Clinical Outcomes of 3D-Printed Titanium Patient-Specific Implants in Lumbar Interbody Fusion: A Prospective Clinical Trial with a Systematic Review of Conventional Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. PSIC Cohort
2.1.1. Study Design
2.1.2. Ethical Approval
2.1.3. Sample Size and Power Analysis
2.1.4. Inclusion and Exclusion Criteria
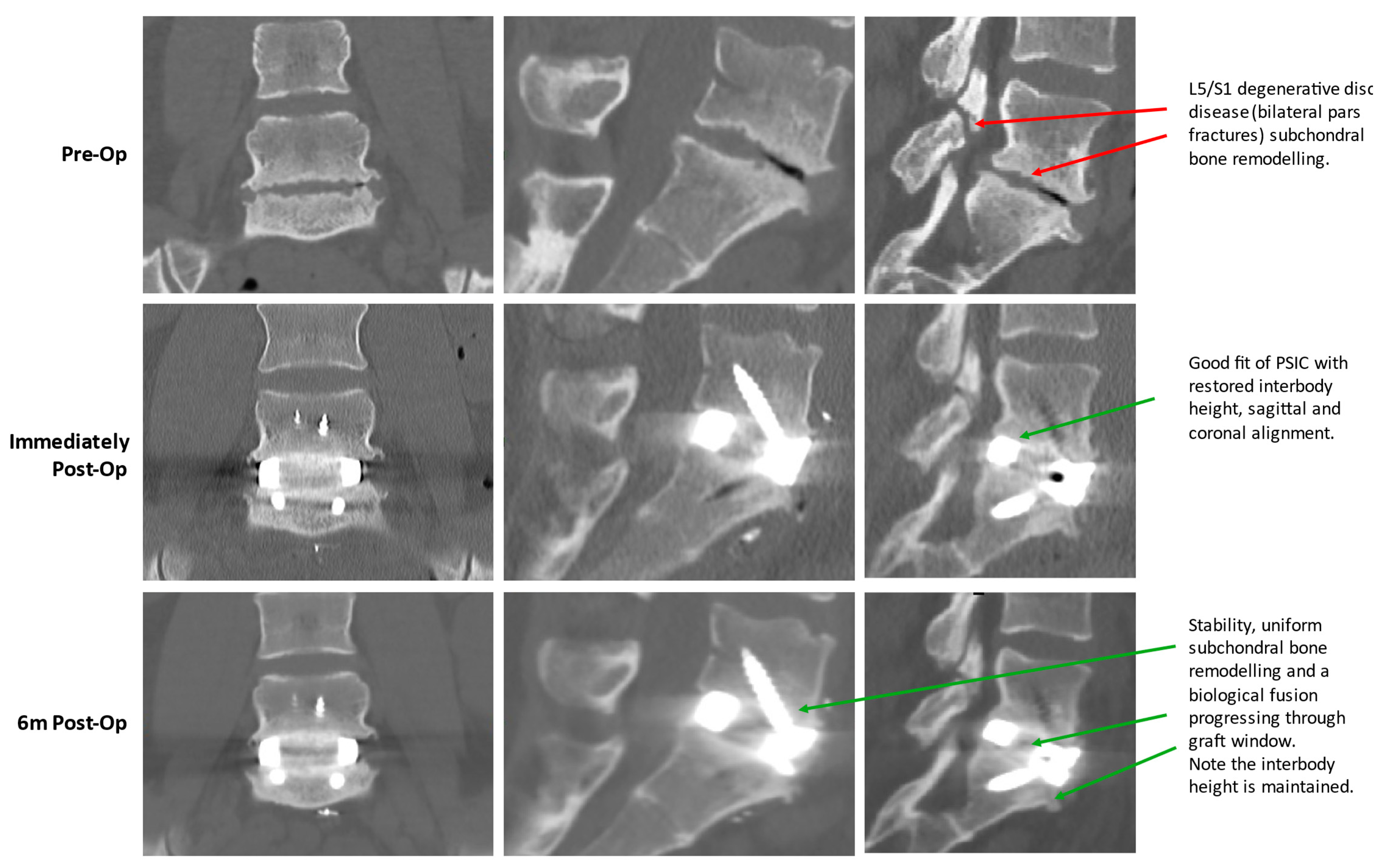
2.1.5. PSIC Design
2.1.6. Operative Technique
2.1.7. Post-Operative Care
2.1.8. Outcome Measures
2.1.9. Follow-Up Protocol
2.2. Systematic Review of OTS Comparator Group
2.2.1. Search Strategy
2.2.2. Systematic Review Inclusion and Exclusion Criteria
2.2.3. Systematic Review Outcome Measures
2.2.4. Risk of Bias Assessment
2.2.5. Data Extraction and Analysis
2.3. Statistical Analyses
3. Results
3.1. Systematic Review
3.2. Patient-Reported Outcome Measures (PROMs)
3.2.1. VAS Clinical Outcomes
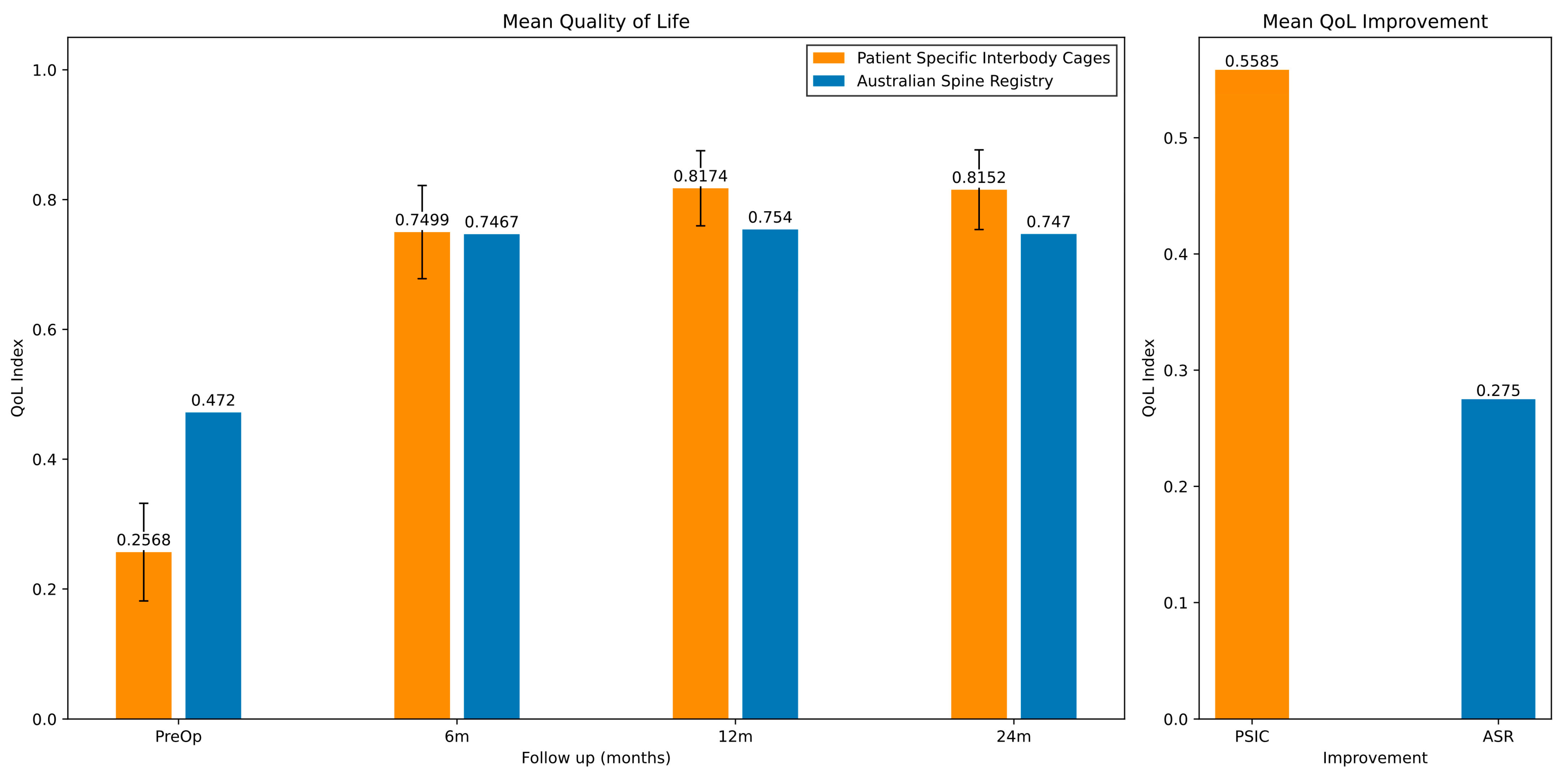
3.2.2. Quality of Life (QoL) Clinical Outcomes
3.3. Revision and Reoperation Results
4. Discussion
4.1. VAS
4.2. QoL
4.3. Revision and Reoperation
4.4. Efficacy
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lee, C.K.; Langrana, N.A. A Review of Spinal Fusion for Degenerative Disc Disease: Need for Alternative Treatment Approach of Disc Arthroplasty? Spine J. 2004, 4, S173–S176. [Google Scholar] [CrossRef] [PubMed]
- Yavin, D.; Casha, S.; Wiebe, S.; Feasby, T.E.; Clark, C.; Isaacs, A.; Holroyd-Leduc, J.; Hurlbert, R.J.; Quan, H.; Nataraj, A. Lumbar Fusion for Degenerative Disease: A Systematic Review and Meta-Analysis. Neurosurgery 2017, 80, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Elias, E.; Sursal, T.; Line, B.; Lafage, V.; Lafage, R.; Klineberg, E.; Kim, H.J.; Passias, P.; Nasser, Z.; et al. How Good Are Surgeons at Achieving Their Preoperative Goal Sagittal Alignment Following Adult Deformity Surgery? Glob. Spine J. 2023, 14, 1924–1936. [Google Scholar] [CrossRef] [PubMed]
- Mummaneni, P.V.; Hussain, I.; Shaffrey, C.I.; Eastlack, R.K.; Mundis, G.M.; Uribe, J.S.; Fessler, R.G.; Park, P.; Robinson, L.; Rivera, J.; et al. The Minimally Invasive Interbody Selection Algorithm for Spinal Deformity. J. Neurosurg. Spine 2021, 34, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.; Mobbs, R.J. Evolution of Design of Interbody Cages for Anterior Lumbar Interbody Fusion. Orthop. Surg. 2016, 8, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, Y.; Zou, D.; Yuan, B.; Ke, H.Z.; Li, W. Therapeutics for Enhancement of Spinal Fusion: A Mini Review. J. Orthop. Transl. 2021, 31, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Lewin, A.M.; Fearnside, M.; Kuru, R.; Jonker, B.P.; Naylor, J.M.; Sheridan, M.; Harris, I.A. Rates, Costs, Return to Work and Reoperation Following Spinal Surgery in a Workers’ Compensation Cohort in New South Wales, 2010–2018: A Cohort Study Using Administrative Data. BMC Health Serv. Res. 2021, 21, 955. [Google Scholar] [CrossRef] [PubMed]
- Giang, G.; Mobbs, R.; Phan, S.; Tran, T.M.; Phan, K. Evaluating Outcomes of Stand-Alone Anterior Lumbar Interbody Fusion: A Systematic Review. World Neurosurg. 2017, 104, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Parr, W.C.H.; Choy, W.J.; Budiono, G.R.; Maharaj, M.; Mathis, X.; Phan, K.; Walsh, W.R.; Mobbs, R.J. Three-Dimensional Morphometric Analysis of Lumbar Vertebral End Plate Anatomy. World Neurosurg. 2020, 135, e321–e332. [Google Scholar] [CrossRef] [PubMed]
