Related Imaging Abnormalities (ARIAs)
5.1. Pathophysiology of ARIAs
5.2. Incidence of ARIA-E and ARIA-H
5.3. Risk Factors Associated with ARIAs
5.4. Clinical Monitoring and Management
5.5. Global Regulatory Perspective
6. Biomarkers and Predictors of Response
6.1. Tau Pathology
6.2. Genetic Factors
6.3. Precision Patient Selection, Cost, and Access Considerations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABCA7 | ATP-binding cassette sub-family A member 7 |
| AD | Alzheimer’s disease |
| ADCOMS | Alzheimer’s Disease Composite Score |
| ADAS-Cog14 | Alzheimer’s Disease Assessment Scale–Cognitive subscale (14 items) |
| ADCS-ADL-MCI | Alzheimer’s Disease Cooperative Study–Activities of Daily Living for mild cognitive impairment |
| Aβ | amyloid-beta |
| APOE | apolipoprotein E |
| APP | amyloid precursor protein |
| ARIAs | amyloid-related imaging abnormalities |
| ARIA-E | ARIA with edema/effusion |
| ARIA-H | ARIA with cerebral microhemorrhages/siderosis |
| BBB | blood–brain barrier |
| CAA | cerebral amyloid angiopathy |
| CDR-SB | Clinical Dementia Rating–Sum of Boxes |
| CSF | cerebrospinal fluid |
| FDA | Food and Drug Administration |
| GFAP | glial fibrillary acidic protein |
| iADRS | Integrated Alzheimer’s Disease Rating Scale |
| MMSE | mini-mental state examination |
| MRI | magnetic resonance imaging |
| NFT | neurofibrillary tangle |
| NIA-AA ATN framework | National Institute on Aging–Alzheimer’s Association amyloid/tau/neurodegeneration framework |
| PET | positron emission tomography |
| p-Tau | phosphorylated tau |
| PSEN1 | presenilin 1 |
| PSEN2 | presenilin 2 |
| SORL1 | sortilin-related receptor |
| Tau | microtubule-associated protein tau |
| TREM2 | triggering receptor expressed on myeloid cells 2 |
References
- Tenchov, R.; Sasso, J.M.; Zhou, Q.A. Alzheimer’s Disease: Exploring the Landscape of Cognitive Decline. ACS Chem. Neurosci. 2024, 15, 3800–3827. [Google Scholar] [CrossRef] [PubMed]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.E.; Yaffe, K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011, 10, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. 2024 Alzheimer’s disease facts and figures. Alzheimers Dement 2024, 20(5), 3708–3821. [Google Scholar] [CrossRef]
- Nasb, M.; Tao, W.; Chen, N. Alzheimer’s Disease Puzzle: Delving into Pathogenesis Hypotheses. Aging Dis. 2024, 15, 43–73. [Google Scholar] [CrossRef]
- Ryan, N.S.; Rossor, M.N.; Fox, N.C. Alzheimer’s disease in the 100 years since Alzheimer’s death. Brain 2015, 138, 3816–3821. [Google Scholar] [CrossRef]
- Chen, Z.-R.; Huang, J.-B.; Yang, S.-L.; Hong, F.-F. Role of cholinergic signaling in Alzheimer’s disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef]
- Francis, P.T.; Palmer, A.M.; Snape, M.; Wilcock, G.K. The cholinergic hypothesis of Alzheimer’s disease: A review of progress. J. Neurol. Neurosurg. Psychiatry 1999, 66, 137–147. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-based therapy for Alzheimer’s disease: Challenges, successes and future. Signal Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [CrossRef]
- Ryman, D.C.; Acosta-Baena, N.; Aisen, P.S.; Bird, T.; Danek, A.; Fox, N.C.; Goate, A.; Frommelt, P.; Ghetti, B.; Langbaum, J.B. Symptom onset in autosomal dominant Alzheimer disease: A systematic review and meta-analysis. Neurology 2014, 83, 253–260. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Kepp, K.P.; Robakis, N.K.; Høilund-Carlsen, P.F.; Sensi, S.L.; Vissel, B. The amyloid cascade hypothesis: An updated critical review. Brain 2023, 146, 3969–3990. [Google Scholar] [CrossRef]
- Corder, E.H.; Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C.; Small, G.; Roses, A.; Haines, J.; Pericak-Vance, M.A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993, 261, 921–923. [Google Scholar] [CrossRef] [PubMed]
- Bellenguez, C.; Küçükali, F.; Jansen, I.E.; Kleineidam, L.; Moreno-Grau, S.; Amin, N.; Naj, A.C.; Campos-Martin, R.; Grenier-Boley, B.; Andrade, V. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 2022, 54, 412–436. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Guerrero, J.; Santiago-Balmaseda, A.; Jeronimo-Aguilar, P.; Vargas-Rodríguez, I.; Cadena-Suárez, A.R.; Sánchez-Garibay, C.; Pozo-Molina, G.; Méndez-Catalá, C.F.; Cardenas-Aguayo, M.D.; Diaz-Cintra, S.; et al. Alzheimer’s Disease: An Updated Overview of Its Genetics. Int. J. Mol. Sci. 2023, 24, 3754. [Google Scholar] [CrossRef]
- Ballard, C.; Mobley, W.; Hardy, J.; Williams, G.; Corbett, A. Dementia in Down’s syndrome. Lancet Neurol. 2016, 15, 622–636. [Google Scholar] [CrossRef]
- Kim, J.; Basak, J.M.; Holtzman, D.M. The role of apolipoprotein E in Alzheimer’s disease. Neuron 2009, 63, 287–303. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J. NIA-AA research framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Alhazmi, H.A.; Albratty, M. An update on the novel and approved drugs for Alzheimer disease. Saudi Pharm. J. 2022, 30, 1755–1764. [Google Scholar] [CrossRef]
- Miculas, D.C.; Negru, P.A.; Bungau, S.G.; Behl, T.; Hassan, S.S.U.; Tit, D.M. Pharmacotherapy Evolution in Alzheimer’s Disease: Current Framework and Relevant Directions. Cells 2022, 12, 131. [Google Scholar] [CrossRef]
