Microvascularization of the Vocal Folds: Molecular Architecture, Functional Insights, and Personalized Research Perspectives
Abstract
1. Background
Purpose of the Study: The Importance and Complexity of Vascularization in the Vocal Apparatus
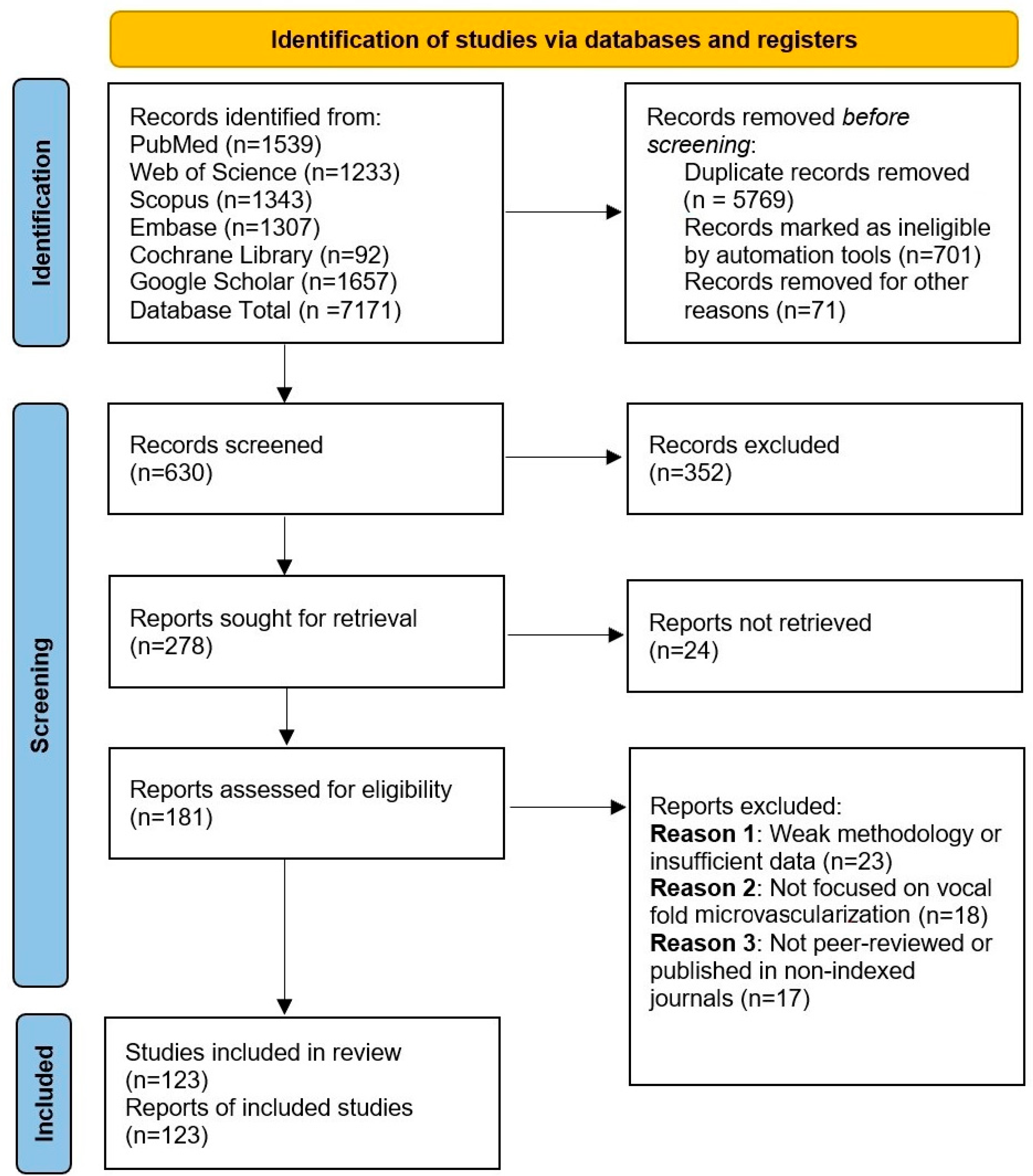
2. Materials and Methods
3. Results
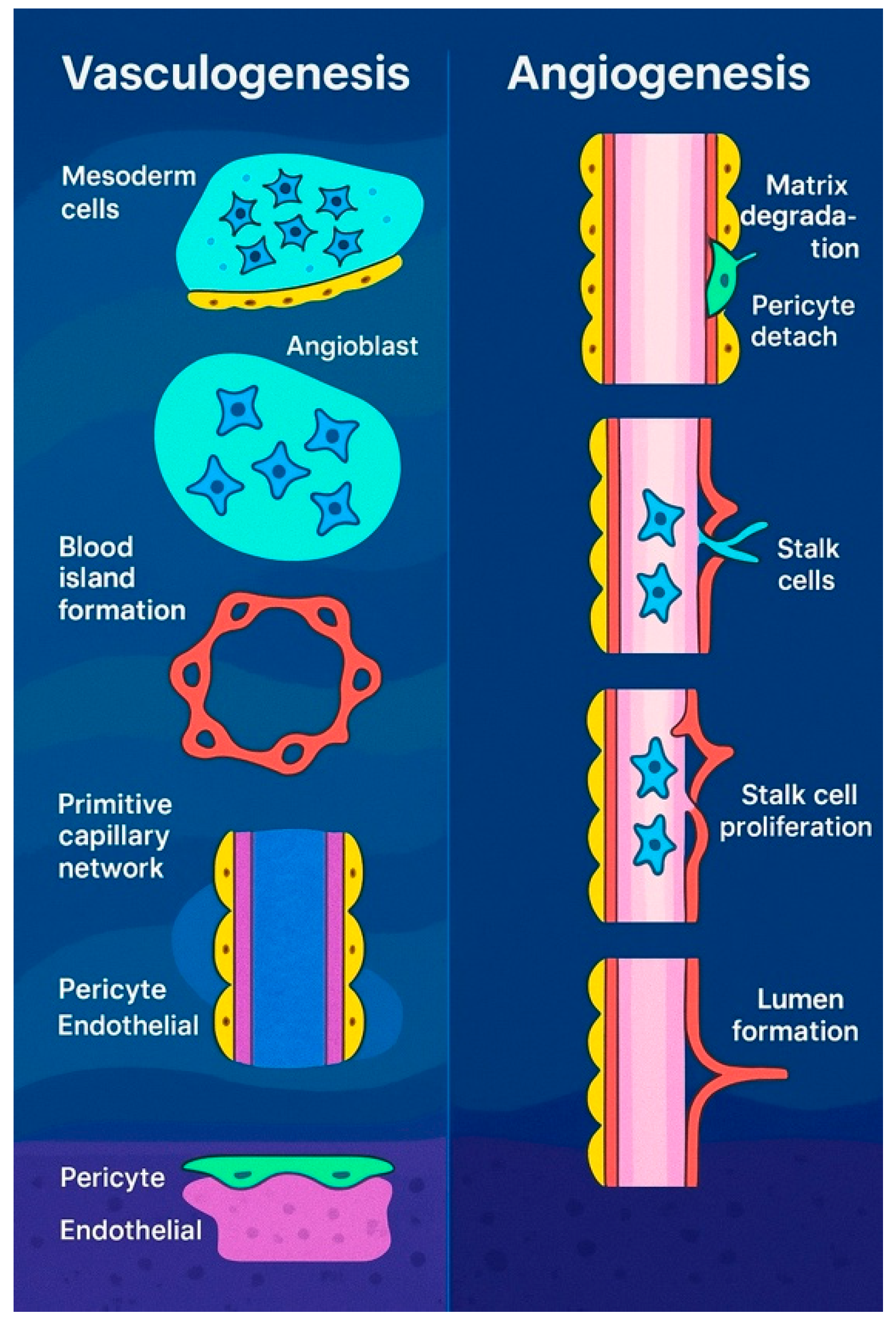
3.1. Personalized Approach on Embryonic Blood Vessel Formation: From Vasculogenesis to Angiogenesis
3.2. Normal Microvascular Structure of the Vocal Folds
3.3. The Microarchitecture of Blood Vessels Within the Mucosa of the Vocal Fold
3.4. Functional Role of the Vascular Network in the Human Vocal Fold Mucosa
3.5. Functional Importance of Capillary Pericytes in the Human Vocal Fold Mucosa
3.6. Key Mechanisms in Blood Vessel Formation: Endothelial Cell–Pericyte Interactions and Vascular Morphogenesis
3.7. The Role of Neuropeptides in the Regulation of Laryngeal Vascularization
- Neuropeptide Y (NPY): A 36-amino-acid peptide originally isolated from the porcine brain, with its molecular structure first elucidated by Tatemoto in 1982 [117]. NPY is co-localized with noradrenaline in sympathetic nerve terminals and is co-released with it. It is believed to play a significant role in angiogenesis during tissue development and repair processes [118,119].