- Suh, P.B.; Puttlitz, C.; Lewis, C.; Bal, B.S.; McGilvray, K. The Effect of Cervical Interbody Cage Morphology, Material Composition, and Substrate Density on Cage Subsidence. J. Am. Acad. Orthop. Surg. 2017, 25, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Hull, C.W. Apparatus for Production of Three-Dimensional Objects by Stereolithography. U.S. Patent 638,905, 8 August 1984. [Google Scholar]
- Ventola, C.L. Medical Applications for 3D Printing: Current and Projected Uses. Pharm. Ther. 2014, 39, 704. [Google Scholar]
- Aimar, A.; Palermo, A.; Innocenti, B. The Role of 3D Printing in Medical Applications: A State of the Art. J. Healthc. Eng. 2019, 2019, 5340616. [Google Scholar] [CrossRef] [PubMed]
- Lador, R.; Regev, G.; Salame, K.; Khashan, M.; Lidar, Z. Use of 3-Dimensional Printing Technology in Complex Spine Surgeries. World Neurosurg. 2020, 133, e327–e341. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, P.S.; Askin, G.; Earwaker, J.S.; Merry, G.S.; Thompson, R.G.; Barker, T.M.; Effeney, D.J. Spinal Biomodeling. Spine 1999, 24, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Guo, W.; Ji, T.; Zhang, Y.; Liang, H. One-Step Reconstruction with a 3D-Printed, Custom-Made Prosthesis after Total En Bloc Sacrectomy: A Technical Note. Eur. Spine J. 2017, 26, 1902–1909. [Google Scholar] [CrossRef] [PubMed]
- Mobbs, R.J.; Choy, W.J.; Wilson, P.; McEvoy, A.; Phan, K.; Parr, W.C.H. L5 En-Bloc Vertebrectomy with Customized Reconstructive Implant: Comparison of Patient-Specific versus off-the-Shelf Implant. World Neurosurg. 2018, 112, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Wallace, N.; Schaffer, N.E.; Aleem, I.S.; Patel, R. 3D-Printed Patient-Specific Spine Implants: A Systematic Review. Clin. Spine Surg. 2020, 33, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Burnard, J.L.; Parr, W.C.H.; Choy, W.J.; Walsh, W.R.; Mobbs, R.J. 3D-Printed Spine Surgery Implants: A Systematic Review of the Efficacy and Clinical Safety Profile of Patient-Specific and off-the-Shelf Devices. Eur. Spine J. 2020, 29, 1248–1260. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.; Gupta, M.; Singh, M.; Kalyanasundaram, D. Outcome and Safety Analysis of 3D-Printed Patient-Specific Pedicle Screw Jigs for Complex Spinal Deformities: A Comparative Study. Spine J. 2019, 19, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.; Ding, H.; Chen, H.; Miao, Q.; Yang, X.; Li, K.; Zhang, K.; Wu, Z.; Tang, Y.; Xia, H. Three-Dimensional–Printed Individualized Guiding Templates for Surgical Correction of Severe Kyphoscoliosis Secondary to Ankylosing Spondylitis: Outcomes of 9 Cases. World Neurosurg. 2019, 130, e961–e970. [Google Scholar] [CrossRef] [PubMed]
- Pijpker, P.A.J.; Kuijlen, J.M.A.; Kraeima, J.; Faber, C. Three-Dimensional Planning and Use of Individualized Osteotomy-Guiding Templates for Surgical Correction of Kyphoscoliosis: A Technical Case Report. World Neurosurg. 2018, 119, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Tredan, D.A.M.; Mobbs, R.J.; Maharaj, M.; Parr, W.C.H. Combining Virtual Surgical Planning and Patient-Specific 3D-Printing as a Solution to Complex Spinal Revision Surgery. J. Pers. Med. 2023, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Apos, E.; Bulmer, S.; Truong, T.; Garduce, P.; Pourghaderi, A.; Cunningham, A.S.; Kuru, R.; Ho, S.; Mt, I.; Stanford, R.; et al. The Australian Spine Registry Annual Report, 2023: Spine Society of Australia and Public Health and Preventive Medicine. Monash University: Melbourne, VIC, Australia April 2024, Report No. 06.