- Thawabteh, A.M.; Ghanem, A.W.; AbuMadi, S.; Thaher, D.; Jaghama, W.; Karaman, D.; Karaman, R. Recent Advances in Therapeutics for the Treatment of Alzheimer’s Disease. Molecules 2024, 29, 5131. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L. Maximizing the benefit and managing the risk of anti-amyloid monoclonal antibody therapy for Alzheimer’s disease: Strategies and research directions. Neurotherapeutics 2025, 22, e00570. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Osse, A.M.L.; Cammann, D.; Powell, J.; Chen, J. Anti-Amyloid Monoclonal Antibodies for the Treatment of Alzheimer’s Disease. BioDrugs 2024, 38, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Lacorte, E.; Ancidoni, A.; Zaccaria, V.; Remoli, G.; Tariciotti, L.; Bellomo, G.; Sciancalepore, F.; Corbo, M.; Lombardo, F.L.; Bacigalupo, I.; et al. Safety and Efficacy of Monoclonal Antibodies for Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Published and Unpublished Clinical Trials. J. Alzheimer’s Dis. 2022, 87, 101–129. [Google Scholar] [CrossRef]
- Ebell, M.H.; Barry, H.C.; Baduni, K.; Grasso, G. Clinically Important Benefits and Harms of Monoclonal Antibodies Targeting Amyloid for the Treatment of Alzheimer Disease: A Systematic Review and Meta-Analysis. Ann. Fam. Med. 2024, 22, 50–62. [Google Scholar] [CrossRef]
- Geerts, H.; Bergeler, S.; Walker, M.; Rose, R.H.; van der Graaf, P.H. Quantitative systems pharmacology-based exploration of relevant anti-amyloid therapy challenges in clinical practice. Alzheimer’s Dement 2024, 10, e12474. [Google Scholar] [CrossRef]
- Drzezga, A.; Barthel, H. Imaging and fluid biomarkers of Alzheimer disease: Complementation rather than competition. J. Nucl. Med. 2025, 66 (Suppl. 2), S32–S44. [Google Scholar] [CrossRef]
- Sato, K.; Niimi, Y.; Ihara, R.; Suzuki, K.; Iwata, A.; Iwatsubo, T. APOE-ε4 allele[s]-associated adverse events reported from placebo arm in clinical trials for Alzheimer’s disease: Implications for anti-amyloid beta therapy. Front. Dement. 2024, 2, 1320329. [Google Scholar] [CrossRef]
- Kane, M. Lecanemab therapy and APOE genotype. In Medical Genetics Summaries [Internet]; National Center for Biotechnology Information: Bethesda, MD, USA, 2024. [Google Scholar]
- Selkoe, D.J. The molecular pathology of Alzheimer’s disease. Neuron 1991, 6, 487–498. [Google Scholar] [CrossRef]
- Hardy, J.; Allsop, D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol. Sci. 1991, 12, 383–388. [Google Scholar] [CrossRef]
- Tanzi, R.E.; Bertram, L. Twenty years of the Alzheimer’s disease amyloid hypothesis: A genetic perspective. Cell 2005, 120, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Quon, D.; Wang, Y.; Catalano, R.; Scardina, J.M.; Murakami, K.; Cordell, B. Formation of β-amyloid protein deposits in brains of transgenic mice. Nature 1991, 352, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.; Schneider, J.; Arvanitakis, Z.; Kelly, J.; Aggarwal, N.; Shah, R.; Wilson, R. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 2006, 66, 1837–1844. [Google Scholar] [CrossRef]
- De Meyer, G.; Shapiro, F.; Vanderstichele, H.; Vanmechelen, E.; Engelborghs, S.; De Deyn, P.P.; Coart, E.; Hansson, O.; Minthon, L.; Zetterberg, H. Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch. Neurol. 2010, 67, 949–956. [Google Scholar] [CrossRef]
- Fagan, A.M.; Roe, C.M.; Xiong, C.; Mintun, M.A.; Morris, J.C.; Holtzman, D.M. Cerebrospinal fluid tau/β-amyloid42 ratio as a prediction of cognitive decline in nondemented older adults. Arch. Neurol. 2007, 64, 343–349. [Google Scholar]
- Gomperts, S.; Rentz, D.; Moran, E.; Becker, J.; Locascio, J.; Klunk, W.; Mathis, C.; Elmaleh, D.; Shoup, T.; Fischman, A. Imaging amyloid deposition in Lewy body diseases. Neurology 2008, 71, 903–910. [Google Scholar] [CrossRef]
- Yang, T.; Li, S.; Xu, H.; Walsh, D.M.; Selkoe, D.J. Large Soluble Oligomers of Amyloid β-Protein from Alzheimer Brain Are Far Less Neuroactive Than the Smaller Oligomers to Which They Dissociate. J. Neurosci. 2017, 37, 152–163. [Google Scholar] [CrossRef]
- Shankar, G.M.; Li, S.; Mehta, T.H.; Garcia-Munoz, A.; Shepardson, N.E.; Smith, I.; Brett, F.M.; Farrell, M.A.; Rowan, M.J.; Lemere, C.A.; et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008, 14, 837–842. [Google Scholar] [CrossRef]
- Knowles, R.B.; Wyart, C.; Buldyrev, S.V.; Cruz, L.; Urbanc, B.; Hasselmo, M.E.; Stanley, H.E.; Hyman, B.T. Plaque-induced neurite abnormalities: Implications for disruption of neural networks in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 1999, 96, 5274–5279. [Google Scholar]
- Meyer-Luehmann, M.; Spires-Jones, T.L.; Prada, C.; Garcia-Alloza, M.; de Calignon, A.; Rozkalne, A.; Koenigsknecht-Talboo, J.; Holtzman, D.M.; Bacskai, B.J.; Hyman, B.T. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature 2008, 451, 720–724. [Google Scholar] [CrossRef]
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.-K.; Xu, X.-L.; Chen, B.-Y.; Su, J.; Du, Y.-Z. Combining nanotechnology with monoclonal antibody drugs for rheumatoid arthritis treatments. J. Nanobiotechnology 2023, 21, 105. [Google Scholar] [CrossRef] [PubMed]