- Vasoactive Intestinal Peptide (VIP): A 28-amino-acid peptide initially identified in porcine intestine in 1974. VIP is widely distributed in both the central and peripheral nervous systems and functions primarily in the relaxation of intestinal smooth muscles, dilation of peripheral blood vessels, and stimulation of salivary secretion [120].
- Calcitonin Gene-Related Peptide (CGRP): A 37-amino-acid peptide derived from alternative splicing of the calcitonin gene, first characterized in the 1980s. CGRP is extensively distributed throughout the central and peripheral nervous systems, where it plays key roles in vasodilation, pain transmission, and inflammatory processes [121].
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Esmaeili, N.; Davaris, N.; Boese, A.; Illanes, A.; Navab, N.; Friebe, M.; Arens, C. Contact Endoscopy—Narrow Band Imaging (CE-NBI) data set for laryngeal lesion assessment. Sci. Data 2023, 10, 733. [Google Scholar] [CrossRef] [PubMed]
- Rzepakowska, A.; Żurek, M.; Grzybowski, J.; Pihowicz, P.; Górnicka, B.; Osuch-Wójcikiewicz, E.; Niemczyk, K. Correlation of narrow band imaging vascular patterns with immunohistological microvessel density in vocal fold lesions. Braz. J. Otorhinolaryngol. 2021, 87, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Seeing the future of histotechnology through its history. J. Histotechnol. 2018, 41, 135–136. [Google Scholar] [CrossRef]
- Thomas, A.M.; Banerjee, A.K. The History of Radiology; OUP Oxford: Oxford, UK, 2013. [Google Scholar]
- Hînganu, M.V.; Cozma, R.S.; Ciochina, P.; Scutariu, I.A.; Asimionoaiei-Simionescu, C.; Hînganu, D. The morphometry of the laryngeal phonatory system—Base of the anatomical study of the voice aptitudes. Rom. J. Morphol. Embryol. 2017, 58, 1365–1369. [Google Scholar]
- Hînganu, M.V.; Hînganu, D.; Cozma, S.R.; Asimionoaiei-Simionescu, C.; Scutariu, I.A.; Ionesi, D.S.; Haba, D. Morphofunctional evaluation of buccopharyngeal space using three-dimensional cone-beam computed tomography (3D-CBCT). Ann. Anat.-Anat. Anz. 2018, 220, 1–8. [Google Scholar] [CrossRef]
- Sakthivel, S.; Prabhu, V. Optimal Deep Learning-Based Vocal Fold Disorder Detection and Classification Model on High-Speed Video Endoscopy. J. Healthc. Eng. 2022, 2022, 4248938. [Google Scholar] [CrossRef]
- Noden, D.M. Embryonic origins and assembly of blood vessels. Am. Rev. Respir. Dis. 1989, 140, 1097–1103. [Google Scholar] [CrossRef]
- Folkman, J.; Haudenschild, C. Angiogenesis in vitro. Nature 1980, 288, 551–556. [Google Scholar] [CrossRef]
- Folkman, J. Toward an understanding of angiogenesis: Search and discovery. Perspect. Biol. Med. 1985, 29, 10–36. [Google Scholar] [CrossRef]
- D’Amore, P.A.; Thompson, R.W. Mechanisms of angiogenesis. Annu. Rev. Physiol. 1987, 49, 453–464. [Google Scholar] [CrossRef]
