Transoral Robotic Surgery for the Salvage of Primarily Irradiated Oropharyngeal Squamous Cell Carcinomas Recurring at the Base of the Tongue: A Small Monoinstitutional Series
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
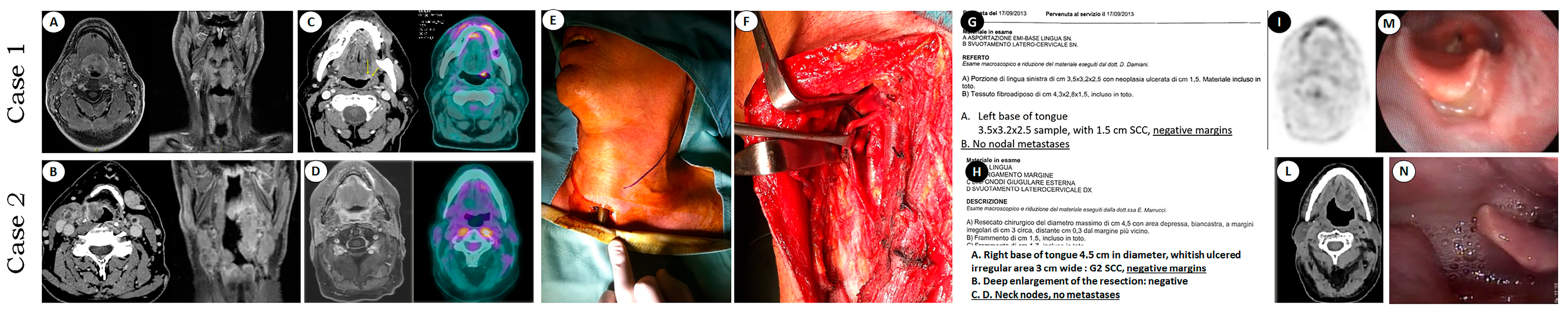
2.2. Diagnostic Work-Up
2.3. Surgery
2.4. Outcomes
2.5. Ethical Considerations
3. Results
4. Discussion
- Is treatment deintensification through primary surgery rational for treating notoriously radiosensitive malignancies, such as HPV-related OPSCC? Is the survival obtained through TORS in this group really equivalent to that obtained with chemoradiation?
- In case of recurrence, is the pattern of spread of the recurrence and therefore the salvageability of patients previously treated through TORS similar to that of those who exclusively underwent radiation?
- How many OPSCC patients treated through TORS will require adjuvant treatment? Is the long-term toxicity from a single modality treatment, even at higher doses, really greater than that from a combined treatment?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer Statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Baskin, R.M.; Boyce, B.J.; Amdur, R.; Mendenhall, W.M.; Hitchcock, K.; Silver, N.; Dziegielewski, P.T. Transoral Robotic Surgery for Oropharyngeal Cancer: Patient Selection and Special Considerations. Cancer Manag. Res. 2018, 10, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka, M.; Pietruszewska, W.; Maciejczyk, A.; Markowski, J. Trends in Incidence and Mortality of Head and Neck Cancer Subsites Among Elderly Patients: A Population-Based Analysis. Cancers 2025, 17, 548. [Google Scholar] [CrossRef]
- DeVita, V.T.; Lawrence, T.S.; Rosenberg, S.A. DeVita, Hellman, and Rosenberg’s Cancer: Principles & Practice of Oncology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; ISBN 9780781772075. [Google Scholar]
- Mahmoud, O.; Sung, K.; Civantos, F.J.; Thomas, G.R.; Samuels, M.A. Transoral Robotic Surgery for Oropharyngeal Squamous Cell Carcinoma in the Era of Human Papillomavirus. Head Neck 2018, 40, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Bussu, F.; Muresu, N.; Crescio, C.; Gallus, R.; Rizzo, D.; Cossu, A.; Sechi, I.; Fedeli, M.; Cossu, A.; Delogu, G.; et al. Low Prevalence of HPV Related Oropharyngeal Carcinogenesis in Northern Sardinia. Cancers 2022, 14, 4205. [Google Scholar] [CrossRef]
- Bussu, F.; Ragin, C.; Boscolo-Rizzo, P.; Rizzo, D.; Gallus, R.; Delogu, G.; Morbini, P.; Tommasino, M. HPV as a Marker for Molecular Characterization in Head and Neck Oncology: Looking for a Standardization of Clinical Use and of Detection Method(s) in Clinical Practice. Head Neck 2019, 41, 1104–1111. [Google Scholar] [CrossRef]
- Gillison, M.L.; Koch, W.M.; Capone, R.B.; Spafford, M.; Westra, W.H.; Wu, L.; Zahurak, M.L.; Daniel, R.W.; Viglione, M.; Symer, D.E.; et al. Evidence for a Causal Association between Human Papillomavirus and a Subset of Head and Neck Cancers. J. Natl. Cancer Inst. 2000, 92, 709–720. [Google Scholar] [CrossRef]
- Mehanna, H.; Taberna, M.; von Buchwald, C.; Tous, S.; Brooks, J.; Mena, M.; Morey, F.; Grønhøj, C.; Rasmussen, J.H.; Garset-Zamani, M.; et al. Prognostic Implications of p16 and HPV Discordance in Oropharyngeal Cancer (HNCIG-EPIC-OPC): A Multicentre, Multinational, Individual Patient Data Analysis. Lancet Oncol. 2023, 24, 239–251. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human Papillomavirus and Rising Oropharyngeal Cancer Incidence in the United States. J. Clin. Oncol. 2023, 41, 3081–3088. [Google Scholar] [CrossRef]