- Rosner, B.A. Fundamentals of Biostatistics; Thomson-Brooks/Cole: Belmont, CA, USA, 2006; Volume 6. [Google Scholar]
- Kelly, A. The Minimum Clinically Significant Difference in Visual Analogue Scale Pain Score Does Not Differ with Severity of Pain. Emerg. Med. J. 2001, 18, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.J.; Loganathan, A.; Yeung, V.; Mobbs, R.J. Outcomes of Anterior Lumbar Interbody Fusion Surgery Based on Indication: A Prospective Study. Neurosurgery 2015, 76, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Hani, U.; Monk, S.H.; Pfortmiller, D.; Stanley, G.; Kim, P.K.; Bohl, M.A.; Holland, C.M.; McGirt, M.J. Effect of Workers’ Compensation Status on Pain, Disability, Quality of Life, and Return to Work after Lumbar Spine Surgery: A 1-Year Propensity-Matched Analysis. J. Neurosurg. Spine 2023, 39, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Parr, W.C.H.; Wroe, S.; Chamoli, U.; Richards, H.S.; McCurry, M.R.; Clausen, P.D.; McHenry, C. Toward Integration of Geometric Morphometrics and Computational Biomechanics: New Methods for 3D Virtual Reconstruction and Quantitative Analysis of Finite Element Models. J. Theor. Biol. 2012, 301, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M.; Boehlert, C.J. Titanium Alloys for Biomedical Applications. In Advances in Metallic Biomaterials: Tissues, Materials and Biological Reactions; Springer: Berlin/Heidelberg, Germany, 2015; pp. 179–213. [Google Scholar]
- Pelletier, M.H.; Cordaro, N.; Punjabi, V.M.; Waites, M.; Lau, A.; Walsh, W.R. PEEK versus Ti Interbody Fusion Devices: Resultant Fusion, Bone Apposition, Initial and 26-Week Biomechanics. Clin. Spine Surg. 2016, 29, E208–E214. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Aoki, K.; Ohya, K. Effects of Surface Roughness of Titanium Implants on Bone Remodeling Activity of Femur in Rabbits. Bone 1997, 21, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Torstrick, F.B.; Lin, A.S.P.; Safranski, D.L.; Potter, D.; Sulchek, T.; Lee, C.S.D.; Gall, K.; Guldberg, R.E. Effects of Surface Topography and Chemistry on Polyether-Ether-Ketone (PEEK) and Titanium Osseointegration. Spine 2020, 45, E417–E424. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.; Jimbo, R.; Kozai, Y.; Sakurai, T.; Kjellin, P.; Currie, F.; Wennerberg, A. Nanosized Hydroxyapatite Coating on PEEK Implants Enhances Early Bone Formation: A Histological and Three-Dimensional Investigation in Rabbit Bone. Materials 2015, 8, 3815–3830. [Google Scholar] [CrossRef] [PubMed]
- Walsh, W.R.; Pelletier, M.H.; Bertollo, N.; Christou, C.; Tan, C. Does PEEK/HA Enhance Bone Formation Compared with PEEK in a Sheep Cervical Fusion Model? Clin. Orthop. Relat. Res. 2016, 474, 2364–2372. [Google Scholar] [CrossRef] [PubMed]
- Yataganbaba, A.; Parr, W.C.H.; Hill, J.C.; Seex, K.A. The Importance of Stabilisation in Enabling Bone Fusion Demonstrated by Successful Revision of Failed Occipitocervical Fusion Using Patient-Specific Atlantoaxial Joint Spacers: A Case Report. J. Spine Surg. 2025, 11, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Group, T.E. EuroQol-a New Facility for the Measurement of Health-Related Quality of Life. Health Policy 1990, 16, 199–208. [Google Scholar]
- Abelson, P. S2 The Value of Life and Health for Public Policy*. Econ. Rec. 2003, 79, 2–13. [Google Scholar] [CrossRef]
- Norman, R.; Mulhern, B.; Lancsar, E.; Lorgelly, P.; Ratcliffe, J.; Street, D.; Viney, R. The Use of a Discrete Choice Experiment Including Both Duration and Dead for the Development of an EQ-5D-5L Value Set for Australia. Pharmacoeconomics 2023, 41, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Viney, R.; Norman, R.; King, M.T.; Cronin, P.; Street, D.J.; Knox, S.; Ratcliffe, J. Time Trade-off Derived EQ-5D Weights for Australia. Value Health 2011, 14, 928–936. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, N.; Kaambwa, B.; Currow, D.C.; Ratcliffe, J. Health-Related Quality of Life Measured Using the EQ-5D-5L: South Australian Population Norms. Health Qual. Life Outcomes 2016, 14, 133. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T. Chapter 8: Assessing Risk of Bias in Included Studies. In Cochrane Handbook for Systematic Reviews of Interventions; The Cochrane Collaboration: London, UK, 2011; Volume 5. [Google Scholar]
- Vali, Y.; Leeflang, M.M.G.; Bossuyt, P.M.M. Application of Weighting Methods for Presenting Risk-of-Bias Assessments in Systematic Reviews of Diagnostic Test Accuracy Studies. Syst. Rev. 2021, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Buttermann, G.R.; Mullin, W.J. Two-Level Circumferential Lumbar Fusion Comparing Midline and Paraspinal Posterior Approach: 5-Year Interim Outcomes of a Randomized, Blinded, Prospective Study. J. Spinal Disord. Tech. 2015, 28, E534–E543. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, W.; McLain, R.F.; Rufo-Smith, C.; Gurd, D.P. Prospective Randomized Controlled Trial of the Stabilis Stand Alone Cage (SAC) versus Bagby and Kuslich (BAK) Implants for Anterior Lumbar Interbody Fusion. Int. J. Spine Surg. 2014, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Zigler, J.; Delamarter, R.; Spivak, J.M.; Linovitz, R.J.; Danielson, G.O.; Haider, T.T.; Cammisa, F.; Zuchermann, J.; Balderston, R.; Kitchel, S.; et al. Results of the Prospective, Randomized, Multicenter Food and Drug Administration Investigational Device Exemption Study of the ProDisc-L Total Disc Replacement Versus Circumferential Fusion for the Treatment of 1-Level Degenerative Disc Disease. Spine 2007, 32, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Delamarter, R.; Zigler, J.E.; Balderston, R.A.; Cammisa, F.P.; Goldstein, J.A.; Spivak, J.M. Prospective, Randomized, Multicenter Food and Drug Administration Investigational Device Exemption Study of the ProDisc-L Total Disc Replacement Compared with Circumferential Arthrodesis for the Treatment of Two-Level Lumbar Degenerative Disc Disease: Results at Twenty-Four Months. J. Bone Jt. Surg. 2011, 93, 705–715. [Google Scholar] [CrossRef]
- Ohtori, S.; Kinoshita, T.; Yamashita, M.; Inoue, G.; Yamauchi, K.; Koshi, T.; Suzuki, M.; Orita, S.; Eguchi, Y.; Nakamura, S.; et al. Results of Surgery for Discogenic Low Back Pain A Randomized Study Using Discography Versus Discoblock for Diagnosis. Spine 2009, 34, 1345–1348. [Google Scholar] [CrossRef] [PubMed]
- Gornet, M.F.; Burkus, J.K.; Dryer, R.F.; Peloza, J.H. Lumbar Disc Arthroplasty with MAVERICK Disc versus Stand-Alone Interbody Fusion: A Prospective, Randomized, Controlled, Multicenter Investigational Device Exemption Trial. Spine 2011, 36, E1600–E1611. [Google Scholar] [CrossRef] [PubMed]
- Christensen, F.B.; Hansen, E.S.; Eiskjaer, S.P.; Høy, K.; Helmig, P.; Neumann, P.; Niedermann, B.; Bünger, C.E. Circumferential Lumbar Spinal Fusion With Brantigan Cage Versus Posterolateral Fusion With Titanium Cotrel-Dubousset Instrumentation A Prospective, Randomized Clinical Study of 146 Patients. Spine 2002, 27, 2674–2683. [Google Scholar] [CrossRef] [PubMed]