- Olah, I.; Medgyes, J.; Glick, B. Origin of aortic cell clusters in the chicken embryo. Anat. Rec. 1988, 222, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Castroviejo, I. The association of extracranial and intracranial vascular malformations in children. Can. J. Neurol. Sci. 1985, 12, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Udan, R.S.; Culver, J.C.; Dickinson, M.E. Understanding vascular development. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 327–346. [Google Scholar] [CrossRef]
- Rezaei, F.; Shakoori, S.; Fazlyab, M.; Esnaashari, E.; Savadkouhi, S.T. Effect of low-level laser on proliferation, angiogenic and dentinogenic differentiation of human dental pulp stem cells. BMC Oral Health 2025, 25, 441. [Google Scholar] [CrossRef]
- Pardanaud, L.; Altmann, C.; Kitos, P.; Dieterlen-Lievre, F.; Buck, C.A. Vasculogenesis in the early quail blastodisc as studied with a monoclonal antibody recognizing endothelial cells. Development 1987, 100, 339–349. [Google Scholar] [CrossRef]
- Aragon, J.W.; Hirschi, K.K. Endothelial Cell Differentiation and Hemogenic Specification. Cold Spring Harb. Perspect. Med. 2022, 12, a041164. [Google Scholar] [CrossRef]
- Canu, G.; Ruhrberg, C. First blood: The endothelial origins of hematopoietic progenitors. Angiogenesis 2021, 24, 199–211. [Google Scholar] [CrossRef]
- Ferkowicz, M.J.; Yoder, M.C. Blood island formation: Longstanding observations and modern interpretations. Exp. Hematol. 2005, 33, 1041–1047. [Google Scholar] [CrossRef]
- Risau, W. Mechanisms of angiogenesis. Nature 1997, 386, 671–674. [Google Scholar] [CrossRef]
- Venketasubramanian, N.; Yeo, T.T.; Chen, C.L.H. Translational Medicine in Acute Ischemic Stroke and Traumatic Brain Injury-NeuroAiD Trials, from Traditional Beliefs to Evidence-Based Therapy. Biomolecules 2024, 14, 680. [Google Scholar] [CrossRef]
- Hudlická, O.; Tyler, K. Angiogenesis: The Growth of the Vascular System; Academic Press: Cambridge, MA, USA, 1986. [Google Scholar]
- Ludzki, A.C.; Krueger, E.M.; Baldwin, T.C.; Schleh, M.W.; Porsche, C.E.; Ryan, B.J.; Muir, L.A.; Singer, K.; Lumeng, C.N.; Horowitz, J.F. Acute Aerobic Exercise Remodels the Adipose Tissue Progenitor Cell Phenotype in Obese Adults. Front. Physiol. 2020, 11, 903. [Google Scholar] [CrossRef] [PubMed]
- Chappell, J.C.; Taylor, S.M.; Ferrara, N.; Bautch, V.L. Local guidance of emerging vessel sprouts requires soluble Flt-1. Dev. Cell 2009, 17, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Phng, L.K.; Gerhardt, H. Angiogenesis: A team effort coordinated by notch. Dev. Cell 2009, 16, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Kurusamy, S.; David, E.L.S.; Khan, K.; Kalyanakrishnan, K.; Ian-Gobo, M.; Kola, T.M.; Wilkinson, R.N.; Kannappan, V.; Wang, W.; et al. Aberrant expression of miR-133a in endothelial cells inhibits angiogenesis by reducing pro-angiogenic but increasing anti-angiogenic gene expression. Sci. Rep. 2022, 12, 14730. [Google Scholar] [CrossRef]
- Czarkwiani, A.; Dylus, D.V.; Carballo, L.; Oliveri, P. FGF signalling plays similar roles in development and regeneration of the skeleton in the brittle star Amphiura filiformis. Development 2021, 148, dev180760. [Google Scholar] [CrossRef]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef]