- Morán-Torres, A.; Pazos-Salazar, N.G.; Téllez-Lorenzo, S.; Jiménez-Lima, R.; Lizano, M.; Reyes-Hernández, D.O.; Marin-Aquino, J.d.J.; Manzo-Merino, J. HPV Oral and Oropharynx Infection Dynamics in Young Population. Braz. J. Microbiol. 2021, 52, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Young, D.; Xiao, C.C.; Murphy, B.; Moore, M.; Fakhry, C.; Day, T.A. Increase in Head and Neck Cancer in Younger Patients due to Human Papillomavirus (HPV). Oral Oncol. 2015, 51, 727–730. [Google Scholar] [CrossRef]
- Alemany, L.; Felsher, M.; Giuliano, A.R.; Waterboer, T.; Mirghani, H.; Mehanna, H.; Roberts, C.; Chen, Y.-T.; Lara, N.; Lynam, M.; et al. Oral Human Papillomavirus (HPV) Prevalence and Genotyping among Healthy Adult Populations in the United States and Europe: Results from the PROGRESS (PRevalence of Oral Hpv Infection, a Global aSSessment) Study. EClinicalMedicine 2025, 79, 103018. [Google Scholar] [CrossRef] [PubMed]
- Bussu, F.; Sali, M.; Gallus, R.; Petrone, G.; Zannoni, G.F.; Autorino, R.; Dinapoli, N.; Santangelo, R.; Vellone, V.G.; Graziani, C.; et al. Human Papillomavirus (HPV) Infection in Squamous Cell Carcinomas Arising from the Oropharynx: Detection of HPV DNA and p16 Immunohistochemistry as Diagnostic and Prognostic Indicators—a Pilot Study. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 1115–1120. [Google Scholar] [CrossRef]
- Morand, G.B.; Diaconescu, A.; Ibrahim, I.; Lamarche, G.; Ruas, J.S.; Dalfen, J.; Hier, M.P.; Alaoui-Jamali, M.A.; Maschietto, M.; da Silva, S.D. Molecular Prognostic Indicators in HPV-Positive Oropharyngeal Cancer: An Updated Review. Clin. Exp. Metastasis 2022, 39, 407–416. [Google Scholar] [CrossRef]
- Wang, M.B.; Liu, I.Y.; Gornbein, J.A.; Nguyen, C.T. HPV-Positive Oropharyngeal Carcinoma: A Systematic Review of Treatment and Prognosis. Otolaryngol. Head Neck Surg. 2015, 153, 758–769. [Google Scholar] [CrossRef]
- Stransky, N.; Egloff, A.M.; Tward, A.D.; Kostic, A.D.; Cibulskis, K.; Sivachenko, A.; Kryukov, G.V.; Lawrence, M.S.; Sougnez, C.; McKenna, A.; et al. The Mutational Landscape of Head and Neck Squamous Cell Carcinoma. Science 2011, 333, 1157–1160. [Google Scholar] [CrossRef]
- Kędzierawski, P.; Huruk-Kuchinka, A.; Radowicz-Chil, A.; Mężyk, R.; Rugała, Z.; Sadowski, J. Human Papillomavirus Infection Predicts a Better Survival Rate in Patients with Oropharyngeal Cancer. Arch. Med. Sci. 2021, 17, 1308–1316. [Google Scholar] [CrossRef]
- Carlander, A.-L.F.; Bendtsen, S.K.; Rasmussen, J.H.; Jakobsen, K.K.; Garset-Zamani, M.; Grønhøj, C.; Friborg, J.; Hutcheson, K.; Johnson, F.M.; Fuller, C.D.; et al. Clinical and Prognostic Differences in Oropharyngeal Squamous Cell Carcinoma in USA and Denmark, Two HPV High-Prevalence Areas. Eur. J. Cancer 2024, 202, 113983. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Karadaghy, O.A.; Doering, M.M.; Tuuli, M.G.; Jackson, R.S.; Haughey, B.H. Survival for HPV-Positive Oropharyngeal Squamous Cell Carcinoma with Surgical versus Non-Surgical Treatment Approach: A Systematic Review and Meta-Analysis. Oral Oncol. 2018, 86, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Colevas, A.D.; Cmelak, A.J.; Pfister, D.G.; Spencer, S.; Adkins, D.; Birkeland, A.C.; Brizel, D.M.; Busse, P.M.; Caudell, J.J.; Durm, G.; et al. NCCN Guidelines® Insights: Head and Neck Cancers, Version 2.2025. J. Natl. Compr. Cancer Netw. 2025, 23, 2–11. [Google Scholar] [CrossRef]