- Zahavi, D.; Weiner, L. Monoclonal antibodies in cancer therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Breedveld, F. Therapeutic monoclonal antibodies. Lancet 2000, 355, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Zhou, Y.; Lee, G.; Zhong, K.; Fonseca, J.; Cheng, F. Alzheimer’s disease drug development pipeline: 2023. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2023, 9, e12385. [Google Scholar] [CrossRef]
- Kim, B.-H.; Kim, S.; Nam, Y.; Park, Y.H.; Shin, S.M.; Moon, M. Second-generation anti-amyloid monoclonal antibodies for Alzheimer’s disease: Current landscape and future perspectives. Transl. Neurodegener. 2025, 14, 6. [Google Scholar] [CrossRef]
- Honig, L.S.; Vellas, B.; Woodward, M.; Boada, M.; Bullock, R.; Borrie, M.; Hager, K.; Andreasen, N.; Scarpini, E.; Liu-Seifert, H.; et al. Trial of Solanezumab for Mild Dementia Due to Alzheimer’s Disease. N. Engl. J. Med. 2018, 378, 321–330. [Google Scholar] [CrossRef]
- Ostrowitzki, S.; Lasser, R.A.; Dorflinger, E.; Scheltens, P.; Barkhof, F.; Nikolcheva, T.; Ashford, E.; Retout, S.; Hofmann, C.; Delmar, P.; et al. A phase III randomized trial of gantenerumab in prodromal Alzheimer’s disease. Alzheimer’s Res. Ther. 2017, 9, 95. [Google Scholar] [CrossRef]
- Ostrowitzki, S.; Bittner, T.; Sink, K.M.; Mackey, H.; Rabe, C.; Honig, L.S.; Cassetta, E.; Woodward, M.; Boada, M.; van Dyck, C.H.; et al. Evaluating the Safety and Efficacy of Crenezumab vs Placebo in Adults with Early Alzheimer Disease: Two Phase 3 Randomized Placebo-Controlled Trials. JAMA Neurol. 2022, 79, 1113–1121. [Google Scholar] [CrossRef]
- Doggrell, S.A. More failure with solanezumab—This time in preclinical Alzheimer’s disease. Expert. Opin. Biol. Ther. 2024, 24, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Johannesson, M.; Söderberg, L.; Zachrisson, O.; Fritz, N.; Kylefjord, H.; Gkanatsiou, E.; Button, E.; Svensson, A.S.; Rachalski, A.; Nygren, P.; et al. Lecanemab demonstrates highly selective binding to Aβ protofibrils isolated from Alzheimer’s disease brains. Mol. Cell Neurosci. 2024, 130, 103949. [Google Scholar] [CrossRef] [PubMed]
- Khartabil, N.; Awaness, A. Targeting Amyloid Pathology in Early Alzheimer’s: The Promise of Donanemab-Azbt. Pharm 2025, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Ashique, S.; Sirohi, E.; Kumar, S.; Rihan, M.; Mishra, N.; Bhatt, S.; Gautam, R.K.; Singh, S.K.; Gupta, G.; Chellappan, D.K.; et al. Aducanumab in Alzheimer’s Disease: A Critical Update. Curr. Med. Chem. 2024, 31, 5004–5026. [Google Scholar] [CrossRef]
- Hampel, H.; Elhage, A.; Cho, M.; Apostolova, L.G.; Nicoll, J.A.R.; Atri, A. Amyloid-related imaging abnormalities (ARIA): Radiological, biological and clinical characteristics. Brain 2023, 146, 4414–4424. [Google Scholar] [CrossRef]
- Usman, M.; Bhardwaj, S.; Roychoudhury, S.; Kumar, D.; Alexiou, A.; Kumar, P.; Ambasta, R.; Prasher, P.; Shukla, S.; Upadhye, V. Immunotherapy for Alzheimer’s disease: Current scenario and future perspectives. J. Prev. Alzheimer’s Dis. 2021, 8, 534–551. [Google Scholar] [CrossRef]
- Bougea, A.; Gourzis, P. Biomarker-Based Precision Therapy for Alzheimer’s Disease: Multidimensional Evidence Leading a New Breakthrough in Personalized Medicine. J. Clin. Med. 2024, 13, 4661. [Google Scholar] [CrossRef]
- Arndt, J.W.; Qian, F.; Smith, B.A.; Quan, C.; Kilambi, K.P.; Bush, M.W.; Walz, T.; Pepinsky, R.B.; Bussière, T.; Hamann, S. Structural and kinetic basis for the selectivity of aducanumab for aggregated forms of amyloid-β. Sci. Rep. 2018, 8, 6412. [Google Scholar] [CrossRef]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef]
- Biogen. Biogen and Eisai Amend Collaboration Agreements on Alzheimer’s Disease Treatments; [Updated 14 March 2022]; Biogen: Cambridge, MA, USA, 2022; Available online: https://investors.biogen.com/news-releases/news-release-details/biogen-and-eisai-amend-collaboration-agreements-alzheimers (accessed on 12 July 2025).
- Vaz, M.; Silva, V.; Monteiro, C.; Silvestre, S. Role of Aducanumab in the Treatment of Alzheimer’s Disease: Challenges and Opportunities. Clin. Interv. Aging 2022, 17, 797–810. [Google Scholar] [CrossRef]
- Heidebrink, J.L.; Paulson, H.L. Lessons learned from approval of aducanumab for Alzheimer’s disease. Annu. Rev. Med. 2024, 75, 99–111. [Google Scholar] [CrossRef]
- Padda, I.S.; Parmar, M. Aducanumab. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Lythgoe, M.P.; Jenei, K.; Prasad, V. Regulatory decisions diverge over aducanumab for Alzheimer’s disease. BMJ 2022, 376, e069780. [Google Scholar] [CrossRef]
- Laura Joszt, M. Biogen abandons aducanumab, pivots focus to Lecanemab for alzheimer disease. Am. J. Manag. Care 2024. [Google Scholar]
- Budd Haeberlein, S.; Aisen, P.S.; Barkhof, F.; Chalkias, S.; Chen, T.; Cohen, S.; Dent, G.; Hansson, O.; Harrison, K.; von Hehn, C.; et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J. Prev. Alzheimer’s Dis. 2022, 9, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Belluck, P.; Robbins, R. Three FDA advisers resign over agency’s approval of Alzheimer’s drug. N. Y. Times 2021, 11. [Google Scholar]
- Lin, G.A.; Whittington, M.D.; Synnott, P.G.; McKenna, A.; Campbell, J.; Pearson, S.D.; Rind, D.M. Aducanumab for Alzheimer’s disease: Effectiveness and value; final evidence report and meeting summary. Inst. Clin. Econ. Rev. 2021, 5. [Google Scholar]
- Englund, H.; Sehlin, D.; Johansson, A.S.; Nilsson, L.N.; Gellerfors, P.; Paulie, S.; Lannfelt, L.; Pettersson, F.E. Sensitive ELISA detection of amyloid-β protofibrils in biological samples. J. Neurochem. 2007, 103, 334–345. [Google Scholar] [CrossRef]
- Nilsberth, C.; Westlind-Danielsson, A.; Eckman, C.B.; Condron, M.M.; Axelman, K.; Forsell, C.; Stenh, C.; Luthman, J.; Teplow, D.B.; Younkin, S.G. The’Arctic’APP mutation (E693G) causes Alzheimer’s disease by enhanced Aβ protofibril formation. Nat. Neurosci. 2001, 4, 887–893. [Google Scholar] [CrossRef]
- Tucker, S.; Möller, C.; Tegerstedt, K.; Lord, A.; Laudon, H.; Sjödahl, J.; Söderberg, L.; Spens, E.; Sahlin, C.; Waara, E.R.; et al. The murine version of BAN2401 (mAb158) selectively reduces amyloid-β protofibrils in brain and cerebrospinal fluid of tg-ArcSwe mice. J. Alzheimer’s Dis. 2015, 43, 575–588. [Google Scholar] [CrossRef]
- Logovinsky, V.; Satlin, A.; Lai, R.; Swanson, C.; Kaplow, J.; Osswald, G.; Basun, H.; Lannfelt, L. Safety and tolerability of BAN2401—A clinical study in Alzheimer’s disease with a protofibril selective Aβ antibody. Alzheimer’s Res. Ther. 2016, 8, 14. [Google Scholar] [CrossRef]
- Swanson, C.J.; Zhang, Y.; Dhadda, S.; Wang, J.; Kaplow, J.; Lai, R.Y.K.; Lannfelt, L.; Bradley, H.; Rabe, M.; Koyama, A.; et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimer’s Res. Ther. 2021, 13, 80. [Google Scholar] [CrossRef]
- Tahami Monfared, A.A.; Tafazzoli, A.; Ye, W.; Chavan, A.; Zhang, Q. Long-Term Health Outcomes of Lecanemab in Patients with Early Alzheimer’s Disease Using Simulation Modeling. Neurol. Ther. 2022, 11, 863–880. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Converts Novel Alzheimer’s Disease Treatment to Traditional Approval; [updated 6 July 2023]; FDA: Silver Spring, MD, USA, 2023. Available online: https://www.fda.gov/news-events/press-announcements/fda-converts-novel-alzheimers-disease-treatment-traditional-approval (accessed on 12 July 2025).
- Eisai, Inc. Leqembi™ (lecanemab-irmb) Prescribing Information; Eisai Inc.: Nutley, NJ, USA, 2023; Available online: https://www.leqembi.com (accessed on 12 July 2025).
- Eisai, B. FDA Approves LEQEMBI™ (Lecanemab-Irmb) Under the Accelerated Approval Pathway for the Treatment of Alzheimer’s Disease; [updated 6 January 2023]; Biogen: Cambridge, MA, USA; Tokyo, Japan, 2023; Available online: https://investors.biogen.com/news-releases/news-release-details/fda-approves-leqembitm-lecanemab-irmb-under-accelerated-approval (accessed on 12 July 2025).
- Yoon, C.H.; Groff, C.; Criss, O. Lecanemab: A second in class therapy for the management of early Alzheimer’s disease. Innov. Pharm. 2024, 15, 10-24926. [Google Scholar] [CrossRef]
- Eli Lilly and Company. Lilly’s Kisunla™ (Donanemab-azbt) Approved by the FDA for the Treatment of Early Symptomatic Alzheimer’s Disease; Eli Lilly and Company: Indianapolis, IN, USA, 2024; Available online: https://investor.lilly.com/news-releases/news-release-details/lillys-kisunlatm-donanemab-azbt-approved-fda-treatment-early (accessed on 8 April 2025).
- Demattos, R.B.; Lu, J.; Tang, Y.; Racke, M.M.; Delong, C.A.; Tzaferis, J.A.; Hole, J.T.; Forster, B.M.; McDonnell, P.C.; Liu, F.; et al. A plaque-specific antibody clears existing β-amyloid plaques in Alzheimer’s disease mice. Neuron 2012, 76, 908–920. [Google Scholar] [CrossRef]
- Rabinovici, G.D.; Selkoe, D.J.; Schindler, S.E.; Aisen, P.; Apostolova, L.G.; Atri, A.; Greenberg, S.M.; Hendrix, S.B.; Petersen, R.C.; Weiner, M.; et al. Donanemab: Appropriate use recommendations. J. Prev. Alzheimer’s Dis. 2025, 12, 100150. [Google Scholar] [CrossRef]
- Eli Lilly and Company. A Study of LY3002813 in Participants with Alzheimer’s Disease; [updated October 4, 2024. Completed Clinical Study Evaluating LY3002813 (Donanemab) in Alzheimer’s Disease]; ClinicalTrials.gov: Bethesda, MD, USA, 2024. Available online: https://clinicaltrials.gov/study/NCT01837641 (accessed on 8 April 2025).
- Eli Lilly and Company. A Study of LY3002813 in Participants with Memory Damage Due to Alzheimer’s Disease (AD) or AD; ClinicalTrials.gov: Bethesda, MD, USA, 2025. [Completed Clinical Trial Investigating LY3002813 (Donanemab) in Alzheimer’s Disease]. Available online: https://clinicaltrials.gov/study/NCT02624778 (accessed on 8 April 2025).
- Eli Lilly and Company. A Study of LY3002813 in Participants with Early Symptomatic Alzheimer’s Disease (TRAILBLAZER-ALZ); [Completed Phase 2 Study of Donanemab (LY3002813) in Early Symptomatic Alzheimer’s Disease]; ClinicalTrials.gov: Bethesda, MD, USA, 2022. Available online: https://clinicaltrials.gov/study/NCT03367403 (accessed on 8 April 2025).
- Eli Lilly and Company. A Study of Donanemab (LY3002813) in Participants with Early Alzheimer’s Disease (TRAILBLAZER-ALZ 2); ClinicalTrials.gov: Bethesda, MD, USA, 2025. Available online: https://clinicaltrials.gov/study/NCT04437511 (accessed on 8 April 2025).
- Eli Lilly and Company. A Donanemab (LY3002813) Study in Participants with Preclinical Alzheimer’s Disease (TRAILBLAZER-ALZ 3); [Ongoing Clinical Trial Evaluating Donanemab in Individuals with Preclinical Alzheimer’s Disease]; ClinicalTrials.gov: Bethesda, MD, USA, 2025. Available online: https://clinicaltrials.gov/study/NCT05026866 (accessed on 8 April 2025).
- Eli Lilly and Company. A Study of Donanemab (LY3002813) Compared with Aducanumab in Participants with Early Symptomatic Alzheimer’s Disease (TRAILBLAZER-ALZ 4); ClinicalTrials.gov: Bethesda, MD, USA, 2024. Available online: https://clinicaltrials.gov/study/NCT05108922 (accessed on 8 April 2025).
- Eli Lilly and Company. A Study of Donanemab (LY3002813) in Participants with Early Symptomatic Alzheimer’s Disease (TRAILBLAZER-ALZ 5); ClinicalTrials.gov: Bethesda, MD, USA, 2025. Available online: https://clinicaltrials.gov/study/NCT05508789 (accessed on 8 April 2025).
- Eli Lilly and Company. A Study of Different Donanemab (LY3002813) Dosing Regimens in Adults with Early Alzheimer’s Disease (TRAILBLAZER-ALZ 6); ClinicalTrials.gov Identifier: NCT05738486; ClinicalTrials.gov: Bethesda, MD, USA, 2025. Available online: https://clinicaltrials.gov/study/NCT05738486 (accessed on 8 April 2025).
- International Collaboration for Real-World Evidence in Alzheimer’s Disease (ICARE AD)—A Prospective Real-World Observational Study of Aducanumab-Avwa in Patients with Alzheimer’s Disease in the US. 2021. Available online: https://clinicaltrials.gov/study/NCT05097131 (accessed on 8 April 2025).
- A Phase 3 Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study to Evaluate the Efficacy and Safety of Aducanumab (BIIB037) in Subjects with Early Alzheimer’s Disease. 2015. Available online: https://clinicaltrials.gov/study/NCT02477800 (accessed on 8 April 2025).
- A Phase 3b/4 Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study to Verify the Clinical Benefit of Aducanumab (BIIB037) in Participants with Alzheimer’s Disease. 2022. Available online: https://clinicaltrials.gov/study/NCT05310071 (accessed on 8 April 2025).
- Shcherbinin, S.; Evans, C.D.; Lu, M.; Andersen, S.W.; Pontecorvo, M.J.; Willis, B.A.; Gueorguieva, I.; Hauck, P.M.; Brooks, D.A.; Mintun, M.A.; et al. Association of Amyloid Reduction After Donanemab Treatment with Tau Pathology and Clinical Outcomes: The TRAILBLAZER-ALZ Randomized Clinical Trial. JAMA Neurol. 2022, 79, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- A Placebo-Controlled, Double-Blind, Parallel-Group, 18-Month Study with an Open-Label Extension Phase to Confirm Safety and Efficacy of BAN2401 in Subjects with Early Alzheimer’s Disease. 2019. Available online: https://clinicaltrials.gov/study/NCT03887455 (accessed on 8 April 2025).
- A Placebo-Controlled, Double-Blind, Parallel-Group, Bayesian Adaptive Randomization Design and Dose Regimen-Finding Study With an Open-Label Extension Phase to Evaluate Safety, Tolerability and Efficacy of BAN2401 in Subjects with Early Alzheimer’s Disease. 2013. Available online: https://clinicaltrials.gov/study/NCT01767311 (accessed on 8 April 2025).
- A Randomized, Double-blind, Placebo-controlled, Combined Single Ascending Dose and Multiple Ascending Dose Study to Assess Safety, Tolerability, Immunogenicity, Pharmacodynamic Response, and Pharmacokinetics of Intravenous Infusions of BAN2401 in Subjects with Mild to Moderate Alzheimer’s Disease. 2010. Available online: https://clinicaltrials.gov/study/NCT01230853 (accessed on 8 April 2025).
- Tarawneh, R.; Pankratz, V.S. The search for clarity regarding “clinically meaningful outcomes” in Alzheimer disease clinical trials: CLARITY-AD and Beyond. Alzheimer’s Res. Ther. 2024, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Cummings, J.; Knox, S.; Potashman, M.; Harrison, J. Clinical trial endpoints and their clinical meaningfulness in early stages of Alzheimer’s disease. J. Prev. Alzheimer’s Dis. 2022, 9, 507–522. [Google Scholar] [CrossRef]
- Berg, L.; Miller, J.P.; Baty, J.; Rubin, E.H.; Morris, J.C.; Figiel, G. Mild senile dementia of the Alzheimer type. 4. Evaluation of intervention. Ann. Neurol. 1992, 31, 242–249. [Google Scholar] [CrossRef]
- Rogers, B.M. No Easy Answers on Clinical Meaningfulness of Alzheimer’s Treatments. Alzforum. 2023. Available online: https://www.alzforum.org/news/research-news/no-easy-answers-clinical-meaningfulness-alzheimers-treatments (accessed on 8 April 2025).
- Kahle-Wrobleski, K.; Ye, W.; Henley, D.; Hake, A.M.; Siemers, E.; Chen, Y.-F.; Liu-Seifert, H. Assessing quality of life in Alzheimer’s disease: Implications for clinical trials. Alzheimer’s Dement. Diagn. Assess. Dis. Monitoring. 2017, 6, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P.; Akehurst, R.; Ballard, C.; Banerjee, S.; Blennow, K.; Bremner, J.; Broich, K.; Cummings, J.; Dening, K.; Dubois, B. Taking stock: A multistakeholder perspective on improving the delivery of care and the development of treatments for Alzheimer’s disease. Alzheimer’s Dement. 2015, 11, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- Alzheimer’s Research UK. AAIC 2025: The Future of Alzheimer’s Research Is Here. Alzheimer’s Research UK News, 2025. Available online: https://alzheimersresearchuk.org/news/aaic-2025-the-future-of-alzheimers-research-is-here/ (accessed on 8 April 2025).
- Eli Lilly and Company. Lilly’s Kisunla (donanemab-azbt) Showed Growing Benefit over Three Years in Early Symptomatic Alzheimer’s Disease. Eli Lilly and Company: Indianapolis, IN, USA, 2025. Available online: https://investor.lilly.com/news-releases/news-release-details/lillys-kisunla-donanemab-azbt-showed-growing-benefit-over-three (accessed on 8 April 2025).
- Jeong, S.Y.; Suh, C.H.; Kim, S.J.; Lemere, C.A.; Lim, J.-S.; Lee, J.-H. Amyloid-related imaging abnormalities in the era of anti-amyloid beta monoclonal antibodies for Alzheimer’s disease: Recent updates on clinical and imaging features and MRI monitoring. Korean J. Radiol. 2024, 25, 726. [Google Scholar] [CrossRef]
- Filippi, M.; Cecchetti, G.; Spinelli, E.G.; Vezzulli, P.; Falini, A.; Agosta, F. Amyloid-Related Imaging Abnormalities and β-Amyloid-Targeting Antibodies: A Systematic Review. JAMA Neurol. 2022, 79, 291–304. [Google Scholar] [CrossRef]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Corriveau, R.A.; Bosetti, F.; Emr, M.; Gladman, J.T.; Koenig, J.I.; Moy, C.S.; Pahigiannis, K.; Waddy, S.P.; Koroshetz, W. The Science of Vascular Contributions to Cognitive Impairment and Dementia (VCID): A Framework for Advancing Research Priorities in the Cerebrovascular Biology of Cognitive Decline. Cell Mol. Neurobiol. 2016, 36, 281–288. [Google Scholar] [CrossRef]
- Scolding, N.J.; Joseph, F.; Kirby, P.A.; Mazanti, I.; Gray, F.; Mikol, J.; Ellison, D.; Hilton, D.A.; Williams, T.L.; MacKenzie, J.M.; et al. Abeta-related angiitis: Primary angiitis of the central nervous system associated with cerebral amyloid angiopathy. Brain 2005, 128 Pt 3, 500–515. [Google Scholar] [CrossRef] [PubMed]
- Eng, J.A.; Frosch, M.P.; Choi, K.; Rebeck, G.W.; Greenberg, S.M. Clinical manifestations of cerebral amyloid angiopathy-related inflammation. Ann. Neurol. 2004, 55, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Sellimi, A.; Schwartze, J.; Humphries, F.; Panteleienko, L.; Mallon, D.; Banerjee, G.; Werring, D.J. Contemporary perspectives in cerebral amyloid angiopathy. Expert. Rev. Neurother. 2025, 25, 887–910. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, S.M.; Bacskai, B.J.; Hernandez-Guillamon, M.; Pruzin, J.; Sperling, R.; van Veluw, S.J. Cerebral amyloid angiopathy and Alzheimer disease—One peptide, two pathways. Nat. Rev. Neurol. 2020, 16, 30–42. [Google Scholar] [CrossRef]
- van Dyck, C.H. Anti-Amyloid-β Monoclonal Antibodies for Alzheimer’s Disease: Pitfalls and Promise. Biol. Psychiatry 2018, 83, 311–319. [Google Scholar] [CrossRef]
- Cogswell, P.M.; Barakos, J.A.; Barkhof, F.; Benzinger, T.S.; Jack, C.R., Jr.; Poussaint, T.Y.; Raji, C.A.; Ramanan, V.K.; Whitlow, C.T. Amyloid-Related Imaging Abnormalities with Emerging Alzheimer Disease Therapeutics: Detection and Reporting Recommendations for Clinical Practice. AJNR Am. J. Neuroradiol. 2022, 43, E19–E35. [Google Scholar] [CrossRef]
- Crespi, G.A.; Hermans, S.J.; Parker, M.W.; Miles, L.A. Molecular basis for mid-region amyloid-β capture by leading Alzheimer’s disease immunotherapies. Sci. Rep. 2015, 5, 9649. [Google Scholar] [CrossRef]
- Salloway, S.; Sperling, R.; Fox, N.C.; Blennow, K.; Klunk, W.; Raskind, M.; Sabbagh, M.; Honig, L.S.; Porsteinsson, A.P.; Ferris, S.; et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N. Engl. J. Med. 2014, 370, 322–333. [Google Scholar] [CrossRef]
- Ketter, N.; Brashear, H.R.; Bogert, J.; Di, J.; Miaux, Y.; Gass, A.; Purcell, D.D.; Barkhof, F.; Arrighi, H.M. Central Review of Amyloid-Related Imaging Abnormalities in Two Phase III Clinical Trials of Bapineuzumab in Mild-To-Moderate Alzheimer’s Disease Patients. J. Alzheimer’s Dis. 2017, 57, 557–573. [Google Scholar] [CrossRef]
- Doody, R.S.; Thomas, R.G.; Farlow, M.; Iwatsubo, T.; Vellas, B.; Joffe, S.; Kieburtz, K.; Raman, R.; Sun, X.; Aisen, P.S.; et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N. Engl. J. Med. 2014, 370, 311–321. [Google Scholar] [CrossRef]
- Landen, J.W.; Cohen, S.; Billing, C.B., Jr.; Cronenberger, C.; Styren, S.; Burstein, A.H.; Sattler, C.; Lee, J.H.; Jack, C.R., Jr.; Kantarci, K.; et al. Multiple-dose ponezumab for mild-to-moderate Alzheimer’s disease: Safety and efficacy. Alzheimer’s Dement 2017, 3, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Biogen, Inc. Lecanemab Confirmatory Phase 3 Clarity AD Study Met Primary Endpoint, Showing Statistically Significant Results in Early Alzheimer’s Disease. 2022. Available online: https://investors.biogen.com/news-releases/news-release-details/lecanemab-confirmatory-phase-3-clarity-ad-study-met-primary (accessed on 8 April 2025).
- Ferrero, J.; Williams, L.; Stella, H.; Leitermann, K.; Mikulskis, A.; O’Gorman, J.; Sevigny, J. First-in-human, double-blind, placebo-controlled, single-dose escalation study of aducanumab (BIIB037) in mild-to-moderate Alzheimer’s disease. Alzheimer’s Dement 2016, 2, 169–176. [Google Scholar] [CrossRef] [PubMed]
- VandeVrede, L.; Gibbs, D.M.; Koestler, M.; La Joie, R.; Ljubenkov, P.A.; Provost, K.; Soleimani-Meigooni, D.; Strom, A.; Tsoy, E.; Rabinovici, G.D.; et al. Symptomatic amyloid-related imaging abnormalities in an APOE ε4/ε4 patient treated with aducanumab. Alzheimer’s Dement 2020, 12, e12101. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA). Aducanumab (marketed as Aduhelm) Information. Available online: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/aducanumab-marketed-aduhelm-information (accessed on 8 April 2025).