- Markiewski, M.M.; Daugherity, E.; Reese, B.; Karbowniczek, M. The Role of Complement in Angiogenesis. Antibodies 2020, 9, 67. [Google Scholar] [CrossRef]
- Nakai, Y.; Masutani, H.; Moriguchi, M.; Matsunaga, K.; Sugita, M. Microvascular structure of the larynx a scanning electron microscopic study of microcorrosion casts. Acta Oto-Laryngol. 1991, 111, 254–263. [Google Scholar] [CrossRef]
- Sato, K. Pericytes in the Human Vocal Fold Mucosa. Adv. Exp. Med. Biol. 2018, 1109, 79–93. [Google Scholar] [CrossRef]
- Splavski, B.; Rotim, K.; Lakičević, G.; Gienapp, A.J.; Boop, F.A.; Arnautović, K.I. Andreas Vesalius, the Predecessor of Neurosurgery: How his Progressive Scientific Achievements Affected his Professional Life and Destiny. World Neurosurg. 2019, 129, 202–209. [Google Scholar] [CrossRef]
- Anatomy, S.S.G.s. The Anatomical Basis of Clinical Practice; Churchill Livingstone: London, UK, 2008; Volume 40, p. 432. [Google Scholar]
- Garg, K.; Corona, B.T.; Walters, T.J. Therapeutic strategies for preventing skeletal muscle fibrosis after injury. Front. Pharmacol. 2015, 6, 87. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Hirano, M. Fine three-dimensional structure of pericytes in the vocal fold mucosa. Ann. Otol. Rhinol. Laryngol. 1997, 106, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Sato, K. Functional Histoanatomy of the Human Larynx; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- McCullagh, K.L.; Shah, R.N.; Huang, B.Y. Anatomy of the Larynx and Cervical Trachea. Neuroimaging Clin. N. Am. 2022, 32, 809–829. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, D. Blood and lymph vascular systems. In A Textbook of Histology, 11th ed.; Saunders: Philadelphia, PA, USA, 1986; pp. 367–405. [Google Scholar]
- Zimmermann, K.W. Der feinere bau der blutcapillaren. Z. Für Anat. Entwicklungsgeschichte 1923, 68, 29–109. [Google Scholar] [CrossRef]
- Rayner, S.G.; Hung, C.F.; Liles, W.C.; Altemeier, W.A. Lung pericytes as mediators of inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 2023, 325, L1–L8. [Google Scholar] [CrossRef]
- Kosztyła-Hojna, B.; Andrzejewska, A.; Moskal, D.; Kasperuk, J.; Falkowski, D.; Rogowski, M. Videostroboscopic and morphological aspects of voice disturbances in patients with larynx atrophy and coexisting hypopharynx cancer. Folia Histochem. Cytobiol./Pol. Acad. Sci. Pol. Histochem. Cytochem. Soc. 2011, 49, 659–663. [Google Scholar] [CrossRef]
- Cao, L.; Zhou, Y.; Chen, M.; Li, L.; Zhang, W. Pericytes for Therapeutic Approaches to Ischemic Stroke. Front. Neurosci. 2021, 15, 629297. [Google Scholar] [CrossRef]
- Dech, S.; Bittmann, F.N.; Schaefer, L.V. Muscle Oxygenation Level Might Trigger the Regulation of Capillary Venous Blood Filling during Fatiguing Isometric Muscle Actions. Diagnostics 2021, 11, 1973. [Google Scholar] [CrossRef]