- Howard, J.; Dwivedi, R.C.; Masterson, L.; Kothari, P.; Quon, H.; Holsinger, F.C. De-Intensified Adjuvant (chemo)radiotherapy versus Standard Adjuvant Chemoradiotherapy Post Transoral Minimally Invasive Surgery for Resectable HPV-Positive Oropharyngeal Carcinoma. Cochrane Libr. 2018, CD012939. [Google Scholar] [CrossRef]
- Pace, G.M.; Costantino, A.; Festa, B.M.; Spriano, G.; Bussu, F.; Pellini, R.; De Virgilio, A. Oropharyngeal Squamous Cell Carcinoma: Treatment (de-) Intensification? Oral Oncol. 2024, 153, 106299. [Google Scholar] [CrossRef]
- Gallus, R.; Nauta, I.H.; Marklund, L.; Rizzo, D.; Crescio, C.; Mureddu, L.; Tropiano, P.; Delogu, G.; Bussu, F. Accuracy of p16 IHC in Classifying HPV-Driven OPSCC in Different Populations. Cancers 2023, 15, 656. [Google Scholar] [CrossRef]
- Golusiński, W. Functional Organ Preservation Surgery in Head and Neck Cancer: Transoral Robotic Surgery and Beyond. Front. Oncol. 2019, 9, 293. [Google Scholar] [CrossRef]
- Kornfeld, B.; Taha, A.; Kyang, L.; Sim, H.-W.; Dewhurst, S.; McCloy, R.; Chin, V.; Earls, P.; Parker, A.; Leavers, B.; et al. Oncological Outcomes Post Transoral Robotic Surgery (TORS) for HPV-Associated Oropharyngeal Squamous Cell Carcinoma, a Single-Centre Retrospective Australian Study. J. Robot. Surg. 2024, 18, 226. [Google Scholar] [CrossRef]
- Chillakuru, Y.; Benito, D.A.; Strum, D.; Mehta, V.; Saini, P.; Shim, T.; Darwish, C.; Joshi, A.S.; Thakkar, P.; Goodman, J.F. Transoral Robotic Surgery versus Nonrobotic Resection of Oropharyngeal Squamous Cell Carcinoma. Head Neck 2021, 43, 2259–2273. [Google Scholar] [CrossRef]
- Amin, D.R.; Philips, R.; Bertoni, D.G.; Mastrolonardo, E.V.; Campbell, D.J.; Agarwal, A.M.; Tekumalla, S.; Urdang, Z.D.; Luginbuhl, A.J.; Cognetti, D.M.; et al. Differences in Functional and Survival Outcomes Between Patients Receiving Primary Surgery vs Chemoradiation Therapy for Treatment of T1-T2 Oropharyngeal Squamous Cell Carcinoma. JAMA Otolaryngol. Head Neck Surg. 2023, 149, 980–986. [Google Scholar] [CrossRef]
- Jefferson, G.D.; Frey, H. Open Versus Robotic Surgery for Oropharyngeal Cancer. Otolaryngol. Clin. N. Am. 2020, 53, 995–1003. [Google Scholar] [CrossRef]
- White, H.; Ford, S.; Bush, B.; Holsinger, F.C.; Moore, E.; Ghanem, T.; Carroll, W.; Rosenthal, E.; Sweeny, L.; Magnuson, J.S. Salvage Surgery for Recurrent Cancers of the Oropharynx: Comparing TORS with Standard Open Surgical Approaches. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 773–778. [Google Scholar] [CrossRef]
- Moore, E.J.; Olsen, K.D.; Kasperbauer, J.L. Transoral Robotic Surgery for Oropharyngeal Squamous Cell Carcinoma: A Prospective Study of Feasibility and Functional Outcomes. Laryngoscope 2009, 119, 2156–2164. [Google Scholar] [CrossRef]
- Palma, D.A.; Prisman, E.; Berthelet, E.; Tran, E.; Hamilton, S.; Wu, J.; Eskander, A.; Higgins, K.; Karam, I.; Poon, I.; et al. Assessment of Toxic Effects and Survival in Treatment Deescalation with Radiotherapy vs Transoral Surgery for HPV-Associated Oropharyngeal Squamous Cell Carcinoma: The ORATOR2 Phase 2 Randomized Clinical Trial. JAMA Oncol. 2022, 8, 845–851. [Google Scholar] [CrossRef]
