Impact of Sex on Rehospitalization Rates and Mortality of Patients with Heart Failure with Preserved Ejection Fraction: Differences Between an Analysis Stratified by Sex and a Global Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
2.2. Patients
2.3. Clinical Variables
2.4. Follow-Up and Events
2.5. Statistical Analysis
3. Results
3.1. Demographics and Clinical Data of Study Patients
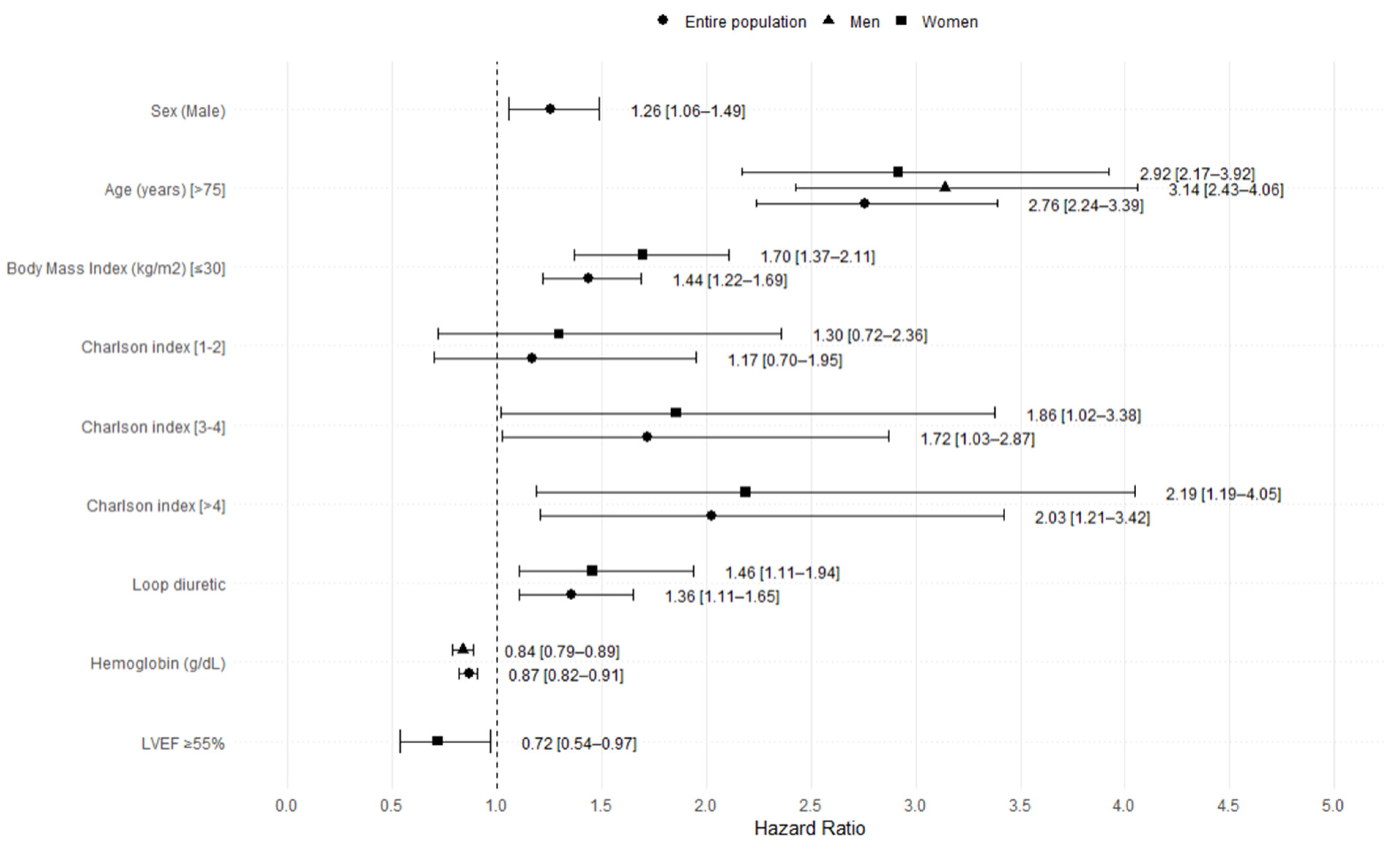
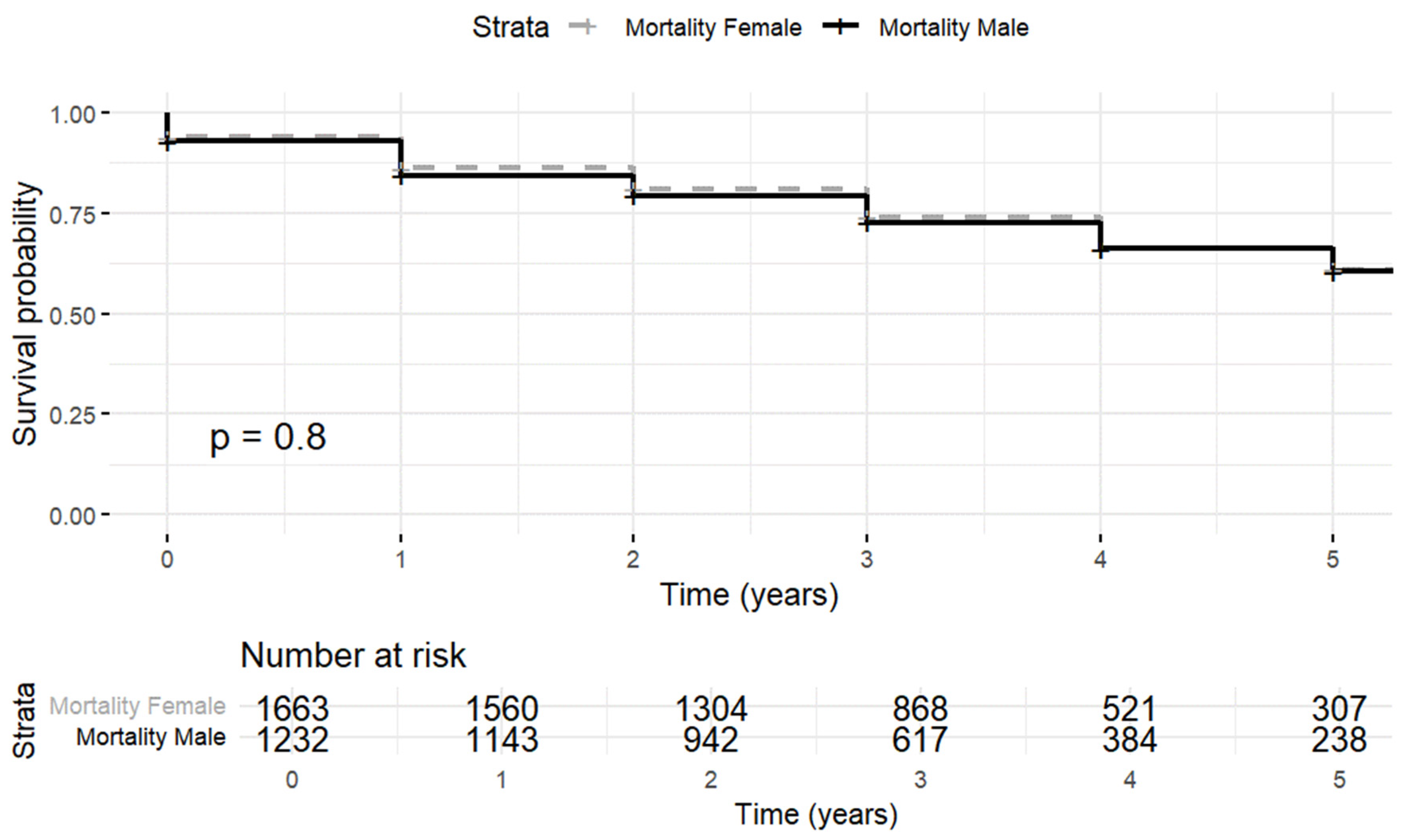
3.2. Mortality
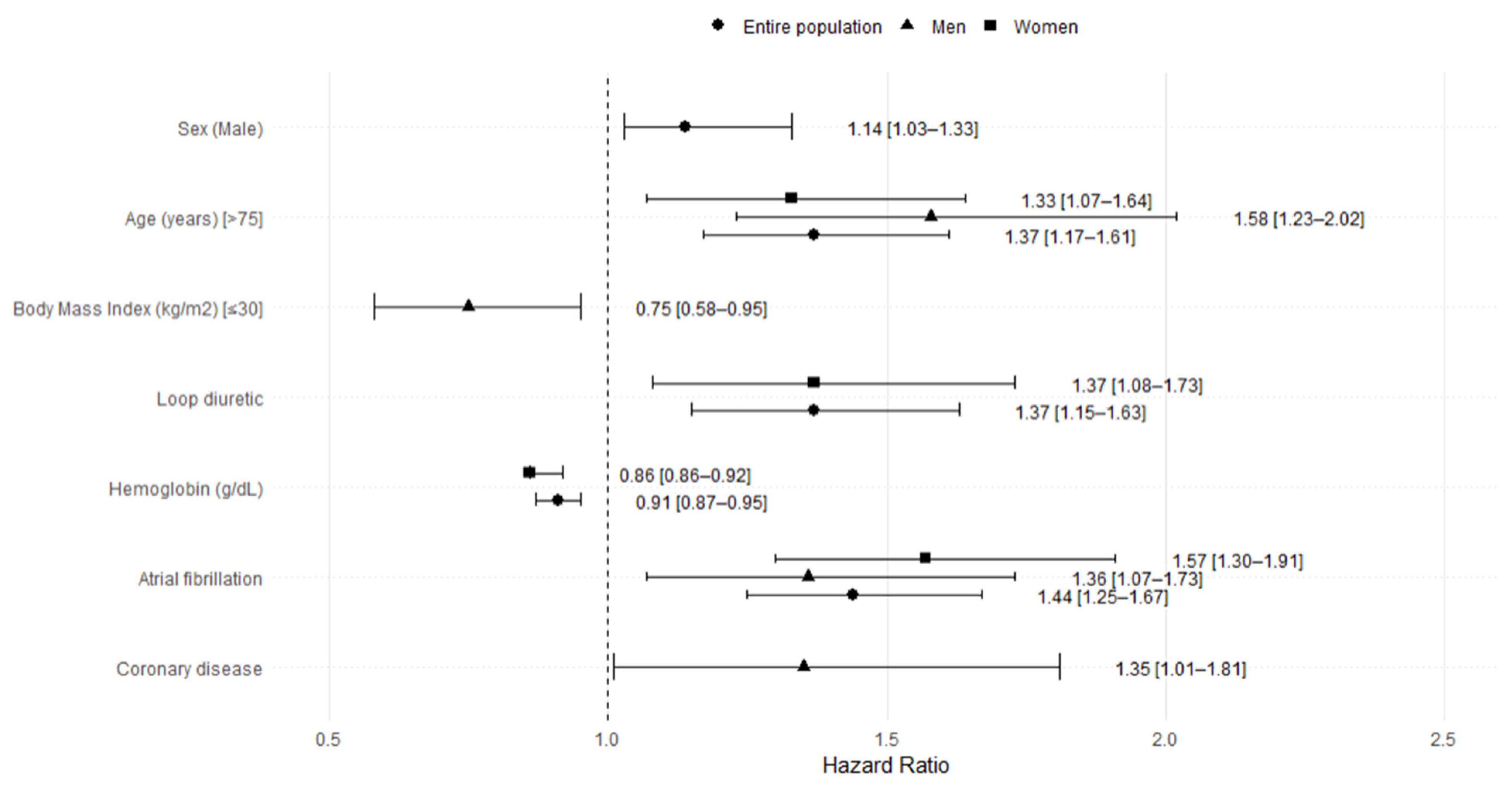
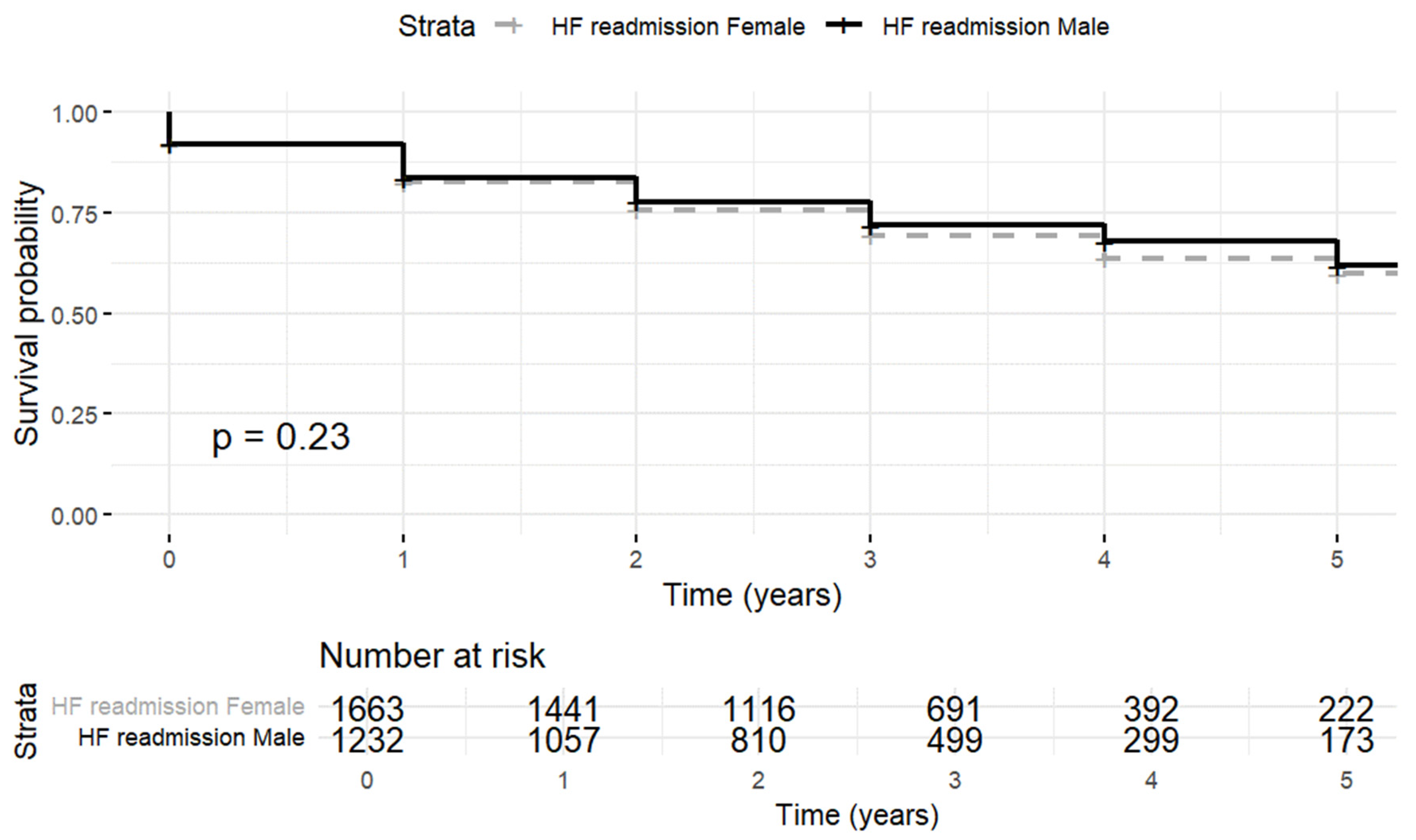
3.3. Heart Failure Rehospitalization
4. Discussion
4.1. Study Limitations
4.2. Implications and Future Lines of Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beghini, A.; Sammartino, A.M.; Papp, Z.; von Haehling, S.; Biegus, J.; Ponikowski, P.; Adamo, M.; Falco, L.; Lombardi, C.M.; Pagnesi, M.; et al. 2024 update in heart failure. ESC Heart Fail. 2024, 12, 8–42. [Google Scholar] [CrossRef] [PubMed]
- Lala, A.; Tayal, U.; Hamo, C.E. Sex Differences in Heart Failure. J. Card. Fail. 2022, 28, 477–498. [Google Scholar] [CrossRef]
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef]
- Anderson, T.; Hummel, S.L.; Konerman, M.C. Epidemiology, diagnosis, pathophysiology, and initial approach to heart failure with preserved ejection fraction. Cardiol Clin. 2022, 40, 397–413. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Santema, B.T.; Ouwerkerk, W.; Tromp, J.; Sama, I.E.; Ravera, A.; Regitz-Zagrosek, V.; Hillege, H.; Samani, N.J.; Zannad, F.; Dickstein, K.; et al. Identifying optimal doses of heart failure medications in men compared with women: A prospective, observational, cohort study. Lancet 2019, 394, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.E.; Unger, E.F.; Jenkins, M.R.; Southworth, M.R.; McDowell, T.-Y.; Geller, R.J.; Elahi, M.; Temple, R.J.; Woodcock, J. Participation of Women in Clinical Trials Supporting FDA Approval of Cardiovascular Drugs. J. Am. Coll. Cardiol. 2018, 71, 1960–1969. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, J.N.; Kaur, G.; Power, J.E.; Barkhordarian, M.; Mares, A.; Goyal, A.; Gulati, M. Underrepresentation of Women in Late-Breaking Cardiovascular Clinical Trials. J. Women’s Health 2023, 32, 635–640. [Google Scholar] [CrossRef]
