Two Machine Learning Models to Economize Glaucoma Screening Programs: An Approach Based on Neural Nets
Abstract
1. Introduction
2. Materials and Methods
2.1. Definition of Definite OAG
2.2. Definition of Endpoints
10 Years Follow-Up
2.3. Statistical Methods and Model Development
2.4. Machine Learning Algorithms, Training and Testing
2.5. Data for Model Training and Testing
2.6. Methods for Model Training
2.6.1. Neural Nets
2.6.2. Other Models
2.7. Feature Selection
2.8. Reject Option
3. Results
3.1. Demographic Data at Baseline
3.2. Overview of Demographic Data at 10-Year Follow-Up
3.3. Illustration of the Models Using Real Data
3.4. Illustration for the Need for a Reject Option
4. Discussion
4.1. Discussion of Model M1
4.2. Discussion of Model M2
4.3. PEX as Risk Factor and How It Was Handled by the Models
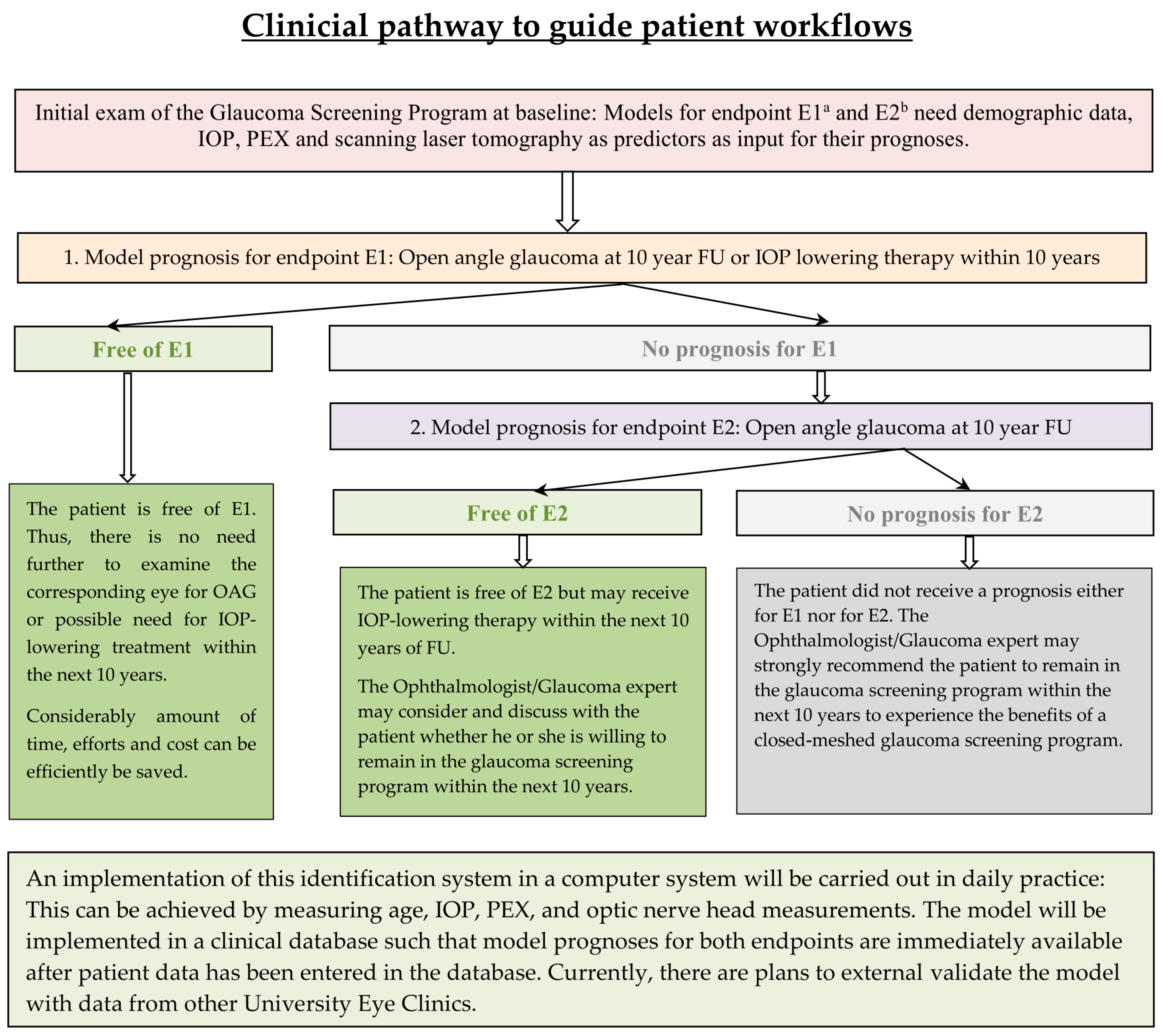
4.4. Implication for Practical Purposes
4.5. Outlook and Further Developments
4.6. Strengths and Limitations of This Study
4.6.1. Strengths
4.6.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vision Loss Expert Group of the Global Burden of Disease Study; GBD 2019 Blindness and Vision Impairment Collaborators. Prevalence of Vision Loss in High-Income Countries and in Eastern and Central Europe in 2020: Magnitude and Temporal Trends. Ophthalmic Epidemiol. 2025, 1–14, ahead of print. [Google Scholar]
- Johns, K.L.; Faralli, J.A.; Filla, M.S.; Shah, N.S.; Sun, Y.Y.; Keller, K.E.; Peters, D.M. Age-Related Dysregulation of α5β1 and αvβ3 Integrin Activity Alters Contractile Properties of Trabecular Meshwork Cells. Investig. Ophthalmol. Vis. Sci. 2025, 66, 31. [Google Scholar] [CrossRef] [PubMed]
- Adelpour, M.; Moghimi, S.; Nishida, T.; Meller, L.; Du, K.H.; Kamalipour, A.; Tansuebchueasai, N.; Mahmoudinezhad, G.; Zangwill, L.M.; Weinreb, R.N. Short-Term Rates of Visual Field Change Predict Glaucoma Progression. Ophthalmol. Glaucoma 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Tonti, E.; Tonti, S.; Mancini, F.; Bonini, C.; Spadea, L.; D’Esposito, F.; Gagliano, C.; Musa, M.; Zeppieri, M. Artificial Intelligence and Advanced Technology in Glaucoma: A Review. J. Pers. Med. 2024, 14, 1062. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Lin, S.; Moghimi, S. Application of artificial intelligence in glaucoma care: An updated review. Taiwan J. Ophthalmol. 2024, 14, 340–351. [Google Scholar] [CrossRef]