- Ribatti, D.; Nico, B.; Crivellato, E. The role of pericytes in angiogenesis. Int. J. Dev. Biol. 2011, 55, 261–268. [Google Scholar] [CrossRef]
- Darden, J.; Payne, L.B.; Zhao, H.; Chappell, J.C. Excess vascular endothelial growth factor-A disrupts pericyte recruitment during blood vessel formation. Angiogenesis 2019, 22, 167–183. [Google Scholar] [CrossRef]
- Hirano, M.; Koike, Y.; Hirose, K.; Kasuya, H. Observation of mucous membrane of human vocal cords under electron miscoscopy. Nihon Jibiinkoka Gakkai Kaiho 1974, 77, 650–656. [Google Scholar] [PubMed]
- Hiroto, I.; Toyozumi, Y.; Tomita, H.; Miyagi, T.; Kuroki, K.; Koike, Y.; Matsushita, H. An experimental study on the hemodynamics of the vocal fold during vibration. J. Otolaryngol. Jpn. 1969, 72, 884–888. [Google Scholar]
- Bowers, S.L.; Meng, C.X.; Davis, M.T.; Davis, G.E. Investigating human vascular tube morphogenesis and maturation using endothelial cell-pericyte co-cultures and a doxycycline-inducible genetic system in 3D extracellular matrices. Methods Mol. Biol. 2015, 1189, 171–189. [Google Scholar] [CrossRef] [PubMed]
- Carresi, C.; Mollace, R.; Macrì, R.; Scicchitano, M.; Bosco, F.; Scarano, F.; Coppoletta, A.R.; Guarnieri, L.; Ruga, S.; Zito, M.C.; et al. Oxidative Stress Triggers Defective Autophagy in Endothelial Cells: Role in Atherothrombosis Development. Antioxidants 2021, 10, 387. [Google Scholar] [CrossRef] [PubMed]
- Bowers, S.L.K.; Kemp, S.S.; Aguera, K.N.; Koller, G.M.; Forgy, J.C.; Davis, G.E. Defining an Upstream VEGF (Vascular Endothelial Growth Factor) Priming Signature for Downstream Factor-Induced Endothelial Cell-Pericyte Tube Network Coassembly. Arter. Thromb. Vasc. Biol. 2020, 40, 2891–2909. [Google Scholar] [CrossRef]
- Sun, Z.; Kemp, S.S.; Lin, P.K.; Aguera, K.N.; Davis, G.E. Endothelial k-RasV12 Expression Induces Capillary Deficiency Attributable to Marked Tube Network Expansion Coupled to Reduced Pericytes and Basement Membranes. Arter. Thromb. Vasc. Biol. 2022, 42, 205–222. [Google Scholar] [CrossRef]
- Yao, Y. Basement membrane and stroke. J. Cereb. Blood Flow. Metab. 2019, 39, 3–19. [Google Scholar] [CrossRef]
- Hinganu, D.; Eva, I.; Stan, C.I.; Hinganu, M.V. Morphological aspects of the rectal neovascularization in colorectal cancer—Anatomical-surgical and imaging implications. Rom. J. Morphol. Embryol. 2016, 57, 161–165. [Google Scholar]
- Song, X.; Yu, Y.; Leng, Y.; Ma, L.; Mu, J.; Wang, Z.; Xu, Y.; Zhu, H.; Qiu, X.; Li, P.; et al. Expanding tubular microvessels on stiff substrates with endothelial cells and pericytes from the same adult tissue. J. Tissue Eng. 2022, 13, 20417314221125310. [Google Scholar] [CrossRef]
- Díaz-Flores, L.; Gutiérrez, R.; Madrid, J.F.; Varela, H.; Valladares, F.; Acosta, E.; Martín-Vasallo, P.; Díaz-Flores, L., Jr. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol. Histopathol. 2009, 24, 909–969. [Google Scholar] [CrossRef]
- van Splunder, H.; Villacampa, P.; Martínez-Romero, A.; Graupera, M. Pericytes in the disease spotlight. Trends Cell Biol. 2024, 34, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Mulay, A.R.; Hwang, J.; Kim, D.H. Microphysiological Blood-Brain Barrier Systems for Disease Modeling and Drug Development. Adv. Healthc. Mater. 2024, 13, e2303180. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; An, M.; Luo, Y.; Diao, X.; Zhong, W.; Pang, M.; Lin, Y.; Chen, J.; Li, Y.; Kong, Y.; et al. PDGFRα+ITGA11+ fibroblasts foster early-stage cancer lymphovascular invasion and lymphatic metastasis via ITGA11-SELE interplay. Cancer Cell 2024, 42, 682–700.E12. [Google Scholar] [CrossRef] [PubMed]
- Paolini, C.; Agarbati, S.; Benfaremo, D.; Mozzicafreddo, M.; Svegliati, S.; Moroncini, G. PDGF/PDGFR: A Possible Molecular Target in Scleroderma Fibrosis. Int. J. Mol. Sci. 2022, 23, 3904. [Google Scholar] [CrossRef]
- Bi, Q.; Wang, C.; Cheng, G.; Chen, N.; Wei, B.; Liu, X.; Li, L.; Lu, C.; He, J.; Weng, Y.; et al. Microglia-derived PDGFB promotes neuronal potassium currents to suppress basal sympathetic tonicity and limit hypertension. Immunity 2022, 55, 1466–1482.E9. [Google Scholar] [CrossRef]
- Jackson, E.L.; Garcia-Verdugo, J.M.; Gil-Perotin, S.; Roy, M.; Quinones-Hinojosa, A.; VandenBerg, S.; Alvarez-Buylla, A. PDGFRα-Positive B Cells Are Neural Stem Cells in the Adult SVZ that Form Glioma-like Growths in Response to Increased PDGF Signaling. Neuron 2006, 51, 187–199. [Google Scholar] [CrossRef]
- Kirdajova, D.; Anderova, M. NG2 cells and their neurogenic potential. Curr. Opin. Pharmacol. 2020, 50, 53–60. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Zhou, H.; Zhou, J. NG2-glia crosstalk with microglia in health and disease. CNS Neurosci. Ther. 2022, 28, 1663–1674. [Google Scholar] [CrossRef]
- Dean, T.; Ghaemmaghami, J.; Corso, J.; Gallo, V. The cortical NG2-glia response to traumatic brain injury. Glia 2023, 71, 1164–1175. [Google Scholar] [CrossRef]
- He, Y.; Li, Z.; Shi, X.; Ding, J.; Wang, X. Roles of NG2 Glia in Cerebral Small Vessel Disease. Neurosci. Bull. 2023, 39, 519–530. [Google Scholar] [CrossRef]
- Robins, S.C.; Kokoeva, M.V. NG2-Glia, a New Player in Energy Balance. Neuroendocrinology 2018, 107, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Song, S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005, 7, 452–464. [Google Scholar] [CrossRef]
- Birbrair, A.; Zhang, T.; Wang, Z.M.; Messi, M.L.; Mintz, A.; Delbono, O. Pericytes at the intersection between tissue regeneration and pathology. Clin. Sci. 2015, 128, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Wasik, A.; Ratajczak-Wielgomas, K.; Badzinski, A.; Dziegiel, P.; Podhorska-Okolow, M. The Role of Periostin in Angiogenesis and Lymphangiogenesis in Tumors. Cancers 2022, 14, 4225. [Google Scholar] [CrossRef]
- Xu, J.; Li, D.; Hsu, C.Y.; Tian, Y.; Zhang, L.; Wang, Y.; Tower, R.J.; Chang, L.; Meyers, C.A.; Gao, Y.; et al. Comparison of skeletal and soft tissue pericytes identifies CXCR4+ bone forming mural cells in human tissues. Bone Res. 2020, 8, 22. [Google Scholar] [CrossRef]
