Simple Summary
Ovarian cancer (OC) diagnosis remains challenging due to the low specificity of CA 125, requiring additional biomarkers for improved accuracy. This prospective multicenter study analyzed 94 patients with adnexal masses, including 31 benign tumors, 42 borderline ovarian tumors (BOTs), and 21 OC cases. We assessed the diagnostic performance of the Systemic Inflammation Response Index (SIRI) and the Systemic Inflammatory Response (SIR) compared to CA 125. Our results demonstrated that SIRI had superior diagnostic accuracy (AUC = 0.71) compared to CA 125 (AUC = 0.59). Regression analysis confirmed SIRI as an independent predictor of non-benign ovarian tumors (p = 0.01). These findings suggest systemic inflammatory indices could enhance risk stratification and early OC detection, offering a cost-effective and accessible alternative to traditional biomarkers. Further research is needed to validate and integrate these results into clinical practice.
Abstract
Objective: Ovarian cancer (OC) remains a diagnostic challenge, often leading to late-stage detection and poor prognosis. CA 125 is widely used in clinical practice, but its limited specificity necessitates complementary biomarkers. This study evaluates the diagnostic performance of systemic inflammatory indices, specifically the Systemic Inflammation Response Index (SIRI) and the Systemic Inflammatory Response (SIR), in differentiating benign ovarian masses, borderline ovarian tumors (BOTs), and OC. Methods: A prospective multicenter observational study was conducted on 94 patients with adnexal masses undergoing primary surgical staging with adnexal masses. The cohort included 31 patients with benign tumors, 42 with BOTs, and 21 with OC. Inclusion criteria encompassed patients with a unilateral adnexal mass identified via transvaginal ultrasound, no suspected extra-ovarian disease, and complete preoperative blood work including CA 125 and systemic inflammatory indices. Exclusion criteria included chronic inflammatory diseases, history of malignancies within the past three years, endometriosis, recent corticosteroid therapy, or incomplete clinical data. Preoperative levels of CA 125, SIRI, and SIR were measured, and their diagnostic performance was assessed using ROC curve analysis. Linear regression models were applied to determine associations between inflammatory indices and ovarian tumor types. Results: SIRI and SIR were significantly higher in OC and BOTs compared to benign tumors (p < 0.001). ROC curve analysis demonstrated that SIRI had a superior diagnostic performance (AUC = 0.71) compared to CA 125 (AUC = 0.59). Regression analysis confirmed that SIRI was independently associated with non-benign ovarian tumors (p = 0.01), while CA 125 did not reach statistical significance. Conclusions: SIRI and SIR show promising diagnostic potential in differentiating ovarian tumor types, outperforming CA 125 in specificity and predictive value. Given their accessibility and cost-effectiveness, these indices could be integrated into preoperative evaluation strategies to enhance risk stratification and early detection of malignancy.
1. Introduction
Ovarian cancer (OC) remains one of the most challenging gynecologic malignancies to diagnose in its early stages, significantly impacting prognosis and treatment options [1]. The lack of reliable early diagnostic tools often leads to delayed intervention, reducing the chances of curative treatment and increasing the need for aggressive therapeutic strategies [2,3]. Distinguishing between benign ovarian masses, borderline ovarian tumors (BOTs), and malignant neoplasms is essential for optimizing patient management and avoiding unnecessary invasive procedures [4].
Several diagnostic models have been developed to enhance preoperative evaluation [5,6,7,8,9], including the Assessment of Different Neoplasias in the Adnexa (ADNEX), formulated by the International Ovarian Tumor Analysis (IOTA) group [10]. While ADNEX has shown high sensitivity in differentiating benign from malignant lesions, its accuracy declines when distinguishing between benign formations, early-stage OC, and BOTs. A similar attempt, with similar results, also arises from MRI analysis [11]. This distinction is particularly relevant as BOTs and OC require different surgical and therapeutic approaches [4], and late diagnosis can severely affect patients’ prognosis [12].
Among the biomarkers explored in ovarian cancer diagnostics, CA 125 remains the most widely used [13,14,15]. However, its limited specificity reduces its clinical utility [16], as elevated levels are also observed in benign conditions such as endometriosis, pelvic inflammatory disease, and other inflammatory and hepatic disorders [17,18,19,20]. To refine diagnostic accuracy, novel biomarkers are needed, particularly those reflecting the complex interplay between cancer progression and the immune response.
Systemic inflammatory indices, such as the Systemic Inflammation Response Index (SIRI) and the Systemic Inflammatory Response (SIR), have shown prognostic and diagnostic value in various malignancies [21,22,23]. These indices are particularly promising because they can be obtained at no additional cost as they are derived from routine blood tests already performed in standard preoperative assessments [24]. In addition, they can potentially reflect the individual’s response to tumoral progression. It is likely that upon the tumor tissue’s acquisition of infiltrative capacity, it comes in contact with the patient’s immune system, resulting in its response, which can potentially be measured. This makes them an accessible and cost-effective tool to complement existing diagnostic methods. If validated, these inflammatory indices could serve as an additional tool in the preoperative monitoring of suspicious adnexal masses, improving risk stratification and potentially reducing the number of unnecessary surgeries. Currently, many patients with benign lesions undergo surgery due to diagnostic uncertainty [25]. By integrating SIRI and SIR into clinical decision-making, it may be possible to better identify patients who require immediate surgical intervention and those who can be safely monitored over time, thereby reducing the burden on surgical teams and minimizing patient exposure to operative risks.
1.1. Objective
This study aims to evaluate whether inflammatory indices (SIRI and SIR) are elevated in patients with ovarian cancer (OC) and borderline ovarian tumors (BOTs), and whether their combination improves diagnostic performance compared to CA 125 alone. To explore this, we conducted a prospective observational multicenter cohort study, enrolling patients with suspected adnexal masses without evidence of extra-ovarian disease. We compared the mean value of inflammatory indices and CA 125 in benign lesions, OC, and BOTs.
1.2. Secondary Objectives
Additionally, we aim to evaluate their diagnostic power by constructing ROC curves and estimating the optimal cut-off values to improve differential diagnosis and refine patient selection for surgery.
2. Material and Methods
2.1. Study Design
We conducted a multicenter prospective observational cohort study utilizing secondary data from a broader clinical registry related to the multicentric study “Ovarian Cyst Enucleation Spillage Score” (NTC05376384). This study focused on patients diagnosed with adnexal masses who underwent primary surgical staging at three specialized gynecologic oncology units: the University of Campania Luigi Vanvitelli in Naples, the Santa Maria della Misericordia University Hospital in Udine, and the University of Messina in Italy.
This study adhered to the STROBE guidelines for observational research [26]. To ensure compliance with ethical and privacy regulations, specific informed consent forms for data processing were required from all participants. According to local regulatory frameworks, this study was approved by the Ethical Committee of the University of Campania Luigi Vanvitelli (Approval No. 0013958/I 5 May 2022). This research was aligned with the principles outlined in the Helsinki Declaration.
The primary aim of this study was to evaluate differences in systemic inflammatory indices (SIR and SIRI) and CA 125 among patients with benign adnexal tumors, BOTs, and OC. To assess the diagnostic potential of these biomarkers, we performed ROC curves analysis and constructed a linear regression model to explore their association with BOT and OC diagnoses.
2.2. Setting
Between January 2023 and January 2025, we prospectively collected data from patients treated for ovarian cysts at three participating centers. Those with a histological diagnosis of OC or BOTs underwent staging surgery, which included hysterectomy, bilateral adnexectomy, omentectomy, peritoneal biopsies, and, when OC was diagnosed, lumboaortic and pelvic lymphadenectomy to confirm early-stage disease. Otherwise, the following were excluded from this study.
Preoperative blood samples were collected within seven days before surgery, including a quantitative white blood cell assay and its characterization. Additionally, serum CA 125 levels were measured within 15 days preoperatively. Based on histological findings, patients were classified as benign, BOT, or OC. Two independent expert pathologists reviewed and confirmed the final diagnosis.
2.3. Participants
Patients were eligible for inclusion if they had a unilateral adnexal mass identified via transvaginal pelvic ultrasound within 30 days before surgery. Additional requirements included a complete blood count performed within 7 days before surgery, a serum CA 125 measurement within 15 days preoperatively, and the completion of surgical staging with a confirmed histological diagnosis. The ADNEX model was used for risk stratification, with patients included only if their probability of disease beyond FIGO Stage I [27] was ≤10%. Furthermore, participants needed to have comprehensive clinical status documentation based on either a preoperative CT scan or a total-body PET scan, showing no evidence of extra-ovarian disease in the case of a diagnosed BOT or OC. Imaging studies must have been conducted within 30 days after surgery and reviewed independently by two blinded radiologists. The ADNEX model [10] was used for risk stratification, with patients included only if their probability of disease beyond FIGO Stage I was ≤10%.
Exclusion criteria included chronic systemic inflammatory conditions such as Crohn’s disease, ulcerative colitis, systemic lupus erythematosus, multiple sclerosis, Hashimoto’s thyroiditis, non-alcoholic fatty liver disease, fibromyalgia, chronic kidney disease, hepatitis, osteoarthritis, or psoriasis. Additionally, patients were excluded if they had a histological diagnosis of ovarian or extra-ovarian endometriosis. Those with a history of other malignancies diagnosed within the last three years, conditions causing excessive corticosteroid production, or who had received steroid therapy in the 30 days preceding blood collection were also not eligible. Finally, patients with incomplete clinical data or an uncertain histological diagnosis of OC or BOT were categorized under an “intention-to-treat” classification, as illustrated in the flowchart (Figure 1).
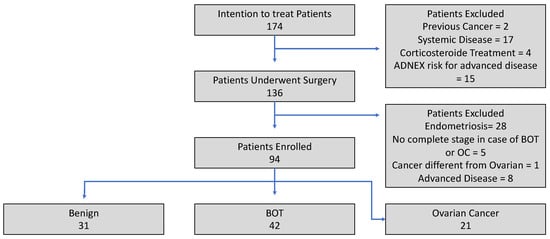
Figure 1.
Enrollment flowchart.
2.4. Variables
This study analyzed several variables, including Body Mass Index (BMI) (kg/m2) and age (years), both considered as continuous variables. The histological classification of ovarian neoplasia was treated as an ordinal variable, divided into three categories: benign, BOT, and OC, with further stratification based on specific histotype, as independently assessed by two pathologists.
Laboratory parameters included neutrophils, monocytes, lymphocytes, and platelets, which were quantified as 103 units/dL and considered continuous variables. Serum CA-125 levels were also measured in IU/dL and treated as continuous variables.
To assess the inflammatory response, two derived indices were calculated: the Systemic Inflammatory Response (SIR), obtained by multiplying the neutrophil count by the platelet count and dividing by the lymphocyte count, and the Systemic Inflammatory Response Index (SIRI), determined by multiplying monocytes by platelets and dividing by lymphocytes. Both were considered continuous variables and were used as the principal outcome of differentiation between histological categories.
2.5. Laboratory
Peripheral blood samples (3.0 mL) were collected from the ulnar vein within seven days before surgery for hematological analysis. To prevent coagulation, samples were drawn using a sterile vacuum collection system and immediately placed in tubes containing ethylenediaminetetraacetic acid (EDTA). After gentle inversion to ensure proper anticoagulation, samples were stored at 4 °C until processing. Within two hours of collection, an automated hematology analyzer was used to measure neutrophils, lymphocytes, monocytes, eosinophils, basophils, and platelet counts, with results reported in 103 units/dL. For CA 125 quantification, additional blood samples were collected within 15 days before surgery. These were drawn into serum separator tubes (SSTs), allowed to clot at room temperature for 30 min, and then centrifuged at 3000× g for 10 min to isolate the serum. The separated serum was then aliquoted and stored at −80 °C until further analysis. Measurements were conducted using an electrochemiluminescence immunoassay (ECLIA) on a Cobas e411 analyzer (Roche Diagnostics, Basel, Switzerland), ensuring high sensitivity and specificity in detecting CA 125 levels.
All participating centers adhered to standardized pre-analytical and analytical procedures for blood sample collection and processing. Complete blood counts and CA 125 levels were analyzed using automated, calibrated instruments, each undergoing daily internal quality checks. Additionally, all laboratories participated in national external quality assessment (EQA) programs to ensure inter-laboratory consistency and diagnostic accuracy. Staff at each site were trained to follow harmonized protocols for sample handling, timing, and storage to minimize variability across centers.
All laboratory analyses were performed on-site at the three participating centers, adhering to standardized protocols to maintain accuracy and reproducibility.
2.6. Statistical Analysis
The normality of continuous variables was assessed using the Kolmogorov–Smirnov test. Categorical variables were summarized as absolute frequencies and percentages, with comparisons between groups performed using Fisher’s exact and Chi-square tests. Continuous variables were presented as medians with interquartile ranges (Q1–Q3) and analyzed using the Wilcoxon test for two-group comparisons. In contrast, the Kruskal–Wallis test compared more than two independent groups.
Based on their histological diagnosis, patients were categorized into benign, BOT, and OC. The null hypothesis (H0) assumed no significant differences in mean SIR and SIRI values among the three groups (H0: µ1 = µ2 = µ3), while the alternative hypothesis (H1) suggested a significant variation (H1: µ1 ≠ µ2 ≠ µ3, two-sided test).
The sample size calculation aimed to detect a minimum difference of 0.76 standard deviations in the mean SIR and SIRI values among the three groups. Due to the lack of prior data, this estimate was based on the assumption of a notable distinction between groups, aligning with biological evidence from other malignancies. To achieve 80% statistical power at a significance level of α = 0.05, a minimum of 21 patients per group was required. The sample size calculation was performed using R (pwr package).
A multivariate linear regression model was applied to assess the association between inflammation indices and relevant clinical parameters. The model’s significance was evaluated using the maximum likelihood method.
ROC curve analysis was conducted for SIR, SIRI, and CA 125 to assess diagnostic performance, differentiating benign from non-benign (BOTs or OC) cases. The area under the curve (AUC) was calculated to determine diagnostic accuracy, applying the 2000 bootstrap resampling method. The Youden Index was used to establish optimal cutoff values for SIR and SIRI, while the CA 125 cutoff was based on clinical standards and set at 39 IU/dL (<40 IU/dL) [28].
Boxplots were generated to visualize the distribution of continuous variables across outcome groups. All statistical analyses were conducted using R software (version 2024.12.0 + 467) and RStudio (version 2024.12.0 + 467). ROC curves and AUC values were derived using the ROC package, with a p-value < 0.05 considered statistically significant.
2.7. Risk of Bias
Multivariate regression analyses were performed to reduce potential confounders, incorporating all available variables. The resulting models were assessed using adjusted R2 and Bayesian Information Criterion (BIC), with the best-fitting model selected based on the lowest BIC value.
CR initially conducted the data analysis, followed by an independent blinded review by MF, who was unaware of this study’s objectives. No missing data were identified in the key outcome variables.
2.8. Handling of Missing Data and Sensitivity Analysis
Missing data were addressed using multiple imputation techniques, ensuring the preservation of statistical power and reducing potential bias. The imputed values were cross-validated using complete case analysis to verify consistency. Sensitivity analyses involved excluding extreme values, testing alternative statistical models, and assessing subgroup-specific trends.
2.9. Declaration of Generative A.I. in Scientific Writing
The authors declare that no A.I. was used to write the original draft. Grammar correction tools (Grammarly, Inc., San Francisco, CA, USA), were used to improve the quality of English and readability. The technology was used under human oversight and control.
3. Results
Between January 2023 and January 2025, 174 patients were screened for inclusion in this study. Of these, 38 were excluded before surgery due to the presence of one or more exclusion criteria, and 136 underwent surgery. Due to the presence of post-surgical exclusion criteria, 42 patients were excluded from this study. A total of 94 patients were in the end enrolled: 31 benign, 42 BOT, and 21 OC, reaching the calculated minimum sample size of 21 patients for the single arm. All exclusion reasons are shown in Figure 1.
There was no statistically significant difference between the three groups regarding age, BMI, or menopausal status. However, patients in the OC group exhibited higher mean levels of CA 125, neutrophils, monocytes, and platelets. Table 1 provides a detailed summary of the population characteristics.

Table 1.
Patient characteristics.
3.1. Outcomes
The primary outcome was to estimate the difference in mean value of SIR and SIRI between benign, BOT, and OC. SIR and SIRI were higher in the OC group (SIR 1347 vs. 699 vs. 557, p = 0.016; SIRI 2.73 vs. 1.36 vs. 0.76 p < 0.001). These results are shown in Table 2.

Table 2.
Inflammation outcomes.
The distribution of the SIR, SIRI, and CA 125 values is graphed as a boxplot in Figure 2.
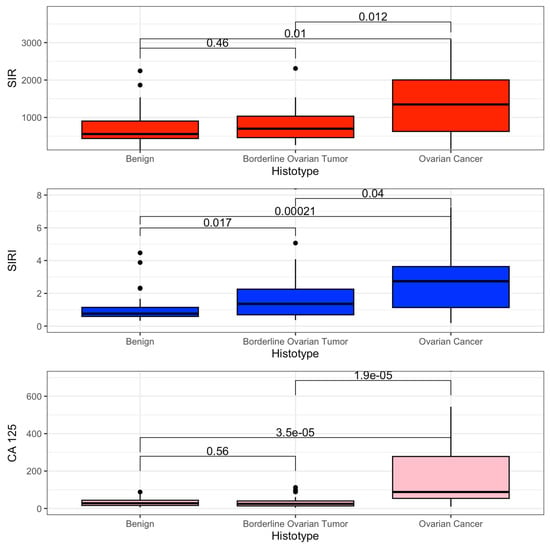
Figure 2.
Boxplot SIR, SIRI, and CA 125.
3.2. Linear Regression
A linear regression model was constructed to evaluate the relationship between non-benign ovarian diagnosis and SIR, SIRI, and CA 125 as independent variables. The analysis identified a statistically significant association between non-benign ovarian diagnosis (BOT or OC) and SIRI (beta coefficient of 1.2 95% CI: 0.3–2.1, p = 0.01). At the same time, SIR and CA 125 failed to reach a statistically significant association. Those data are shown in Table 3.

Table 3.
Linear regression.
3.3. ROC Curve
We constructed ROC specifications for SIR, SIRI, and CA 125 to compare the potential diagnostic of non-benign adnexal neoplasia (Figure 3).
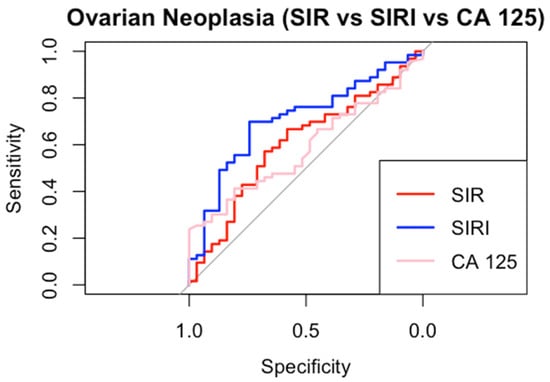
Figure 3.
ROC curves.
SIRI demonstrated the highest AUC (0.71, CI 95% 0.59–0.81) and CA 125 the lowest AUC (0.59, CI 95% 0.46–0.70). SIR showed intermediate performance (AUC 0.60, CI 95% 0.48–0.72). Using Youden’s method, the optimal diagnostic cut-off values were determined as 710 for SIR and 1 for SIRI, while for CA 125, the commonly used positivity threshold of 40 IU/dL was applied [28]. SIRI achieved the highest specificity (0.74) and sensitivity (0.70). Both the inflammatory indices outperformed CA 125 in sensitivity, positive predictive value (PPV), and negative predictive value (NPV). SIR, on the other hand, demonstrated specificity (0.68) equal to that of CA 125. All detailed cut-off performance metrics are provided in Table 4.

Table 4.
Predictive value of ovarian neoplasia.
4. Discussion
4.1. Interpretation of Results
Our study demonstrates that SIR and SIRI levels progressively increase along the spectrum from benign neoplasms to BOTs and OC. This trend is primarily driven by a rise in neutrophils, monocytes, and platelets, while lymphocyte levels remain stable. From a biological perspective, we hypothesize that elevated inflammatory markers reflect a pro-inflammatory tumor microenvironment, which is known to promote tumor progression [29]. Additionally, tumor metabolism, driven by hypoxia and oxidative stress, may further modulate the host immune response [30]. Notably, our study exclusively included patients with ovarian-confined disease, minimizing confounding factors related to metastatic dissemination. This strengthens the hypothesis that the observed inflammatory response is an intrinsic characteristic of tumor biology rather than a secondary effect of systemic disease spread. Therefore, the mechanisms underlying malignant transformation appear to drive distinct immune responses in benign neoplasms, BOTs, and OC. In line with this observation, SIRI was also shown to differ significantly between benign neoformations and BOTs. Given their potential role in the early diagnosis of ovarian neoplasms, we conducted a linear regression analysis to confirm the association between systemic inflammation indices and non-benign adnexal masses, encompassing both BOTs and OC. The need to accurately distinguish benign from non-benign adnexal masses stems from the different clinical management these conditions provide and the different prognostic impacts [4,12]. Interestingly, our findings revealed that SIRI was the only marker to exhibit a statistically significant association in differentiating benign from non-benign formations. Surprisingly, CA 125, the most widely used preoperative biomarker for ovarian neoplasms, did not show a similar correlation. Consistently, ROC curve analysis also identified SIRI as the best-performing index, while both inflammation-based indices (SIR and SIRI) outperformed CA 125, although the diagnostic performance levels shown remain perfectible. Furthermore, the optimal cut-off values derived for these markers demonstrated higher positive predictive value (PPV) and specificity than the conventional 39 IU/dL threshold of CA 125, which remains the most widely used positivity cut-off for this tumor marker.
4.2. Clinical Implication
The SIR and SIRI inflammatory indices have demonstrated greater diagnostic accuracy than CA 125 in distinguishing benign from non-benign adnexal formations (BOTs and OC). This finding suggests that these indices could serve as valuable diagnostic tools for gynecologic oncologists. Interestingly, CA 125 remains the most commonly requested biomarker for adnexal masses despite its questionable diagnostic performance in this field [31]. Moreover, its widespread use burdens healthcare systems financially [32]. In the United States, a CA 125 assay costs between USD 20 and USD 105, with an average price of USD 68.
In contrast, SIR and SIRI can be derived from a standard complete blood count (CBC), an inexpensive test already included in preoperative evaluations. This makes them highly accessible and practical for clinical implementation, potentially offering a more cost-effective approach to risk stratification in patients with adnexal masses. Other groups of scholars have shown that this method is largely reproducible and cost-effective. Preoperative NLR combined with CA 125 has been shown to be a simple and cost-effective approach for identifying ovarian cancer [33]. Similarly, platelet to lymphocyte ratio (PLR) is considered an accessible and low cost biomarker for diagnosis and staging, especially when used adjunctively with other diagnostic tools [34].
Beyond cost-effectiveness, these inflammatory indices provide broader clinical information than CA 125. Recent evidence has further supported this approach. In particular, Opławski et al. demonstrated that systemic inflammatory biomarkers, alongside molecular signatures, are associated with treatment response and prognosis in ovarian cancer, highlighting their relevance in biomarker-driven clinical decision-making [35].
It is important to note that this study was conducted under highly stringent conditions, excluding patients with pre-existing pro-inflammatory conditions to avoid potential confounding factors. Consequently, 46% of “intention-to-treat” patients were excluded for failing to meet inclusion criteria, which may limit the real-world applicability of these indices.
Furthermore, while the absolute diagnostic performance of these indices is not exceptional, with the highest AUC reaching only 70%, the primary objective of this study was to demonstrate that inflammatory indices increase as ovarian neoplasms progress, which was clearly confirmed.
From a clinical perspective, an important observation is the poor performance of CA 125, a biomarker that is frequently overused in daily practice despite its limited diagnostic reliability. Although CA 125 is often integrated with imaging techniques to develop diagnostic models, similar applications could extend to inflammatory indices if validated by further research.
Ultimately, SIR and, more notably, SIRI could serve as valuable complementary diagnostic tools for monitoring suspicious adnexal formations and improving the early detection of malignant transformation.
4.3. Comparison with Existing Literature
The interplay between systemic inflammation and tumor progression has been widely studied across various malignancies, with increasing evidence supporting the role of inflammatory biomarkers as potential diagnostic and prognostic tools [21,22,23]. Like many other solid tumors, OC triggers a complex immune response that can be reflected in systemic inflammatory indices such as SIRI and SIR. Our findings demonstrate a progressive elevation of these indices from benign to malignant ovarian lesions, which are consistent with broader oncological research highlighting the link between inflammation and tumor biology [36].
Previous studies have shown that systemic inflammatory markers are already elevated at the earliest stages of tumor development, likely due to the tumor’s ability to manipulate the host immune system [34]. This is particularly relevant in gynecologic malignancies. In endometrial cancer, for instance, our research group previously identified a similar pattern, where an increase in inflammatory indices accompanied the transition from atypical endometrial hyperplasia to carcinoma [37,38]. This aligns with the current understanding, suggesting that inflammation is both an early warning signal and a key player in tumor progression.
Recent research has further refined our understanding of the immune landscape in OC. Blanc-Durand et al. have demonstrated that in the initial phases of ovarian cancer, a robust immune response is mediated by natural killer (NK) cells and tumor-infiltrating lymphocytes [39]. However, as the disease progresses, the chronicity of inflammation fosters an immunosuppressive environment, facilitating tumor angiogenesis, metastasis, and immune evasion. This shift is thought to be mediated by increased production of cytokines such as IL-6 and TGF-β1, which are also implicated in the modulation of extracellular vesicles in OC [40]. These findings underscore the dynamic nature of the immune response in ovarian malignancies and provide a biological rationale for the observed variations in SIRI and SIR levels in our study.
The significance of systemic inflammation in BOTs and OC extends beyond diagnosis to prognosis. Several studies have demonstrated that elevated inflammatory indices correlate with poorer overall survival and shorter platinum-free intervals in OC patients [41,42]. While our study focused primarily on the diagnostic implications of SIRI and SIR, this broader body of evidence suggests that these indices could also serve as valuable prognostic markers. Systemic inflammation’s ability to predict malignancy and stratify patient risk highlights its potential as a multifaceted tool in oncologic decision-making.
Our results also contribute to the ongoing discussion about the limitations of CA 125 as a standalone biomarker for ovarian tumor diagnostics. Despite being the most widely used serum marker, CA 125 has well-documented limitations, particularly in differentiating malignant from benign ovarian conditions [43]. Our findings reinforce this notion, as CA 125 exhibited lower sensitivity and specificity than SIRI, suggesting that inflammation-based indices may provide additional discriminatory power when incorporated into diagnostic models.
From a clinical standpoint, these insights emphasize the need for a more integrated approach to ovarian tumor diagnostics.
In conclusion, our findings align with and expand upon the existing literature demonstrating the diagnostic and potential prognostic utility of systemic inflammatory markers in ovarian masses. By reaffirming the link between inflammation and malignancy, our study supports the growing recognition of inflammatory indices as promising tools for early detection and risk stratification in ovarian tumors.
4.4. Strengths and Limitations
Our study finds its strength in the strong statistical significance of the results, methodological rigor, and its prospective nature. In addition, this study is among the first to compare indices of systemic inflammation (SIR and SIRI) with CA 125 in the differential diagnosis of benign formations, BOTs and OC, offering new diagnostic perspectives. Moreover, the multicenter design ensures that the results are more generalizable than single-center studies. We minimized potential selection biases by including multiple institutions and increased our findings’ external validity. Additionally, the use of strict inclusion criteria allowed us to analyze a well-defined and homogeneous population, ensuring that the observed differences were genuinely related to ovarian tumor biology rather than confounding inflammatory conditions. However, it has limitations that cannot be avoided. One weakness is related to the very strictness of the inclusion criteria, which limits applicability in everyday clinical practice.