- Hoff, E.K.; Strube, P.; Pumberger, M.; Zahn, R.K.; Putzier, M. ALIF and Total Disc Replacement versus 2-Level Circumferential Fusion with TLIF: A Prospective, Randomized, Clinical and Radiological Trial. Eur. Spine J. 2016, 25, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Rickert, M.; Fleege, C.; Papachristos, I.; Makowski, M.R.; Rauschmann, M.; Arabmotlagh, M. Clinical Outcome After Anterior Lumbar Interbody Fusion With a New Osteoinductive Bone Substitute Material A Randomized Clinical Pilot Study. Clin. Spine Surg. 2019, 32, E319–E325. [Google Scholar] [CrossRef] [PubMed]
- Menezes, C.M.; Lacerda, G.C.; do Valle, G.S.O.; de Oliveira Arruda, A.; Menezes, E.G. Ceramic Bone Graft Substitute vs Autograft in XLIF: A Prospective Randomized Single-Center Evaluation of Radiographic and Clinical Outcomes. Eur. Spine J. 2022, 31, 2262–2269. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, B.; Rauschmann, M.; Fleege, C.; Arabmotlagh, M.; Schmidt, S.; Martin, K.; Rickert, M. A Prospective, Randomized Study Evaluating Clinical and Radiographic Efficacy of Lumbar Interbody Fusion Performed Using a Truss Technology–Based Interbody Fusion Device with Homologous Bone or Bone Marrow Aspirate. Int. J. Spine Surg. 2020, 14, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, S.; Mcafee, P.C.; Guyer, R.D.; Hochschuler, S.H.; Geisler, F.H.; Holt, R.T.; Garcia, R.; Regan, J.J.; Ohnmeiss, D.D. A Prospective, Randomized, Multicenter Food and Drug Administration Investigational Device Exemptions Study of Lumbar Total Disc Replacement With the CHARITÉTM Artificial Disc Versus Lumbar Fusion Part I: Evaluation of Clinical Outcomes. Spine 2005, 30, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Thalgott, J.S.; Fogarty, M.E.; Giuffre, J.M.; Christenson, S.D.; Epstein, A.K.; Aprill, C. A Prospective, Randomized, Blinded, Single-Site Study to Evaluate the Clinical and Radiographic Differences Between Frozen and Freeze-Dried Allograft When Used as Part of a Circumferential Anterior Lumbar Interbody Fusion Procedure. Spine 2009, 34, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
- McKenna, P.J.; Freeman, B.J.C.; Mulholland, R.C.; Grevitt, M.P.; Webb, J.K.; Mehdian, S.H. A Prospective, Randomised Controlled Trial of Femoral Ring Allograft versus a Titanium Cage in Circumferential Lumbar Spinal Fusion with Minimum 2-Year Clinical Results. Eur. Spine J. 2005, 14, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Guyer, R.D.; Zigler, J.E.; Blumenthal, S.L.; Shellock, J.L.; Ohnmeiss, D.D. Evaluation of Anterior Lumbar Interbody Fusion Performed Using a Stand-Alone, Integrated Fusion Cage. Int. J. Spine Surg. 2023, 17, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jacob, K.C.; Patel, M.R.; Ribot, M.A.; Parsons, A.W.; Vanjani, N.N.; Pawlowski, H.; Prabhu, M.C.; Singh, K. Single-Level Minimally Invasive Transforaminal Lumbar Interbody Fusion versus Anterior Lumbar Interbody Fusion with Posterior Instrumentation at L5/S1. World Neurosurg. 2022, 157, e111–e122. [Google Scholar] [CrossRef] [PubMed]
- Kashlan, O.; Frerich, J.M.; Malcolm, J.G.; Gary, M.F.; Rodts, G.E.; Refai, D. Safety Profile and Radiographic and Clinical Outcomes of Stand-Alone 2-Level Anterior Lumbar Interbody Fusion: A Case Series of 41 Consecutive Patients. Cureus 2020, 12, e11684. [Google Scholar] [CrossRef] [PubMed]
- Kuang, L.; Wang, B.; Lü, G. Transforaminal Lumbar Interbody Fusion Versus Mini-Open Anterior Lumbar Interbody Fusion with Oblique Self-Anchored Stand-Alone Cages for the Treatment of Lumbar Disc Herniation. Spine 2017, 42, E1259–E1265. [Google Scholar] [CrossRef] [PubMed]
- Allain, J.; Delecrin, J.; Beaurain, J.; Poignard, A.; Vila, T.; Flouzat-Lachaniette, C.H. Stand-Alone ALIF with Integrated Intracorporeal Anchoring Plates in the Treatment of Degenerative Lumbar Disc Disease: A Prospective Study on 65 Cases. Eur. Spine J. 2014, 23, 2136–2143. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, P.; Mundis, G.M.; Eastlack, R.K.; Bagheri, R.; Vargas, E.; Tran, S.; Akbarnia, B.A. Preliminary Results of Anterior Lumbar Interbody Fusion, Anterior Column Realignment for the Treatment of Sagittal Malalignment. Neurosurg. Focus. 2017, 43, E6. [Google Scholar] [CrossRef] [PubMed]
- Siepe, C.J.; Stosch-Wiechert, K.; Heider, F.; Amnajtrakul, P.; Krenauer, A.; Hitzl, W.; Szeimies, U.; Stäbler, A.; Michael Mayer, H. Anterior Stand-Alone Fusion Revisited: A Prospective Clinical, X-Ray and CT Investigation. Eur. Spine J. 2015, 24, 838–851. [Google Scholar] [CrossRef] [PubMed]
- Noriega, D.C.; Eastlack, R.K.; Hernández, G.L.; Kafchitsas, K.; Ryang, Y.M.; Spitz, S.M.; Lite, I.S. Does the Interfacing Angle between Pedicle Screws and Support Rods Affect Clinical Outcomes after Posterior Thoracolumbar Fusion? A Retrospective Clinical Study. Spine J. 2024, 24, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Malham, G.M.; Wang, W.; McGuckin, J.P.; Mahoney, J.M.; Biddau, D.T.; Bucklen, B.S. Maximizing Screw Length in Expandable Lateral Lumbar Interbody Spacers with Integrated Fixation May Obviate the Need for Supplemental Pedicle Screws. Spine J. 2025, 25, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Buckland, A.J.; Ashayeri, K.; Leon, C.; Manning, J.; Eisen, L.; Medley, M.; Protopsaltis, T.S.; Thomas, J.A. Single Position Circumferential Fusion Improves Operative Efficiency, Reduces Complications and Length of Stay Compared with Traditional Circumferential Fusion. Spine J. 2021, 21, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, E.A.M.; Rath, A.; Antoine, S.L.; Eikermann, M.; Seidel, D.; Koenen, C.; Jacobs, E.; Pieper, D.; Laville, M.; Pitel, S.; et al. Specific Barriers to the Conduct of Randomised Clinical Trials on Medical Devices. Trials 2017, 18, 427. [Google Scholar] [CrossRef] [PubMed]
- Schuler, T.C.; Burkus, J.K.; Gornet, M.F.; Subach, B.R.; Zdeblick, T.A. The Correlation Between Preoperative Disc Space Height and Clinical Outcomes After Anterior Lumbar Interbody Fusion. J. Spinal Disord. Tech. 2005, 18, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, P.; Gerlach, R.; Schär, B.; Cain, C.M.J.; Achatz, W.; Pflugmacher, R.; Haas, N.P.; Kandziora, F. Biomechanical Comparison of Two Different Concepts for Stand Alone Anterior Lumbar Interbody Fusion. Eur. Spine J. 2008, 17, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Chiang, M.C.; Lin, J.F.; Lin, S.C.; Hung, C.H. Biomechanical Comparison of Three Stand-Alone Lumbar Cages—A Three-Dimensional Finite Element Analysis. BMC Musculoskelet. Disord. 2013, 14, 281. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, L.; Chohreh Partovian, M.; Lin, Z.; Herrin, J.; Grady, J.; Mitchell Conover, M.; Julia Montague, B.; Chloe Dillaway, M.; Kathleen Bartczak, B.; Lisa Suter, B.; et al. Hospital-Wide All-Cause Unplanned Readmission Measure Final Technical Report Submitted by Yale New Haven Health Services Corporation/Center for Outcomes Research & Evaluation (YNHHSC/CORE). 2012. Available online: https://www.cms.gov/priorities/innovation/files/fact-sheet/bpciadvanced-fs-nqf1789.pdf (accessed on 3 July 2025).
- Smith, J.S.; Mundis, G.M.; Osorio, J.A.; Nicolau, R.J.; Temple-Wong, M.; Lafage, R.; Bess, S.; Ames, C.P. Analysis of Personalized Interbody Implants in the Surgical Treatment of Adult Spinal Deformity. Glob. Spine J. 2023, 15, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Ames, C.P.; Smith, J.S.; Nicolau, R.J. Tomographic Assessment of Fusion Rate, Implant-Endplate Contact Area, Subsidence, and Alignment With Lumbar Personalized Interbody Implants at 1-Year Follow-Up. Int. J. Spine Surg. 2024, 18, S41–S49. [Google Scholar] [CrossRef] [PubMed]
- Walsh, W.R.; Pelletier, M.H.; Bertollo, N.; Lovric, V.; Wang, T.; Morberg, P.; Parr, W.C.H.; Bergadano, D. Bone Ongrowth and Mechanical Fixation of Implants in Cortical and Cancellous Bone. J. Orthop. Surg. Res. 2020, 15, 177. [Google Scholar] [CrossRef] [PubMed]
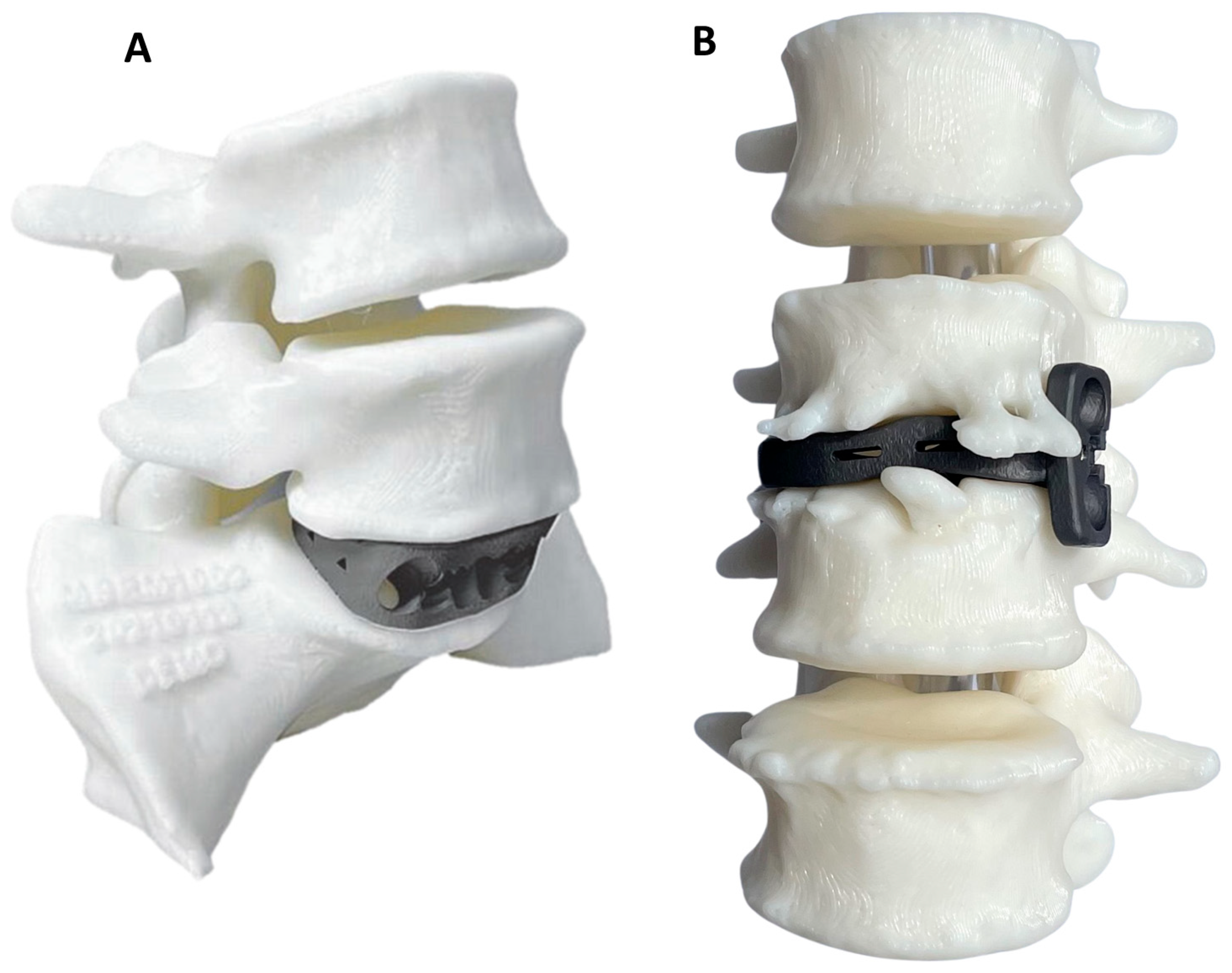
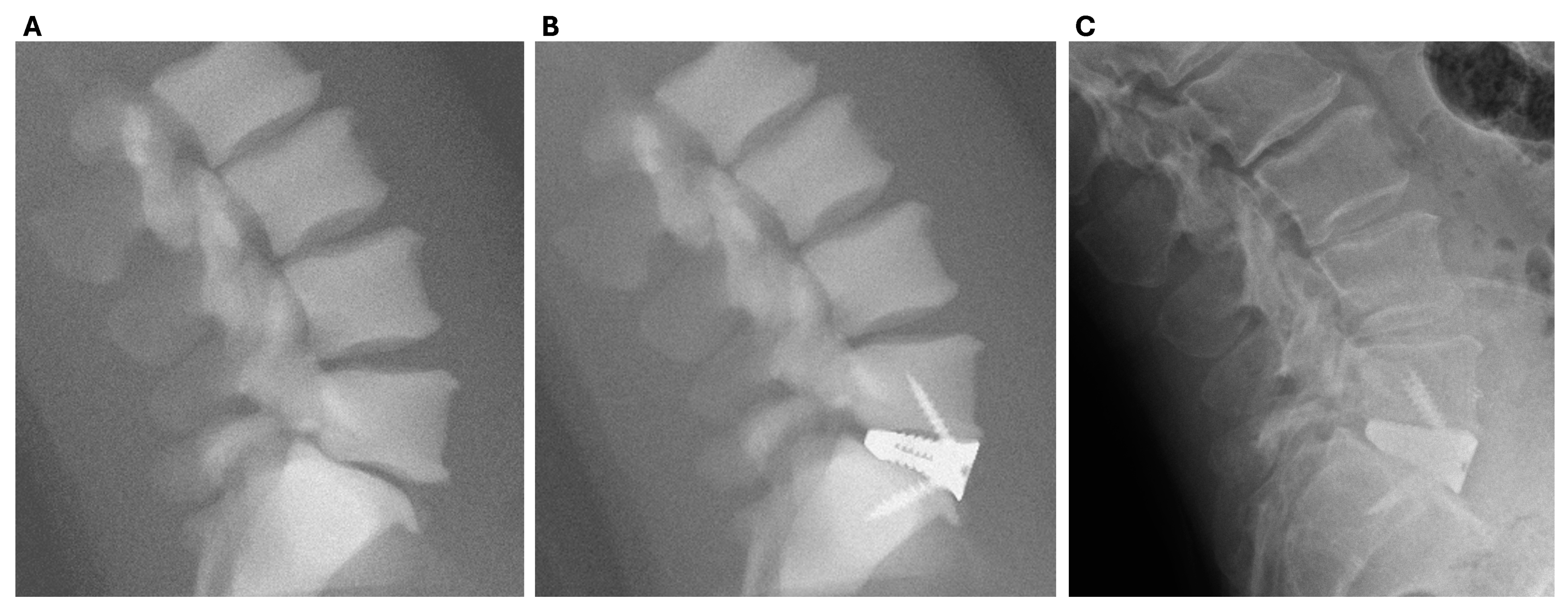
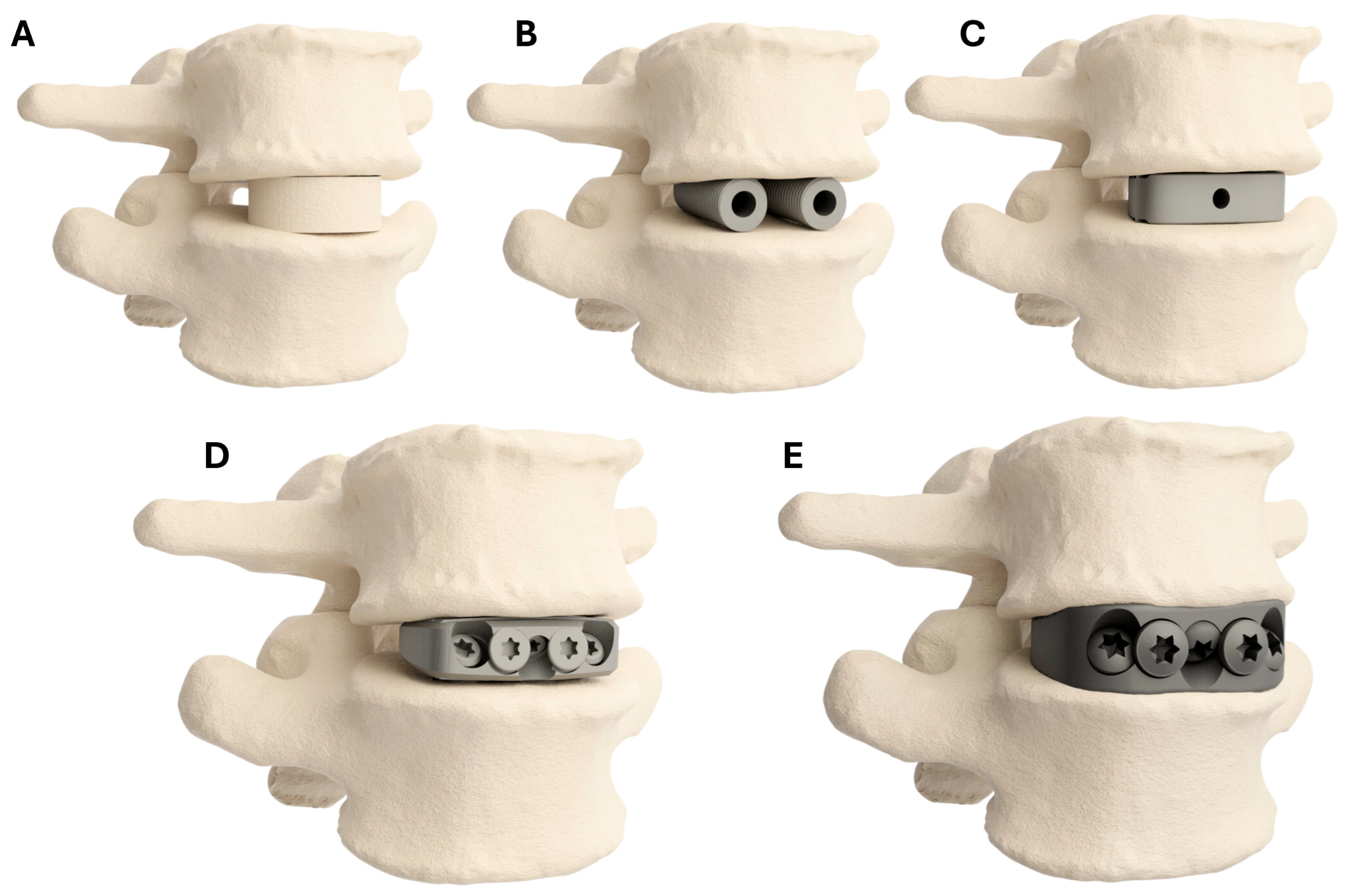


| PSIC | OTS—ISF | OTS—NISF | OTS—FRA | OTS—BAK | |
|---|---|---|---|---|---|
| Sample size (n) | 78 | 508 | 242 | 274 | 344 |
| Age (mean) | 57.7 | 52.9 | 55.6 | 42.6 | 42.1 |
| % Male/female | 56% | 43% | 38% | 42% | 46% |
| % Circumferential fusion | 59% | 24% | 95% | 100% | 0% |
| No. of operated levels (mean) | 1.4 | 1.2 | 1.2 | 1.6 | 1.1 |
| Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding of Participants and Personnel (Performance Bias) | Blinding of Outcome Assessment (Detection Bias) | Incomplete Outcome Data (Attrition Bias) | Selective Reporting (Reporting Bias) | No Other Bias | |
|---|---|---|---|---|---|---|---|
| McKenna et al. [59] |  |  |  |  |  |  |  |
| Chatterjee et al. [56] |  |  |  |  |  |  |  |
| Thalgott et al. [58] |  |  |  |  |  |  |  |
| Blumenthal et al. [57] |  |  |  |  |  |  |  |
| Hoff et al. [53] |  |  |  |  |  |  |  |
| Menezes et al. [55] |  |  |  |  |  |  |  |
| Christensen et al. [52] |  |  |  |  |  |  |  |
| Rickert et al. [54] |  |  |  |  |  |  |  |
| Gornet et al. [51] |  |  |  |  |  |  |  |
| Lavelle et al. [47] |  |  |  |  |  |  |  |
| Delamarter et al. [49] |  |  |  |  |  |  |  |
| Ziegler et al. [48] |  |  |  |  |  |  |  |
| Ohtori et al. [50] |  |  |  |  |  |  |  |
| Butterman et al. [46] |  |  |  |  |  |  |  |
 = Low risk,
= Low risk,  = Unclear/some risk,
= Unclear/some risk,  = High risk.
= High risk.| Author | Study Design | Year | Cohort Characteristics | Intervention/Comparator Arm | Mean (Age) | Sample Size | Follow-Up |
|---|---|---|---|---|---|---|---|
| McKenna et al. [59] | RCT | 2005 | DDD between L3 and S1 with pain/functional deficit for 6 months and failure to respond to conservative measures for at least 3 months. | Circumferential (ALIF) with SynCage | 41.4 (29–65) * | 41 | >24 months |
| Circumferential (ALIF) with FRA | 39 (24–53) * | 37 | |||||
| Chatterjee et al. [56] | RCT | 2020 | Patients were treated for one or more of the following diagnoses: painful DDD, postlaminectomy syndrome, spondylolisthesis, stenosis, failed discectomy, and recurrent disc herniation. | ALIF (bone marrow aspirate graft) | 58.95 (14.6) ** | 20 | 12 months |
| ALIF (cancellous homologous bone chips) | 58.33 (33.99) ** | 21 | |||||
| Thalgott et al. [58] | RCT | 2009 | Primary diagnosis of internal disc disruption diagnosed via discography, DDD, or herniated nucleus pulposus at 1 or 2 levels between L3 and S1. | ALIF (freeze dried femoral ring allograft) | 44.8 (6.87) ** | 19 | 24 months |
| ALIF (frozen allograft) | 42.29 (10.14) ** | 21 | |||||
| Blumenthal et al. [57] | RCT | 2005 | Symptomatic single-level DDD confirmed by discography, back pain, and/or leg pain with no nerve root compression. | ALIF | 39.6 (20–60) * | 99 | 24 months |
| Total Disc Replacement (TDR) | 39.6 (19–60) * | 205 | |||||
| Hoff et al. [53] | RCT | 2015 | Two-level DDD with severe low back pain with or without non-radicular leg pain. | Hybrid (ALIF)) | 45.4 (38–57) * | 26 | >24 months |
| Transforaminal LIF (TLIF | 47.6 (36–61) * | 24 | |||||
| Menezes et al. [55] | RCT | 2022 | Degenerative conditions of the spine. | Extreme LIF (XLIF) | 57.04 (3.8) ** | 30 | 24 months |
| 15 | |||||||
| Christensen et al. [52] | RCT | 2002 | Severe chronic low back/leg pain, static or dynamic, resulting from localized lumbar or lumbosacral segmental instability caused by isthmic spondylolisthesis (grades 1 and 2), primary degeneration (no previous surgery), secondary degeneration after decompressive surgery, or accelerating degeneration after decompressive surgery. | Circumferential (ALIF) | 45.4 (20–63) * | 75 | 24 months |
| Posterolateral fusion | 45.5 (23–65) * | 73 | |||||
| Rickert et al. [54] | RCT | 2019 | Chronic low back pain, radiculopathy, and spinal claudication symptoms caused by degenerative changes in the lumbar spine. | ALIF (NH-SiO2 Nanobone putty) | 60.6 (12.5) ** | 20 | 12 months |
| ALIF (Homologous bone graft) | 66.1 (9.6) ** | 20 | |||||
| Gornet et al. [51] | RCT | 2011 | DDD symptomatic of chronic low back pain. | ALIF | 40.2 (18–65) | 172 | 24 months |
| TDR | 39.9 (18–70) | 405 | |||||
| Lavelle et al. [47] | RCT | 2014 | Primary diagnosis of DDD at one or two contiguous levels in patients between 21 and 65 years of age; and chronic, disabling low back pain. | ALIF (Stabilis stand alone cage) | 43 (NR) | 41 | 24 months |
| ALIF (anterior threaded BAK cage) | 45.6 (NR) | 32 | |||||
| Delamarter et al. [49] | RCT | 2011 | DDD at two contiguous vertebral levels from L3 to S1 with or without leg pain. | Circumferential (ALIF) | 41.8 (7.81) ** | 72 | 24 months |
| TDR | 41.8 (7.73) ** | 165 | |||||
| Ziegler et al. [48] | RCT | 2007 | Single-level DDD at L3-S1 diagnosed by (i) back and/or leg pain AND (ii) radiographic confirmation. | Circumferential (ALIF) | 40.4 (7.6) ** | 75 | 24 months |
| TDR | 38.7 (8) ** | 161 | |||||
| Ohtori et al. [50] | RCT | 2009 | Patients had low back pain continuing for at least 3 years. | ALIF (Discography) | 33 (18–43) * | 15 | >24 months |
| ALIF (Discoblock) | 36 (20–46) * | 15 | |||||
| Butterman et al. [46] | RCT | 2015 | Two-level DDD with axial back pain greater than leg symptoms. | Circumferential (ALIF) with posterior open midline approach | 45.8 (12.6) ** | 25 | >24 months |
| Circumferential (ALIF) with posterior open paraspinal muscle splitting approach | 44 (10.6) ** | 25 | |||||
| Guyer et al. [60] | Cohort Study | 2023 | Persistent symptomatic disc degeneration. | ALIF | 47.8 (20–77) * | 58 | >24 months |
| Hosseini et al. [65] | Cohort Study | 2017 | Adult Spinal Deformity | ALIF | 66.1 (34–85) * | 39 | 12 months |
| Kashlan et al. [62] | Cohort Study | 2020 | Degenerative lumbar disease | ALIF | 51.9 | 23 | >9 months |
| Jacob et al. [61] | Cohort Study | 2022 | DDD | ALIF | 51.3 (13.3) ** | 59 | 12 months |
| TLIF | 46.7 (11.0) ** | 346 | |||||
| Kuang et al. [63] | Cohort Study | 2017 | Back or leg pain unresponsive to conservative treatment, noncalcified disc herniation compressing neural structures as confirmed by imaging. | ALIF | 52.9 (8.6) ** | 42 | 24 months |
| TLIF | 53.6 (7.4) ** | 40 | |||||
| Rao et al. [27] | Cohort Study | 2014 | ALIF for multiple indications including DDD, Spondylolisthesis, Failed Posterior Fusion, adjacent segment disease, and scoliosis. | ALIF | 57 (25–86) * | 125 | >24 months |
| Siepe et al. [66] | Cohort Study | 2014 | Patients were treated for predominant (>80%) low back pain resulting from degenerative disc disease at the L5/S1 level. | ALIF | 47 (18.7–73.9) * | 71 | 24 months |
| Allain et al. [64] | Cohort Study | 2014 | Back/leg pain unresponsive to conservative treatment, requiring 1 level surgery for degenerative disc disease | ALIF | 57.1 (11.1) ** | 65 | 12 months |
| Author | Cage Information | Cage Subgroup Reference 1 | Pre- Operative | 6M | 12M | 24M |
| McKenna et al. [59] | SynCage (Synthes) | NISF-SC | 7.1 | 5.8 | 6.4 | 6 |
| FRA | FRA | 7.2 | 5 | 4.8 | 5.2 | |
| Chatterjee et al. [56] | 4WEB Anterior Spine Truss System | NISF-4W | 6.85 | 3.88 | 4.05 | NR |
| 7.04 | 4.14 | 3.5 | NR | |||
| Thalgott et al. [58] | FRA | FRA | 8.2 | 6.7 | 6.2 | 6 |
| 8 | 6 | 4.8 | 4.9 | |||
| Blumenthal et al. [57] | BAK Fusion Cage (Zimmer Spine, Minneapolis, MN, USA) | BAK | 7.18 | 4.39 | 4.04 | 3.75 |
| TDR (CHARITE Artificial Disc) | NA | 7.2 | 3.31 | 3.29 | 3.12 | |
| Hoff et al. [53] | ALIF (PEEK Cage—SynFix LR, Depuy-Synthes); TDR (Maverick, Medtronic, Brampton, Canada) | ISF-SF | 6.8 | NR | 2.7 | 2.5 |
| TLIF (PEEK Cage—Capstone, Medtronic) | NA | 6.5 | NR | 3.1 | 3.8 | |
| Menezes et al. [55] | XLIF (PEEK, Nuvasive, San Diego, CA, USA) | NISF-XLIF | 7.3 | NR | NR | 1.6 |
| 6.1 | NR | NR | 1.5 | |||
| Christensen et al. [52] | ALIF (Radiolucent Brantigan Cage) | NISF | 7 | NR | 3 | 3 |
| PLF (Cotrel–Dubousset Instrumentation) | NA | 7 | NR | 5 | 5 | |
| Rickert et al. [54] | Carbon Fibre-Reinforced Polymer ALIF Cage (DePuy Synthes) | NISF-CF | 7.5 | 3 | 2 | NR |
| 7 | 3 | 3 | NR | |||
| Gornet et al. [51] | Tapered Fusion Cages (Lumbar Tapered Fusion Device, Medtronic) | BAK | 7.33 | 2.41 | 2.47 | 2.36 |
| Maverick Cobalt Chrome Alloy (Medtronic) | NA | 7.17 | 1.81 | 1.76 | 1.8 | |
| Lavelle et al. [47] | Stabilis Stand-Alone Cage | BAK | 6.9 | 4.9 | 4.4 | 3.6 |
| Anterior Threaded BAK cages | BAK | 7.4 | 4.8 | 4.4 | 3.7 | |
| Delamarter et al. [49] | FRA with Posterolateral Fusion | FRA | 7.47 | 4.43 | 4.03 | 3.84 |
| ProDisc-L (Synthes USA) | NA | 7.57 | 3.79 | 3.56 | 3.19 | |
| Ziegler et al. [48] | FRA with Posterolateral Fusion | FRA | 7.5 | 4.2 | 4.2 | 4.3 |
| ProDisc-L (Synthes, Warsaw, IN, USA) | NA | 7.6 | 4 | 3.9 | 3.7 | |
| Ohtori et al. [50] | Iliac Bone. No Instrumentation | NA | 7 | NR | 3.2 | 3.6 |
| 6.5 | NR | 1 | 1.6 | |||
| Butterman et al. [46] | Structural Femoral Cortical Ring Allograft | FRA | 7.4 | NR | 3.4 | 3.5 |
| 7.3 | NR | 3.5 | 2.8 | |||
| Guyer et al. [60] | STALIF Midline, STALIF M, and STALIF M-Ti Interbody Cage (Centinel Spine) | ISF | 6 | NR | NR | 2.5 |
| Hosseini et al. [65] | ALIF ACR (Nuvasive) | ISF | 6.8 | NR | 2.1 | NR |
| Kashlan et al. [62] | PEEK Cages (Globus, St. Wendel, Germany) | ISF | 5.8 | 3 | NR | NR |
| Jacob et al. [61] | Device not reported. Integral fixation screws used. | ISF | 5.8 | 3.5 | 2.6 | NR |
| TLIF | NA | 6.5 | 3.3 | 3.3 | 3.6 | |
| Kuang et al. [63] | ROI-A Oblique MO-ALIF devices (Zimmer Biomet) | ISF | 6.4 | 2.4 | 2.5 | 2.3 |
| TLIF | NA | 6.7 | 3 | 2.7 | 2.6 | |
| Rao et al. [27] | Device not reported. Integral fixation screws used. | ISF | 7.31 | NR | NR | 2.7 |
| Siepe et al. [66] | SynFix LR Device used (DePuy Synthes, Warsaw, IN, USA) | ISF | 7.52 | 3.85 | 4.2 | 3.9 |
| Allain et al. [64] | ROI-A cage with self-locking plates (Zimmer Biomet, Warsaw, IN, USA) | ISF | 5.9 | 3 | 2.6 | NR |
| Mean VAS | Pre-Operative (SD) | 6 Months (SD) | 12 Months (SD) | 24 Months (SD) | Improvement |
|---|---|---|---|---|---|
| Non-Integral Screw Fixation | 7.03 | 4.28 * | 3.77 * | 3.36 * | 3.67 |
| BAK Cages | 7.24 | 3.5 * | 3.33 * | 3.03 * | 4.21 |
| FRA | 7.51 | 4.79 * | 4.28 * | 4.25 * | 3.26 |
| Integral Screw Fixation | 6.46 | 3.25 * | 2.91 * | 2.93 * | 3.53 |
| 3DMorphic PSIC | 7.85 (1.50) n = 78 | 2.53 (2.26) * n = 50 | 2.62 (2.12) * n = 39 | 2.03 (2.13) * n = 44 | 5.82 |
| Mean QoL | Pre-Operative (SD) | 6 Months (SD) | 12 Months (SD) | 24 Months (SD) | Improvement |
|---|---|---|---|---|---|
| Australian Spine Registry | 0.472 | 0.747 | 0.754 | 0.747 | 0.275 |
| 3DMorphic PSIC | 0.257 (SD 0.332) n = 78 | 0.750 (0.243) n = 44 | 0.817 (0.182) n = 38 | 0.815 (0.208) n = 44 | 0.558 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seex, K.A.; Mobbs, R.J.; Coughlan, M.; Pelletier, M.; Walsh, W.R.; Hill, J.C.; Parr, W.C.H. Clinical Outcomes of 3D-Printed Titanium Patient-Specific Implants in Lumbar Interbody Fusion: A Prospective Clinical Trial with a Systematic Review of Conventional Techniques. J. Pers. Med. 2025, 15, 320. https://doi.org/10.3390/jpm15070320
Seex KA, Mobbs RJ, Coughlan M, Pelletier M, Walsh WR, Hill JC, Parr WCH. Clinical Outcomes of 3D-Printed Titanium Patient-Specific Implants in Lumbar Interbody Fusion: A Prospective Clinical Trial with a Systematic Review of Conventional Techniques. Journal of Personalized Medicine. 2025; 15(7):320. https://doi.org/10.3390/jpm15070320
Chicago/Turabian StyleSeex, Kevin A., Ralph J. Mobbs, Marc Coughlan, Matthew Pelletier, William R. Walsh, Jackson C. Hill, and William C. H. Parr. 2025. "Clinical Outcomes of 3D-Printed Titanium Patient-Specific Implants in Lumbar Interbody Fusion: A Prospective Clinical Trial with a Systematic Review of Conventional Techniques" Journal of Personalized Medicine 15, no. 7: 320. https://doi.org/10.3390/jpm15070320
APA StyleSeex, K. A., Mobbs, R. J., Coughlan, M., Pelletier, M., Walsh, W. R., Hill, J. C., & Parr, W. C. H. (2025). Clinical Outcomes of 3D-Printed Titanium Patient-Specific Implants in Lumbar Interbody Fusion: A Prospective Clinical Trial with a Systematic Review of Conventional Techniques. Journal of Personalized Medicine, 15(7), 320. https://doi.org/10.3390/jpm15070320