- Sperling, R.A.; Jack, C.R., Jr.; Black, S.E.; Frosch, M.P.; Greenberg, S.M.; Hyman, B.T.; Scheltens, P.; Carrillo, M.C.; Thies, W.; Bednar, M.M.; et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: Recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimer’s Dement. 2011, 7, 367–385. [Google Scholar] [CrossRef]
- Salloway, S.; Chalkias, S.; Barkhof, F.; Burkett, P.; Barakos, J.; Purcell, D.; Suhy, J.; Forrestal, F.; Tian, Y.; Umans, K.; et al. Amyloid-Related Imaging Abnormalities in 2 Phase 3 Studies Evaluating Aducanumab in Patients with Early Alzheimer Disease. JAMA Neurol. 2022, 79, 13–21. [Google Scholar] [CrossRef]
- Bohrmann, B.; Baumann, K.; Benz, J.; Gerber, F.; Huber, W.; Knoflach, F.; Messer, J.; Oroszlan, K.; Rauchenberger, R.; Richter, W.F.; et al. Gantenerumab: A novel human anti-Aβ antibody demonstrates sustained cerebral amyloid-β binding and elicits cell-mediated removal of human amyloid-β. J. Alzheimer’s Dis. 2012, 28, 49–69. [Google Scholar] [CrossRef]
- Caselli, R.J.; Walker, D.; Sue, L.; Sabbagh, M.; Beach, T. Amyloid load in nondemented brains correlates with APOE e4. Neurosci. Lett. 2010, 473, 168–171. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, V.; Brahmbhatt, P.; Desai, A.; Vibhute, P.; Joseph-Mathurin, N.; Bathla, G. Amyloid-related Imaging Abnormalities in Alzheimer Disease Treated with Anti-Amyloid-β Therapy. Radiographics 2023, 43, e230009. [Google Scholar] [CrossRef]
- Arrighi, H.M.; Barakos, J.; Barkhof, F.; Tampieri, D.; Jack, C., Jr.; Melançon, D.; Morris, K.; Ketter, N.; Liu, E.; Brashear, H.R. Amyloid-related imaging abnormalities-haemosiderin (ARIA-H) in patients with Alzheimer’s disease treated with bapineuzumab: A historical, prospective secondary analysis. J. Neurol. Neurosurg. Psychiatry 2016, 87, 106–112. [Google Scholar] [CrossRef]
- Kallmes, D.F.; Hui, F.K.; Mugler, J.P., 3rd. Suppression of cerebrospinal fluid and blood flow artifacts in FLAIR MR imaging with a single-slab three-dimensional pulse sequence: Initial experience. Radiology 2001, 221, 251–255. [Google Scholar] [CrossRef]
- Barakos, J.; Purcell, D.; Suhy, J.; Chalkias, S.; Burkett, P.; Marsica Grassi, C. Detection and management of amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with anti-amyloid beta therapy. J. Prev. Alzheimer’s Dis. 2022, 9, 211–220. [Google Scholar] [CrossRef]
- Withington, C.G.; Turner, R.S. Amyloid-Related Imaging Abnormalities With Anti-amyloid Antibodies for the Treatment of Dementia Due to Alzheimer’s Disease. Front. Neurol. 2022, 13, 862369. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Apostolova, L.; Rabinovici, G.; Atri, A.; Aisen, P.; Greenberg, S.; Hendrix, S.; Selkoe, D.; Weiner, M.; Petersen, R. Lecanemab: Appropriate use recommendations. J. Prev. Alzheimer’s Dis. 2023, 10, 362–377. [Google Scholar] [CrossRef] [PubMed]
- Dodel, R.; Frölich, L. Donanemab for Alzheimer’s Disease: From Preclinical Research to the Clinical Application. Expert Rev. Neurother. 2025, 25(8), 563–574. [Google Scholar] [CrossRef]
- Alzheimer’s Association; AaRDTW. Donanemab for Early-Stage Alzheimer’s Disease: Recommendations for Clinical Practice; [Summary Document Outlining Recommendations for the Safe and Effective Clinical Use of Donanemab (Kisunla™) in Patients with Early-Stage Alzheimer’s Disease, Including Candidate Selection, MRI Monitoring, and Management of ARIA Risk.], Alzheimer’s Association: Chicago, IL, USA, 2024; Available online: https://pro.alz.org/files/download/DBB34343-2D0C-42BF-9101-42A32583CA37.pdf (accessed on 8 April 2025).
- Europe, A. Clinical and Research Experts Respond to the EMA Refusal of Donanemab in a Letter to the Lancet Editors Luxembourg; [A News Article Summarizing the Response of Clinical and Research Experts to the EMA Refusal of Donanemab, Published as a Letter in The Lancet.]; Alzheimer Europe: Luxembourg, 2025; Available online: https://www.alzheimer-europe.org/news/clinical-and-research-experts-respond-ema-refusal-donanemab-letter-lancet-editors?language_content_entity=en#:~:text=Gothenburg%2C%20Sweden,ALZ%206 (accessed on 8 April 2025).
- De Strooper, B.; Haass, C.; Hardy, J.; Zetterberg, H. The regulatory rollercoaster continues—EMA refuses donanemab. Lancet 2025, 405, 1810–1812. [Google Scholar] [CrossRef]
- Pokhrel, P.; Jindal, A.; Ahmed, Q. FDA vs. EMA: Evaluating donanemab and the global debate on accelerated approvals. J. Neurol. 2025, 272, 378. [Google Scholar] [CrossRef]
- Mahase, E. Aducanumab: European agency rejects Alzheimer’s drug over efficacy and safety concerns. Br. Med. J. Publ. Group 2021, 375. [Google Scholar] [CrossRef]
- Watt, J.A.; Marple, R.; Hemmelgarn, B.; Straus, S.E. Should Canadian patients look forward to aducanumab for Alzheimer disease? Can. Med. Assoc. J. 2021, 193, E1430–E1431. [Google Scholar] [CrossRef]
- Tanzi, R.E. FDA Approval of Aduhelm Paves a New Path for Alzheimer’s Disease; ACS Publications: Washington, DC, USA, 2021; Volume 12, pp. 2714–2715. [Google Scholar]
- Gurud, T.; Jadhav, M.; Bhosale, A.; Wadkar, S.; Balid, A.; Fulari, S.; Narawade, T. Lecanemab: A Novel Therapeutic Approach for Alzheimer’s Disease. Int. J. Sci. Res. Technol. 2024, 1(11). [Google Scholar] [CrossRef]
- Agency, E.M. Leqembi Recommended for Treatment of Early Alzheimer’s Disease; European Medicines Agency: Amsterdam, The Netherlands, 2024; Available online: https://www.ema.europa.eu/en/news/leqembi-recommended-treatment-early-alzheimers-disease#:~:text=After%20re%2Dexamining%20its%20initial,patients%20with%20early%20Alzheimer’s%20disease (accessed on 8 April 2025).
- Wall, J. Results from Lilly’s Landmark Phase 3 Trial of Donanemab Presented. Eli Lilly and Company: Indianapolis, IN, USA, 2023. Available online: https://investor.lilly.com/news-releases/news-release-details/results-lillys-landmark-phase-3-trial-donanemab-presented (accessed on 8 April 2025).
- Iwatsubo, T. Clinical implementation of lecanemab: Challenges, questions and solutions. J. Prev. Alzheimer’s Dis. 2023, 10, 353–355. [Google Scholar] [CrossRef]
- Niidome, T.; Ishikawa, Y.; Ogawa, T.; Nakagawa, M.; Nakamura, Y. Mechanism of action and clinical trial results of Lecanemab (Leqembi® 200 mg, 500 mg for Intravenous Infusion), a novel treatment for Alzheimer’s disease. Folia Pharmacol. Jpn. 2024, 159, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Ameen, T.b.; Ali, U.; Salma, O.; Abdul Samee, M.; Iraj Abbas, S.M.; Naveera Kashif, S.; Arif Arifi, M.; Ali, M.; Khowaja, M.; Sinaan Ali, S.M. Amyloid solutions: Lecanemab, gantenerumab, and donanemab in the treatment of Alzheimer’s disease. Egypt. J. Neurol. Psychiatry Neurosurg. 2025, 61, 37. [Google Scholar] [CrossRef]
- Goedert, M. Tau protein and the neurofibrillary pathology of Alzheimer’s disease. Trends Neurosci. 1993, 16, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef]
- Parhizkar, S.; Holtzman, D.M. (Eds.) APOE mediated neuroinflammation and neurodegeneration in Alzheimer’s disease. In Seminars in Immunology; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Park, G.; Nhan, H.S.; Tyan, S.-H.; Kawakatsu, Y.; Zhang, C.; Navarro, M.; Koo, E.H. Caspase activation and caspase-mediated cleavage of APP is associated with amyloid β-protein-induced synapse loss in Alzheimer’s disease. Cell Rep. 2020, 31, 107839. [Google Scholar] [CrossRef]
- Mintun, M.A.; Lo, A.C.; Duggan Evans, C.; Wessels, A.M.; Ardayfio, P.A.; Andersen, S.W.; Shcherbinin, S.; Sparks, J.; Sims, J.R.; Brys, M.; et al. Donanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2021, 384, 1691–1704. [Google Scholar] [CrossRef]
- Pontecorvo, M.J.; Lu, M.; Burnham, S.C.; Schade, A.E.; Dage, J.L.; Shcherbinin, S.; Collins, E.C.; Sims, J.R.; Mintun, M.A. Association of donanemab treatment with exploratory plasma biomarkers in early symptomatic Alzheimer disease: A secondary analysis of the TRAILBLAZER-ALZ randomized clinical trial. JAMA Neurol. 2022, 79, 1250–1259. [Google Scholar] [CrossRef]
- Vemuri, P.; Wiste, H.J.; Weigand, S.D.; Knopman, D.S.; Shaw, L.M.; Trojanowski, J.Q.; Aisen, P.S.; Weiner, M.; Petersen, R.C.; Jack, C.R., Jr. Effect of apolipoprotein E on biomarkers of amyloid load and neuronal pathology in Alzheimer disease. Ann. Neurol. 2010, 67, 308–316. [Google Scholar] [CrossRef]
- McQuade, A.; Blurton-Jones, M. Microglia in Alzheimer’s Disease: Exploring How Genetics and Phenotype Influence Risk. J. Mol. Biol. 2019, 431, 1805–1817. [Google Scholar] [CrossRef]
- Eisai Co., Ltd. Eisai’s Approach to U.S. Pricing for Leqembi™ (Lecanemab), a Treatment for Early Alzheimer’s Disease, Sets Forth Our Concept of “Societal Value of Medicine” in Relation to “Price of Medicine.” 2023. Available online: https://media-us.eisai.com/2023-01-06-EISAIS-APPROACH-TO-U-S-PRICING-FOR-LEQEMBI-TM-LECANEMAB-,-A-TREATMENT-FOR-EARLY-ALZHEIMERS-DISEASE,-SETS-FORTH-OUR-CONCEPT-OF-SOCIETAL-VALUE-OF-MEDICINE-IN-RELATION-TO-PRICE-OF-MEDICINE#:~:text=This%20yearly%20estimate%20of%20$37%2C600,pricing%20may%20vary%20by%20patient (accessed on 4 April 2025).
- Medicare, C.; Services, C.f.M. Monoclonal Antibodies Directed Against Amyloid for the Treatment of Alzheimer’s Disease (AD); Centers for Medicare & Medicaid Services: Baltimore, MD, USA, 2022. Available online: https://www.cms.gov/medicare/coverage/coverage-evidence-development/monoclonal-antibodies-directed-against-amyloid-treatment-alzheimers-disease-ad (accessed on 4 April 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhalifa, A.E.; Al Mokhlf, A.; Ali, H.; Al-Ghraiybah, N.F.; Syropoulou, V. Anti-Amyloid Monoclonal Antibodies for Alzheimer’s Disease: Evidence, ARIA Risk, and Precision Patient Selection. J. Pers. Med. 2025, 15, 437. https://doi.org/10.3390/jpm15090437
Alkhalifa AE, Al Mokhlf A, Ali H, Al-Ghraiybah NF, Syropoulou V. Anti-Amyloid Monoclonal Antibodies for Alzheimer’s Disease: Evidence, ARIA Risk, and Precision Patient Selection. Journal of Personalized Medicine. 2025; 15(9):437. https://doi.org/10.3390/jpm15090437
Chicago/Turabian StyleAlkhalifa, Amer E., Abdulrahman Al Mokhlf, Hande Ali, Nour F. Al-Ghraiybah, and Vasiliki Syropoulou. 2025. "Anti-Amyloid Monoclonal Antibodies for Alzheimer’s Disease: Evidence, ARIA Risk, and Precision Patient Selection" Journal of Personalized Medicine 15, no. 9: 437. https://doi.org/10.3390/jpm15090437
APA StyleAlkhalifa, A. E., Al Mokhlf, A., Ali, H., Al-Ghraiybah, N. F., & Syropoulou, V. (2025). Anti-Amyloid Monoclonal Antibodies for Alzheimer’s Disease: Evidence, ARIA Risk, and Precision Patient Selection. Journal of Personalized Medicine, 15(9), 437. https://doi.org/10.3390/jpm15090437