Additionally, while the sample size was statistically adequate, larger-scale studies are needed to confirm the reliability of these inflammatory indices across diverse patient populations. Another important consideration is that this study was conducted exclusively in Italy, which may limit the applicability of the findings to other geographic regions. Genetic, environmental, and healthcare system differences could influence inflammatory responses and diagnostic performance. The generalizability of the results to other geographic regions and healthcare systems should be considered with caution. Additionally, subgroup analyses may prove that BMI and comorbidities, such as metabolic syndrome and autoimmune disorders, may hypothetically impact systemic inflammatory indices and their diagnostic accuracy. Age and BMI were initially included as covariates in exploratory multivariable logistic regression models to control for potential confounding effects. However, these variables were not retained in the final models, as their inclusion did not improve diagnostic performance.
Similarly, transient and subclinical proinflammatory states can also potentially influence the results obtained. Further research is needed to determine whether these variables affect the robustness of SIRI and SIR as diagnostic tools in broader clinical settings, with multicenter collaborations that ensure maximum variability in the study population. Furthermore, our study focused on short-term diagnostic accuracy without evaluating the prognostic value of SIR and SIRI over time. A longitudinal follow-up would be necessary to determine whether these indices can also serve as prognostic markers for disease progression or treatment response.
Lastly, despite excluding patients with known chronic inflammatory diseases, transient inflammatory conditions (such as subclinical infections or physiological stress) could still have influenced SIR and SIRI values, introducing a potential confounding factor.
4.5. Future Perspectives
The results obtained from our study would benefit from validation with cohorts of patients more varied in ethnicity and underlying clinical conditions. In addition, the highlighted laboratory data could be explored in combination with other imaging data, such as MRI or data related to organ function, to expand the perspectives with which to approach the differential diagnosis of adnexal neoformations. In addition, the advancement of technologies and artificial intelligence could provide an opportunity to design synthesis studies that encapsulate all the individual pieces useful for improving diagnostic power.
5. Conclusions
Our study highlights the potential diagnostic role of systemic inflammatory indices (SIR and SIRI) in differentiating benign from non-benign adnexal masses (BOTs and OC). Compared to CA 125, these markers demonstrated higher specificity and diagnostic accuracy, making them valuable tools in preoperative assessment. The ability of SIRI to outperform CA 125 in distinguishing benign from non-benign conditions suggests that systemic inflammation plays a key role in tumor progression. Furthermore, SIR and SIRI are derived from a simple complete blood count (CBC), so they offer a cost-effective and widely accessible alternative to traditional biomarkers. However, our study was conducted under strict inclusion criteria, limiting its generalizability, and more extensive multicenter studies are required to validate these findings. Future research should explore the integration of inflammatory indices with imaging techniques to improve diagnostic models further. Only after further results have been obtained could these indices be useful in practice.
Author Contributions
C.R.: conceptualization, data curation, investigation, project administration, software, writing—original draft; writing—review and editing; S.R.: data curation; M.L.: data curation, validation; G.V.: validation; M.C.D.D.: investigation; G.C.: validation; M.C.S.: data curation; C.S.: validation; P.D.F.: supervision; validation; M.F.: data curation, software, investigation; S.C.: data curation, validation; V.C.: supervision, validation. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethical Committee of the University of Campania Luigi Vanvitelli (Approval No. 0013958/I, on 5 May 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data regarding any of the subjects in this study has not been previously published. Data will be made available to the editors of the journal pre- and/or post-publication for review or query upon request. This study used the STROBE statement for observational studies [26]. All data and the methodological process for their calculation can be supplied under explicit request to the corresponding author and provided as an ‘R’ file.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ADNEX | Assessment of Different NEoplasias in the adneXa |
| ART | Assisted Reproductive Technology |
| AUC | Area Under the Curve |
| BIC | Bayesian Information Criteria |
| BMI | Body Mass Index |
| BOT | Borderline Ovarian Tumor |
| CA 125 | Cancer Antigen 125 |
| CT | Computed Tomography |
| ECLIA | Electrochemiluminescence Immunoassay |
| FIGO | International Federation of Gynecology and Obstetrics |
| HGSOC | High-Grade Serous Ovarian Cancer |
| IOTA | International Ovarian Tumor Analysis |
| IRB | Institutional Review Board |
| LGSOC | Low-Grade Serous Ovarian Cancer |
| MRI | Magnetic Resonance Imaging |
| NK | Natural Killer (cells) |
| NPV | Negative Predictive Value |
| OC | Ovarian Carcinoma |
| PET | Positron Emission Tomography |
| PPV | Positive Predictive Value |
| ROC | Receiver Operating Characteristic |
| SIR | Systemic Inflammatory Response |
| SIRI | Systemic Inflammation Response Index |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
References
- Liberto, J.M.; Chen, S.Y.; Shih, I.M.; Wang, T.H.; Wang, T.L.; Pisanic, T.R., 2nd. Current and Emerging Methods for Ovarian Cancer Screening and Diagnostics: A Comprehensive Review. Cancers 2022, 14, 2885. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Erol, A.; Niemira, M.; Krętowski, A.J. Novel Approaches in Ovarian Cancer Research against Heterogeneity, Late Diagnosis, Drug Resistance, and Transcoelomic Metastases. Int. J. Mol. Sci. 2019, 20, 2649. [Google Scholar] [CrossRef]
- Menon, U.; Karpinskyj, C.; Gentry-Maharaj, A. Ovarian Cancer Prevention and Screening. Obstet. Gynecol. 2018, 131, 909–927. [Google Scholar] [CrossRef]
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef]
- Xiao, Y.; Bi, M.; Guo, H.; Li, M. Multi-omics approaches for biomarker discovery in early ovarian cancer diagnosis. EBioMedicine 2022, 79, 104001. [Google Scholar] [CrossRef]
- Karlsen, M.A.; Sandhu, N.; Høgdall, C.; Christensen, I.J.; Nedergaard, L.; Lundvall, L.; Engelholm, S.A.; Pedersen, A.T.; Hartwell, D.; Lydolph, M.; et al. Evaluation of HE4, CA125, risk of ovarian malignancy algorithm (ROMA) and risk of malignancy index (RMI) as diagnostic tools of epithelial ovarian cancer in patients with a pelvic mass. Gynecol. Oncol. 2012, 127, 379–383. [Google Scholar] [CrossRef]
- Van Calster, B.; Timmerman, D.; Bourne, T.; Testa, A.C.; Van Holsbeke, C.; Domali, E.; Jurkovic, D.; Neven, P.; Van Huffel, S.; Valentin, L. Discrimination between benign and malignant adnexal masses by specialist ultrasound examination versus serum CA-125. J. Natl. Cancer Inst. 2007, 99, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, D.; Ameye, L.; Fischerova, D.; Epstein, E.; Melis, G.B.; Guerriero, S.; Van Holsbeke, C.; Savelli, L.; Fruscio, R.; Lissoni, A.A.; et al. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: Prospective validation by IOTA group. BMJ 2010, 341, c6839. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, D.; Valentin, L.; Bourne, T.H.; Collins, W.P.; Verrelst, H.; Vergote, I.; International Ovarian Tumor Analysis (IOTA) Group. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: A consensus opinion from the International Ovarian Tumor Analysis (IOTA) Group. Ultrasound Obstet. Gynecol. 2000, 16, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Van Calster, B.; Van Hoorde, K.; Valentin, L.; Testa, A.C.; Fischerova, D.; Van Holsbeke, C.; Savelli, L.; Franchi, D.; Epstein, E.; Kaijser, J.; et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: Prospective multicentre diagnostic study. BMJ 2014, 349, g5920. [Google Scholar] [CrossRef]
- Thomassin-Naggara, I.; Poncelet, E.; Jalaguier-Coudray, A.; Guerra, A.; Fournier, L.S.; Stojanovic, S.; Millet, I.; Bharwani, N.; Juhan, V.; Cunha, T.M.; et al. Ovarian-Adnexal Reporting Data System Magnetic Resonance Imaging (O-RADS MRI) Score for Risk Stratification of Sonographically Indeterminate Adnexal Masses. JAMA Netw. Open 2020, 3, e1919896. [Google Scholar] [CrossRef]
- Rampes, S.; Choy, S.P. Early diagnosis of symptomatic ovarian cancer in primary care in the UK: Opportunities and challenges. Prim. Health Care Res. Dev. 2022, 23, e52. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, M.; Cheng, S.; Jin, Y.; Zhao, Y.; Wang, Y. Roles of CA125 in diagnosis, prediction, and oncogenesis of ovarian cancer. Biochimica et biophysica acta. Rev. Cancer 2021, 1875, 188503. [Google Scholar] [CrossRef]
- Weiland, F.; Martin, K.; Oehler, M.K.; Hoffmann, P. Deciphering the molecular nature of ovarian cancer biomarker CA125. Int. J. Mol. Sci. 2012, 13, 10568–10582. [Google Scholar] [CrossRef] [PubMed]
- Punzón-Jiménez, P.; Lago, V.; Domingo, S.; Simón, C.; Mas, A. Molecular Management of High-Grade Serous Ovarian Carcinoma. Int. J. Mol. Sci. 2022, 23, 13777. [Google Scholar] [CrossRef]
- Valentin, L.; Jurkovic, D.; Van Calster, B.; Testa, A.; Van Holsbeke, C.; Bourne, T.; Vergote, I.; Van Huffel, S.; Timmerman, D. Adding a single CA 125 measurement to ultrasound imaging performed by an experienced examiner does not improve preoperative discrimination between benign and malignant adnexal masses. Ultrasound Obstet. Gynecol. 2009, 34, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Patton, P.E.; Field, C.S.; Harms, R.W.; Coulam, C.B. CA-125 levels in endometriosis. Fertil. Steril. 1986, 45, 770–773. [Google Scholar] [CrossRef] [PubMed]
- Paavonen, J.; Miettinen, A.; Heinonen, P.K.; Aaran, R.K.; Teisala, K.; Aine, R.; Punnonen, R.; Laine, S.; Kallioniemi, O.P.; Lehtinen, M. Serum CA 125 in acute pelvic inflammatory disease. Br. J. Obstet. Gynaecol. 1989, 96, 574–579. [Google Scholar] [CrossRef]
- Iavarone, I.; Padovano, M.; Pasanisi, F.; Della Corte, L.; La Mantia, E.; Ronsini, C. Meigs Syndrome and Elevated CA-125: Case Report and Literature Review of an Unusual Presentation Mimicking Ovarian Cancer. Medicina 2023, 59, 1684. [Google Scholar] [CrossRef]
- Cheng, D.L.; Xu, H.; Lv, W.F.; Hua, R.; Du, H.; Zhang, Q.Q. The Significance of Serum CA-125 Elevation in Chinese Patients with Primary Budd-Chiari Syndrome: A Multicenter Study. Gastroenterol. Res. Pract. 2015, 2015, 121060. [Google Scholar] [CrossRef]
- Fest, J.; Ruiter, R.; Mulder, M.; Groot Koerkamp, B.; Ikram, M.A.; Stricker, B.H.; van Eijck, C.H.J. The systemic immune-inflammation index is associated with an increased risk of incident cancer-A population-based cohort study. Int. J. Cancer 2020, 146, 692–698. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meng, L.; Yang, Y.; Hu, X.; Zhang, R.; Li, X. Prognostic value of the pretreatment systemic immune-inflammation index in patients with prostate cancer: A systematic review and meta-analysis. J. Transl. Med. 2023, 21, 79. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Z.; Qian, L. Prognostic and clinicopathological significance of C-reactive protein in patients with ovarian cancer: A meta-analysis. World J. Surg. Oncol. 2024, 22, 8. [Google Scholar] [CrossRef]
- Ghobadi, H.; Mohammadshahi, J.; Javaheri, N.; Fouladi, N.; Mirzazadeh, Y.; Aslani, M.R. Role of leukocytes and systemic inflammation indexes (NLR, PLR, MLP, dNLR, NLPR, AISI, SIR-I, and SII) on admission predicts in-hospital mortality in non-elderly and elderly COVID-19 patients. Front. Med. 2022, 9, 916453. [Google Scholar] [CrossRef]
- Nezhat, C.; Cho, J.; King, L.P.; Hajhosseini, B.; Nezhat, F. Laparoscopic management of adnexal masses. Obstet. Gynecol. Clin. N. Am. 2011, 38, 663–676. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the reporting of observational studies in epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef]
- Prat, J.; FIGO Committee on Gynecologic Oncology. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynaecol. Obstet. 2014, 124, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bast, R.C.; Klug, T.L., Jr.; St John, E.; Jenison, E.; Niloff, J.M.; Lazarus, H.; Berkowitz, R.S.; Leavitt, T.; Griffiths, C.T.; Parker, L.; et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N. Engl. J. Med. 1983, 309, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Garlisi, B.; Lauks, S.; Aitken, C.; Ogilvie, L.M.; Lockington, C.; Petrik, D.; Eichhorn, J.S.; Petrik, J. The Complex Tumor Microenvironment in Ovarian Cancer: Therapeutic Challenges and Opportunities. Curr. Oncol. 2024, 31, 3826–3844. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lior, C.; Barki, D.; Halperin, C.; Iacobuzio-Donahue, C.A.; Kelsen, D.; Shouval, R.S. Mapping the tumor stress network reveals dynamic shifts in the stromal oxidative stress response. Cell Rep. 2024, 43, 114236. [Google Scholar] [CrossRef]
- Zhang, R.; Siu, M.K.Y.; Ngan, H.Y.S.; Chan, K.K.L. Molecular Biomarkers for the Early Detection of Ovarian Cancer. Int. J. Mol. Sci. 2022, 23, 12041. [Google Scholar] [CrossRef] [PubMed]
- Margoni, A.; Gargalionis, A.N.; Papavassiliou, A.G. CA-125:CA72-4 ratio—Towards a promising cost-effective tool in ovarian cancer diagnosis and monitoring of post-menopausal women under hormone treatment. J. Ovarian Res. 2024, 17, 164. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Cho, H.; Hur, H.W.; Kim, S.W.; Kim, S.H.; Kim, J.H.; Kim, Y.T.; Lee, K. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol. Immunother. CII 2009, 58, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Opławski, M.; Średnicka, A.; Niewiadomska, E.; Boroń, D.; Januszyk, P.; Grabarek, B.O. Clinical and molecular evaluation of patients with ovarian cancer in the context of drug resistance to chemotherapy. Front. Oncol. 2022, 12, 954008. [Google Scholar] [CrossRef]
- Bizon, M.; Olszewski, M.; Krason, B.; Kochanowicz, E.; Safiejko, K.; Borowka, A.; Sekita-Krzak, J.; Pruc, M.; Drozd, A.; Feduniw, S.; et al. The Diagnostic Role of the Platelet-to-Lymphocyte Ratio in Ovarian Cancer: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 1841. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Ronsini, C.; Iavarone, I.; Vastarella, M.G.; Della Corte, L.; Andreoli, G.; Bifulco, G.; Cobellis, L.; De Franciscis, P. SIR-EN-New Biomarker for Identifying Patients at Risk of Endometrial Carcinoma in Abnormal Uterine Bleeding at Menopause. Cancers 2024, 16, 3567. [Google Scholar] [CrossRef]
- Ronsini, C.; Iavarone, I.; Braca, E.; Vastarella, M.G.; Della Corte, L.; Vitale, C.; Andreoli, G.; La Mantia, E.; Cobellis, L.; de Franciscis, P. Deep Myometrial Infiltration leads to a Measurable Inflammatory Response in Endometrial Cancer. A Prospective Observational Study. Semin. Oncol. 2024, 51, 149–153. [Google Scholar] [CrossRef]
- Blanc-Durand, F.; Clemence Wei Xian, L.; Tan, D.S.P. Targeting the immune microenvironment for ovarian cancer therapy. Front. Immunol. 2023, 14, 1328651. [Google Scholar] [CrossRef]
- Lucidi, A.; Buca, D.; Ronsini, C.; Tinari, S.; Bologna, G.; Buca, D.; Leombroni, M.; Liberati, M.; D’Antonio, F.; Scambia, G.; et al. Role of Extracellular Vesicles in Epithelial Ovarian Cancer: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 8762. [Google Scholar] [CrossRef] [PubMed]
- Dinca, A.L.; Diaconu, A.; Birla, R.D.; Coculescu, B.I.; Dinca, V.G.; Manole, G.; Marica, C.; Tudorache, I.S.; Panaitescu, E.; Constantinoiu, S.M.; et al. Systemic inflammation factors as survival prognosis markers in ovarian neoplasm and the relationship with cancer-associated inflammatory mediators-a review. Int. J. Immunopathol. Pharmacol. 2023, 37, 3946320231178769. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yin, S.; Chen, K. Predictive value of the systemic immune-inflammation index for the efficacy of neoadjuvant chemotherapy and prognosis in patients with stage III ovarian cancer-a retrospective cohort study. Gland Surg. 2022, 11, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).