- Yver, C.M.; Shimunov, D.; Weinstein, G.S.; Rajasekaran, K.; Cannady, S.B.; Lukens, J.N.; Lin, A.; Swisher-McClure, S.; Cohen, R.B.; Aggarwal, C.; et al. Oncologic and Survival Outcomes for Resectable Locally-Advanced HPV-Related Oropharyngeal Cancer Treated with Transoral Robotic Surgery. Oral Oncol. 2021, 118, 105307. [Google Scholar] [CrossRef]
- Kang, S.K.; Qamar, S.N.; Khan, I.M.; Crosbie, R.; Tikka, T. 10-Year Experience with the Modified Pectoralis Major Flap: The Use of the Deltopectoral Flap to Reduce Skin Tension. Indian J. Otolaryngol. Head Neck Surg. 2023, 75, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Virós Porcuna, D.; Viña Soria, C.; Vila Poyatos, J.; Palau Viarnès, M.; Malagon López, P.; Gonzàlez Lluch, C.; Higueras Suñe, C.; Pollán Guisasola, C.M.; Carrasco López, C. Oropharyngeal Free Flap Reconstruction: Transoral Robotic Surgery versus Open Approach. Laryngoscope Investig. Otolaryngol. 2023, 8, 1564–1570. [Google Scholar] [CrossRef] [PubMed]
- Kaki, P.C.; Sangal, N.R.; Lam, D.; Carey, R.M.; Rajasekaran, K.; Chalian, A.; Brody, R.M.; Weinstein, G.S.; Cannady, S.B. Functional Outcomes of Free Flap Reconstruction After TORS in Early-Stage HPV-Positive Oropharyngeal Cancer. Otolaryngol. Head Neck Surg. 2025, 173, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Melan, J.-B.; Philouze, P.; Pradat, P.; Benzerdjeb, N.; Blanc, J.; Ceruse, P.; Fuchsmann, C. Functional Outcomes of Soft Palate Free Flap Reconstruction Following Oropharyngeal Cancer Surgery. Eur. J. Surg. Oncol. 2021, 47, 2265–2271. [Google Scholar] [CrossRef]
- Dowthwaite, S.; Jackson, J.; Dzienis, M.; Khoo, E.; Cronin, M.; Guazzo, E. Management of Recurrent HPV-Positive Oropharyngeal Squamous Cell Carcinoma: A Contemporary Review. Curr. Oncol. Rep. 2023, 25, 501–510. [Google Scholar] [CrossRef]
- Achim, V.; Bolognone, R.K.; Palmer, A.D.; Graville, D.J.; Light, T.J.; Li, R.; Gross, N.; Andersen, P.E.; Clayburgh, D. Long-Term Functional and Quality-of-Life Outcomes After Transoral Robotic Surgery in Patients with Oropharyngeal Cancer. JAMA Otolaryngol. Head Neck Surg. 2018, 144, 18–27. [Google Scholar] [CrossRef]
- Richmon, J.D.; Feng, A.L.; Yang, W.; Starmer, H.; Quon, H.; Gourin, C.G. Feasibility of Rapid Discharge after Transoral Robotic Surgery of the Oropharynx. Laryngoscope 2014, 124, 2518–2525. [Google Scholar] [CrossRef]
- Meldgaard Justesen, M.; Kronberg Jakobsen, K.; Fenger Carlander, A.-L.; Hjordt Holm Larsen, M.; Wessel, I.; Kiss, K.; Friborg, J.; Ibrahim Channir, H.; Rubek, N.; Grønhøj, C.; et al. Outcomes of Transoral Robotic Surgery for Early-Stage Oropharyngeal Squamous Cell Carcinoma with Low Rates of Adjuvant Therapy: A Consecutive Single-Institution Study from 2013 to 2020. Oral Oncol. 2024, 152, 106783. [Google Scholar] [CrossRef]
- Niewinski, P.; Golusiński, W.J. Current Indications and Patient Selection for Transoral Robotic Surgery in Head and Neck Cancer—A Brief Review. Contemp. Oncol. 2022, 26, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Mella, M.H.; Chabrillac, E.; Dupret-Bories, A.; Mirallie, M.; Vergez, S. Transoral Robotic Surgery for Head and Neck Cancer: Advances and Residual Knowledge Gaps. J. Clin. Med. 2023, 12, 2303. [Google Scholar] [CrossRef] [PubMed]
- Kwan, B.Y.M.; Khan, N.M.; de Almeida, J.R.; Goldstein, D.; Paleri, V.; Forghani, R.; Yu, E. Transoral Robotic Surgery for Head and Neck Malignancies: Imaging Features in Presurgical Workup. Head Neck 2019, 41, 4018–4025. [Google Scholar] [CrossRef] [PubMed]
| Selection Criteria According to White et al. (2013) [30]—Whole Oropharynx | Proposed Modified Criteria—Base of Tongue Only |
|---|---|
| No bone involvement | No bone involvement |
| Anterior extension not considered | No involvement of hard palate, limited (2 cm) extension beyond the lingual V |
| Tumor spread to be considered not stated (cTNM, rTNM, both?) | Surgery planned on the recurrence (rTNM) |
| No significant trismus | No significant trismus |
| Adequate mouth opening | Adequate mouth opening |
| Base of tongue tumors that cross the midline | At least one lingual artery has to be spareable according to preoperative imaging |
| Difficult visualization and/or palpation of margins | Preoperative definition through morphological and functional imaging and, in some cases, with histological mapping even when visualization and/or palpation of margins are difficult |
| Case | Age at Diagnosis (Years) | cT | cN | HPV Status (E6/E7 mRNA on Fresh Sample) | Time to TORS Salvage (Months) | Pre-Op Composite MDADI | rT # | rN # | G, PNI | Post-Op Composite MDADI | Post- TORS Relapse | Dead/Alive | Last Follow-Up After TORS (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 * | 49 | 4 | 2c | Positive | 65 | 69 | 1 | 0 | II, - | 73 | No | Alive | 105 |
| 2 * | 60 | 4 | 2c | Negative | 24 | 68 | 2 | 0 | III, - | 66 | No | DOC | 72 |
| 3 | 69 | 1 | 2b | Negative | 18 | 66 | 2 | 0 | III, + | - | Yes (time to relapse 3 months) | DOD | 11 |
| 4 | 74 | 3 | 2c | Negative | 9 | 68 | 2 | 2c | II, - | 70 | No | Alive | 96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frasconi, S.; Rizzo, D.; Gallus, R.; Machouchas, N.; Cannova, S.; Fliss, D.M.; Galli, J.; Bussu, F. Transoral Robotic Surgery for the Salvage of Primarily Irradiated Oropharyngeal Squamous Cell Carcinomas Recurring at the Base of the Tongue: A Small Monoinstitutional Series. J. Pers. Med. 2025, 15, 419. https://doi.org/10.3390/jpm15090419
Frasconi S, Rizzo D, Gallus R, Machouchas N, Cannova S, Fliss DM, Galli J, Bussu F. Transoral Robotic Surgery for the Salvage of Primarily Irradiated Oropharyngeal Squamous Cell Carcinomas Recurring at the Base of the Tongue: A Small Monoinstitutional Series. Journal of Personalized Medicine. 2025; 15(9):419. https://doi.org/10.3390/jpm15090419
Chicago/Turabian StyleFrasconi, Samuele, Davide Rizzo, Roberto Gallus, Nikolaos Machouchas, Sergio Cannova, Dan Marian Fliss, Jacopo Galli, and Francesco Bussu. 2025. "Transoral Robotic Surgery for the Salvage of Primarily Irradiated Oropharyngeal Squamous Cell Carcinomas Recurring at the Base of the Tongue: A Small Monoinstitutional Series" Journal of Personalized Medicine 15, no. 9: 419. https://doi.org/10.3390/jpm15090419
APA StyleFrasconi, S., Rizzo, D., Gallus, R., Machouchas, N., Cannova, S., Fliss, D. M., Galli, J., & Bussu, F. (2025). Transoral Robotic Surgery for the Salvage of Primarily Irradiated Oropharyngeal Squamous Cell Carcinomas Recurring at the Base of the Tongue: A Small Monoinstitutional Series. Journal of Personalized Medicine, 15(9), 419. https://doi.org/10.3390/jpm15090419







