Computational and Imaging Approaches for Precision Characterization of Bone, Cartilage, and Synovial Biomolecules
Abstract
1. Introduction
2. Methodology
3. Biomolecular Architecture of Joint Tissues
3.1. Bone, Cartilage, and Synovium Composition and Molecular Interactions
3.2. Implications for Imaging and Computer Modeling
4. Challenges of Conventional Diagnostics
5. Advanced Imaging Modalities for Joint Tissue Characterization
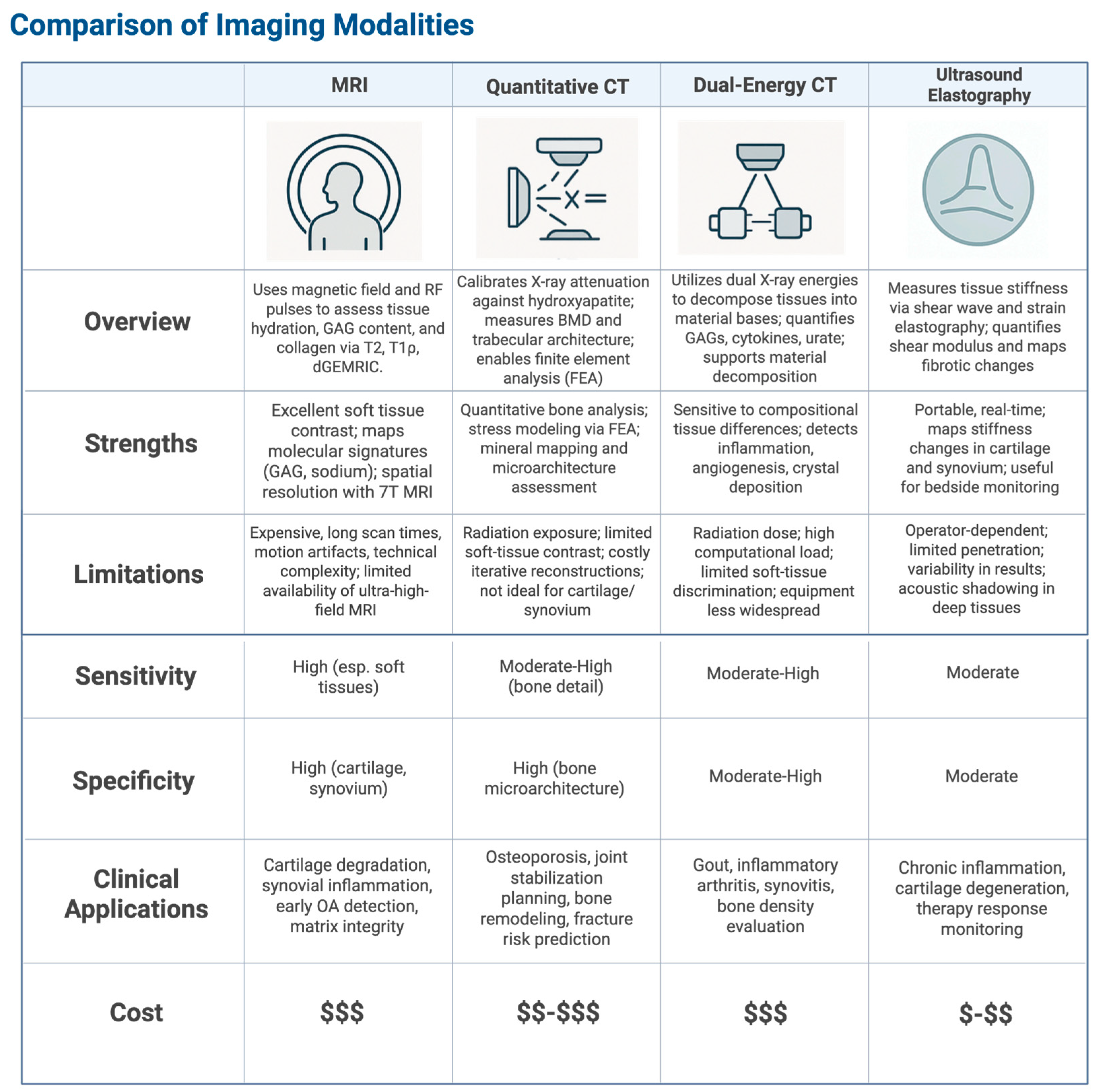
5.1. High-Resolution Magnetic Resonance Imaging (MRI)
5.2. Quantitative Computed Tomography (qCT)
5.3. Dual-Energy Computed Tomography (DECT)
5.4. Ultrasound Elastography
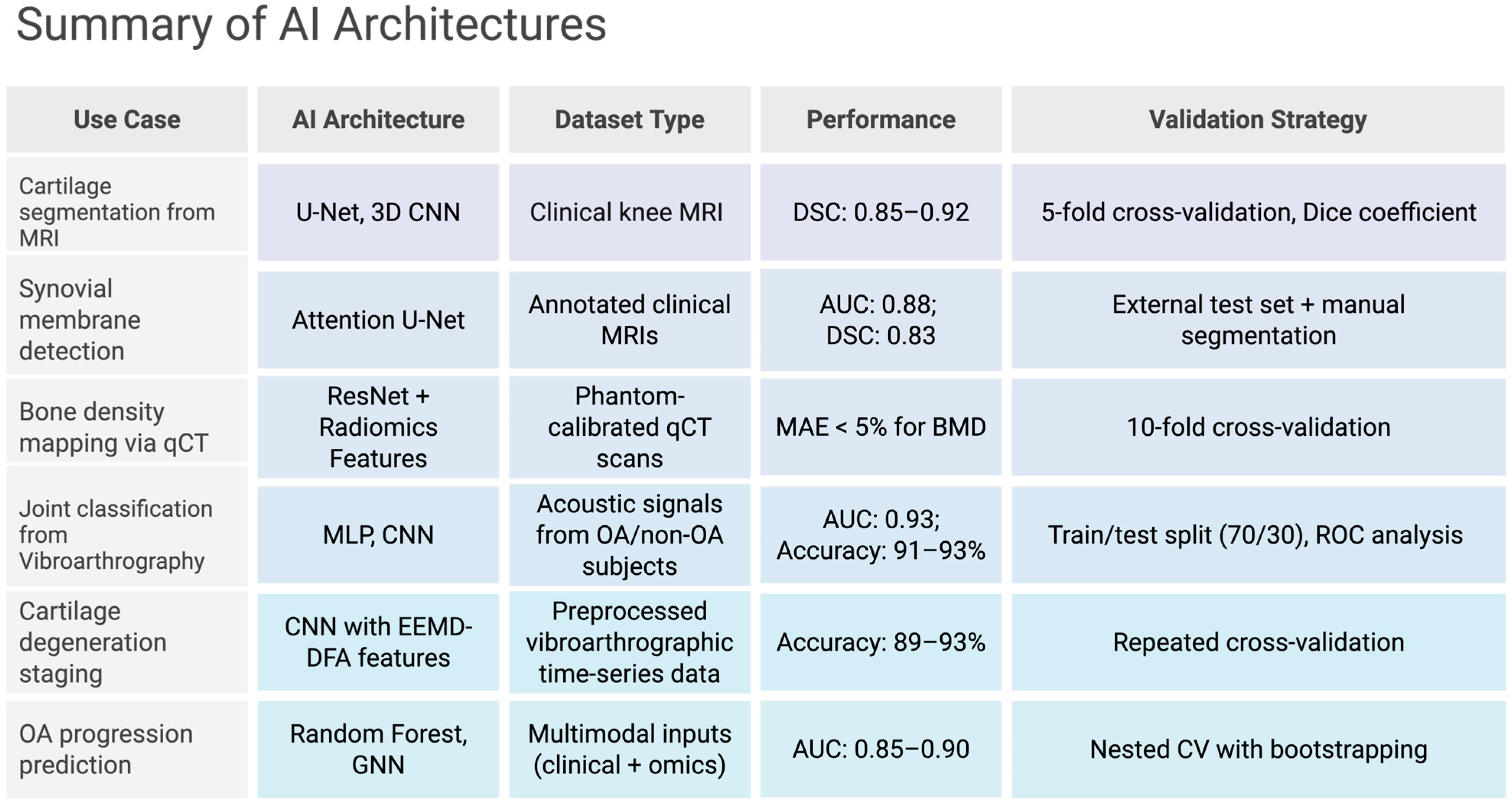
5.5. Vibroarthrography
6. Computational Frameworks for Biomolecular Analysis
6.1. Radiomic Feature Extraction
6.2. Deep Learning Pipelines
7. Multimodal Data Fusion and Omics Integration
7.1. Transcriptomic Integration
7.2. Proteomic Integration
7.3. AI-Augmented Segmentation
7.4. Multi-Scale Modeling
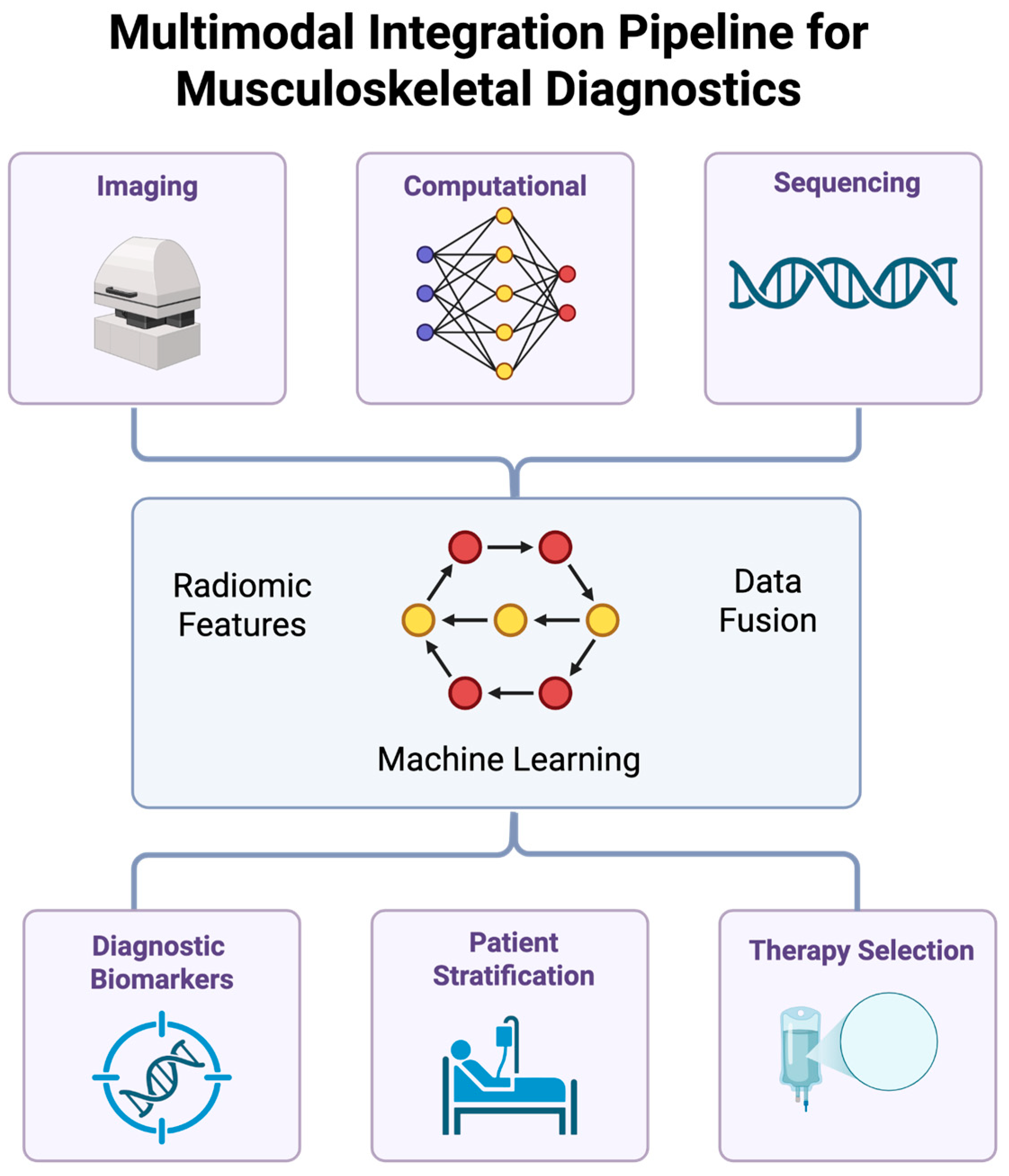
8. Translational Applications in Musculoskeletal Care
8.1. Image-Guided Molecular Profiling
8.2. Predictive Modeling of Tissue Remodeling
8.3. Clinical Integration and Workflow Optimization
9. Discussion
10. Conclusions
10.1. Clinical Implications
10.2. Future Directions
10.3. Key Takeaways
- Multimodal integration of imaging and computational technologies enables molecular-level evaluation of musculoskeletal systems with significant depth.
- Advanced imaging modalities, particularly MRI, qCT, DECT, ultrasound elastography, and vibroarthrography provide complementary structural and functional insights.
- Radiomics and deep learning models (e.g., CNNs, U-Net, GNNs) enhance tissue characterization and are able to predict disease progression with increasing accuracy.
- Transcriptomic and proteomic integration with imaging data supports the identification of tissue-specific biomarkers and offers mechanistic insight into joint degeneration.
- Clinical translation is limited by challenges in reproducibility and explainability of AI.
- Future directions should emphasize explainable AI and cross-platform coordination for the incorporation of these technologies into clinical workflows and education.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| MRI | Magnetic Resonance Imaging |
| qCT | Quantitative Computed Tomography |
| DECT | Dual-Energy Computed Tomography |
| RNA-seq | RNA Sequencing |
| GAG | Glycosaminoglycan |
| MMP | Matrix Metalloproteinase |
| AI | Artificial Intelligence |
| ML | Machine Learning |
| CNN | Convolutional Neural Network |
| T1ρ | T1 Rho (Spin-lattice relaxation in the rotating frame) |
References
- Goldring, S.R.; Goldring, M.B. The Role of Cytokines in Cartilage Matrix Degeneration in Osteoarthritis. Clin. Orthop. Relat. Res. 2004, 427, S27–S36. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A.; Mankin, H.J.; Grodzinsky, A.J. Articular Cartilage and Osteoarthritis. Instr. Course Lect. 2005, 54, 465–480. [Google Scholar] [PubMed]
- Heinegård, D.; Saxne, T. The Role of the Cartilage Matrix in Osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F. Molecular Mechanisms of Cartilage Destruction in Osteoarthritis. J. Musculoskelet. Neuronal Interact. 2008, 8, 303–306. [Google Scholar] [PubMed]
- Pelletier, J.P.; Martel-Pelletier, J.; Abramson, S.B. Osteoarthritis, an Inflammatory Disease: Potential Implication for the Selection of New Therapeutic Targets. Arthritis Rheum. 2001, 44, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Felson, D.T. Osteoarthritis. BMJ 2006, 332, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Scanzello, C.R.; Goldring, S.R. The Role of Synovitis in Osteoarthritis Pathogenesis. Bone 2012, 51, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Sellam, J.; Berenbaum, F. The Role of Synovitis in Pathophysiology and Clinical Symptoms of Osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, F.; Burstein, D.; Link, T.M. Quantitative MRI of Cartilage and Bone: Degenerative Changes in Osteoarthritis. NMR Biomed. 2006, 19, 822–854. [Google Scholar] [CrossRef] [PubMed]
- Guermazi, A.; Roemer, F.W.; Burstein, D.; Hayashi, D. Why Radiography Should No Longer Be Considered a Surrogate Outcome Measure for Longitudinal Assessment of Cartilage in Knee Osteoarthritis. Arthritis Res. Ther. 2011, 13, 247. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yin, J.; Gao, J.; Cheng, T.S.; Pavlos, N.J.; Zhang, C.; Zheng, M.H. Subchondral Bone in Osteoarthritis: Insight into Risk Factors and Microstructural Changes. Arthritis Res. Ther. 2013, 15, 223. [Google Scholar] [CrossRef] [PubMed]
- Peterfy, C.G.; Guermazi, A.; Zaim, S.; Tirman, P.F.; Miaux, Y.; White, D.; Kothari, M.; Lu, Y.; Fye, K.; Zhao, S.; et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the Knee in Osteoarthritis. Osteoarthr. Cartil. 2004, 12, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Roemer, F.W.; Crema, M.D.; Trattnig, S.; Guermazi, A. Advances in Imaging of Osteoarthritis and Cartilage. Radiology 2011, 260, 332–354. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, D.; Roemer, F.W.; Katur, A.; Felson, D.T.; Yang, S.O.; Alomran, F.; Guermazi, A. Imaging of Synovitis in Osteoarthritis: Current Status and Outlook. Semin. Arthritis Rheum. 2011, 41, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Crema, M.D.; Roemer, F.W.; Marra, M.D.; Burstein, D.; Gold, G.E.; Eckstein, F.; Baum, T.; Mosher, T.J.; Carrino, J.A.; Guermazi, A. Articular Cartilage in the Knee: Current MR Imaging Techniques and Applications in Clinical Practice and Research. Radiographics 2011, 31, 37–61. [Google Scholar] [CrossRef] [PubMed]
- Potter, H.G.; Black, B.R.; Chong, L.R. New Techniques in Articular Cartilage Imaging. Clin. Sports Med. 2009, 28, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Sofka, C.M.; Potter, H.G.; Adler, R.S.; Pavlov, H. Musculoskeletal Imaging Update: Current Applications of Advanced Imaging Techniques to Evaluate the Early and Long-Term Complications of Patients with Orthopedic Implants. HSS J. 2006, 2, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Link, T.M.; Neumann, J.; Li, X. Prestructural Cartilage Assessment Using MRI. J. Magn. Reson. Imaging 2017, 45, 949–965. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T.; Hodgson, R. Identifying and Treating Preclinical and Early Osteoarthritis. Rheum. Dis. Clin. N. Am. 2014, 40, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L.; Imbert, L. Boskey AL, Imbert L. Bone quality changes associated with aging and disease: A review. Ann. N. Y. Acad. Sci. 2017, 1410, 1915–1925. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Komori, T. Regulation of Bone Development and Maintenance by Runx2. Front. Biosci. 2008, 13, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Mow, V.C.; Guo, X.E. Mechano-Electrochemical Properties of Articular Cartilage: Their Inhomogeneities and Anisotropies. Annu. Rev. Biomed. Eng. 2002, 4, 175–209. [Google Scholar] [CrossRef] [PubMed]
