Abstract
Actinic keratosis (AK) is considered the early phase of a squamous cell carcinoma (SCC) and represents one of the most common epithelial skin lesions, with an estimated global prevalence of approximately 14%. An estimated annual risk of progression has been reported with a range from 0 to 0.53%. Although spontaneous regression of individual AK lesions has been described in approximately 23% of cases, the frequent presence of multiple lesions, usually in the broader context of field cancerization, significantly diminishes the likelihood of regression and contributes to a higher cumulative risk of progression to SCC. The aim of the present narrative review was to provide an overview of the current evidence of the most effective available lesion-directed and field-directed treatments for actinic keratoses, on the personalized, combined, or sequential approach, as well as on the emerging therapeutic options.
1. Introduction
Actinic keratosis (AK) is considered the early phase of a cutaneous squamous cell carcinoma (cSCC) and represents one of the most common epithelial skin lesions, with an estimated global prevalence of approximately 14% [1].
Risk factors for AK development include age >45 years, male gender, a history of non-melanoma skin cancer, outdoor work for more than 6 h a day, skin type I/II, solar lentigines, immunosuppression, use of tanning beds, baldness, use of photosensitizing drugs in chronic therapies, alcohol consumption and immunosuppression [2,3]. In particular, solid organ transplant recipients treated with chronic immunosuppressive therapy have an 80% lifetime risk of developing AK and a 30% risk of having at least five AKs [4].
Clinically, AK presents as an erythematous papule or plaque, scaly, usually <1 mm thick, located in sun-exposed areas; it can be asymptomatic, pruritic, or occasionally tender to palpation [5]. AKs are frequently classified according to the Olsen grading system, wherein grade I lesions are characterized by minimal hyperkeratosis, being more readily appreciable by tactile examination than visual inspection; grade II lesions exhibit moderate hyperkeratosis with both clear visual demarcation and palpable thickening; and grade III lesions demonstrate pronounced hyperkeratosis, often with a verruciform or markedly indurated surface architecture [2,5]. Diagnosis is essentially clinical and dermoscopic and may be aided by noninvasive diagnostic techniques, such as confocal microscopy and line-field optical coherence tomography (LC-OCT), which have also been applied to monitor treatment efficacy of Aks [6,7,8]. Histological confirmation is required only if progression to invasive cSCC is suspected.
An estimated annual risk of progression has been reported with a range from 0 to 0.53% [2,9]. Although spontaneous regression of individual AK lesions has been described in approximately 23% of cases [10], the frequent presence of multiple lesions, usually in the broader context of field cancerization, significantly diminishes the likelihood of regression and contributes to a higher cumulative risk of progression to cSCC [9,11].
These areas represent a subclinical reservoir of atypical keratinocytes with molecular alterations similar to those found in overt lesions, underscoring the need for therapeutic strategies that address not only clinically visible AKs but also the surrounding UV-damaged field to reduce the risk of malignant transformation.
The aim of the present narrative review was to provide an overview of the current evidence on the most effective available treatments for actinic keratoses and emerging therapeutic options.
2. Materials and Methods
This narrative review provides state-of-the-art information on AK treatment, incorporating the best available evidence up to 1 April 2025. Primary sources were sought in the PubMed, Scopus, and Cochrane Library databases. Google Scholar was consulted to broaden the search. Search terms included “actinic keratosis” and “field cancerization” along with keywords related to various therapies and treatment techniques (e.g., cryotherapy, cryosurgery, 5-fluorouracil (5-FU), imiquimod, tirbanibulin). The review includes randomized controlled trials (RCTs), scientific society guidelines, interventional studies, and expert consensus statements. Only papers published in English were considered. Case reports and studies that do not meet quality criteria, defined as lacking peer review, including very small or heterogeneous patient cohorts, or failing to provide clear methodological details or clinically relevant outcomes, were excluded. The inclusion of English-language studies only and the lack of systematic cost-effectiveness analyses may limit the applicability of the findings for clinical decision-making.
3. Relevant Sections
3.1. General Considerations
The management of AKs encompasses two primary therapeutic strategies: lesion-directed and field-directed approaches [2]. Lesion-directed treatments are designed to target individual, clinically visible lesions. However, the majority of AKs develop within areas of chronic sun damage, referred to as field cancerization. These fields represent zones of photodamage and widespread genetic alterations where numerous atypical keratinocytes coexist, along with both clinically apparent and subclinical lesions. While field-directed therapies are often associated with greater local skin reactions and may be less well tolerated by patients, they are increasingly recognized as essential due to the potential for cSCC to develop from subclinical lesions.
Therapeutic modalities can further be categorized based on the setting of administration. ‘In-office’ treatments are performed under the supervision of a healthcare provider, allowing for real-time adjustment of the procedure based on patient’s response and tolerability. Examples include cryotherapy, photodynamic therapy (PDT), and laser therapy. On the other hand, ‘at-home’ treatments are self-administered by patients according to a prescribed regimen, and include topical agents such as 5-FU, imiquimod, diclofenac and tirbanibulin. While offering convenience and cost-effectiveness, at-home therapies require adherence and proper patient education to ensure efficacy and minimize adverse effects.
Importantly, therapeutic choice also varies with clinical grade: most topical agents (Imiquimod and 5-FU) are licensed for non-hyperkeratotic/non-hypertrophic lesions (Olsen grades I–II), and tirbanibulin is EU-approved specifically for Olsen grade I on the face/scalp [2]; by contrast, hyperkeratotic (Olsen grade III) lesions are generally managed with lesion-directed procedures (curettage and/or cryosurgery) and, when appropriate, sequential regimens combining initial debulking with subsequent field-directed therapy [2].
3.2. Lesion-Directed Treatments
3.2.1. Cryosurgery
Cryosurgery involves the application of a cryogenic agent to induce targeted tissue destruction through multiple mechanisms [12]. The primary effect is the rapid freezing of intracellular and extracellular fluids, which disrupts cellular membranes and results in mechanical cell rupture. This process triggers the release of intracellular components and pro-inflammatory cytokines, leading to localized immune activation. In addition, cryo-induced vascular stasis, including thrombosis and microthrombosis, causes ischemic necrosis, further contributing to tissue destruction. Being a destructive technique, cryosurgery prevents histopathological analysis of lesions and should be avoided in cases of uncertain diagnosis.
The most widely available and commonly used cryogenic agent is liquid nitrogen (LN), which can be delivered through various methods. The most common involves application with an LN-soaked cotton swab or a pressurized container that releases a continuous LN spray.
Application techniques reported in the literature vary considerably. One protocol suggests continuous applications of LN cryospray lasting 5–10 s, until complete freezing is achieved [13]. The procedure uses an 18-gauge spray tip held 1–2 cm from the lesion at an angle of 90° to the skin. Treatment is repeated after 120 days. The authors report a 63% and 54% reduction in AK lesions on the face and forearms, respectively. However, this was associated with a high incidence of mild complications such as pain, formation of epidermal blisters, local superinfection, and residual hypopigmentation (58%).
Comparison of the effects of one application cycle versus two consecutive cycles to the same lesions found a high lesion clearance rate after a single cycle (≃80%), without significant differences in the presence of cytological atypia, hyperkeratosis, dyskeratosis, dermal fibrosis, and inflammatory cells in the dermis as assessed by confocal microscopy [14].
Treatment with an LN-soaked cotton swab is both cost-effective and widely available. According to some authors, the commonly used protocol involves direct contact of the swab with the lesion for 10–20 s, until a peripheral frozen rim of 1–2 mm is achieved [15]. At 90 days, a complete response rate of ≃71% and a good-to-excellent cosmetic outcome of ≃81% of cases have been described.
Although LN cryosurgery is a lesion-oriented technique, some authors suggest a broader application of LN to cover the entire field cancerization (or cryopeeling). However, as of today, standardized protocols and robust data on its efficacy and safety are still lacking [16,17].
3.2.2. Ablative Lasers
The use of ablative lasers, most commonly CO2 lasers and erbium:YAG lasers, is a highly operator-dependent procedure that is also affected by the lack of standardized protocols for AK treatment. In a small case series, facial resurfacing with a CO2 laser resulted in a 92% reduction in AKs at 3 months, without adverse events [18]. However, in a larger sample size the results were less favorable: at 3 months, CO2 laser ablation achieved complete clearance of ≃65% of lesions, but recurrence rates at 12 months were ≃78%, substantially inferior to cryosurgery albeit with similar tolerability [15].
In one RCT comparing 5-FU with laser resurfacing, a protocol involving epidermal laser ablation of the entire scalp achieved a clearance rate of ≃94% at 6 months and ≃90% at 12 months [19]. Though superior to the 5-FU outcome, this result was however associated with frequent and significant adverse effects, including erythema, edema, crusting and infection, despite prophylactic antibiotic therapy.
Ablative laser devices may also be combined to Methyl aminolevulinate (MAL) or Aminolevulinic acid (ALA)-PDT as a lesion or field-targeted pre-treatment by (laser-assisted PDT) [20]. An ablative fractional laser has direct cytotoxic effect and creates microscopic vertical channels that may facilitate the penetration and enrichment of ALA or MAL in dysplastic cells, a concept that has been termed “laser-assisted drug delivery”.
In a randomized split-scalp study, 56 patients were treated with ablative fractional CO2 laser using a protocol involving 19 W power, 1000 µm spacing, triple stacking, and a scan time of 1800 µs, followed by PDT with MAL [21]. At 6 months, the combination with laser pretreatment showed better outcomes in terms of complete clearance, partial clearance, and lesion-specific clearance rates than PDT alone, as well as a comparable tolerability. These findings are supported by other clinical studies [22,23,24].
3.3. Surgery
AKs do not generally require excisional biopsy; however, current guidelines recommend histopathological examination when the diagnosis is uncertain or in cases of treatment resistance, particularly in high-risk areas with an increased likelihood of progression to cSCC, such as the lips, ears, dorsal hands, and lower limbs [2]. In such cases, scalpel excision is recommended to obtain histopathological confirmation and to accurately define the surgical margins.
Curettage is primarily used in combination with other treatments, particularly PDT. The authors question the necessity of curettage as a pre-treatment for PDT, suggesting that PDT may be effective even without it [25,26,27].
3.4. Field-Directed Treatments
Photodynamic Therapy
PDT is based on photochemical reactions, where activation of a photosensitizing molecule by light leads to the generation of reactive oxygen species (ROS) through energy transfer to oxygen [28]. The ROS induce cellular damage, ultimately resulting in the destruction of targeted cells.
Currently, the most commonly used photosensitizing molecules are 5-aminolevulinic acid (5-ALA) and MAL [29,30]. These compounds, derivatives of ALA, are metabolized into protoporphyrin IX (PpIX), an intermediate in hemoglobin biosynthesis that also has photosensitizing properties. Exogenous application of 5-ALA and MAL induces intracellular accumulation of PpIX, which, upon exposure to a light source, exerts its photosensitizing effect. Neoplastic cells are particularly susceptible to this mechanism due to their metabolic switch, which leads to increased and uncontrolled hemoglobin production as well as enhanced enzyme activity, which sustains the process. MAL, the agent used most frequently in Europe, is available in a cream formulation, typically applied under occlusion to the target area, and is converted to PpIX. Additionally, the disruption of the epithelial barrier characteristic of AKs facilitates vehicle penetration. The other essential component of the photodynamic reaction is light irradiation. This can be achieved through exposure of the treatment area either to natural sunlight, i.e., daylight PDT (dlPDT), or to a predefined-wavelength light source in a clinical setting, i.e., conventional PDT (cPDT) [29,30]. PpIX exhibits multiple absorption peaks in the visible spectrum, leading to photoexcitation and ROS generation. An effective activation range is between 400 and 430 nm, in the blue-violet spectrum, which can be achieved using a blue light source [31]. However, use of this protocol is limited by its shallow penetration depth. In clinical practice cPDT is more performed commonly, using a lamp emitting red light at a wavelength of 630–635 nm, which provides greater tissue penetration.
In practical terms, MAL-cPDT requires cleansing of the treatment area followed by the application of MAL cream under occlusion, which must remain in place for approximately 3 h. Before red light exposure, the cream is gently removed from the treated area and irradiation at a power of 37 J/cm2 is performed for about 8 min [32].
The duration of irradiation has recently been questioned by a split-face trial comparing irradiation times of 8 and 4 min. Besides slightly greater pain with the longer irradiation during exposure, the study found comparable efficacy (70% vs. 65%, respectively) and similar adverse event rates at 6 months [33].
Alternative protocols of total light dose and fluence rate were evaluated in a prospective randomized trial, in which Olsen grade I and II AKs were treated with cPDT using BF-200 ALA [34]. The study demonstrated that protocols employing either a halved total dose and/or a reduced fluence rate achieved comparable overall clearance rates at 3 months post-treatment, while significantly reducing both mean and maximum pain intensity in the reduced fluence rate groups, and lowering phototoxicity scores across all modified protocols [34].
Reports of the efficacy of MAL-cPDT vary (AK clearance: 37.1–94.6%) [35,36,37]. Although cPDT is widely available, the requirement for patients to remain in the clinical setting for at least 3 h and the pain experienced during the procedure, particularly when treating AKs, have been reported to limit its applicability and to lead to a preference for dlPDT [31].
Treatment with dlPDT can be initiated soon after MAL application [38]. After cleansing the treatment area and applying a chemical sunscreen, MAL is applied without occlusion. Light exposure should begin within 30 min of photosensitizer application [38]. The shorter the interval between MAL application and light exposure, the lower the perceived pain. Sunlight exposure duration must be at least 2 h: a shorter duration may be ineffective, while a longer duration may increase the incidence of adverse events. A key limitation of dlPDT is the need for optimal weather conditions, as it requires clear days ensuring uninterrupted sunlight exposure while should not be performed during rain [38].
RCTs comparing the efficacy and tolerability of dlPDT and cPDT are few. According to a recent RCT, efficacy was substantially similar (90.0% and 94.6% AK reduction, respectively, 6 months post-treatment) although cPDT was associated with significantly higher pain scores [37]. Similarly, another RCT has reported a comparable efficacy at 3 months (89.2% vs. 92.8%, respectively) and overall higher patient satisfaction, with lower pain for dlPDT [39]. An Italian RCT found a lack of significant difference in efficacy for grade I AKs at 3 months, whereas dlPDT was less effective than cPDT for grade II and III Aks [40]. Nevertheless, the vast majority of patients (88%) still preferred dlPDT. Given the efficacy and tolerability of dlPDT, various indoor systems have been developed to replicate and standardize daylight illumination conditions in an indoor setting. These approaches are collectively referred to as simulated dlPDT (sdlPDT) and include the use of operating rooms or specific devices [41,42]. SdlPDT has shown high efficacy, with direct lesion clearance rates of approximately 85% at 3 months after treatment [42].
A formulation known as BF-200 ALA gel, incorporating a nanoemulsion of 7.8% ALA that enhances tissue penetration, was recently evaluated in a multicenter trial as a field-directed therapy for mild-to-moderate AKs [43]. Following treatment, approximately 91% of patients achieved complete clearance, with 75% and 63% of these maintaining a complete response at 6 and 12 months of follow-up, respectively [43].
3.5. Tirbanibulin Ointment
Tirbanibulin 1% ointment is a topical treatment for grade I AKs on the face and scalp, with a recommended application area of up to 25 cm2 [44]. Tirbanibulin acts as an antiproliferative agent by binding to intracellular tubulin and inhibiting its polymerization, thereby disrupting cytoskeletal formation during mitosis [45]. The treatment regimen consists of once-daily application for five consecutive days. Adverse events are predominantly mild, with erythema reported in 91% and scaling in 82% of patients [46].
A notable limitation of tirbanibulin is the fact that is approved for areas up to 25 cm2. However, a recent multicenter, single-arm study evaluated the safety of extending the application area up to 100 cm2 [47]. As with earlier trials, hyperkeratotic and hypertrophic AKs, as well as lesions on the lips, eyelids, and ears, were excluded. Most adverse events were mild to moderate, with severe erythema and severe scaling reported in 5.8% and 8.7% of cases, respectively. Adverse reactions peaked between days 5 and 8 and resolved within two months.
In a single-center trial, tirbanibulin was shown to improve multiple parameters of actinic damage, including facial dyschromia and erythema, suggesting its potential future application for esthetic purposes and overall skin quality enhancement [48].
3.6. 5-Fluorouracil
5-FU is a cytostatic antimetabolite structurally similar to thymine. It interferes with thymine utilization, disrupting DNA and RNA synthesis and thereby inhibiting cellular replication [49]. The drug is contraindicated during pregnancy and breastfeeding [50]. Concurrent use with certain antiviral agents such as brivudine and sorivudine should be avoided due to the risk of elevated plasma 5-FU levels from enzymatic competition [51].
Available formulations include 5% cream (with or without 0.005% calcipotriol), 4% cream, and 0.5% cream (with or without 10% salicylic acid). The 4% aqueous cream in peanut oil is approved for grade I and II AKs on the face, ears, and scalp in adults, without surface area limitations. Due to peanut oil content, it is contraindicated in patients with peanut or soy allergies [46].
An RCT comparing 4% and 5% formulations found that daily application for 4 weeks resulted in 80% complete lesion clearance and 100% partial clearance with both concentrations [52]. Skin irritation occurred in 30% of patients, with the 4% formulation demonstrating better tolerability. Real-world studies confirmed similar efficacy, reporting complete clearance in 74.5% of AKs and no recurrences at 6 months in 65% of cases [53]. The most common side effects included moderate erythema (51%) and a burning sensation (moderate in 22%, severe in 1%), both resolving by follow-up [53].
Although adverse events may lead some patients to discontinue treatment with 4% 5-FU prematurely, a dose-ranging study reported high clearance rates of AKs even in patients treated with once-daily applications for two weeks, with improved tolerability [54].
Another RCT comparing 5% 5-FU applied twice daily for 4 weeks to imiquimod (IMIQ), MAL-PDT, and ingenol mebutate showed a therapeutic success rate of 74.7% at 12 months [55]. However, 91% of patients experienced adverse effects, including moderate-to-severe erythema (81.5%), vesicles/blisters (24%), severe pain (16%), burning (21%), and pruritus (61%) [55]. Two weeks post-treatment, moderate-to-severe erythema persisted in 58%, blisters in 21%, and severe pain in 7% of patients.
A 5% 5-FU and 10% salicylic acid (5-FU/SA) formulation is marketed for lesion-directed therapy of grade I and II AKs on the face, scalp, neck, and extremities in immunocompetent adults. Treatment is limited to a maximum of 10 lesions and 25 cm2. Applied once daily for up to 12 weeks, it demonstrated 49.5% complete and 69.5% partial clearance at 8 weeks in a phase III trial [56]. Adverse events, though slightly more common than placebo (99% vs. 83%), were mostly mild, including erythema (88.9%), mild pain (69.4%), and irritation (59.3%). Systemic effects such as nasopharyngitis (11.1%) and headache (3.7%) were also reported [56].
Calcipotriol, a vitamin D analog, has recently been tested in combination with 0.5% 5-FU [57]. This formulation may enhance immune-mediated clearance of AKs by promoting thymic stromal lymphopoietin expression and CD4+ T-cell activation [57].
Another combination protocol was evaluated in a single-center, split-face study including AKs of all grades. The regimen consisted of 4% 5-FU applied twice daily for 7 days, followed by a session of dlPDT [58]. This approach demonstrated a slightly higher efficacy compared to dlPDT alone at 12 months, with complete response rates of 79.2% versus 70.4% [58].
3.7. Imiquimod
Imiquimod is an immunomodulatory drug that binds to and activates toll-like receptors (TLRs), particularly TLR7, on antigen-presenting cells. This interaction triggers the production of nuclear factor kappa-B and promotes the secretion of pro-inflammatory cytokines, such as tumor necrosis factor-α and interferon-α. Overall, this mechanism enhances cell-mediated antineoplastic activity at the site of application [59].
The most commonly used cream formulations are currently the 5% and 3.75% IMIQ [2]. The 5% cream has been approved to treat AKs on the face and scalp on a maximum surface area of 25 cm2 [2].
A randomized study comparing 5% 5-FU, cryotherapy, and 5% IMIQ (applied 3 times weekly for 4 weeks) found a complete clearance rate of 73% for imiquimod-treated lesions, which was sustained at 12 months. This outcome was superior to both comparator arms and was associated with better esthetic results [60]. In an RCT comparing 5% imiquimod, 5% 5-FU, MAL-PDT, and ingenol mebutate, the therapeutic success rate of the IMIQ formulation was 71%, higher than that of MAL-PDT and ingenol mebutate but lower than the one of 5-FU [55]. Adverse events occurred in 85% of cases, mainly consisting of moderate-to-severe erythema (72%), moderate-to-severe crusting (68%), and moderate-to-severe pruritus (62%), which mostly resolved within 2 weeks.
The 3.75% IMIQ cream is approved for the treatment of AKs of the face and scalp over a surface area of up to 200 cm2, with a regimen of daily application for 2 weeks followed by a 2-week treatment-free interval and by another 2-week treatment period [2]. Its complete lesion clearance rate was 34.0–35.6% at 8 weeks [61,62]. Adverse events were mainly related to local reactions, including pruritus (9.3%) and pain (9.3%). Systemic adverse events were also reported, such as flu-like syndrome (8.0%), headache (4.9%), and fatigue (4.9%) [61].
3.8. Other Topical Therapies
Diclofenac sodium 3% in 2.5% hyaluronic acid gel is approved for the treatment of AKs and field cancerization on face and scalp areas up to 200 cm2 and is applied twice daily for 60 to 90 days [2]. Its mechanism of action involves the inhibition of cyclo-oxygenase-2 and the induction of cell apoptosis, likely mediated by caspase activation and the fragmentation of intracellular nucleic acids [63].
Its efficacy and safety have been investigated in several clinical trials. In one comparative trial with ingenol mebutate, the endpoint of complete clearance at the end of treatment was achieved in 23.5% of patients. Adverse events were mild and local, including erythema (11.5%), scaling (4.7%), and pain (3.4%) [64].
In one RCT, 3% diclofenac sodium plus hyaluranon gel was compared with 5% imiquimod cream using the Total Thickness Score to assess the clinical severity of AKs based on palpation and visual inspection. Significant differences between the treatments emerged only at 24-week follow-up, with complete clearance rates of 14.3% and 45%, respectively [65]. Despite the advantage of being applicable to large skin areas, treatment with 3% diclofenac gel appears to have a relatively limited efficacy and is generally not recommended as first-line field therapy; it may be considered when an excellent tolerability profile is prioritized over clearance rates.
Also, topical retinoids (e.g., tretinoin, adapalene, tazarotene) have been studied for AK, given their role in modulating keratinocyte proliferation and differentiation [66]. However, evidence for topical is heterogeneous and mostly older; small, controlled trials reported modest AK count reductions but with frequent irritation, and contemporary guidelines do not recommend topical retinoids as standard AK treatment, because of inconsistent data on efficacy compared with approved field therapies [2,66].
3.9. Emerging Therapies
Given the need for a highly individualized approach in managing AKs and the considerable variability in indications among existing treatments, novel active compounds are being investigated to broaden the therapeutic options.
An ongoing multicenter, randomized, open-label phase 2 study is evaluating the safety and efficacy of Bimiralisib 2% gel, a pan-PI3K/mTOR inhibitor, for the treatment of AKs on the face, scalp, and/or back of the hands [67]. The product is applied to target lesions for either 2 or 4 weeks. Results are expected later this year.
Resiquimod elicits its effect by binding to and activating TLR7/8, which are primarily expressed on immune cells such as dendritic cells, macrophages, and B lymphocytes [68,69]. Their activation triggers the nuclear translocation of the transcription factor NF-κB and the induction of other transcriptional pathways, leading to an increased production of cytokines, particularly interferon-α, and promoting Th1-type immune responses. Resiquimod gel has been evaluated in a prospective, randomized, partly placebo-controlled, double-blind phase II dose-finding study to assess its safety, tolerability, and efficacy in patients with multiple Aks [69]. However, head-to-head comparisons with other topical therapies have not yet been published.
Colchicine (COL) is a natural alkaloid extracted from the corm of Colchicum autumnale. Its mechanism of action is based on the inhibition of mitotic spindle microtubule polymerization during cell division. In a randomized, open-label, self-controlled clinical trial, 0.5% COL cream or 5% 5-FU cream was applied on forearm skin twice daily for 7 days [70]. After 14 days, complete and partial remission were recorded in 17% and 78% of patients treated with COL, respectively, although adverse events (such as erythema, desquamation, and crusting) were more intense than those reported in the 5-FU group.
4. Conclusions
In conclusion, a wide range of effective therapies are available for managing AKs, each offering distinct advantages and limitations in terms of efficacy, tolerability, and practicality. A practical proposal for a therapeutic algorithm for AKs is illustrated in Figure 1. Achieving optimal outcomes requires a personalized, patient-centered approach that considers lesion grade, extent of field cancerization, patient preferences, local adverse reactions, treatment cost and duration, comorbidities, lifestyle factors, and individual tolerability. Tailoring therapy to the unique needs of each patient not only improves adherence but also enhances clinical outcomes and supports long-term disease control and prevention [71,72].
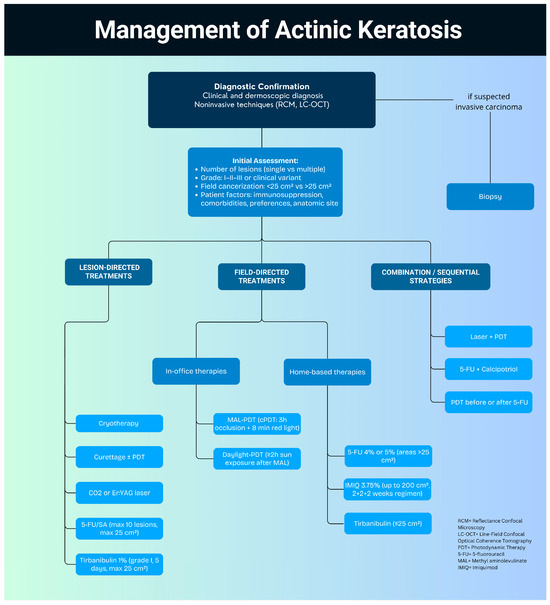
Figure 1.
Proposed algorithm for the management of actinic keratosis.
Emerging evidence highlights the value of multimodal strategies, such as combination or sequential therapies, that integrate different treatment modalities for synergistic benefit [73]. Examples include laser-assisted PDT, the novel 5-FU/calcipotriol combination, or a practical sequential approach combining a physical treatment for single hyperkeratotic lesions (i.e., cryotherapy or ablative CO2 laser) followed one month later by a field cancer treatment. A very recent randomized intraindividual comparative clinical trial [74] compared the efficacy and safety of sequential treatment with cryotherapy followed by 1% topical tirbanibulin versus cryotherapy monotherapy, showing an improved lesion clearance and a reduction in new AK development for the sequential cryotherapy and tirbanibulin approach.
When adapted to individual patient profiles, the sequential combined approaches can boost efficacy, broaden treatment scope, and help overcome the limitations of monotherapy [75].
Nevertheless, to date, relatively few studies have investigated or established standardized protocols for personalized, combination, and rotational treatment strategies of AK, particularly for patients with extensive field cancerization [75]. Ultimately, the future of AK and field cancerization management lies in dynamic, adaptive strategies that integrate diverse therapeutic options, precisely tailored to each patient’s unique clinical presentation and personal needs, ensuring sustained efficacy and enhanced quality of life.
Author Contributions
Conceptualization, A.P. and E.B.; methodology, E.B.; software, D.O.T.; validation, A.P., E.B. and G.G.; formal analysis, E.B.; investigation, E.B. and M.M.; resources, A.D. and D.O.T.; data curation, E.B. and D.O.T.; writing—original draft preparation, A.P. and E.B.; writing—review and editing, K.P., A.D., D.O.T., M.M. and G.G.; visualization, D.O.T.; supervision, A.P. and K.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- George, C.D.; Lee, T.; Hollestein, L.M.; Asgari, M.M.; Nijsten, T. Global epidemiology of actinic keratosis in the general population: A systematic review and meta-analysis. Br. J. Dermatol. 2024, 190, 465–476. [Google Scholar] [CrossRef]
- Kandolf, L.; Peris, K.; Malvehy, J.; Mosterd, K.; Heppt, M.V.; Fargnoli, M.C.; Berking, C.; Arenberger, P.; Bylaite-Bučinskiene, M.; Del Marmol, V.; et al. European consensus-based interdisciplinary guideline for diagnosis, treatment and prevention of actinic keratoses, epithelial UV-induced dysplasia and field cancerization on behalf of European Association of Dermato-Oncology, European Dermatology Forum, European Academy of Dermatology and Venereology and Union of Medical Specialists (Union Européenne des Médecins Spécialistes). J. Eur. Acad. Dermatol. Venereol. 2024, 38, 1024–1047. [Google Scholar]
- Fargnoli, M.C.; Altomare, G.; Benati, E.; Borgia, F.; Broganelli, P.; Carbone, A.; Chimenti, S.; Donato, S.; Girolomoni, G.; Micali, G.; et al. Prevalence and risk factors of actinic keratosis in patients attending Italian dermatology clinics. Eur. J. Dermatol. 2017, 27, 599–608. [Google Scholar] [CrossRef]
- Thamm, J.R.; Schuh, S.; Welzel, J. Epidemiology and Risk Factors of Actinic Keratosis. What is New for The Management for Sun-Damaged Skin. Dermatol. Pract. Concept. 2024, 14 (Suppl. S1), e2024146S. [Google Scholar]
- Conforti, C.; Ambrosio, L.; Retrosi, C.; Cantisani, C.; Di Lella, G.; Fania, L.; Rotunno, R.; Zalaudek, I.; Pellacani, G. Clinical and Dermoscopic Diagnosis of Actinic Keratosis. Dermatol. Pract. Concept. 2024, 14 (Suppl. S1), e2024147S. [Google Scholar] [CrossRef]
- Suppa, M.; Palmisano, G.; Tognetti, L.; Lenoir, C.; Cappilli, S.; Fontaine, M.; Orte Cano, C.; Diet, G.; Perez-Anker, J.; Schuh, S.; et al. Line-field confocal optical coherence tomography in melanocytic and non-melanocytic skin tumors. Ital. J. Dermatol. Venerol. 2023, 158, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Pellacani, G.; Longo, C. Reflectance confocal microscopy: A crucial role for actinic keratosis treatment monitoring. J. Eur. Acad. Dermatolo Venereol. 2018, 32, 1055. [Google Scholar] [CrossRef] [PubMed]
- Ertop Doğan, P.; Akay, B.N.; Okçu Heper, A.; Rosendahl, C.; Erdem, C. Dermatoscopic findings and dermatopathological correlates in clinical variants of actinic keratosis, Bowen’s disease, keratoacanthoma, and squamous cell carcinoma. Dermatol. Ther. 2021, 34, e14877. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Figueras, M.T. From actinic keratosis to squamous cell carcinoma: Pathophysiology revisited. J. Eur. Acad. Dermatol. Venereol. 2017, 31 (Suppl. S2), 5–7. [Google Scholar] [CrossRef]
- Steeb, T.; Petzold, A.; Hornung, A.; Wessely, A.; Berking, C.; Heppt, M.V. Spontaneous regression rates of actinic keratosis: A systematic review and pooled analysis of randomized controlled trials. Sci. Rep. 2022, 12, 5884. [Google Scholar] [CrossRef]
- Figueras Nart, I.; Cerio, R.; Dirschka, T.; Dréno, B.; Lear, J.T.; Pellacani, G.; Peris, K.; Ruiz de Casas, A.; Progressing Evidence in AK (PEAK) Working Group. Defining the actinic keratosis field: A literature review and discussion. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 544–563. [Google Scholar] [CrossRef]
- Arisi, M.; Guasco Pisani, E.; Calzavara-Pinton, P.; Zane, C. Cryotherapy for Actinic Keratosis: Basic Principles and Literature Review. Clin. Cosmet. Investig. Dermatol. 2022, 15, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Ianhez, M.; Miot, H.A.; Bagatin, E. Liquid nitrogen for the treatment of actinic keratosis: A longitudinal assessment. Cryobiology 2014, 69, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Mota, A.N.C.M.; De Carvalho, N.; Pellacani, G.; de Faria, P.C.P.; Melo, D.F.; Pineiro-Maceira, J.M.; Barcaui, C.B. Reflectance confocal microscopy in actinic keratosis-Comparison of efficacy between cryotherapy protocols. Skin Res. Technol. 2020, 26, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Zane, C.; Facchinetti, E.; Rossi, M.; Specchia, C.; Ortel, B.; Calzavara-Pinton, P. Cryotherapy is preferable to ablative CO2 laser for the treatment of isolated actinic keratoses of the face and scalp: A randomized clinical trial. Br. J. Dermatol. 2014, 170, 1114–1121. [Google Scholar] [CrossRef]
- Deonizio, J.; Werner, B.; Mulinari-Brenner, F.A. Histological comparison of two cryopeeling methods for photodamaged skin. ISRN Dermatol. 2014, 2014, 950754. [Google Scholar] [CrossRef]
- Chiarello, S.E. Cryopeeling (extensive cryosurgery) for treatment of actinic keratoses: An update and comparison. Dermatol. Surg. 2000, 26, 728–732. [Google Scholar] [CrossRef]
- Hantash, B.M.; Stewart, D.B.; Cooper, Z.A.; Rehmus, W.E.; Koch, R.J.; Swetter, S.M. Facial resurfacing for nonmelanoma skin cancer prophylaxis. Arch. Dermatol. 2006, 142, 976–982. [Google Scholar] [CrossRef]
- Ostertag, J.U.; Quaedvlieg, P.J.; van der Geer, S.; Nelemans, P.; Christianen, M.E.; Neumann, M.H.; Krekels, G.A. A clinical comparison and long-term follow-up of topical 5-fluorouracil versus laser resurfacing in the treatment of widespread actinic keratoses. Lasers Surg. Med. 2006, 38, 731–739. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, K.H.; Song, K.H. Effect of Methyl Aminolevulinate Photodynamic Therapy with and without Ablative Fractional Laser Treatment in Patients with Microinvasive Squamous Cell Carcinoma: A Randomized Clinical Trial. JAMA Dermatol. 2017, 153, 289–295. [Google Scholar] [CrossRef]
- Lindholm, V.; Salmivuori, M.; Hahtola, S.; Mäkelä, K.; Pitkänen, S.; Isoherranen, K. Ablative Fractional Laser Enhances Artificial or Natural Daylight Photodynamic Therapy of Actinic Field Cancerization: A Randomized and Investigator-initiated Half-side Comparative Study. Acta Derm. Venereol. 2023, 103, adv6579. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-R.; Seo, J.-W.; Kim, H.-J.; Song, K.-H. A comparison of the efficacy of ablative fractional laser-assisted photodynamic therapy according to the density of the ablative laser channel in the treatment of actinic keratosis: A prospective, randomized, controlled trial. J. Am. Acad. Dermatol. 2021, 85, 750–752. [Google Scholar] [CrossRef] [PubMed]
- Wenande, E.; Phothong, W.; Bay, C.; Karmisholt, K.E.; Haedersdal, M.; Togsverd-Bo, K. Efficacy and safety of daylight photodynamic therapy after tailored pretreatment with ablative fractional laser or microdermabrasion: A randomized, side-by-side, single-blind trial in patients with actinic keratosis and large-area field cancerization. Br. J. Dermatol. 2019, 180, 756–764. [Google Scholar] [CrossRef]
- Togsverd-Bo, K.; Lei, U.; Erlendsson, A.M.; Taudorf, E.H.; Philipsen, P.A.; Wulf, H.C.; Skov, L.; Haedersdal, M. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients—A randomized controlled trial. Br. J. Dermatol. 2015, 172, 467–474. [Google Scholar] [CrossRef]
- Heerfordt, I.M.; Wulf, H.C. Daylight photodynamic therapy of actinic keratosis without curettage is as effective as with curettage: A randomized clinical trial. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2058–2061. [Google Scholar] [CrossRef]
- Heerfordt, I.M.; Bieliauskiene, G.; Wulf, H.C. Protoporphyrin IX formation after application of methyl aminolevulinate on the face and scalp with and without prior curettage. Photodiag. Photodyn. Ther. 2018, 22, 155–157. [Google Scholar] [CrossRef]
- Caccavale, S.; Boccellino, M.P.; Brancaccio, G.; Alfano, R.; Argenziano, G. Keratolytics can replace curettage in daylight photodynamic therapy for actinic keratosis on the face/scalp: A randomized clinical trial. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Balakirski, G.; Lehmann, P.; Szeimies, R.; Hofmann, S.C. Photodynamic therapy in dermatology: Established and new indications. J. Dtsch. Dermatol. Ges. 2024, 22, 1651–1662. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Tyrrell, J.; Paterson, C.; Curnow, A. Regression Analysis of Protoporphyrin IX Measurements Obtained During Dermatological Photodynamic Therapy. Cancers 2019, 11, 72. [Google Scholar] [CrossRef]
- García-Rodrigo, C.G.; Pellegrini, C.; Piccioni, A.; Maini, M.; Fargnoli, M.C. Long-term efficacy data for daylight-PDT. G. Ital. Dermatol. Venereol. 2018, 153, 800–805. [Google Scholar] [CrossRef]
- Szeimies, R.-M.; Dirschka, T.; Fargnoli, M.C.; Gilaberte, Y.; Hædersdal, M.; Chavda, R.; Calzavara-Pinton, P. A Review of MAL-PDT for the Treatment Strategy of Actinic Keratosis: Broader Clinical Perspectives Beyond the Data and Guideline Recommendations. Dermatol. Ther. 2023, 13, 1409–1421. [Google Scholar] [CrossRef]
- Fernández-Guarino, M.; Pascual, P.F.; Gomez, P.L.; Castaño, A.H.; Olasolo, P.J. Split-face study comparing conventional MAL photodynamic therapy in multiple actinic keratosis with complete time vs. half-time red light LED conventional illumination. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1529–1534. [Google Scholar] [CrossRef]
- Tanew, A.; Ristl, R.; Trattner, H.; Hacker, V.; Kroyer, B.; Radakovic, S. Impact of light dose and fluence rate on the efficacy and tolerability of topical 5-ALA photodynamic therapy for actinic keratoses: A randomized, controlled, observer-blinded intrapatient comparison study. J. Eur. Acad. Dermatol. Venereol. 2025, 39, 1460–1467. [Google Scholar] [CrossRef]
- Dirschka, T.; Radny, P.; Dominicus, R.; Mensing, H.; Brüning, H.; Jenne, L.; Karl, L.; Sebastian, M.; Oster-Schmidt, C.; Klövekorn, W.; et al. Photodynamic therapy with BF-200 ALA for the treatment of actinic keratosis: Results of a multicentre, randomized, observer-blind phase III study in comparison with a registered methyl-5-aminolaevulinate cream and placebo. Br. J. Dermatol. 2012, 166, 137–146. [Google Scholar] [CrossRef]
- Räsänen, J.E.; Neittaanmäki, N.; Ylitalo, L.; Hagman, J.; Rissanen, P.; Ylianttila, L.; Salmivuori, M.; Snellman, E.; Grönroos, M. 5-aminolaevulinic acid nanoemulsion is more effective than methyl-5-aminolaevulinate in daylight photodynamic therapy for actinic keratosis: A nonsponsored randomized double-blind multicentre trial. Br. J. Dermatol. 2019, 181, 265–274. [Google Scholar] [CrossRef]
- Assikar, S.; Labrunie, A.; Kerob, D.; Couraud, A.; Bédane, C. Daylight photodynamic therapy with methyl aminolevulinate cream is as effective as conventional photodynamic therapy with blue light in the treatment of actinic keratosis: A controlled randomized intra-individual study. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- See, J.-A.; Shumack, S.; Murrell, D.F.; Rubel, D.M.; Fernández-Peñas, P.; Salmon, R.; Hewitt, D.; Foley, P.; Spelman, L. Consensus recommendations on the use of daylight photodynamic therapy with methyl aminolevulinate cream for actinic keratoses in Australia. Australas. J. Dermatol. 2016, 57, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Rubel, D.M.; Spelman, L.; Murrell, D.F.; See, J.A.; Hewitt, D.; Foley, P.; Bosc, C.; Kerob, D.; Kerrouche, N.; Wulf, H.C.; et al. Daylight photodynamic therapy with methyl aminolevulinate cream as a convenient, similarly effective, nearly painless alternative to conventional photodynamic therapy in actinic keratosis treatment: A randomized controlled trial. Br. J. Dermatol. 2014, 171, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Fargnoli, M.; Piccioni, A.; Neri, L.; Tambone, S.; Pellegrini, C.; Peris, K. Conventional vs. daylight methyl aminolevulinate photodynamic therapy for actinic keratosis of the face and scalp: An intra-patient, prospective, comparison study in Italy. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1926–1932. [Google Scholar] [CrossRef]
- Mordon, S.; Vignion-Dewalle, A.S.; Thecua, E.; Vicentini, C.; Maire, C.; Deleporte, P.; Baert, G.; Lecomte, F.; Mortier, L. Can daylight-PDT be performed indoor? G. Ital. Dermatol. Venereol. 2018, 153, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Bai-Habelski, J.C.; Medrano, K.; Palacio, A.; Reinhold, U. No room for pain: A prospective study showing effective and nearly pain-free treatment of actinic keratosis with simulated daylight photodynamic therapy (SDL-PDT) using the IndoorLux® System in combination with BF-200 ALA (Ameluz®). Photodiag. Photodyn. Ther. 2022, 37, 102692. [Google Scholar] [CrossRef]
- Reinhold, U.; Philipp-Dormston, W.G.; Dirschka, T.; Ostendorf, R.; Aschoff, R.; Berking, C.; Jäger, A.; Schmitz, B.; Foguet, M.; Szeimies, R.M. Long-term follow-up of a randomized, double-blind, phase III, multi-centre study to evaluate the safety and efficacy of field-directed photodynamic therapy (PDT) of mild to moderate actinic keratosis using BF-200 ALA versus placebo and the BF-RhodoLED® lamp. J. Eur. Acad. Dermatol. Venereol. 2025, 39, 1449–1459. [Google Scholar]
- Blauvelt, A.; Kempers, S.; Lain, E.; Schlesinger, T.; Tyring, S.; Forman, S.; Ablon, G.; Martin, G.; Wang, H.; Cutler, D.L.; et al. Phase 3 Trials of Tirbanibulin Ointment for Actinic Keratosis. N. Engl. J. Med. 2021, 384, 512–520. [Google Scholar] [CrossRef]
- Oh, Y.; An, D.E.; Park, J.; Koh, B.; Cho, K.-J.; Jeon, H. Synthesis and evaluation of KX-01 analogs with an exploration of linker attachment points for antibody-drug conjugates. Bioorg. Med. Chem. Lett. 2025, 120, 130114. [Google Scholar] [CrossRef]
- Kirchberger, M.C.; Gfesser, M.; Erdmann, M.; Schliep, S.; Berking, C.; Heppt, M.V. Tirbanibulin 1% Ointment Significantly Reduces the Actinic Keratosis Area and Severity Index in Patients with Actinic Keratosis: Results from a Real-World Study. J. Clin. Med. 2023, 12, 4837. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, N.; Lain, E.; Jarell, A.; DuBois, J.; Tamarit, M.L.; Falques, M.; Kiyasova, V.; Padullés, L.; Otero, R.; Blauvelt, A. Safety and tolerability of tirbanibulin ointment 1% treatment on 100 cm2 of the face or scalp in patients with actinic keratosis: A phase 3 study. JAAD Int. 2024, 17, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Kopera, D. Enhancement of sun-damaged skin qualities with tirbanibulin: A prospective Phase 4 study (SunDamage Study). J. Eur. Acad. Dermatol. Venereol. 2025, 39, e464–e466. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Naren, G.; Guo, J.; Bai, Q.; Fan, N.; Nashun, B. Reproductive and developmental toxicities of 5-fluorouracil in model organisms and humans. Expert. Rev. Mol. Med. 2022, 24, e9. [Google Scholar] [CrossRef]
- De Clercq, E. The development of BVDU: An odyssey. Antivir. Chem. Chemother. 2023, 31, 20402066231152971. [Google Scholar] [CrossRef] [PubMed]
- Dohil, M.A. Efficacy, Safety, and Tolerability of 4% 5-Fluorouracil Cream in a Novel Patented Aqueous Cream Containing Peanut Oil Once Daily Compared With 5% 5-Fluorouracil Cream Twice Daily: Meeting the Challenge in the Treatment of Actinic Keratosis. J. Drugs Dermatol. 2016, 15, 1218–1224. [Google Scholar]
- Briatico, G.; Brancaccio, G.; Scharf, C.; Di Brizzi, E.V.; Pellerone, S.; Caccavale, S.; Giorgio, C.M.; Procaccini, E.M.; Moscarella, E.; Argenziano, G. Real-World Experience with Topical 5-Fluorouracil 4% (40 mg/g) Cream for the Treatment of Actinic Keratosis. Dermatol. Pract. Concept. 2023, 13, e2023151. [Google Scholar] [CrossRef] [PubMed]
- Heppt, M.V.; Ulrich, C. Does early discontinuation of topical 4% 5-fluorouracil affect lesion clearance for actinic keratosis? Results from a dose-ranging study. J. Eur. Acad. Dermatol. Venereol. 2025, 39, e675–e677. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.H.E.; Kessels, J.P.H.M.; Nelemans, P.J.; Kouloubis, N.; Arits, A.H.M.M.; van Pelt, H.P.A.; Quaedvlieg, P.J.F.; Essers, B.A.B.; Steijlen, P.M.; Kelleners-Smeets, N.W.J.; et al. Randomized Trial of Four Treatment Approaches for Actinic Keratosis. N. Engl. J. Med. 2019, 380, 935–946. [Google Scholar] [CrossRef]
- Stockfleth, E.; von Kiedrowski, R.; Dominicus, R.; Ryan, J.; Ellery, A.; Falqués, M.; Ivanoff, N.; Azeredo, R.R. Efficacy and Safety of 5-Fluorouracil 0.5%/Salicylic Acid 10% in the Field-Directed Treatment of Actinic Keratosis: A Phase III, Randomized, Double-Blind, Vehicle-Controlled Trial. Dermatol. Ther. 2017, 7, 81–96. [Google Scholar] [CrossRef]
- Cunningham, T.J.; Tabacchi, M.; Eliane, J.-P.; Tuchayi, S.M.; Manivasagam, S.; Mirzaalian, H.; Turkoz, A.; Kopan, R.; Schaffer, A.; Saavedra, A.P.; et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J. Clin. Investig. 2017, 127, 106–116. [Google Scholar] [CrossRef]
- Wiegell, S.R.; Fredman, G.; Andersen, F.; Bjerring, P.; Paasch, U.; Haedersdal, M. Is the benefit of sequential 5-fluorouracil and daylight photodynamic therapy versus daylight photodynamic therapy alone sustained over time?—12-month follow-up of a randomized controlled trial. Photodiagn. Photodyn. Ther. 2025, 51, 104445. [Google Scholar] [CrossRef] [PubMed]
- Bernal Masferrer, L.; Gracia Cazaña, T.; Bernad Alonso, I.; Álvarez-Salafranca, M.; Almenara Blasco, M.; Gallego Rentero, M.; Juarranz de la Fuente, Á.; Gilaberte, Y. Topical Immunotherapy for Actinic Keratosis and Field Cancerization. Cancers 2024, 16, 1133. [Google Scholar] [CrossRef]
- Krawtchenko, N.; Roewert-Huber, J.; Ulrich, M.; Mann, I.; Sterry, W.; Stockfleth, E. A randomised study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: A comparison of clinical and histological outcomes including 1-year follow-up. Br. J. Dermatol. 2007, 157 (Suppl. S2), 34–40. [Google Scholar]
- Hanke, C.W.; Beer, K.R.; Stockfleth, E.; Wu, J.; Rosen, T.; Levy, S. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: Results of two placebo-controlled studies of daily application to the face and balding scalp for two 3-week cycles. J. Am. Acad. Dermatol. 2010, 62, 573–581. [Google Scholar] [CrossRef]
- Swanson, N.; Smith, C.C.; Kaur, M.; Goldenberg, G. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: Two phase 3, multicenter, randomized, double-blind, placebo-controlled studies. J. Drugs Dermatol. 2014, 13, 166–169. [Google Scholar]
- Fecker, L.F.; Stockfleth, E.; Nindl, I.; Ulrich, C.; Forschner, T.; Eberle, J. The role of apoptosis in therapy and prophylaxis of epithelial tumours by nonsteroidal anti-inflammatory drugs (NSAIDs). Br. J. Dermatol. 2007, 156 (Suppl. S3), 25–33. [Google Scholar] [CrossRef]
- Stockfleth, E.; Harwood, C.A.; Serra-Guillén, C.; Larsson, T.; Østerdal, M.; Skov, T. Phase IV head-to-head randomized controlled trial comparing ingenol mebutate 0·015% gel with diclofenac sodium 3% gel for the treatment of actinic keratosis on the face or scalp. Br. J. Dermatol. 2018, 178, 433–442. [Google Scholar] [CrossRef]
- Akarsu, S.; Aktan, Ş.; Atahan, A.; Koç, P.; Özkan, Ş. Comparison of topical 3% diclofenac sodium gel and 5% imiquimod cream for the treatment of actinic keratoses. Clin. Exp. Dermatol. 2011, 36, 479–484. [Google Scholar] [CrossRef]
- Ianhez, M.; Fleury, L.F., Jr.; Miot, H.A.; Bagatin, E. Retinoids for prevention and treatment of actinic keratosis. An. Bras. Dermatol. 2013, 88, 585–593. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. A Study of SNA-001 in Subjects with Actinic Keratosis. Identifier: NCT06319794. Updated June 2024. Available online: https://clinicaltrials.gov/study/NCT06319794 (accessed on 1 April 2025).
- Del Regno, L.; Catapano, S.; Di Stefani, A.; Cappilli, S.; Peris, K. A Review of Existing Therapies for Actinic Keratosis: Current Status and Future Directions. Am. J. Clin. Dermatol. 2022, 23, 339–352. [Google Scholar] [CrossRef]
- Stockfleth, E.; Hofbauer, G.F.L.; Reinhold, U.; Popp, G.; Hengge, U.R.; Szeimies, R.M.; Brüning, H.; Anliker, M.; Hunger, T.; Dummer, R.; et al. Topical resiquimod dosing regimens in patients with multiple actinic keratoses: A multicentre, partly placebo-controlled, double-blind clinical trial. Br. J. Dermatol. 2019, 180, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.S.; Martins, I.M.d.C.; Miola, A.C.; Miot, H.A. Efficacy and safety of 0.5% colchicine cream versus 5% 5-fluorouracil cream in the treatment of cutaneous field cancerization: A randomized clinical trial. An. Bras. Dermatol. 2024, 99, 527–534. [Google Scholar] [CrossRef]
- Morton, C.; Baharlou, S.; Basset-Seguin, N.; Calzavara-Pinton, P.; Dirschka, T.; Gilaberte, Y.; Haedersdal, M.; Hofbauer, G.; Sapra, S.; Waalboer-Spuij, R.; et al. Expert Recommendations on Facilitating Personalized Approaches to Long-term Management of Actinic Keratosis: The Personalizing Actinic Keratosis Treatment (PAKT) Project. Acta. Derm.-Venereol. 2023, 103, adv6229. [Google Scholar] [CrossRef] [PubMed]
- Lang, B.M.; Zielbauer, S.; Stege, H.; Grabbe, S.; Staubach, P. If patients had a choice—Treatment satisfaction and patients’ preference in therapy of actinic keratoses. J. Dtsch. Dermatol. Ges. 2024, 22, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Piaserico, S.; Mazzetto, R.; Sartor, E.; Bortoletti, C. Combination-Based Strategies for the Treatment of Actinic Keratoses with Photodynamic Therapy: An Evidence-Based Review. Pharmaceutics 2022, 14, 1726. [Google Scholar] [CrossRef] [PubMed]
- Morelló-Vicente, A.; Oteiza-Rius, I.; Gómez-González, E.M.; Carrera-Gabilondo, A.; Marcos-Muñagorri, D.; Antoñanzas-Pérez, J.; Rodríguez-Garijo, N.; Pilar-Gil, M.; España, A.; Nuñez-Córdoba, J.M.; et al. Efficacy and safety of sequential treatment with cryotherapy followed by 1% topical tirbanibulin for actinic keratoses in organ transplant recipients: A randomized clinical trial. J. Am. Acad. Dermatol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Pariser, D.M. Approaches to Field Therapy for Actinic Keratoses: Relating Clinical Trial Results to Real-world Practice—A Commentary. J. Clin. Aesthet. Dermatol. 2022, 15, 40–43. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).