- Ravindranath, R.; Stein, J.D.; Hernandez-Boussard, T.; Fisher, A.C.; Wang, S.Y.; SOURCE Consortium. The Impact of Race, Ethnicity, and Sex on Fairness in Artificial Intelligence for Glaucoma Prediction Models. Ophthalmol. Sci. 2024, 5, 100596. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Wu, J.; Cao, J.; Feng, Y.; Zhou, J.; Luo, Z.; Song, P.; Rudan, I.; Global Health Epidemiology Research Group (GHERG). Global incidence and risk factors for glaucoma: A systematic review and meta-analysis of prospective studies. J. Glob. Health 2024, 14, 04252. [Google Scholar] [CrossRef] [PubMed]
- Mastronikolis, S.; Pagkalou, M.; Baroutas, G.; Kyriakopoulou, K.; Makri, O.E.; Georgakopoulos, C.D. Pseudoexfoliation syndrome: The critical role of the extracellular matrix in pathogenesis and treatment. IUBMB Life 2022, 74, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari Sharaf, M.; Damji, K.F.; Unsworth, L.D. Recent advances in risk factors associated with ocular exfoliation syndrome. Acta Ophthalmol. 2020, 98, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.Y.; Wang, S.Y. Standardizing the diagnosis and treatment of ocular hypertension based on evidence-based medicine. Chin. Med. J. 2024, 104, 2195–2199. [Google Scholar]
- Svedberg, E.; Ekström, C. Distribution of cup-disc ratio in a Swedish population. Upsala J. Med. Sci. 2023, 128, 10-48101. [Google Scholar] [CrossRef] [PubMed]
- Ekström, C.; Carlsson, C. Incidence of blindness in open-angle glaucoma in Sweden: A long-term follow-up study. Upsala J. Med. Sci. 2024, 129, 10-48101. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Yang, H.; Wei, C.C.; Xu, L.; Wei, W.B.; Jonas, J.B. High myopia as risk factor for the 10-year incidence of open-angle glaucoma in the Beijing Eye Study. Br. J. Ophthalmol. 2023, 107, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Spaeth, G.L. European Glaucoma Society Terminology and Guidelines for Glaucoma, 5th edition. Br. J. Ophthalmol. 2021, 105, 1–169. [Google Scholar] [CrossRef]
- Wolfram Research, Inc. Mathematica, version 13.3; Wolfram Research: Champaign, IL, USA, 2023.