- Mathiessen, A.; Conaghan, P.G. Synovitis in Osteoarthritis: Current Understanding with Therapeutic Implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-Grade Inflammation as a Key Mediator of the Pathogenesis of Osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of Proinflammatory Cytokines in the Pathophysiology of Osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Burr, D.B.; Gallant, M.A. Bone Remodelling in Osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Seibel, M.J.; Robins, S.P.; Bilezikian, J.P. Dynamics of Bone and Cartilage Metabolism. Clin. Biochem. Rev. 2006, 27, 49–56. [Google Scholar] [PubMed][Green Version]
- Cohen, N.P.; Foster, R.J.; Mow, V.C. Composition and Dynamics of Articular Cartilage: Structure, Function, and Maintaining Healthy State. J. Orthop. Sports Phys. Ther. 1998, 28, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Boileau, C.; Pelletier, J.P.; Roughley, P.J. Cartilage in Normal and Osteoarthritis Conditions. Best Pract. Res. Clin. Rheumatol. 2008, 22, 351–384. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A Disease of the Joint as an Organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Recht, M.P.; Goodwin, D.W.; Winalski, C.S.; White, L.M. MRI of Articular Cartilage: Revisiting Current Status and Future Directions. AJR Am. J. Roentgenol. 2005, 185, 899–914. [Google Scholar] [CrossRef] [PubMed]
- Kijowski, R.; Blankenbaker, D.G.; Davis, K.W.; Shinki, K.; Kaplan, L.D.; De Smet, A.A. Comparison of Quantitative and Qualitative Magnetic Resonance Imaging Measures of Cartilage Degeneration in Osteoarthritis. J. Magn. Reson. Imaging 2010, 32, 1390–1398. [Google Scholar] [CrossRef] [PubMed]
- Kazakia, G.J.; Majumdar, S. New Imaging Technologies in the Diagnosis of Osteoporosis. Rev. Endocr. Metab. Disord. 2006, 7, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Garnero, P.; Rousseau, J.C.; Delmas, P.D. Molecular Basis and Clinical Use of Biochemical Markers of Bone, Cartilage, and Synovium in Joint Diseases. Arthritis Rheum. 2000, 43, 953–968. [Google Scholar] [CrossRef] [PubMed]
- Kraus, V.B.; Burnett, B.; Coindreau, J.; Cottrell, S.; Eyre, D.; Gendreau, M.; Gardiner, J.; Garnero, P.; Hardin, J.; Henrotin, Y.; et al. Application of Biomarkers in the Development of Drugs Intended for the Treatment of Osteoarthritis. Osteoarthr. Cartil. 2011, 19, 515–542. [Google Scholar] [CrossRef] [PubMed]
- Bijlsma, J.W.; Berenbaum, F.; Lafeber, F.P. Osteoarthritis: An Update with Relevance for Clinical Practice. Lancet 2011, 377, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Aigner, T.; Söder, S.; Gebhard, P.M.; McAlinden, A.; Haag, J. Mechanisms of Disease: Role of Chondrocytes in the Pathogenesis of Osteoarthritis—Structure, Chaos and Senescence. Nat. Clin. Pract. Rheumatol. 2007, 3, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Hügle, T.; Geurts, J. What Drives Osteoarthritis?—Synovial Versus Subchondral Bone Pathology. Rheumatology 2017, 56, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Fonseka, M.; Ramasamy, R.; Geczy, C.L. Spatial Analysis of the Osteoarthritis Microenvironment: Techniques, Insights, and Applications. Bone Res. 2024, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Brandt, K.D.; Dieppe, P.; Radin, E.L. Etiopathogenesis of Osteoarthritis. Rheum. Dis. Clin. N. Am. 2008, 34, 531–559. [Google Scholar] [CrossRef] [PubMed]
- Kellgren, J.H.; Lawrence, J.S. Radiological Assessment of Osteo-Arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.D.; Gold, G.E. Atlas of Individual Radiographic Features in Osteoarthritis, Revised. Osteoarthr. Cartil. 2007, 15 (Suppl. A), A1–A56. [Google Scholar] [CrossRef] [PubMed]
- Pritzker, K.P.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis Cartilage Histopathology Grading and Staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef] [PubMed]
- van der Kraan, P.M.; van den Berg, W.B. Chondrocyte Hypertrophy and Osteoarthritis: Role in Initiation and Progression of Cartilage Degeneration? Osteoarthr. Cartil. 2012, 20, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F. Osteoarthritis as an Inflammatory Disease (Osteoarthritis Is Not Osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Dieppe, P.A.; Lohmander, L.S. Pathogenesis and Management of Pain in Osteoarthritis. Lancet 2005, 365, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Glyn-Jones, S.; Palmer, A.J.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Winalski, C.S.; Rajiah, P. The Evolution of Musculoskeletal MRI: From Low to Ultra-High Field Strengths. J. Magn. Reson. Imaging 2018, 47, 1453–1472. [Google Scholar] [CrossRef] [PubMed]
- Mosher, T.J.; Dardzinski, B.J. Cartilage MRI T2 Relaxation Time Mapping: Overview and Applications. Semin. Musculoskelet. Radiol. 2004, 8, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Regatte, R.R.; Akella, S.V.; Wheaton, A.J.; Lech, G.; Borthakur, A.; Kneeland, J.B.; Reddy, R. 3D-T1rho-Relaxation Mapping of Articular Cartilage: In Vivo Assessment of Early Degenerative Changes in Symptomatic Osteoarthritic Subjects. Acad. Radiol. 2004, 11, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Tiderius, C.J.; Olsson, L.E.; Leander, P.; Ekberg, O.; Dahlberg, L. Delayed Gadolinium-Enhanced MRI of Cartilage (dGEMRIC) in Early Knee Osteoarthritis. Magn. Reson. Med. 2003, 49, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Burstein, D.; Velyvis, J.; Scott, K.T.; Stock, K.W.; Kim, Y.J.; Jaramillo, D.; Boutin, R.D.; Gray, M.L. Protocol Issues for Delayed Gd(DTPA)(2-)-Enhanced MRI (dGEMRIC) for Clinical Evaluation of Articular Cartilage. Magn. Reson. Med. 2001, 45, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Bieri, O.; Mamisch, T.C.; Trattnig, S.; Kraff, O.; Ladd, M.E.; Scheffler, K. Optimized 3D Magnetization-Prepared Rapid Gradient-Echo Imaging for Knee Cartilage at 3T and 7T. Magn. Reson. Med. 2012, 67, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Madelin, G.; Regatte, R.R. Biomedical Applications of Sodium MRI In Vivo. J. Magn. Reson. Imaging 2013, 38, 511–529. [Google Scholar] [CrossRef] [PubMed]
- Zbýň, Š.; Mlynárik, V.; Juras, V.; Szomolanyi, P.; Trattnig, S. Sodium MR Imaging of Articular Cartilage Pathologies. Curr. Radiol. Rep. 2014, 2, 41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chung, M.S.; Choi, J.Y.; Hong, S.H.; Kang, H.S. Susceptibility Artifacts in High-Field MRI: Mechanisms and Solutions. J. Magn. Reson. Imaging 2019, 50, 1043–1057. [Google Scholar] [CrossRef] [PubMed]
- Kidoh, M.; Shinoda, K.; Kitajima, M.; Isogawa, K.; Nambu, M.; Uetani, H.; Morita, K.; Nakaura, T.; Tateishi, M.; Yamashita, Y. Deep Learning Based Noise Reduction for Brain MR Imaging: Tests on Phantoms and Healthy Volunteers. Magn. Reson. Med. Sci. 2020, 19, 195–206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lustig, M.; Donoho, D.; Pauly, J.M. Sparse MRI: The Application of Compressed Sensing for Rapid MR Imaging. Magn. Reson. Med. 2007, 58, 1182–1195. [Google Scholar] [CrossRef] [PubMed]
- Gold, G.E.; Suh, B.; Sawyer-Glover, A.; Beaulieu, C. Musculoskeletal MRI at 3.0 T: Initial Clinical Experience. AJR Am. J. Roentgenol. 2004, 183, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pedoia, V.; Heilmeier, U.; Ku, E.; Su, F.; Khanna, S.; Imboden, J.; Graf, J.; Link, T.; Li, X. Quantitative Susceptibility Mapping in the Evaluation of Osteoporosis. J. Magn. Reson. Imaging 2019, 49, 1726–1734. [Google Scholar] [CrossRef] [PubMed]
- Deniz, C.M.; Xiang, S.; Hallyburton, R.S.; Welbeck, A.; Babb, J.S.; Honig, S.; Cho, K.; Chang, G. Segmentation of the Proximal Femur from MR Images Using Deep Convolutional Neural Networks. Sci. Rep. 2018, 8, 16485. [Google Scholar] [CrossRef] [PubMed]
- MacKay, J.W.; Low, S.B.L.; Smith, T.O.; Toms, A.P.; McCaskie, A.W.; Gilbert, F.J. Systematic review and meta-analysis of the reliability and discriminative validity of cartilage compositional MRI in knee osteoarthritis. Osteoarthr. Cartil. 2018, 26, 1140–1152. [Google Scholar] [CrossRef] [PubMed]
- Pipe, J.G. Motion Correction with PROPELLER MRI: Application to Head Motion and Free-Breathing Cardiac Imaging. Magn. Reson. Med. 1999, 42, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Ugurbil, K.; Auerbach, E.; Moeller, S.; Grant, A.; Wu, X.; Van de Moortele, P.F.; Olman, C.A.; DelaBarre, L.; Schillak, S.; Radder, J.; et al. Brain Imaging at Ultrahigh Field: Pushing the Limits of Sensitivity and Specificity. Neuroimage 2018, 168, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Juras, V.; Zbýň, Š.; Pressl, C.; Valkovič, L.; Szomolanyi, P.; Frollo, I.; Trattnig, S. Regional Variations of T2* in Healthy and Pathologic Achilles Tendon In Vivo at 7 T: Preliminary Results. Magn. Reson. Med. 2012, 68, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Rahmim, A.; Huang, P.; Shenkov, N.; Fotouhi, S.; Davoodi-Bojd, E.; Lu, L.; Mari, Z.; Soltanian-Zadeh, H.; Sossi, V. Improved Prediction of Outcome in Parkinson’s Disease Using Radiomics and Machine Learning Applied to DaTscan SPECT. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Bashir, A.; Gray, M.L.; Hartke, J.; Burstein, D. Nondestructive Imaging of Human Cartilage Glycosaminoglycan Concentration by MRI. Magn. Reson. Med. 1999, 41, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Engelke, K.; Adams, J.E.; Armbrecht, G.; Augat, P.; Bogado, C.E.; Bouxsein, M.L.; Felsenberg, D.; Ito, M.; Prevrhal, S.; Hans, D.B.; et al. Clinical Use of Quantitative Computed Tomography and Peripheral Quantitative Computed Tomography in the Management of Osteoporosis in Adults: The 2007 ISCD Official Positions. J. Clin. Densitom. 2008, 11, 123–162. [Google Scholar] [CrossRef] [PubMed]
- Genant, H.K.; Engelke, K.; Prevrhal, S. Advanced CT Bone Imaging in Osteoporosis. Rheumatology 2008, 47 (Suppl. S4), iv9–iv16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bouxsein, M.L.; Seeman, E. Quantifying the Material and Structural Determinants of Bone Strength. Best. Pract. Res. Clin. Rheumatol. 2009, 23, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Keyak, J.H.; Kaneko, T.S.; Tehranzadeh, J.; Skinner, H.B. Predicting Proximal Femoral Strength Using Structural Engineering Models. Clin. Orthop. Relat. Res. 2005, 437, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Baim, S.; Leonard, M.B.; Bianchi, M.L.; Hans, D.B.; Kalkwarf, H.J.; Langman, C.B.; Rauch, F. Official Positions of the International Society for Clinical Densitometry and Executive Summary of the 2007 ISCD Pediatric Position Development Conference. J. Clin. Densitom. 2008, 11, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.W.; Thomas, B.J.; Brown, J.K.; Finkelstein, J.S. Simulated Increases in Body Fat and Errors in Bone Mineral Density Measurements by DXA and QCT. J. Bone Miner. Res. 2012, 27, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.R.; Krauss, B.; Sedlmair, M.; Grasruck, M.; Bruder, H.; Morhard, D.; Fink, C.; Weckbach, S.; Lenhard, M.; Schmidt, B.; et al. Material Differentiation by Dual Energy CT: Initial Experience. Eur. Radiol. 2007, 17, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Willemink, M.J.; Noël, P.B. The Evolution of Image Reconstruction for CT-from Filtered Back Projection to Artificial Intelligence. Eur. Radiol. 2019, 29, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, S.S.; De Beer, J.F.; Barth, J.R.; Cresswell, T.; Roberts, C.; Richards, D.P. Results of Modified Latarjet Reconstruction in Patients with Anteroinferior Instability and Significant Bone Loss. Arthroscopy 2007, 23, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Pickhardt, P.J.; Pooler, B.D.; Lauder, T.; del Rio, A.M.; Bruce, R.J.; Binkley, N. Opportunistic Screening for Osteoporosis Using Abdominal Computed Tomography Scans Obtained for Other Indications. Ann. Intern. Med. 2013, 158, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Graffy, P.M.; Lee, S.J.; Ziemlewicz, T.J.; Pickhardt, P.J. Prevalence of Vertebral Compression Fractures on Routine CT Scans According to L1 Trabecular Attenuation: Determining Relevant Thresholds for Opportunistic Osteoporosis Screening. AJR Am. J. Roentgenol. 2017, 209, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Schwaiger, B.J.; Gersing, A.S.; Baum, T.; Noël, P.B.; Zimmer, C.; Bauer, J.S. Bone Mineral Density Values Derived from Routine Lumbar Spine Multidetector Row CT Predict Osteoporotic Vertebral Fractures and Screw Loosening. AJNR Am. J. Neuroradiol. 2014, 35, 1628–1633. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.; Boll, D.T.; Mileto, A.; Nelson, R.C. State of the Art: Dual-Energy CT of the Abdomen. Radiology 2014, 271, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.E.; Macovski, A. Energy-Selective Reconstructions in X-ray Computerized Tomography. Phys. Med. Biol. 1976, 21, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, S.; Liang, T.; Murphy, D.T.; Korzan, J.R.; Ouellette, H.; Munk, P. Dual-Energy CT: A Promising New Technique for the Evaluation of the Musculoskeletal System. AJR Am. J. Roentgenol. 2012, 199, S78–S86. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Umezawa, Y.; Asahina, A.; Nakagawa, H.; Furuya, K.; Fukuda, K. Dual Energy CT Iodine Map for Delineating Inflammation of Inflammatory Arthritis. Eur. Radiol. 2017, 27, 5034–5040. [Google Scholar] [CrossRef] [PubMed]
- McCollough, C.H.; Leng, S.; Yu, L.; Fletcher, J.G. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology 2015, 276, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Leng, S.; McCollough, C.H. Dual-Energy CT-based Monochromatic Imaging. AJR Am. J. Roentgenol. 2012, 199, S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Patino, M.; Prochowski, A.; Agrawal, M.D.; Simeone, F.J.; Gupta, R.; Hahn, P.F.; Sahani, D.V. Material Separation Using Dual-Energy CT: Current and Emerging Applications. Radiographics 2016, 36, 1087–1105. [Google Scholar] [CrossRef] [PubMed]
- Mallinson, P.I.; Coupal, T.M.; McLaughlin, P.D.; Nicolaou, S.; Munk, P.L.; Ouellette, H.A. Dual-Energy CT for the Musculoskeletal System. Radiology 2016, 281, 690–707. [Google Scholar] [CrossRef] [PubMed]
- Diekhoff, T.; Scheel, M.; Hermann, S.; Mews, J.; Hamm, B.; Hermann, K.G.A. Osteitis: A Retrospective Feasibility Study Comparing Single-Source Dual-Energy CT to MRI in Selected Patients with Suspected Acute Gout. Skelet. Radiol. 2017, 46, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Omoumi, P.; Becce, F.; Racine, D.; Ott, J.G.; Andreisek, G.; Verdun, F.R. Dual-Energy CT: Basic Principles, Technical Approaches, and Applications in Musculoskeletal Imaging (Part 1). Semin. Musculoskelet. Radiol. 2015, 19, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Gallo-Bernal, S.; Peña-Trujillo, V.; Gee, M.S. Dual-energy computed tomography: Pediatric considerations. Pediatr. Radiol. 2024, 54, 2112–2126. [Google Scholar] [CrossRef]
- Sigrist, R.M.S.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef] [PubMed]
- Saarakkala, S.; Julkunen, P.; Mäkitalo, J.; Töyräs, J.; Jurvelin, J.S. Ultrasound Backscatter and Attenuation in Human Cartilage with Osteoarthritis: Ex Vivo Study. Ultrasound Med. Biol. 2006, 32, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.T.; Chen, C.L.; Jou, I.M.; Kuo, L.C.; Su, F.C. Ultrasonography for Assessment of Synovitis in Osteoarthritis: A Reliability Exercise. Mod. Rheumatol. 2016, 26, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Sarvazyan, A.P.; Rudenko, O.V.; Swanson, S.D.; Fowlkes, J.B.; Emelianov, S.Y. Shear Wave Elasticity Imaging: A New Ultrasonic Technology of Medical Diagnostics. Ultrasound Med. Biol. 1998, 24, 1419–1435. [Google Scholar] [CrossRef] [PubMed]
- Taljanovic, M.S.; Gimber, L.H.; Becker, G.W.; Latt, L.D.; Klauser, A.S.; Melville, D.M.; Gao, L.; Witte, R.S. Shear-Wave Elastography: Basic Physics and Musculoskeletal Applications. Radiographics 2017, 37, 855–870. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Lin, Y.; Zhang, J. Reliability of shear wave elastography for the assessment of gastrocnemius fascia elasticity in healthy individual. Sci. Rep. 2022, 12, 8698. [Google Scholar] [CrossRef]
- Ozcan, M.; Copuroglu, C.; Ciftdemir, M.; Duran, F.N.; Erem, M.; Ozcan, M.; Yilmaz, B.; Yilmaz, K.B.; Saridogan, K. The Efficiency of Ultrasound Elastography in the Differential Diagnosis of Soft Tissue Masses. J. Ultrasound Med. 2019, 38, 2055–2062. [Google Scholar] [CrossRef] [PubMed]
- Venne, G.; Rasquinha, B.J.; Kunz, M.; Ellis, R.E. Quantifying Subchondral Bone Stiffness Using Shear Wave Elastography: A Pilot Study. J. Ultrasound Med. 2020, 39, 2023–2031. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Barr, R.G.; Farrokh, A.; Dighe, M.; Hocke, M.; Jenssen, C.; Dong, Y.; Saftoiu, A.; Havre, R.F. Strain Elastography—How To Do It? Ultrasound Int. Open 2017, 3, E137–E149. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhou, S.; Zhu, J.; Wang, Q.; Zhu, Y.; Zhang, X. Quantitative Ultrasound Elastography Combined with RNA-Seq for Detection of Early-Stage Osteoarthritis. J. Orthop. Res. 2021, 39, 2588–2596. [Google Scholar] [CrossRef] [PubMed]
- Befrui, N.; Elsner, J.; Flesser, A.; Huvanandana, J.; Jarrousse, O.; Le, T.N.; Müller, M.; Schulze, W.H.W.; Taing, S.; Weidert, S. Vibroarthrography For Early Detection of Knee Osteoarthritis Using Normalized Frequency Features. Med. Biol. Eng. Comput. 2018, 56, 1499–1514. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, R.; Prus, A.; Jonak, K.; Krakowski, P. Vibroarthrography as a Noninvasive Screening Method for Early Diagnosis of Knee Osteoarthritis: A Review of Current Research. Appl. Sci. 2025, 15, 279. [Google Scholar] [CrossRef]
- Machrowska, A.; Karpiński, R.; Maciejewski, M.; Jonak, J.; Krakowski, P. Application of EEMD-DFA Algorithms and ANN Classification for Detection of Knee Osteoarthritis Using Vibroarthrography. Appl. Comput. Sci. 2024, 20, 90–108. [Google Scholar] [CrossRef]
- Karpiński, R.; Prus, A.; Jonak, K.; Krakowski, P. Application of Recurrence Quantification Analysis in the Detection of Osteoarthritis of the Knee with the Use of Vibroarthrography. Adv. Sci. Technol. Res. J. 2024, 18, 19–31. [Google Scholar] [CrossRef]
- Machrowska, A.; Karpiński, R.; Maciejewski, M.; Jonak, J.; Krakowski, P.; Syta, A. Multi-Scale Analysis of Knee Joint Acoustic Signals for Cartilage Degeneration Assessment. Sensors 2025, 25, 706. [Google Scholar] [CrossRef]
- Xuan, A.; Chen, H.; Chen, T.; Li, J.; Lu, S.; Fan, T.; Zeng, D.; Wen, Z.; Ma, J.; Hunter, D.; et al. The Application of Machine Learning in Early Diagnosis of Osteoarthritis: A Narrative Review. Ther. Adv. Musculoskelet. Dis. 2023, 15, 1759720X231158198. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.; Granton, P.; Zegers, C.M.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting More Information from Medical Images Using Advanced Feature Analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef] [PubMed]
- van Timmeren, J.E.; Cester, D.; Tanadini-Lang, S.; Alkadhi, H.; Baessler, B. Radiomics in Medical Imaging—“How-To” Guide and Critical Reflection. Insights Imaging 2020, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Mayerhoefer, M.E.; Materka, A.; Langs, G.; Häggström, I.; Szczypiński, P.; Gibbs, P.; Cook, G. Introduction to Radiomics. J. Nucl. Med. 2020, 61, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Gu, Y.; Basu, S.; Berglund, A.; Eschrich, S.A.; Schabath, M.B.; Forster, K.; Aerts, H.J.; Dekker, A.; Fenstermacher, D.; et al. Radiomics: The Process and the Challenges. Magn. Reson. Imaging 2012, 30, 1234–1248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tian, J.; Dong, D.; Gu, D.; Dong, Y.; Zhang, L.; Lian, Z.; Liu, J.; Zhao, X.; Mo, X.; et al. Radiomics Features of Multiparametric MRI as Novel Prognostic Factors in Advanced Nasopharyngeal Carcinoma. Clin. Cancer Res. 2017, 23, 4259–4269. [Google Scholar] [CrossRef] [PubMed]
- van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H.J.W.L. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef] [PubMed]
- Parmar, C.; Grossmann, P.; Bussink, J.; Lambin, P.; Aerts, H.J.W.L. Machine Learning Methods for Quantitative Radiomic Biomarkers. Sci. Rep. 2015, 5, 13087. [Google Scholar] [CrossRef] [PubMed]
- Lao, J.; Chen, Y.; Li, Z.C.; Li, Q.; Zhang, J.; Liu, J.; Zhai, G. A Deep Learning-Based Radiomics Model for Prediction of Survival in Glioblastoma Multiforme. Sci. Rep. 2017, 7, 10353. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, S.; Dong, D.; Wei, J.; Fang, C.; Zhou, X.; Sun, K.; Li, L.; Li, B.; Wang, M.; et al. The Applications of Radiomics in Precision Diagnosis and Treatment of Oncology: Opportunities and Challenges. Theranostics 2019, 9, 1303–1322. [Google Scholar] [CrossRef] [PubMed]
- Peuna, A.; Hekkala, J.; Haapea, M.; Podlipská, J.; Guermazi, A.; Saarakkala, S.; Nieminen, M.T.; Lammentausta, E. Texture Analysis of Quantitative Susceptibility Maps to Differentiate Type of Knee Cartilage. J. Magn. Reson. Imaging 2021, 54, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Ashinsky, B.G.; Coletta, C.E.; Bouhrara, M.; Lukas, V.A.; Boyle, J.M.; Reiter, D.A.; Neu, C.P.; Goldberg, I.G.; Spencer, R.G. Machine Learning Classification of Articular Cartilage Integrity Using MRI Data. Osteoarthr. Cartil. 2021, 29, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-Based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Qian, X.H.; Hu, J.; Zhang, Y.; Lin, X.; Hai, W.; Shi, K.; Jiang, X.; Li, Y.; Tang, H.D. Integrating TSPO PET imaging and transcriptomics to unveil the role of neuroinflammation and amyloid-β deposition in Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 455–467. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pan, F.; Feng, L.; Liu, B.; Hu, Y.; Wang, Q. Application of radiomics in diagnosis and treatment of lung cancer. Front. Pharmacol. 2023, 14, 1295511. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Litjens, G.; Kooi, T.; Bejnordi, B.E.; Setio, A.A.A.; Ciompi, F.; Ghafoorian, M.; van der Laak, J.A.W.M.; van Ginneken, B.; Sánchez, C.I. A Survey on Deep Learning in Medical Image Analysis. Med. Image Anal. 2017, 42, 60–88. [Google Scholar] [CrossRef] [PubMed]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. Med. Image Comput. Comput. Assist. Interv. 2015, 9351, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Raghu, M.; Zhang, C.; Kleinberg, J.; Bengio, S. Transfusion: Understanding Transfer Learning for Medical Imaging. Adv. Neural Inf. Process. Syst. 2019, 32, 3347–3357. [Google Scholar] [PubMed]
- Cheplygina, V.; de Bruijne, M.; Pluim, J.P.W. Not-So-Supervised: A Survey of Semi-Supervised, Multi-Instance, and Transfer Learning in Medical Image Analysis. Med. Image Anal. 2019, 54, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Walia, E.; Babyn, P. Generative Adversarial Network in Medical Imaging: A Review. Med. Image Anal. 2019, 58, 101552. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, N.; Tan, T.; Kang, Y.; Sun, C.; Xie, G.; Verdonschot, N.; Sprengers, A. Knee Bone and Cartilage Segmentation Based on a 3D Deep Neural Network Using Adversarial Loss for Prior Shape Constraint. Front. Med. 2022, 9, 792900. [Google Scholar] [CrossRef] [PubMed]
- Wirth, W.; Eckstein, F.; Kemnitz, J.; Baumgartner, C.F.; Konukoglu, E.; Fuerst, D.; Chaudhari, A.S. Accuracy and Longitudinal Reproducibility of Quantitative Femorotibial Cartilage Measures Derived from Automated U-Net-Based Segmentation of Two Different MRI Contrasts: Data from the Osteoarthritis Initiative Healthy Reference Cohort. Magn. Reson. Mater. Phy. 2021, 34, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Schlemper, J.; Oktay, O.; Schaap, M.; Heinrich, M.; Kainz, B.; Glocker, B.; Rueckert, D. Attention Gated Networks: Learning to Leverage Salient Regions in Medical Images. Med. Image Anal. 2019, 53, 197–207. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; Zhao, R.; Hao, L.; Chai, W.; Jiying, C.; Feng, Z.; Ji, Q.; Zhang, G. The Application of Artificial Intelligence in Periprosthetic Joint Infection. J. Adv. Res. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Chen, Y.; Hu, Y.; Zeng, F.; Wang, P.; Xu, L.; Wu, J.; Li, J.; Zhu, J.; Xiang, M.; et al. Development and Validation of a Machine Learning-Derived Radiomics Model for Diagnosis of Osteoporosis and Osteopenia Using Quantitative Computed Tomography. BMC Med. Imaging 2022, 22, 140. [Google Scholar] [CrossRef] [PubMed]
- Tiulpin, A.; Klein, S.; Bierma-Zeinstra, S.M.A.; Thevenot, J.; Rahtu, E.; van Meurs, J.; Oei, E.H.G.; Saarakkala, S. Multimodal Machine Learning-Based Knee Osteoarthritis Progression Prediction from Plain Radiographs and Clinical Data. Sci. Rep. 2019, 9, 20038. [Google Scholar] [CrossRef]
- Trapnell, C.; Pachter, L.; Salzberg, S.L. TopHat: Discovering Splice Junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Stuart, T.; Butler, A.; Hoffman, P.; Hafemeister, C.; Papalexi, E.; Mauck, W.M.; Hao, Y.; Stoeckius, M.; Smibert, P.; Satija, R. Comprehensive Integration of Single-Cell Data. Cell 2019, 177, 1888–1902.e21. [Google Scholar] [CrossRef] [PubMed]
- Ståhl, P.L.; Salmén, F.; Vickovic, S.; Lundmark, A.; Navarro, J.F.; Magnusson, J.; Giacomello, S.; Asp, M.; Westholm, J.O.; Huss, M.; et al. Visualization and Analysis of Gene Expression in Tissue Sections by Spatial Transcriptomics. Science 2016, 353, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Wang, M.; Li, A. Canonical Correlation Analysis for Multi-Omics Data Integration. Methods Mol. Biol. 2019, 1910, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Lê Cao, K.A.; González, I.; Déjean, S. integrOmics: An R Package to Unravel Relationships Between Two Omics Datasets. Bioinformatics 2009, 25, 2855–2856. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Olshen, A.B.; Ladanyi, M. Integrative Clustering of Multiple Genomic Data Types Using a Joint Latent Variable Model with Application to Breast and Lung Cancer Subtype Analysis. Bioinformatics 2009, 25, 2906–2912. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Baladandayuthapani, V.; Morris, J.S.; Broom, B.M.; Manyam, G.; Do, K.A. iBAG: Integrative Bayesian Analysis of High-Dimensional Multiplatform Genomics Data. Bioinformatics 2013, 29, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Barkley, D.; França, G.S.; Yanai, I. Exploring Tissue Architecture Using Spatial Transcriptomics. Nature 2021, 596, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Bergen, V.; Lange, M.; Peidli, S.; Wolf, F.A.; Theis, F.J. Generalizing RNA Velocity to Transient Cell States Through Dynamical Modeling. Nat. Biotechnol. 2020, 38, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Moses, L.; Pachter, L. Museum of Spatial Transcriptomics. Nat. Methods 2022, 19, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.E.; Li, C.; Rabinovic, A. Adjusting Batch Effects in Microarray Expression Data Using Empirical Bayes Methods. Biostatistics 2007, 8, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Hao, S.; Andersen-Nissen, E.; Mauck, W.M.; Zheng, S.; Butler, A.; Lee, M.J.; Wilk, A.J.; Darby, C.; Zager, M.; et al. Integrated Analysis of Multimodal Single-Cell Data. Cell 2021, 184, 3573–3587.e29. [Google Scholar] [CrossRef] [PubMed]
- Way, G.P.; Greene, C.S. Extracting a Biologically Relevant Latent Space from Cancer Transcriptomes with Variational Autoencoders. Pac. Symp. Biocomput. 2018, 23, 80–91. [Google Scholar] [PubMed]
- Aebersold, R.; Mann, M. Mass Spectrometry-Based Proteomics. Nature 2003, 422, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Cox, J. The MaxQuant Computational Platform for Mass Spectrometry-Based Shotgun Proteomics. Nat. Protoc. 2016, 11, 2301–2319. [Google Scholar] [CrossRef] [PubMed]
- Mateos, J.; Lourido, L.; Fernández-Puente, P.; Calamia, V.; Fernández-López, C.; Oreiro, N.; Ruiz-Romero, C.; Blanco, F.J. Differential Protein Profiling of Synovial Fluid from Rheumatoid Arthritis and Osteoarthritis Patients Using LC-MS/MS. J. Proteomics 2012, 75, 2869–2878. [Google Scholar] [CrossRef] [PubMed]
- Rohart, F.; Gautier, B.; Singh, A.; Lê Cao, K.A. mixOmics: An R Package for ‘omics Feature Selection and Multiple Data Integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef] [PubMed]
- Gillet, L.C.; Navarro, P.; Tate, S.; Röst, H.; Selevsek, N.; Reiter, L.; Bonner, R.; Aebersold, R. Targeted Data Extraction of the MS/MS Spectra Generated by Data-Independent Acquisition: A New Concept for Consistent and Accurate Proteome Analysis. Mol. Cell. Proteom. 2012, 11, O111.016717. [Google Scholar] [CrossRef] [PubMed]
- Demichev, V.; Messner, C.B.; Vernardis, S.I.; Lilley, K.S.; Ralser, M. DIA-NN: Neural Networks and Interference Correction Enable Deep Proteome Coverage in High Throughput. Nat. Methods 2020, 17, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Mann, M. MaxQuant Enables High Peptide Identification Rates, Individualized p.p.b.-Range Mass Accuracies and Proteome-Wide Protein Quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xing, Z.; Liu, T.; Zhou, J.; Liang, Q.; Tang, T.; Cui, H.; Peng, W.; Xiong, X.; Wang, Y. Synovial tissue quantitative proteomics analysis reveals paeoniflorin decreases LIFR and ASPN proteins in experimental rheumatoid arthritis. Drug. Des. Devel. Ther. 2018, 12, 463–473. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takashima, Y.; Kawakami, Y.; Maeda, Y.; Ohuchi, H.; Takami, Y.; Yamaguchi, R.; Hayashi, S.; Imoto, S.; Miyano, S.; Miyamoto, T. Integrative Transcriptomic and Proteomic Analysis of Osteoarthritic Cartilage and Synovium. J. Orthop. Res. 2021, 39, 1802–1813. [Google Scholar] [CrossRef] [PubMed]
- Machida, Y.J.; Machida, Y.; Chen, Y.; Gurtan, A.M.; Kupfer, G.M.; D’Andrea, A.D.; Dutta, A. UBE2T Is the E2 in the Fanconi Anemia Pathway and Undergoes Negative Autoregulation. Mol. Cell 2006, 23, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, J.; Wang, X.; Zhu, J.; Liu, Q.; Shi, Z.; Chambers, M.C.; Zimmerman, L.J.; Shaddox, K.F.; Kim, S.; et al. Proteogenomic Characterization of Human Colon and Rectal Cancer. Nature 2014, 513, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Bantscheff, M.; Schirle, M.; Sweetman, G.; Rick, J.; Kuster, B. Quantitative Mass Spectrometry in Proteomics: A Critical Review. Anal. Bioanal. Chem. 2007, 389, 1017–1031. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.; Schlegl, J.; Hahne, H.; Gholami, A.M.; Lieberenz, M.; Savitski, M.M.; Ziegler, E.; Butzmann, L.; Gessulat, S.; Marx, H.; et al. Mass-Spectrometry-Based Draft of the Human Proteome. Nature 2014, 509, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Boykov, Y.; Kolmogorov, V. An Experimental Comparison of Min-Cut/Max-Flow Algorithms for Energy Minimization in Vision. IEEE Trans. Pattern Anal. Mach. Intell. 2004, 26, 1124–1137. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Shelhamer, E.; Darrell, T. Fully Convolutional Networks for Semantic Segmentation. In Proceedings of the 2015 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Boston, MA, USA, 7–12 June 2015; pp. 3431–3440. [Google Scholar] [CrossRef] [PubMed]
- Vaswani, A.; Shazeer, N.; Parmar, N.; Uszoreit, J.; Jones, L.; Gomez, A.N.; Kaiser, Ł.; Polosukhin, I. Attention Is All You Need. Adv. Neural Inf. Process. Syst. 2017, 30, 5998–6008. [Google Scholar] [PubMed]
- Çiçek, Ö.; Abdulkadir, A.; Lienkamp, S.S.; Brox, T.; Ronneberger, O. 3D U-Net: Learning Dense Volumetric Segmentation from Sparse Annotation. Med. Image Comput. Comput. Assist. Interv. 2016, 9901, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, W.; Ourselin, S.; Vercauteren, T. Automatic Brain Tumor Segmentation Using Cascaded Anisotropic Convolutional Neural Networks. Brainlesion 2018, 11383, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Tajbakhsh, N.; Shin, J.Y.; Gurudu, S.R.; Hurst, R.T.; Kendall, C.B.; Gotway, M.B.; Liang, J. Convolutional Neural Networks for Medical Image Analysis: Full Training or Fine Tuning? IEEE Trans. Med. Imaging 2016, 35, 1299–1312. [Google Scholar] [CrossRef] [PubMed]
- Kamnitsas, K.; Ledig, C.; Newcombe, V.F.J.; Simpson, J.P.; Kane, A.D.; Menon, D.K.; Rueckert, D.; Glocker, B. Efficient Multi-Scale 3D CNN with Fully Connected CRF for Accurate Brain Lesion Segmentation. Med. Image Anal. 2017, 36, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Palla, K.; Wadsworth, M.H.; Ståhl, P.L.; Lundeberg, J.; Weibrecht, I.; Butler, A.; Satija, R.; Regev, A.; Rozenblatt-Rosen, O.; Macosko, E.Z.; et al. Spatial Transcriptomics and In Situ Sequencing to Study Alzheimer’s Disease. Cell 2020, 182, 976–991.e19. [Google Scholar] [CrossRef] [PubMed]
- Senior, A.W.; Evans, R.; Jumper, J.; Kirkpatrick, J.; Sifre, L.; Green, T.; Qin, C.; Žídek, A.; Nelson, A.W.R.; Bridgland, A.; et al. Improved Protein Structure Prediction Using Potentials from Deep Learning. Nature 2020, 577, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Oktay, O.; Schlemper, J.; Folgoc, L.L.; Lee, M.; Heinrich, M.; Misawa, K.; Mori, K.; McDonagh, S.; Hammerla, N.Y.; Kainz, B.; et al. Attention U-Net: Learning Where to Look for the Pancreas. Med. Image Comput. Comput. Assist. Interv. 2018, 11070, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Havaei, M.; Davy, A.; Warde-Farley, D.; Biard, A.; Courville, A.; Bengio, Y.; Pal, C.; Jodoin, P.M.; Larochelle, H. Brain Tumor Segmentation with Deep Neural Networks. Med. Image Anal. 2017, 35, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Kohavi, R. A Study of Cross-Validation and Bootstrap for Accuracy Estimation and Model Selection. In Proceedings of the 14th International Joint Conference on Artificial Intelligence, Montreal, QC, Canada, 20–25 August 1995; Volume 2, pp. 1137–1143. [Google Scholar]
- Lundberg, S.M.; Lee, S.I. A Unified Approach to Interpreting Model Predictions. Adv. Neural Inf. Process. Syst. 2017, 30, 4765–4774. [Google Scholar]
- Hosny, A.; Parmar, C.; Quackenbush, J.; Schwartz, L.H.; Aerts, H.J.W.L. Artificial Intelligence in Radiology. Nat. Rev. Cancer 2018, 18, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.K.; Miyake, T. All for One and One for All: Condensations and the Initiation of Skeletal Development. Bioessays 2000, 22, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Huiskes, R.; Ruimerman, R.; van Lenthe, G.H.; Janssen, J.D. Effects of mechanical forces on maintenance and adaptation of form in trabecular bone. Nature 2000, 405, 704–706. [Google Scholar] [CrossRef] [PubMed]
- Tyson, J.J.; Chen, K.C.; Novak, B. Sniffers, Buzzers, Toggles and Blinkers: Dynamics of Regulatory and Signaling Pathways in the Cell. Curr. Opin. Cell Biol. 2003, 15, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Ideker, T.; Galitski, T.; Hood, L. A New Approach to Decoding Life: Systems Biology. Annu. Rev. Genom. Hum. Genet. 2001, 2, 343–372. [Google Scholar] [CrossRef] [PubMed]
- Viceconti, M.; Clapworthy, G.; Van Sint Jan, S.; Denis, K. The VPH—Physiome Project: Standards and Tools for Multiscale Modelling in Clinical Applications. IEEE Rev. Biomed. Eng. 2008, 1, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Macosko, E.Z.; Basu, A.; Satija, R.; Nemesh, J.; Shekhar, K.; Goldman, M.; Tirosh, I.; Bialas, A.R.; Kamitaki, N.; Martersteck, E.M.; et al. Highly Parallel Genome-Wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell 2015, 161, 1202–1214. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Mobasheri, A.; Marty, M. Is There Any Scientific Evidence for the Use of Glucosamine in the Management of Human Osteoarthritis? Arthritis Res. Ther. 2012, 14, 201. [Google Scholar] [CrossRef] [PubMed]
- Cristino, S.; Grassi, F.; Toneguzzi, S.; Lisignoli, G.; Piacentini, A.; Magrini, E.; Facchini, A.; Belardinelli, R.; Melchiorri, C. Analysis of Chondrocyte Functional Marker Expression in Human Chondrocyte Cultures. Cell Tissue Res. 2000, 300, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Bashir, A.; Gray, M.L.; Boutin, R.D.; Burstein, D. Glycosaminoglycan in Articular Cartilage: In Vivo Assessment with Delayed Gd(DTPA)(2-)-Enhanced MR Imaging. Radiology 1997, 205, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Trattnig, S.; Marlovits, S.; Gebetsroither, S.; Szomolanyi, P.; Welsch, G.H.; Salomonowitz, E.; Watanabe, A.; Deimling, M.; Mamisch, T.C. Three-Dimensional Delayed Gadolinium-Enhanced MRI of Cartilage (dGEMRIC) for In Vivo Evaluation of Reparative Cartilage After Matrix-Associated Autologous Chondrocyte Transplantation at 3.0T: Preliminary Results. J. Magn. Reson. Imaging 2007, 26, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Boileau, P.; Villalba, M.; Hery, J.Y.; Balg, F.; Ahrens, P.; Neyton, L. Risk Factors for Recurrence of Shoulder Instability After Arthroscopic Bankart Repair. J. Bone Jt. Surg. Am. 2006, 88, 1755–1763. [Google Scholar] [CrossRef] [PubMed]
- Glaser, C.; Tins, B.J.; Trumm, C.G.; Stoller, D.W.; Reiser, M.F. Imaging of Synovial Inflammation: State of the Art and Future Perspectives. Eur. J. Radiol. 2008, 65, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Merrill, R.K.; Arvind, V.; Kaji, D.; Pasik, S.D.; Nwachukwu, C.C.; Nguyen, J.; Lebl, D.R. Examining the Ability of Artificial Neural Networks Machine Learning Models to Accurately Predict Complications Following Posterior Lumbar Spine Fusion. Spine 2018, 43, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A Revolutionary Tool for Transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Klauser, A.S.; Faschingbauer, R.; Jaschke, W.R. Is Sonoelastography of Value in Assessing Tendons? Semin. Musculoskelet. Radiol. 2010, 14, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.S.; Varmus, H. A New Initiative on Precision Medicine. N. Engl. J. Med. 2015, 372, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Topol, E.J. High-Performance Medicine: The Convergence of Human and Artificial Intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Parmar, C.; Rios Velazquez, E.; Leijenaar, R.; Jermoumi, M.; Carvalho, S.; Mak, R.H.; Tyagi, N.; Yan, B.P.; Lambin, P.; Aerts, H.J. Robust Radiomics Feature Quantification Using Semiautomatic Volumetric Segmentation. PLoS ONE 2014, 9, e102107. [Google Scholar] [CrossRef] [PubMed]
- Pearl, J. Bayesian Networks: A Model of Self-Activated Memory for Evidential Reasoning. Proc. Cogn. Sci. Soc. 1985, 7, 329–334. [Google Scholar]
- Scarselli, F.; Gori, M.; Tsoi, A.C.; Hagenbuchner, M.; Monfardini, G. The Graph Neural Network Model. IEEE Trans. Neural Netw. 2009, 20, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Lu, M.Y.; Chen, T.Y.; Williamson, D.F.K.; Mahmood, F. Synthetic Data in Machine Learning for Medicine and Healthcare. Nat. Biomed. Eng. 2021, 5, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Gal, Y.; Ghahramani, Z. Dropout as a Bayesian Approximation: Representing Model Uncertainty in Deep Learning. In Proceedings of the 33rd International Conference on Machine Learning, New York, NY, USA, 20–22 June 2016; Volume 48, pp. 1050–1059. [Google Scholar]
- Chavez, L.; Meguro, J.; Chen, S.; de Paiva, V.N.; Zambrano, R.; Eterno, J.M.; Kumar, R.; Duncan, M.R.; Benny, M.; Young, K.C.; et al. Circulating extracellular vesicles activate the pyroptosis pathway in the brain following ventilation-induced lung injury. J. Neuroinflamm. 2021, 18, 310. [Google Scholar] [CrossRef] [PubMed]
- Pap, T.; Müller-Ladner, U.; Gay, R.E.; Gay, S. Fibroblast Biology in Rheumatoid Arthritis: Synovial Fibroblasts as Effector Cells in Inflammation and Tissue Destruction. Arthritis Res. 2000, 2, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Mow, V.C.; Wang, C.C.; Hung, C.T. The Extracellular Matrix, Interstitial Fluid and Ions as a Mechanical Signal Transducer in Articular Cartilage. Osteoarthr. Cartil. 1999, 7, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C. Stop Explaining Black Box Machine Learning Models for High Stakes Decisions and Use Interpretable Models Instead. Nat. Mach. Intell. 2019, 1, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Abadi, M.; Agarwal, A.; Barham, P.; Brevdo, E.; Chen, Z.; Citro, C.; Corrado, G.S.; Davis, A.; Dean, J.; Devin, M.; et al. TensorFlow: Large-Scale Machine Learning on Heterogeneous Distributed Systems. arXiv 2016, arXiv:1603.04467. [Google Scholar]
- Bates, D.W.; Saria, S.; Ohno-Machado, L.; Shah, A.; Escobar, G. Big Data In Health Care: Using Analytics To Identify And Manage High-Risk And High-Cost Patients. Health Aff. 2014, 33, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Ratib, O.; Rosset, A.; Heuberger, J. Open Source Software and Social Networks: Disruptive Alternatives for Medical Imaging. Eur. J. Radiol. 2011, 78, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, D.; Roemer, F.W.; Guermazi, A. Osteoarthritis Year in Review 2024: Imaging. Osteoarthr. Cartil. 2025, 33, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Mündermann, A.; Nüesch, C.; Ewald, H.; Jonkers, I. Osteoarthritis Year in Review 2024: Biomechanics. Osteoarthr. Cartil. 2024, 32, 1530–1541. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, H.F.; Birmingham, T.B.; Moyer, R.F.; Yacoub, D.; Kanko, L.E.; Bryant, D.M.; Thiessen, J.D.; Thompson, R.T. MRI T2 and T1ρ Relaxation in Patients at Risk for Knee Osteoarthritis: A Systematic Review and Meta-Analysis. BMC Musculoskelet. Disord. 2019, 20, 182. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, H.; Xie, X.; Tang, H.; Liu, X.; Wen, Y.; Xiao, X.; Ye, L.; Tang, Y.; Dai, G.; et al. Dual-Energy CT Virtual Non-Calcium: An Accurate Method for Detection of Knee Osteoarthritis-Related Edema-like Marrow Signal Intensity. Insights Imaging 2023, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Eskelinen, A.S.A.; Tanska, P.; Florea, C.; Orozco, G.A.; Julkunen, P.; Grodzinsky, A.J.; Korhonen, R.K. Mechanobiological Model for Simulation of Injured Cartilage Degradation via Pro-Inflammatory Cytokines and Mechanical Stimulus. PLoS Comput. Biol. 2020, 16, e1007998. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Uldin, H.; Khan, Z.; Panchal, H.; Iyengar, K.P.; Botchu, R. Role of Artificial Intelligence in Musculoskeletal Interventions. Cancers 2025, 17, 1615. [Google Scholar] [CrossRef] [PubMed]
- Gitto, S.; Serpi, F.; Albano, D.; Risoleo, G.; Fusco, S.; Messina, C.; Sconfienza, L. AI Applications in Musculoskeletal Imaging: A Narrative Review. Eur. Radiol. Exp. 2024, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Demircioğlu, A. Reproducibility and Interpretability in Radiomics: A Critical Assessment. Diagn. Interv. Radiol. 2025, 31, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.M.; Mir, G.M.; Raina, N.T.; Mir, S.M.; Mir, S.M.; Miskeen, E.; Alharthi, M.H.; Alamri, M.M.S. Application of Artificial Intelligence in Medical Education: Current Scenario and Future Perspectives. J. Adv. Med. Educ. Prof. 2023, 11, 133–140. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R.; Sporn, K.; Prabhakar, V.; Alnemri, A.; Khanna, A.; Paladugu, P.; Gowda, C.; Clarkson, L.; Zaman, N.; Tavakkoli, A. Computational and Imaging Approaches for Precision Characterization of Bone, Cartilage, and Synovial Biomolecules. J. Pers. Med. 2025, 15, 298. https://doi.org/10.3390/jpm15070298
Kumar R, Sporn K, Prabhakar V, Alnemri A, Khanna A, Paladugu P, Gowda C, Clarkson L, Zaman N, Tavakkoli A. Computational and Imaging Approaches for Precision Characterization of Bone, Cartilage, and Synovial Biomolecules. Journal of Personalized Medicine. 2025; 15(7):298. https://doi.org/10.3390/jpm15070298
Chicago/Turabian StyleKumar, Rahul, Kyle Sporn, Vibhav Prabhakar, Ahab Alnemri, Akshay Khanna, Phani Paladugu, Chirag Gowda, Louis Clarkson, Nasif Zaman, and Alireza Tavakkoli. 2025. "Computational and Imaging Approaches for Precision Characterization of Bone, Cartilage, and Synovial Biomolecules" Journal of Personalized Medicine 15, no. 7: 298. https://doi.org/10.3390/jpm15070298
APA StyleKumar, R., Sporn, K., Prabhakar, V., Alnemri, A., Khanna, A., Paladugu, P., Gowda, C., Clarkson, L., Zaman, N., & Tavakkoli, A. (2025). Computational and Imaging Approaches for Precision Characterization of Bone, Cartilage, and Synovial Biomolecules. Journal of Personalized Medicine, 15(7), 298. https://doi.org/10.3390/jpm15070298