- Pocock, S.J.; Ariti, C.A.; McMurray, J.J.; Maggioni, A.; Køber, L.; Squire, I.B.; Swedberg, K.; Dobson, J.; Poppe, K.K.; Whalley, G.A.; et al. Meta-Analysis Global Group in Chronic Heart Failure. Predicting survival in heart failure: A risk score based on 39 372 patients from 30 studies. Eur. Heart J. 2013, 34, 1404–1413. [Google Scholar] [CrossRef]
- Massie, B.M.; Carson, P.E.; McMurray, J.J. Irbesartan in patients with heart failure and preserved ejection fraction. N. Engl. J. Med. 2008, 359, 2456–2467. [Google Scholar] [CrossRef]
- Gracia Gutiérrez, A.; Poblador-Plou, B.; Prados-Torres, A.; Laiglesia, F.J.R.; Gimeno-Miguel, A. Sex Differences in Comorbidity, Therapy, and Health Services’ Use of Heart Failure in Spain: Evidence from Real-World Data. Int. J. Environ. Res. Public Health 2020, 17, 2136. [Google Scholar] [CrossRef] [PubMed]
- Cendrós, V.; Domingo, M.; Navas, E.; Muñoz, M.Á.; Bayés-Genís, A.; Verdú-Rotellar, J.M. Rehospitalization, mortality and associated variables in primary care patients with heart failure and preserved ejection fraction after first hospitalization. Int. J. Cardiol. Cardiovasc. Risk Prev. 2025, 25, 200391. [Google Scholar] [CrossRef] [PubMed]
- Recalde, M.; Rodríguez, C.; Burn, E.; Far, M.; García, D.; Carrere-Molina, J.; Benítez, M.; Moleras, A.; Pistillo, A.; Bolíbar, B.; et al. Data Resource Profile: The Information System for Research in Primary Care (SIDIAP). Int. J. Epidemiol. 2022, 51, e324–e336. [Google Scholar] [CrossRef]
- Bolíbar, B.; Avilés, F.F.; Morros, R.; Del Mar Garcia-Gil, M.; Hermosilla, E.; Ramos, R.; Rosell, M.; Rodríguez, J.; Medina, M.; Calero, S.; et al. Base de datos SIDIAP: La historia clínica informatizada de Atención Primaria como fuente de información para la investigación epidemiológica [SIDIAP database: Electronic clinical records in primary care as a source of information for epidemiologic research]. Med. Clin. 2012, 138, 617–621. [Google Scholar] [CrossRef]
- Hobbs, F.D.; Jones, M.I.; Allan, T.F.; Wilson, S.; Tobias, R. European survey of primary care physician perceptions on heart failure diagnosis and management (Euro-HF). Eur. Heart J. 2000, 21, 1877–1887. [Google Scholar] [CrossRef]
- Paulus, W.J.; Tschope, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef]
- Kaur, G.; Lau, E. Sex differences in heart failure with preserved ejection fraction: From traditional risk factors to sex-specific risk factors. Women’s Health 2022, 18, 17455057221140209. [Google Scholar] [CrossRef]
- Beale, A.L.; Meyer, P.; Marwick, T.H.; Lam, C.S.P.; Kaye, D.M. Sex differences in cardiovascular pathophysiology:why women are overrepresented in heart failure with preserved ejection fraction. Circulation 2018, 138, 198–205. [Google Scholar] [CrossRef]
- Sotomi, Y.; Hikoso, S.; Nakatani, D.; Mizuno, H.; Okada, K.; Dohi, T.; Kitamura, T.; Sunaga, A.; Kida, H.; PURSUIT-HFpEF Investigators; et al. Sex Differences in Heart Failure with Preserved Ejection Fraction. J. Am. Heart Assoc. 2021, 10, e018574. [Google Scholar] [CrossRef]
- Gori, M.; Lam, C.S.P.; Gupta, D.K.; Santos, A.B.S.; Cheng, S.; Shah, A.M.; Claggett, B.; Zile, M.R.; Kraigher-Krainer, E.; Pieske, B.; et al. Sex-specific cardio-vascular structure and function in heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2014, 16, 535–542. [Google Scholar] [CrossRef]
- Solomon, S.D.; Vaduganathan, M.; Claggett, B.L.; Packer, M.; Zile, M.; Swedberg, K.; Rouleau, J.; Pfeffer, M.A.; Desai, A.; Lund, L.H.; et al. Sacubitril/valsartan across the spectrum of ejection fraction in heart failure. Circulation 2020, 141, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Dewan, P.; Anand, I.S.; Desai, A.S.; Packer, M.; Zile, M.R.; Pfeffer, M.A.; Solomon, S.D.; Abraham, W.T.; Shah, S.J.; et al. Clinical characteristics and outcomes in patients with heart failure: Are there thresholds and inflection points in left ventricular ejection fraction and thresholds justifying a clinical classification? Circulation 2023, 148, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Wehner, G.J.; Jing, L.; Haggerty, C.M.; Suever, J.D.; Leader, J.B.; Hartzel, D.N.; Kirchner, H.L.; A Manus, J.N.; James, N.; Ayar, Z.; et al. Routinely reported ejection fraction and mortality in clinical practice: Where does the nadir of risk lie? Eur. Heart J. 2020, 41, 1249–1257. [Google Scholar] [CrossRef]
- Pitt, B.; Pfeffer, M.A.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Claggett, B.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; et al. Spironolactone for heart failure with preserved ejection fraction. N. Engl. J. Med. 2014, 370, 1383–1392. [Google Scholar] [CrossRef]
- Chung, J.; Kim, H.-L.; Kim, M.-A.; Choi, D.-J.; Han, S.; Jeon, E.-S.; Cho, M.-C.; Kim, J.-J.; Yoo, B.-S.; Shin, M.-S.; et al. Sex Differences in Long-Term Clinical Outcomes in Patients Hospitalized for Acute Heart Failure: A Report from the Korean Heart Failure Registry. J. Women’s Health 2019, 28, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Opasich, C.; Tavazzi, L.; Lucci, D.; Gorini, M.; Albanese, M.C.; Cacciatore, G.; Maggioni, A.P. Comparison of one year outcome in women versus men with chronic congestive heart failure. Am. J. Cardiol. 2000, 86, 353–357. [Google Scholar] [CrossRef]
- Iorio, A.; Senni, M.; Barbati, G.; Greene, S.J.; Poli, S.; Zambon, E.; Di Nora, C.; Cioffi, G.; Tarantini, L.; Gavazzi, A.; et al. Prevalence and prognostic impact of non-cardiac co-morbidities in heart failure outpatients with preserved and reduced ejection fraction: A community-based study. Eur. J. Heart Fail. 2018, 9, 1257–1266. [Google Scholar] [CrossRef]
| NO Mortality Event | Mortality Event | |||||||
|---|---|---|---|---|---|---|---|---|
| [ALL] (N = 2031) | Female (N = 1170) | Male (N = 861) | p-Value | [ALL] (N = 864) | Female (N = 493) | Male (N = 371) | p-Value | |
| Age (years), mean (SD) | 75.4 (9.9) | 77.4 (8.9) | 72.7 (10.6) | <0.001 | 80.9 (8.1) | 81.7 (7.5) | 79.8 (8.8) | 0.001 |
| Tobacco, N (%) | <0.001 | <0.001 | ||||||
| Non-smoker | 1834 (91.8%) | 1118 (97.0%) | 716 (84.8%) | 810 (95.1%) | 472 (97.9%) | 338 (91.4%) | ||
| Smoker | 163 (8.1%) | 35 (3.0%) | 128 (15.2%) | 42 (4.9%) | 10 (2.0%) | 32 (8.6%) | ||
| Clinical variables | ||||||||
| Body mass index, mean (SD) | 31.6 (6.0) | 32.3 (6.4) | 30.6 (5.4) | <0.001 | 29.9 (6.0) | 30.5 (6.7) | 29.1 (4.8) | 0.001 |
| a Obesity, N (%) | 906 (54.8%) | 566 (60.0%) | 340 (48.0%) | <0.001 | 289 (43.7%) | 173 (47.0%) | 116 (39.6%) | 0.067 |
| Hypertension, N (%) | 1648 (81.1%) | 981 (83.8%) | 667 (77.5%) | <0.001 | 706 (81.7%) | 419 (85.0%) | 287 (77.4%) | 0.005 |
| Diabetes Mellitus, N (%) | 905 (44.6%) | 486 (41.5%) | 419 (48.7%) | 0.002 | 397 (45.9%) | 227 (46.0%) | 170 (45.8%) | 1.000 |
| Microalbuminuria, N (%) | 406 (20.0%) | 206 (17.6%) | 200 (23.2%) | 0.002 | 183 (21.2%) | 93 (18.9%) | 90 (24.3%) | 0.066 |
| Dyslipidemia, N (%) | 1067 (52.5%) | 630 (53.8%) | 437 (50.8%) | 0.182 | 431 (49.9%) | 257 (52.1%) | 174 (46.9%) | 0.146 |
| Coronary artery disease, N (%) | 314 (15.5%) | 132 (11.3%) | 182 (21.1%) | <0.001 | 159 (18.4%) | 75 (15.2%) | 84 (22.6%) | 0.007 |
| Valvular heart disease, N (%) | 587 (28.9%) | 368 (31.5%) | 219 (25.4%) | 0.004 | 279 (32.3%) | 169 (34.3%) | 110 (29.6%) | 0.172 |
| Atrial fibrillation, N (%) | 974 (48.0%) | 600 (51.3%) | 374 (43.4%) | 0.001 | 480 (55.6%) | 279 (56.6%) | 201 (54.2%) | 0.524 |
| Stroke, N (%) | 210 (10.3%) | 119 (10.2%) | 91 (10.6%) | 0.828 | 102 (11.8%) | 60 (12.2%) | 42 (11.3%) | 0.782 |
| Peripheral artery disease, N (%) | 165 (8.1%) | 51 (4.3%) | 114 (13.2%) | <0.001 | 95 (11.0%) | 30 (6.0%) | 65 (17.5%) | <0.001 |
| b Anemia, N (%) | 326 (17.2%) | 168 (15.2%) | 158 (20.2%) | 0.006 | 261 (32.8%) | 131 (29.0%) | 130 (37.8%) | 0.011 |
| c Chronic kidney disease, N (%) | 548 (27.0%) | 345 (29.5%) | 203 (23.6%) | 0.004 | 358 (41.4%) | 215 (43.6%) | 143 (38.5%) | 0.154 |
| COPD, N (%) | 626 (30.8%) | 310 (26.5%) | 316 (36.7%) | <0.001 | 317 (36.7%) | 135 (27.4%) | 182 (49.1%) | <0.001 |
| Charlson index, N (%) | 0.001 | <0.001 | ||||||
| 0 | 139 (6.8%) | 88 (7.5%) | 51 (5.9%) | 30 (3.4%) | 26 (5.2%) | 4 (1.0%) | ||
| (1,2) | 860 (42.3%) | 525 (44.9%) | 335 (38.9%) | 261 (30.2%) | 165 (33.5%) | 96 (25.9%) | ||
| (3,5) | 688 (33.9%) | 389 (33.2%) | 299 (34.7%) | 350 (40.5%) | 197 (40.0%) | 153 (41.2%) | ||
| >4 | 344 (16.9%) | 168 (14.4%) | 176 (20.4%) | 223 (25.8%) | 105 (21.3%) | 118 (31.8%) | ||
| LVFE, mean (SD) | 61.7 (7.3) | 62.5 (7.3) | 60.6 (7.1) | <0.001 | 61.0 (7.2) | 61.6 (7.0) | 60.3 (7.4) | 0.015 |
| LVFE, N (%) | <0.001 | 0.025 | ||||||
| ≥55% | 1726 (85.0%) | 1035 (88.5%) | 691 (80.3%) | 708 (81.9%) | 417 (84.6%) | 291 (78.4%) | ||
| <55% | 305 (15.0%) | 135 (11.5%) | 170 (19.7%) | 156 (18.1%) | 76 (15.4%) | 80 (21.6%) | ||
| Treatment variables | ||||||||
| ACEi/ARB, N (%) | 1431 (70.5%) | 839 (71.7%) | 592 (68.8%) | 0.164 | 576 (66.7%) | 340 (69.0%) | 236 (63.6%) | 0.114 |
| Beta-blockers, N (%) | 1042 (51.3%) | 601 (51.4%) | 441 (51.2%) | 0.983 | 383 (44.3%) | 228 (46.2%) | 155 (41.8%) | 0.215 |
| MRA, N (%) | 198 (9.7%) | 113 (9.6%) | 85 (9.8%) | 0.932 | 79 (9.1%) | 43 (8.7%) | 36 (9.7%) | 0.707 |
| Loop diuretic, N (%) | 1395 (68.7%) | 843 (72.1%) | 552 (64.1%) | <0.001 | 670 (77.5%) | 400 (81.1%) | 270 (72.8%) | 0.005 |
| Tiazide, N (%) | 186 (9.1%) | 115 (9.8%) | 71 (8.2%) | 0.252 | 84 (9.7%) | 51 (10.3%) | 33 (8.8%) | 0.551 |
| Digoxin, N (%) | 268 (13.2%) | 182 (15.6%) | 86 (9.9%) | <0.001 | 138 (16.0%) | 98 (19.9%) | 40 (10.8%) | <0.001 |
| Calcium channel blockers, N (%) | 617 (30.4%) | 346 (29.6%) | 271 (31.5%) | 0.383 | 272 (31.5%) | 161 (32.7%) | 111 (29.9%) | 0.433 |
| Anti-platelet drugs, N (%) | 736 (36.2%) | 357 (30.5%) | 379 (44.0%) | <0.001 | 344 (39.8%) | 199 (40.4%) | 145 (39.1%) | 0.756 |
| Anticoagulants, N (%) | 812 (40.0%) | 480 (41.0%) | 332 (38.6%) | 0.282 | 345 (39.9%) | 200 (40.6%) | 145 (39.1%) | 0.711 |
| Statins, N (%) | 1071 (52.7%) | 576 (49.2%) | 495 (57.5%) | <0.001 | 388 (44.9%) | 228 (46.2%) | 160 (43.1%) | 0.399 |
| Laboratory variables | ||||||||
| Hemoglobin, mean (SD) | 13.7 (1.6) | 13.2 (1.3) | 14.4 (1.7) | <0.001 | 13.1 (1.6) | 12.8 (1.5) | 13.5 (1.7) | <0.001 |
| Estimated GFR, mean (SD) | 70.2 (19.0) | 69.1 (18.6) | 71.8 (19.4) | 0.002 | 62.6 (20.2) | 61.9 (19.7) | 63.5 (20.8) | 0.272 |
| Serum potassium, mean (SD) | 4.7 (0.5) | 4.6 (0.5) | 4.7 (0.5) | 0.003 | 4.7 (0.5) | 4.7 (0.5) | 4.8 (0.5) | 0.110 |
| NO Heart Failure Readmission | Heart Failure Readmission | |||||||
|---|---|---|---|---|---|---|---|---|
| [ALL] (N = 2064) | Female (N = 1169) | Male (N = 895) | p-Value | [ALL] (N = 831) | Female (N = 494) | Male (N = 337) | p-Value | |
| Age (years), mean (SD) | 76.7 (10.1) | 78.5 (9.1) | 74.4 (10.9) | <0.001 | 77.9 (8.7) | 79.2 (7.9) | 76.1 (9.5) | <0.001 |
| Tobacco, N (%) | <0.001 | <0.001 | ||||||
| Non-smoker | 1869 (92.0%) | 1112 (96.8%) | 757 (85.8%) | 775 (94.7%) | 478 (98.4%) | 297 (89.5%) | ||
| Smoker | 162 (7.9%) | 37 (3.2%) | 125 (14.2%) | 43 (5.2%) | 8 (1.6%) | 35 (10.5%) | ||
| Clinical variables | ||||||||
| Body mass index, mean (SD) | 30.9 (6.1) | 31.8 (6.6) | 29.8 (5.2) | <0.001 | 31.5 (6.0) | 31.9 (6.4) | 30.9 (5.2) | 0.033 |
| a Obesity, N (%) | 834 (50.7%) | 519 (56.4%) | 315 (43.4%) | <0.001 | 361 (54.0%) | 220 (56.1%) | 141 (51.1%) | 0.227 |
| Hypertension, N (%) | 1671 (81.0%) | 979 (83.7%) | 692 (77.3%) | <0.001 | 683 (82.2%) | 421 (85.2%) | 262 (77.7%) | 0.007 |
| Diabetes Mellitus, N (%) | 858 (41.6%) | 454 (38.8%) | 404 (45.1%) | 0.005 | 444 (53.4%) | 259 (52.4%) | 185 (54.9%) | 0.529 |
| Microalbuminuria, N (%) | 390 (18.9%) | 191 (16.3%) | 199 (22.2%) | 0.001 | 199 (23.9%) | 108 (21.9%) | 91 (27.0%) | 0.105 |
| Dyslipidemia, N (%) | 1056 (51.2%) | 608 (52.0%) | 448 (50.1%) | 0.403 | 442 (53.2%) | 279 (56.5%) | 163 (48.4%) | 0.026 |
| Coronary artery disease, N (%) | 323 (15.6%) | 134 (11.5%) | 189 (21.1%) | <0.001 | 150 (18.1%) | 73 (14.8%) | 77 (22.8%) | 0.004 |
| Valvular heart disease, N (%) | 613 (29.7%) | 364 (31.1%) | 249 (27.8%) | 0.113 | 253 (30.4%) | 173 (35.0%) | 80 (23.7%) | 0.001 |
| Atrial fibrillation, N (%) | 974 (47.2%) | 577 (49.4%) | 397 (44.4%) | 0.027 | 480 (57.8%) | 302 (61.1%) | 178 (52.8%) | 0.021 |
| Stroke, N (%) | 225 (10.9%) | 128 (10.9%) | 97 (10.8%) | 0.993 | 87 (10.5%) | 51 (10.3%) | 36 (10.7%) | 0.960 |
| Peripheral artery disease, N (%) | 186 (9.0%) | 49 (4.1%) | 137 (15.3%) | <0.001 | 74 (8.9%) | 32 (6.4%) | 42 (12.5%) | 0.004 |
| b Anemia, N (%) | 379 (19.8%) | 183 (16.6%) | 196 (24.0%) | <0.001 | 208 (27.0%) | 116 (25.4%) | 92 (29.4%) | 0.258 |
| c Chronic kidney disease, N (%) | 623 (30.2%) | 379 (32.4%) | 244 (27.3%) | 0.013 | 283 (34.1%) | 181 (36.6%) | 102 (30.3%) | 0.067 |
| COPD, N (%) | 661 (32.0%) | 305 (26.1%) | 356 (39.8%) | <0.001 | 282 (33.9%) | 140 (28.3%) | 142 (42.1%) | <0.001 |
| Charlson index, N (%) | <0.001 | 0.020 | ||||||
| 0 | 130 (6.3%) | 87 (7.4%) | 43 (4.8%) | 39 (4.6%) | 27 (5.4%) | 12 (3.5%) | ||
| [1,2] | 817 (39.6%) | 514 (44.0%) | 303 (33.9%) | 304 (36.6%) | 176 (35.6%) | 128 (38.0%) | ||
| [3,5] | 729 (35.3%) | 387 (33.1%) | 342 (38.2%) | 309 (37.2%) | 199 (40.3%) | 110 (32.6%) | ||
| >4 | 388 (18.8%) | 181 (15.5%) | 207 (23.1%) | 179 (21.5%) | 92 (18.6%) | 87 (25.8%) | ||
| LVFE, mean (SD) | 61.6 (7.2) | 62.3 (7.2) | 60.7 (7.1) | <0.001 | 61.2 (7.3) | 61.9 (7.3) | 60.1 (7.3) | 0.001 |
| LVFE, N (%) | <0.001 | <0.001 | ||||||
| ≥55% | 1746 (84.6%) | 1023 (87.5%) | 723 (80.8%) | 688 (82.8%) | 429 (86.8%) | 259 (76.9%) | ||
| <55% | 318 (15.4%) | 146 (12.5%) | 172 (19.2%) | 143 (17.2%) | 65 (13.2%) | 78 (23.1%) | ||
| Treatment variables | ||||||||
| ACEi/ARB, N (%) | 1412 (68.4%) | 818 (70.0%) | 594 (66.4%) | 0.089 | 595 (71.6%) | 361 (73.1%) | 234 (69.4%) | 0.287 |
| Beta-blockers, N (%) | 1006 (48.7%) | 581 (49.7%) | 425 (47.5%) | 0.341 | 419 (50.4%) | 248 (50.2%) | 171 (50.7%) | 0.935 |
| MRA, N (%) | 189 (9.1%) | 101 (8.6%) | 88 (9.8%) | 0.393 | 88 (10.6%) | 55 (11.1%) | 33 (9.7%) | 0.616 |
| Loop diuretic, N (%) | 1414 (68.5%) | 840 (71.9%) | 574 (64.1%) | <0.001 | 651 (78.3%) | 403 (81.6%) | 248 (73.6%) | 0.008 |
| Tiazide, N (%) | 192 (9.3%) | 118 (10.1%) | 74 (8.2%) | 0.181 | 78 (9.3%) | 48 (9.7%) | 30 (8.9%) | 0.784 |
| Digoxin, N (%) | 261 (12.6%) | 185 (15.8%) | 76 (8.4%) | <0.001 | 145 (17.4%) | 95 (19.2%) | 50 (14.8%) | 0.122 |
| Calcium channel blockers, N (%) | 606 (29.4%) | 337 (28.8%) | 269 (30.1%) | 0.577 | 283 (34.1%) | 170 (34.4%) | 113 (33.5%) | 0.850 |
| Anti-platelet drugs, N (%) | 760 (36.8%) | 378 (32.3%) | 382 (42.7%) | <0.001 | 320 (38.5%) | 178 (36.0%) | 142 (42.1%) | 0.089 |
| Anticoagulants, N (%) | 794 (38.5%) | 455 (38.9%) | 339 (37.9%) | 0.661 | 363 (43.7%) | 225 (45.5%) | 138 (40.9%) | 0.215 |
| Statins, N (%) | 1026 (49.7%) | 552 (47.2%) | 474 (53.0%) | 0.011 | 433 (52.1%) | 252 (51.0%) | 181 (53.7%) | 0.488 |
| Laboratory variables | ||||||||
| Hemoglobin, mean (SD) | 13.6 (1.6) | 13.2 (1.4) | 14.1 (1.8) | <0.001 | 13.3 (1.7) | 12.9 (1.4) | 13.9 (1.8) | <0.001 |
| Estimated GFR, mean (SD) | 68.4 (19.9) | 67.5 (19.3) | 69.6 (20.5) | 0.021 | 66.9 (19.1) | 65.9 (18.9) | 68.4 (19.2) | 0.075 |
| Serum potassium, mean (SD) | 4.7 (0.5) | 4.6 (0.5) | 4.7 (0.5) | 0.002 | 4.7 (0.5) | 4.7 (0.5) | 4.8 (0.6) | 0.130 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cendrós, V.; Domingo, M.; Navas, E.; Muñoz, M.Á.; Bayés-Genís, A.; Verdú-Rotellar, J.M. Impact of Sex on Rehospitalization Rates and Mortality of Patients with Heart Failure with Preserved Ejection Fraction: Differences Between an Analysis Stratified by Sex and a Global Analysis. J. Pers. Med. 2025, 15, 297. https://doi.org/10.3390/jpm15070297
Cendrós V, Domingo M, Navas E, Muñoz MÁ, Bayés-Genís A, Verdú-Rotellar JM. Impact of Sex on Rehospitalization Rates and Mortality of Patients with Heart Failure with Preserved Ejection Fraction: Differences Between an Analysis Stratified by Sex and a Global Analysis. Journal of Personalized Medicine. 2025; 15(7):297. https://doi.org/10.3390/jpm15070297
Chicago/Turabian StyleCendrós, Victoria, Mar Domingo, Elena Navas, Miguel Ángel Muñoz, Antoni Bayés-Genís, and José María Verdú-Rotellar. 2025. "Impact of Sex on Rehospitalization Rates and Mortality of Patients with Heart Failure with Preserved Ejection Fraction: Differences Between an Analysis Stratified by Sex and a Global Analysis" Journal of Personalized Medicine 15, no. 7: 297. https://doi.org/10.3390/jpm15070297
APA StyleCendrós, V., Domingo, M., Navas, E., Muñoz, M. Á., Bayés-Genís, A., & Verdú-Rotellar, J. M. (2025). Impact of Sex on Rehospitalization Rates and Mortality of Patients with Heart Failure with Preserved Ejection Fraction: Differences Between an Analysis Stratified by Sex and a Global Analysis. Journal of Personalized Medicine, 15(7), 297. https://doi.org/10.3390/jpm15070297