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes regulate the blood–brain barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef]
- Ding, H.; Chen, J.; Qin, J.; Chen, R.; Yi, Z. TGF-β-induced α-SMA expression is mediated by C/EBPβ acetylation in human alveolar epithelial cells. Mol. Med. 2021, 27, 22. [Google Scholar] [CrossRef]
- Younesi, F.S.; Son, D.O.; Firmino, J.; Hinz, B. Myofibroblast Markers and Microscopy Detection Methods in Cell Culture and Histology. Methods Mol. Biol. 2021, 2299, 17–47. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Q.; Cai, X.; Jiang, S.; Xu, N.; Zhou, Q.; Cao, X.; Hultström, M.; Tian, J.; Lai, E.Y. Osthole Ameliorates Renal Fibrosis in Mice by Suppressing Fibroblast Activation and Epithelial-Mesenchymal Transition. Front. Physiol. 2018, 9, 1650. [Google Scholar] [CrossRef]
- Yin, S.; Ma, X.Y.; Sun, Y.F.; Yin, Y.Q.; Long, Y.; Zhao, C.L.; Ma, J.W.; Li, S.; Hu, Y.; Li, M.T.; et al. RGS5 augments astrocyte activation and facilitates neuroinflammation via TNF signaling. J. Neuroinflammation 2023, 20, 203. [Google Scholar] [CrossRef]
- Kong, P.; Wang, X.; Gao, Y.K.; Zhang, D.D.; Huang, X.F.; Song, Y.; Zhang, W.D.; Guo, R.J.; Li, H.; Han, M. RGS5 maintaining vascular homeostasis is altered by the tumor microenvironment. Biol. Direct 2023, 18, 78. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.; Gaceb, A.; Enström, A.; Padel, T.; Genové, G.; Özen, I.; Paul, G. Regulator of G-protein signaling 5 regulates the shift from perivascular to parenchymal pericytes in the chronic phase after stroke. Faseb J. 2019, 33, 8990–8998. [Google Scholar] [CrossRef] [PubMed]
- Chang-Panesso, M.; Humphreys, B.D. CD248/Endosialin: A Novel Pericyte Target in Renal Fibrosis. Nephron 2015, 131, 262–264. [Google Scholar] [CrossRef]
- Lu, S.; Gan, L.; Lu, T.; Zhang, K.; Zhang, J.; Wu, X.; Han, D.; Xu, C.; Liu, S.; Yang, F.; et al. Endosialin in Cancer: Expression Patterns, Mechanistic Insights, and Therapeutic Approaches. Theranostics 2024, 14, 379–391. [Google Scholar] [CrossRef]
- Ash, S.L.; Orha, R.; Mole, H.; Dinesh-Kumar, M.; Lee, S.P.; Turrell, F.K.; Isacke, C.M. Targeting the activated microenvironment with endosialin (CD248)-directed CAR-T cells ablates perivascular cells to impair tumor growth and metastasis. J. Immunother. Cancer 2024, 12, e008608. [Google Scholar] [CrossRef]
- Wang, L.T.; Rajah, A.; Brown, C.M.; McCaffrey, L. CD13 orients the apical-basal polarity axis necessary for lumen formation. Nat. Commun. 2021, 12, 4697. [Google Scholar] [CrossRef]
- Anderluzzi, G.; Ghitti, M.; Gasparri, A.M.; Taiè, G.; Sacchi, A.; Gori, A.; Andolfo, A.; Pozzi, F.; Musco, G.; Curnis, F.; et al. A novel aminopeptidase N/CD13 inhibitor selectively targets an endothelial form of CD13 after coupling to proteins. Cell Mol. Life Sci. 2024, 81, 68. [Google Scholar] [CrossRef]
- Domínguez, J.M.; Pérez-Chacón, G.; Guillén, M.J.; Muñoz-Alonso, M.J.; Somovilla-Crespo, B.; Cibrián, D.; Acosta-Iborra, B.; