The Role of Neutrophil-to-Lymphocyte Ratio as a Predictor of Orchiectomy or Testicular Atrophy After Torsion in Children: A Multicentric Study
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Data Analyzed
2.3. Ethical Aspects
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drlík, M.; Kočvara, R. Torsion of spermatic cord in children: A review. J. Pediatr. Urol. 2013, 9, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Sharp, V.J.; Kieran, K.; Arlen, A.M. Testicular torsion: Diagnosis, evaluation, and management. Am. Fam. Physician 2013, 88, 835–840. [Google Scholar] [PubMed]
- Pogorelić, Z.; Anand, S.; Artuković, L.; Krishnan, N. Comparison of the outcomes of testicular torsion among children presenting during the Coronavirus Disease 2019 (COVID-19) pandemic versus the pre-pandemic period: A systematic review and meta-analysis. J. Pediatr. Urol. 2022, 18, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Howe, A.S.; Vasudevan, V.; Kongnyuy, M.; Rychik, K.; Thomas, L.A.; Matuskova, M.; Friedman, S.C.; Gitlin, J.S.; Reda, E.F.; Palmer, L.S. Degree of twisting and duration of symptoms are prognostic factors of testis salvage during episodes of testicular torsion. Transl. Androl. Urol. 2017, 6, 1159–1166. [Google Scholar] [CrossRef]
- Ramachandra, P.; Palazzi, K.L.; Holmes, N.M.; Marietti, S. Factors influencing rate of testicular salvage in acute testicular torsion at a tertiary pediatric center. West. J. Emerg. Med. 2015, 16, 190–194. [Google Scholar] [CrossRef]
- Dias, A.C.F.; Alves, J.R.; Buson, H.F.; Oliveira, P.G. The amount of spermatic cord rotation magnifies the timerelated orchidectomy risk in intravaginal testicular torsion. Int. Braz. J. Urol. 2016, 42, 1210–1219. [Google Scholar] [CrossRef]
- Boettcher, M.; Bergholz, R.; Krebs, T.F.; Wenke, K.; Aronson, D.C. Clinical predictors of testicular torsion in children. Urology 2012, 79, 670–674. [Google Scholar] [CrossRef]
- He, M.; Zhang, W.; Sun, N. Can haematologic parameters be used to predict testicular viability in testicular torsion? Andrologia 2019, 51, e13357. [Google Scholar] [CrossRef]
- Yilmaz, M.; Sahin, Y.; Hacibey, I.; Ozkuvanci, U.; Suzan, S.; Muslumanoglu, A.Y. Should haematological inflammatory markers be included as an adjuvant in the differential diagnosis of acute scrotal pathologies? Andrologia 2022, 54, e14374. [Google Scholar] [CrossRef]
- Karadağ, Ş.G.; Çakmak, F.; Çil, B.; Tanatar, A.; Sönmez, H.E.; Kıyak, A.; Yavuz, S.; Çakan, M.; Ayaz, N.A. The relevance of practical laboratory markers in predicting gastrointestinal and renal involvement in children with Henoch-Schönlein Purpura. Postgrad. Med. 2021, 133, 272–277. [Google Scholar] [CrossRef]
- Güneş, M.; Umul, M.; Altok, M.; Akyuz, M.; İşoğlu, C.S.; Uruc, F.; Aras, B.; Akbaş, A.; Baş, E. Predictive role of hematologic parameters in testicular torsion. Korean J. Urol. 2015, 56, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Miguel, C.; García, A.; Delgado, B.; Muñoz-Serrano, A.J.; Miguel-Ferrero, M.; Camps, J.; Lopez-Santamaria, M.; Martinez, L. Neutrophil-to-Lymphocyte Ratio as a Predictor of the Need for Surgical Treatment in Children’s Intussusception. Eur. J. Pediatr. Surg. 2023, 33, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Nissen, M.; Sander, V.; Rogge, P.; Alrefai, M.; Tröbs, R.B. Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio Might Predict Pediatric Ovarian Torsion: A Single-Institution Experience and Review of the Literature. J. Pediatr. Adolesc. Gynecol. 2021, 34, 334–340. [Google Scholar] [CrossRef]
- Delgado-Miguel, C.; García, A.; Muñoz-Serrano, A.J.; López-Pereira, P.; Martínez-Urrutia, M.J.; Martínez, L. The role of neutrophil-to-lymphocyte ratio as a predictor of testicular torsion in children. J. Pediatr. Urol. 2022, 18, 697.e1–697.e6. [Google Scholar] [CrossRef]
- Arce, J.D.; Cortés, M.; Vargas, J.C. Sonographic diagnosis of acute spermatic cord torsion. Rotation of the cord: A key to the diagnosis. Pediatr. Radiol. 2002, 32, 485–491. [Google Scholar] [CrossRef]
- Zvizdic, Z.; Milisic, E.; Halimic, A.; Zvizdic, D.; Zubovic, S.V. Testicular volume and testicular atrophy index as predictors of functionality of unilaterally cryptorchid testis. Med. Arch. 2014, 68, 79–82. [Google Scholar] [CrossRef]
- Moore, S.L.; Chebbout, R.; Cumberbatch, M.; Bondad, J.; Forster, L.; Hendry, J.; Lamb, B.; MacLennan, S.; Nambiar, A.; Shah, T.T.; et al. Orchidopexy for Testicular Torsion: A Systematic Review of Surgical Technique. Eur. Urol. Focus 2021, 7, 1493–1503. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden Index and its associated cutoff point. Biom. J. 2005, 47, 458–472. [Google Scholar] [CrossRef]
- Taskinen, S.; Makela, E.; Raivio, T. Effect of pediatric testicular torsion on testicular function in the short term. J. Pediatr. Surg. 2020, 55, 1613–1615. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.G.; Farias, J.G.; Henriquez-Olavarrieta, S.; Madrid, E.; Parraga, M.; Zepeda, A.B.; Moreno, R.D. The hypoxic testicle: Physiology and pathophysiology. Oxidative Med. Cell Longev. 2012, 2012, 929285. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Li, H.; Suson, K.D.; Majumder, K.; Sedki, M.; Abdollah, F.; Sammon, J.D.; Friedman, A.; Löppenberg, B.; Lakshmanan, Y.; et al. Treatment patterns, testicular loss and disparities in inpatient surgical management of testicular torsion in boys: A population-based study 1998–2010. BJU Int. 2016, 118, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Sun, L.; Lei, W.; Li, S.; Guo, H. Management of testicular torsion <360° in children: A single-center, retrospective study. J. Int. Med. Res. 2020, 48, 300060519895861. [Google Scholar]
- Tian, X.M.; Tan, X.H.; Shi, Q.L.; Wen, S.; Lu, P.; Liu, X.; Li, X.-L.; He, D.-W.; Lin, T.; Wei, G.-H. Risk Factors for Testicular Atrophy in Children With Testicular Torsion Following Emergent Orchiopexy. Front. Pediatr. 2020, 8, 584796. [Google Scholar] [CrossRef]
- Yu, C.J.; Zhao, J.; Luo, J.; Hong, Y.F.; Zhao, T.X.; Wen, S.; Jiang, L.; Lin, T.; He, D.-W.; Wei, G.-H.; et al. Long-term follow-up results of testicular torsion in children. Asian J. Androl. 2022, 24, 653–659. [Google Scholar] [CrossRef]
- Lian, B.S.; Ong, C.C.; Chiang, L.W.; Rai, R.; Nah, S.A. Factors predicting testicular atrophy after testicular salvage following torsion. Eur. J. Pediatr. Surg. 2016, 26, 17–21. [Google Scholar] [CrossRef]
- Pogorelić, Z.; Mrklić, I.; Jurić, I. Do not forget to include testicular torsion in differential diagnosis of lower acute abdominal pain in young males. J. Pediatr. Urol. 2013, 9 Pt 6, 1161–1165. [Google Scholar] [CrossRef]
- Boettcher, M.; Krebs, T.; Bergholz, R.; Wenke, K.; Aronson, D.; Reinshagen, K. Clinical and sonographic features predict testicular torsion in children: A prospective study. BJU Int. 2013, 112, 1201–1206. [Google Scholar] [CrossRef]
- Feng, S.; Yang, H.; Lou, Y.; Ru, W.; Wang, A.; Liu, W. Clinical Characteristics of Testicular Torsion and Identification of Predictors of Testicular Salvage in Children: A Retrospective Study in a Single Institution. Urol. Int. 2020, 104, 878–883. [Google Scholar] [CrossRef]
- Chen, P.; Huang, W.; He, Y.; Sun, M.; Sun, X.; Huang, Y.; Li, S. A nomogram for predicting risk factors of testicular salvage after testicular torsion in children. Int. J. Urol. 2024, 31, 568–574. [Google Scholar] [CrossRef]
- Grimsby, G.M.; Schlomer, B.J.; Menon, V.S.; Ostrov, L.; Keays, M.; Sheth, K.R.; Villanueva, C.; Granberg, C.; Dajusta, D.; Hill, M.; et al. Prospective Evaluation of Predictors of Testis Atrophy After Surgery for Testis Torsion in Children. Urology 2018, 116, 150–155. [Google Scholar] [CrossRef]

| Total (n = 455) | Non-Lost Testicles (n = 334) | Orchiectomy (n = 87) | Testicular Atrophy (n = 34) | p-Value * | |
|---|---|---|---|---|---|
| Age (years); median (IQR) | 13.2 (10.6–14.4) | 13.3 (11.8–14.6) | 11.9 (3.9–14.2) | 13.2 (11.4–14.3) | 0.018 |
| Weight (kg); median (IQR) | 48.5 (33.4–57.1) | 50.1 (42.3–58.0) | 44 (30.5–53.5) | 51 (38.2–60.1) | 0.002 |
| Testicle involved; n (%) | 0.276 | ||||
| • Right | 209 (45.9) | 155 (46.4) | 38 (43.7) | 16 (47.1) | |
| • Left | 246 (54.1) | 179 (53.6) | 49 (56.3) | 18 (52.9) | |
| Associated symptoms; n (%) | |||||
| • Abdominal pain | 93 (20.4) | 70 (21.0) | 15 (17.2) | 8 (23.5) | 0.051 |
| • Vomiting | 133 (29.2) | 104 (31.1) | 20 (23.0) | 9 (26.5) | 0.184 |
| • Dizziness | 16 (3.5) | 15 (4.5) | 0 | 1 (2.9) | 0.065 |
| • Disuria | 4 (0.9) | 3 (0.9) | 0 | 1 (2.9) | 0.329 |
| • Fever | 8 (1.8) | 5 (1.5) | 2 (2.3) | 1 (2.9) | 0.079 |
| Clinical findings; n (%) | |||||
| • Scrotal swelling | 249 (54.7) | 192 (57.5) | 37 (42.5) | 20 (58.8) | 0.059 |
| • Absent cremasteric reflex | 175 (38.5) | 130 (38.9) | 33 (37.9) | 13 (38.2) | 0.543 |
| • Negative Prehn’s sign | 143 (31.4) | 104 (31.1) | 28 (32.1) | 11 (32.4) | 0.215 |
| Time since symptoms onset (hours); median (IQR) | 5 (3–12) | 4 (2–8) | 24 (10–48) | 8 (6–12) | <0.001 |
| Ultrasound findings; | |||||
| • Absence of intratesticular Doppler flow; n (%) | 411 (90.3) | 295 (88.3) | 84 (96.6) | 32 (94.1) | 0.013 |
| • Whirlpool sign; n (%) | 129 (28.4) | 91 (27.2) | 26 (29.9) | 12 (35.3) | 0.079 |
| • Testicular volume (cm3); median (IQR) | 12 (6–19) | 12 (6–18) | 15 (8–20) | 13 (7–19) | 0.095 |
| Degree of twisted cord (degrees); median (IQR) | 360 (360–720) | 360 (180–720) | 720 (360–720) | 720 (360–720) | <0.001 |
| Non-Lost Testicles (n = 334) | Orchiectomy (n = 87) | Testicular Atrophy (n = 34) | p-Value | |
|---|---|---|---|---|
| Leukocytes (×109/L) | 9.26 (7.5–11.9) | 12.1 (10.0–14.6) | 13.2 (10.1–15.1) | <0.001 |
| Neutrophils (×109/L) | 5.6 (3.6–8.3) | 9.61 (8.2–12.8) | 9.68 (6.60–11.71) | <0.001 |
| Lymphocytes (×109/L) | 2.4 (1.8–3.4) | 1.2 (1.12–1.64) | 1.65 (1.16–2.59) | <0.001 |
| Monocytes (×109/L) | 0.55 (0.43–0.76) | 0.57 (0.39–0.74) | 0.90 (0.64–1.24) | <0.001 |
| Platelets (×109/L) | 270 (228–320) | 269 (235–300) | 305 (234–373) | 0.052 |
| NLR | 2.0 (1.1–4.2) | 7.5 (6.1–9.2) | 6.4 (3.2–8.7) | <0.001 |
| PLR | 107 (81–152) | 209 (164.3–277.3) | 175.3 (109.8–268.6) | <0.001 |
| SIRI (×109/L) | 1.2 (0.6–2.64) | 3.9 (2.7–5.75) | 4.4 (1.9–9.2) | <0.001 |
| SII (×109/L) | 557 (326–1251) | 2104 (1561–2624) | 1738 (875–2876) | <0.001 |
| CRP (mg/L) | 0.5 (0.2–2.9) | 5.5 (0.9–7.4) | 2.9 (0.5–9.8) | <0.001 |
| Glucose (mg/dL) | 102 (91–119) | 109 (101–120) | 100 (84–114) | 0.056 |
| Urea (mg/dL) | 29 (25–34) | 31 (25–35) | 27 (22.5–32.5) | 0.095 |
| Creatinine (mg/dL) | 0.64 (0.5–0.76) | 0.63 (0.5–0.71) | 0.6 (0.4–0.74) | 0.509 |
| Fibrinogen (mg/dL) | 338 (302–398) | 358 (300–463) | 365 (314–481) | 0.215 |
| Ionogram | ||||
| • Na+ | 139 (137.2–104.4) | 138.4 (137–140.1) | 140 (137–141) | 0.145 |
| • K+ | 4.0 (3.7–4.3) | 4.3 (3.7–4.4) | 4.2 (3.8–4.4) | 0.072 |
| • Cl− | 104 (102–106) | 103 (102–105.7) | 102 (101–104) | 0.121 |
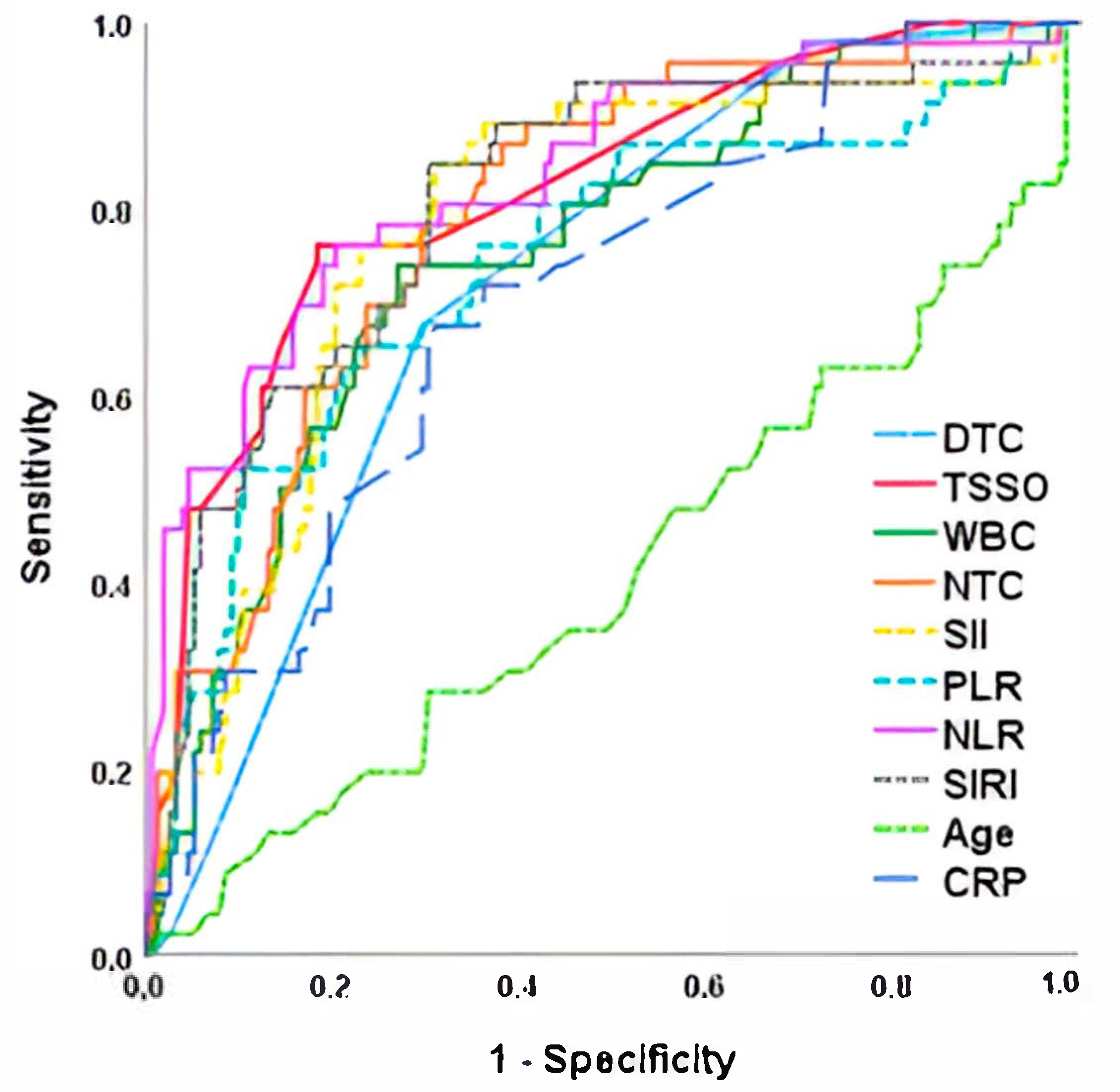
| AUC (CI 95%) | Cut-Off Point | Sensitivity | Specificity | p Value | |
|---|---|---|---|---|---|
| NLR | 0.834 (0.763–0.905) | 5.1 | 78.5 | 77.4 | <0.001 |
| TSSO | 0.820 (0.749–0.891) | 8.5 | 76.1 | 78.0 | <0.001 |
| SII | 0.806 (0.731–0.881) | 1176 | 78.3 | 70.2 | <0.001 |
| SIRI | 0.794 (0.723–0.865) | 2545 | 76.1 | 77.3 | <0.001 |
| NTC | 0.781 (0.704–0.860) | 8.1 | 78.3 | 71.2 | <0.001 |
| WBC | 0.753 (0.675–0.832) | 11.3 | 73.9 | 73.0 | <0.001 |
| PLR | 0.741 (0.653–0.830) | 137.9 | 71.7 | 68.5 | 0.005 |
| DTC | 0.720 (0.644–0.797) | 2 | 67.4 | 71.2 | 0.012 |
| CRP | 0.702 (0.619–0.784) | 1.5 | 67.4 | 68.6 | 0.057 |
| Age | 0.411 (0.311–0.510) | 10.4 | 54.2 | 48.7 | 0.066 |
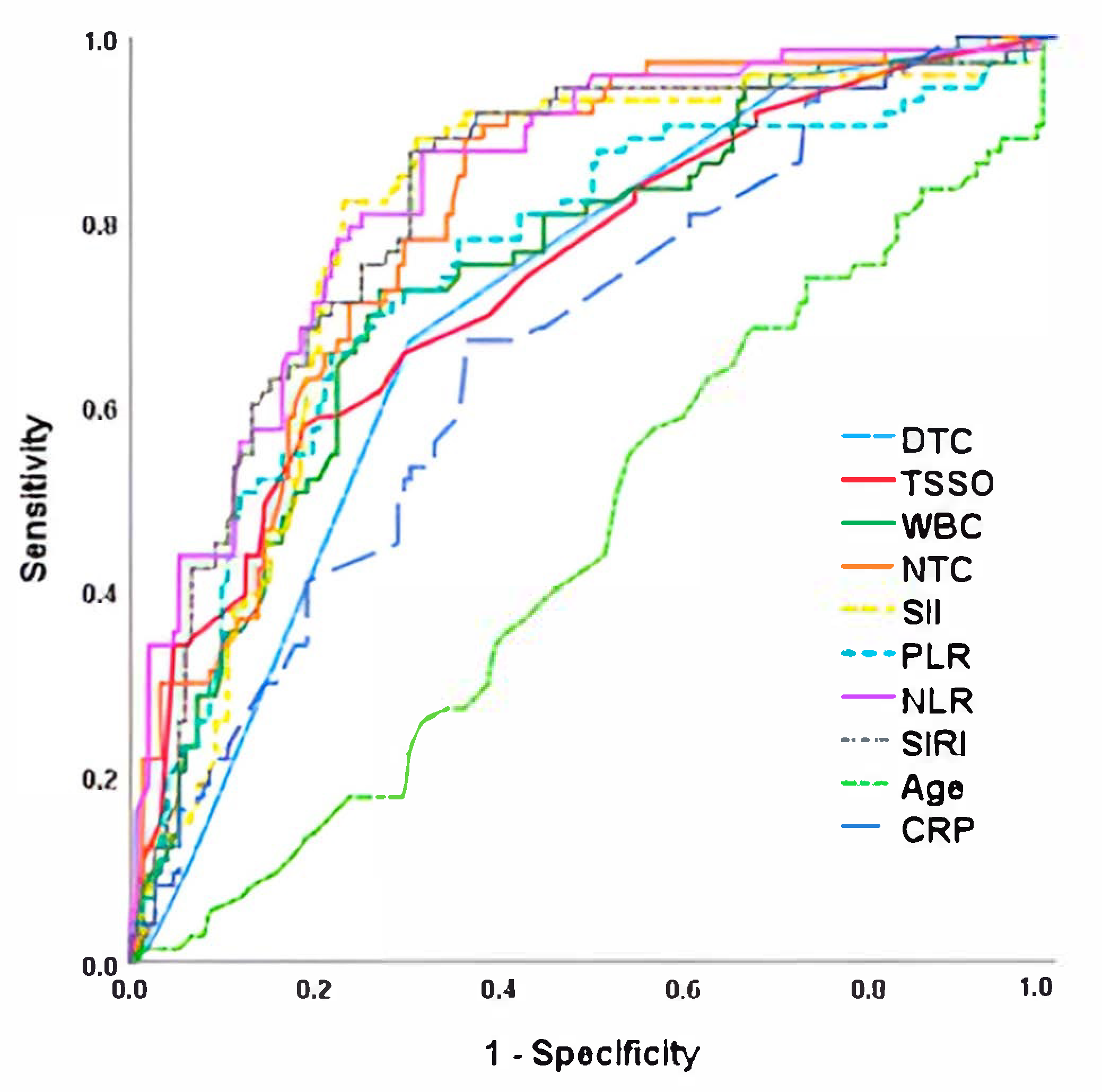
| AUC (CI 95%) | Cut-Off Point | Sensitivity | Specificity | p Value | |
|---|---|---|---|---|---|
| NLR | 0.849 (0.792–0.906) | 5.4 | 88.9 | 77.4 | <0.001 |
| SIRI | 0.840 (0.769–0.896) | 2655 | 85.2 | 77.2 | <0.001 |
| SII | 0.833 (0.770–0.913) | 1355 | 85.2 | 75.0 | <0.001 |
| NTC | 0.829 (0.762–0.895) | 2545 | 77.8 | 71.1 | <0.001 |
| PLR | 0.779 (0.688–0.870) | 158 | 74.1 | 75.0 | <0.001 |
| WBC | 0.733 (0.635–0.830) | 11.3 | 73.9 | 73.0 | <0.001 |
| DTC | 0.697 (0.595–0.798) | 1.5 | 64.5 | 70.2 | 0.005 |
| TSSO | 0.607 (0.497–0.717) | 2 | 61.2 | 58.3 | 0.012 |
| CRP | 0.593 (0.484–0.703) | 1.5 | 60.4 | 57.6 | 0.057 |
| Age | 0.539 (0.448–0.630) | 10.4 | 56.2 | 51.9 | 0.066 |
| AUC (CI 95%) | Cut-Off Point | Sensitivity | Specificity | p Value | |
|---|---|---|---|---|---|
| NLR | 0.838 (0.784–0.893) | 5.2 | 82.2 | 77.0 | <0.001 |
| SII | 0.817 (0.757–0.877) | 1224 | 78.1 | 74.0 | <0.001 |
| NTC | 0.806 (0.749–0.864) | 8.1 | 75.3 | 72.0 | <0.001 |
| SIRI | 0.799 (0.737–0.861) | 2456 | 74.2 | 72.3 | <0.001 |
| PLR | 0.753 (0.683–0.824) | 148 | 73.6 | 73.1 | <0.001 |
| WBC | 0.745 (0.678–0.813) | 11.4 | 72.6 | 73.0 | 0.007 |
| TSSO | 0.741 (0.671–0.811) | 137.9 | 71.7 | 68.5 | 0.012 |
| DTC | 0.709 (0.640–0.778) | 5.5 | 69.6 | 66.2 | 0.025 |
| CRP | 0.663 (0.589–0.737) | 0.7 | 67.1 | 64.3 | 0.038 |
| Age | 0.458 (0.378–0.537) | 11.7 | 53.4 | 47.3 | 0.303 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Miguel, C.; Arredondo-Montero, J.; Moreno-Alfonso, J.C.; Garavis Montagut, I.; San Basilio, M.; Hernández, I.; Carrera, N.; Martínez, L.; Iraola, E.; Ruiz Jiménez, I.; et al. The Role of Neutrophil-to-Lymphocyte Ratio as a Predictor of Orchiectomy or Testicular Atrophy After Torsion in Children: A Multicentric Study. J. Pers. Med. 2025, 15, 310. https://doi.org/10.3390/jpm15070310
Delgado-Miguel C, Arredondo-Montero J, Moreno-Alfonso JC, Garavis Montagut I, San Basilio M, Hernández I, Carrera N, Martínez L, Iraola E, Ruiz Jiménez I, et al. The Role of Neutrophil-to-Lymphocyte Ratio as a Predictor of Orchiectomy or Testicular Atrophy After Torsion in Children: A Multicentric Study. Journal of Personalized Medicine. 2025; 15(7):310. https://doi.org/10.3390/jpm15070310
Chicago/Turabian StyleDelgado-Miguel, Carlos, Javier Arredondo-Montero, Julio César Moreno-Alfonso, Isabella Garavis Montagut, María San Basilio, Irene Hernández, Noela Carrera, Leopoldo Martínez, Estíbalitz Iraola, Inmaculada Ruiz Jiménez, and et al. 2025. "The Role of Neutrophil-to-Lymphocyte Ratio as a Predictor of Orchiectomy or Testicular Atrophy After Torsion in Children: A Multicentric Study" Journal of Personalized Medicine 15, no. 7: 310. https://doi.org/10.3390/jpm15070310
APA StyleDelgado-Miguel, C., Arredondo-Montero, J., Moreno-Alfonso, J. C., Garavis Montagut, I., San Basilio, M., Hernández, I., Carrera, N., Martínez, L., Iraola, E., Ruiz Jiménez, I., Aguado Roncero, P., Fuentes, E., Díez, R., & Hernández-Oliveros, F. (2025). The Role of Neutrophil-to-Lymphocyte Ratio as a Predictor of Orchiectomy or Testicular Atrophy After Torsion in Children: A Multicentric Study. Journal of Personalized Medicine, 15(7), 310. https://doi.org/10.3390/jpm15070310