- Franc, V.; Prusa, D.; Voracek, V. Optimal strategies for reject option classifiers. J. Mach. Learn. Res. 2023, 24, 1–49. [Google Scholar]
- Cloud Software Group, Inc. Spotfire, version 14. Data Science Workbench. Cloud Software Group: Fort Lauderdale, FL, USA, 2023. Available online: https://www.spotfire.com/ (accessed on 28 October 2024).
- Lan, C.H.; Chiu, T.H.; Yen, W.T.; Lu, D.W. Artificial Intelligence in Glaucoma: Advances in Diagnosis, Progression Forecasting, and Surgical Outcome Prediction. Int. J. Mol. Sci. 2025, 26, 4473. [Google Scholar] [CrossRef]
- Carlà, M.M.; Gambini, G.; Giannuzzi, F.; Boselli, F.; De Luca, L.; Rizzo, S. Testing the Reliability of ChatGPT Assistance for Surgical Choices in Challenging Glaucoma Cases. J. Pers. Med. 2025, 15, 97. [Google Scholar] [CrossRef]
- Wąż, P.; Zorena, K.; Murawska, A.; Bielińska-Wąż, D. Classification Maps: A New Mathematical Tool Supporting the Diagnosis of Age-Related Macular Degeneration. J. Pers. Med. 2023, 13, 1074. [Google Scholar] [CrossRef] [PubMed]
- Demir, F.; Burak, T. An Effective and Robust Approach Based on R-CNN+LSTM Model and NCAR Feature Selection for Ophthalmological Disease Detection from Fundus Images. J. Pers. Med. 2021, 11, 1276. [Google Scholar] [CrossRef] [PubMed]
- Govindaiah, A.; Baten, A.; Smith, R.T.; Balasubramanian, S.; Bhuiyan, A. Optimized Prediction Models from Fundus Imaging and Genetics for Late Age-Related Macular Degeneration. J. Pers. Med. 2021, 11, 1127. [Google Scholar] [CrossRef] [PubMed]
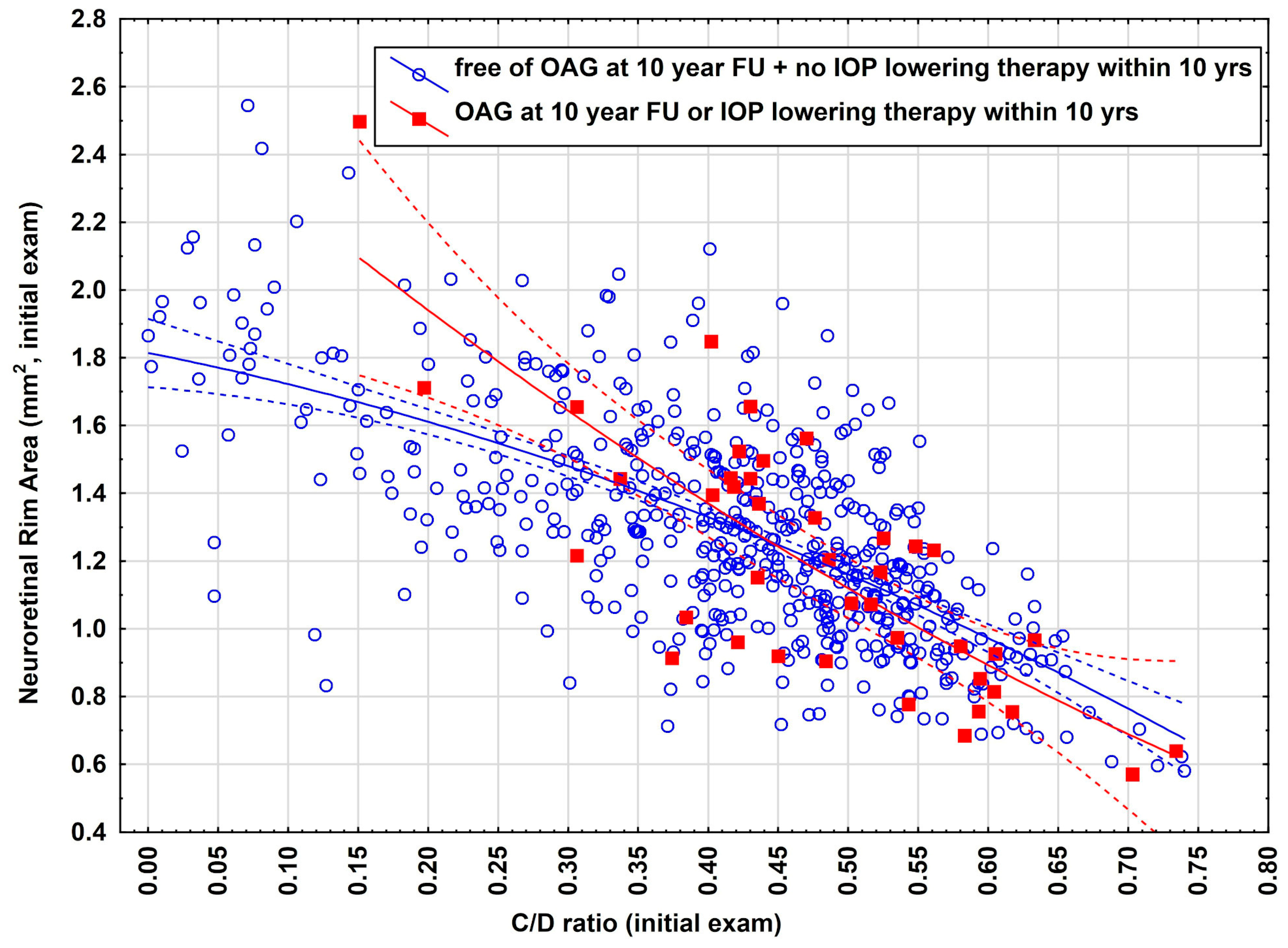
| OAG-Free and no IOP Lowering Therapy 10 yr FU (n = 544) | OAG or IOP-Lowering Therapy at 10 yr FU (n = 41) | |||||
|---|---|---|---|---|---|---|
| Mean | Std | Mean | Std | Odds Ratio | p-Value | |
| Age (yrs) | 58.84 | 7.96 | 60.7 | 7.29 | 1.02 (0.99–1.08) | 0.16 1 |
| IOP (mmHg) | 15.1 | 2.83 | 18.5 | 4.27 | 1.33 (1.21–1.46) | <0.0001 1,* |
| PEX | 12/532 | 2.2% | 3/38 | 7.3% | 3.5 (0.6–13.7) | 0.027 2,* |
| Effective Area (mm2) | 0.95 | 0.40 | 1.08 | 0.32 | 2.37 (1.02–5.5) | 0.042 1,* |
| Neuroretinal Rim Area (mm2) | 1.29 | 0.32 | 1.19 | 0.18 | 0.38 (0.13–1.09) | 0.07 1 |
| Half Depth Area (mm2) | 0.37 | 0.21 | 0.44 | 0.20 | 4.0 (1.01–15.8) | 0.047 1,* |
| Half Depth Volume (mm3) | −0.06 | 0.06 | −0.07 | 0.05 | 0.08 (0.008–7.09) | 0.26 1 |
| Volume Below (mm3) | −0.25 | 0.18 | −0.31 | 0.20 | 0.23 (0.05–0.996) | 0.047 1,* |
| Cup-To-Disc Ratio/0.1 unit change | 0.42 | 0.14 | 0.48 | 0.12 | 1.46 (1.11–1.92) | 0.006 1,* |
| Mean | SD | −95% CI | +95% CI | |
|---|---|---|---|---|
| Age (y) | 58.9 | 8.1 | 58.8 | 59.6 |
| Follow-up (years) | 11.1 | 1.1 | 10.99 | 11.2 |
| IOP (mmHg) | 15.3 | 3.2 | 15.1 | 15.6 |
| Total Contour Area (mm2) | 2.23 | 0.44 | 2.20 | 2.27 |
| Effective Area (mm2) | 0.96 | 0.39 | 0.93 | 0.99 |
| Neuroretinal Rim Area (mm2) | 1.28 | 0.33 | 1.25 | 1.31 |
| Half Depth Area (mm2) | 0.38 | 0.21 | 0.36 | 0.40 |
| Half Depth Volume (mm3) | −0.06 | 0.057 | −0.066 | −0.057 |
| Volume Below (mm3) | −0.26 | 0.19 | −0.27 | −0.24 |
| Cup-To-Disc Ratio | 0.42 | 0.14 | 0.41 | 0.43 |
| N | Percentage | −95% CI | 95% CI | |
| Endpoint E1: OAG 2 at 10-year FU or IOP lowering therapy within 10 years | 41/585 | 7% | 5.1% | 9.4% |
| Endpoint E2: OAG 2 at 10-year FU | 21/585 | 3.6% | 2.23% | 5.43% |
| Model Performance | Support Vector Machine | Nearest Neighbors | Random Forest | Bayes Classifier | Neural Network |
|---|---|---|---|---|---|
| (NPV/PPV)% | 93.2/92.3% | 93.2/92.3% | 93.2/92.4% | 90.4/85.5% | 93.5/93.2% |
| Endpoint | Percentage of Eyes Filtered Out/Received a Prediction (%) | Performances of the Models 3 (n/%) | Advantages | Disadvantages | Remarks |
|---|---|---|---|---|---|
| E1 2 | 57%/43% | 253/253 (100%) | In total, 43% of all eyes can safely be excluded from a glaucoma screening program for up to 10 years if one wants to be certain that the eye remains OAG-free and will not require IOP-lowering therapy. This also significantly reduces the screening amount. | 57% did not receive a prediction. | Eyes can safely be excluded from the OAG screening program, especially if the patient wants to leave the screening program. |
| E2 1 | 53.6%/46.4% | 271/271 (100%) | Overall, 46.4% of all eyes can safely be excluded from a glaucoma screening program for up to 10 years if one wants to be certain that the eye remains OAG-free. This significantly reduces the screening workload of ophthalmologists. | Overall, 53.6% did not receive a prediction. Eyes receiving IOP-lowering therapy were not filtered out. | This model is designed for patients in a glaucoma screening setting within a hospital, assuming these patients want to remain within the screening program and continue with IOP-lowering therapy. |
| Initial Exam | Predictions of Endpoint E1 Made at Initial Exam | Observed Endpoint E1 at the 10 yr FU | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | IOP 1 | EA 2 | NR 3 | VB 4 | HDA 5 | HD 6 | C/D 7 | PEX 8 | ||
| 62 | 12 | 0.85 | 1.22 | −0.15 | 0.19 | −0.02 | 0.41 | No | No prediction | OAG-free/no IOP low. therapy |
| 63 | 14 | 0.66 | 1.46 | −0.15 | 0.24 | −0.03 | 0.31 | No | OAG-free/no IOP low. therapy | OAG-free/no IOP low. therapy |
| 56 | 15 | 1.49 | 1.18 | −0.35 | 0.60 | −0.07 | 0.56 | No | OAG-free/no IOP low. therapy | OAG-free/no IOP low. therapy |
| 61 | 15 | 1.22 | 0.76 | −0.52 | 0.48 | −0.11 | 0.62 | No | No prediction | OAG |
| 66 | 14 | 1.41 | 1.10 | −0.34 | 0.50 | −0.09 | 0.56 | No | OAG-free/no IOP low. therapy | OAG-free/no IOP low. therapy |
| 42 | 17 | 0.51 | 1.73 | −0.10 | 0.19 | −0.03 | 0.23 | No | OAG-free/no IOP low. therapy | OAG-free/no IOP low. therapy |
| 54 | 14 | 0.85 | 0.91 | −0.13 | 0.32 | −0.02 | 0.49 | No | No prediction | OAG |
| 69 | 13 | 0.96 | 1.14 | −0.18 | 0.39 | −0.05 | 0.46 | No | OAG-free/no IOP low. therapy | OAG-free/no IOP low. therapy |
| 66 | 14 | 1.13 | 1.21 | −0.31 | 0.34 | −0.06 | 0.48 | No | OAG-free/no IOP low. therapy | OAG-free/no IOP low. therapy |
| 70 | 14 | 1.09 | 1.44 | −0.17 | 0.27 | −0.028 | 0.43 | Yes | No prediction | IOP low. therapy |
| 59 | 18 | 0.83 | 1.18 | −0.21 | 0.22 | −0.04 | 0.41 | No | OAG-free/no IOP low. therapy | OAG-free/no IOP low. therapy |
| 62 | 15 | 0.99 | 1.21 | −0.45 | 0.39 | −0.12 | 0.45 | No | No prediction | OAG-free/no IOP low. therapy |
| 57 | 12 | 0.50 | 1.39 | −0.06 | 0.20 | −0.01 | 0.27 | No | OAG-free/no IOP low. therapy | OAG-free/no IOP low. therapy |
| 58 | 13 | 1.16 | 0.97 | −0.65 | 0.66 | −0.22 | 0.54 | No | No prediction | OAG-free/no IOP low. therapy |
| 57 | 16 | 1.25 | 0.94 | −0.41 | 0.69 | −0.12 | 0.57 | No | No prediction | OAG-free/no IOP low. therapy |
| 62 | 12 | 1.21 | 1.25 | −0.64 | 0.70 | −0.19 | 0.49 | No | No prediction | OAG-free/no IOP low. therapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hitzl, W.; Lenzhofer, M.; Hohensinn, M.; Reitsamer, H.A. Two Machine Learning Models to Economize Glaucoma Screening Programs: An Approach Based on Neural Nets. J. Pers. Med. 2025, 15, 361. https://doi.org/10.3390/jpm15080361
Hitzl W, Lenzhofer M, Hohensinn M, Reitsamer HA. Two Machine Learning Models to Economize Glaucoma Screening Programs: An Approach Based on Neural Nets. Journal of Personalized Medicine. 2025; 15(8):361. https://doi.org/10.3390/jpm15080361
Chicago/Turabian StyleHitzl, Wolfgang, Markus Lenzhofer, Melchior Hohensinn, and Herbert Anton Reitsamer. 2025. "Two Machine Learning Models to Economize Glaucoma Screening Programs: An Approach Based on Neural Nets" Journal of Personalized Medicine 15, no. 8: 361. https://doi.org/10.3390/jpm15080361
APA StyleHitzl, W., Lenzhofer, M., Hohensinn, M., & Reitsamer, H. A. (2025). Two Machine Learning Models to Economize Glaucoma Screening Programs: An Approach Based on Neural Nets. Journal of Personalized Medicine, 15(8), 361. https://doi.org/10.3390/jpm15080361






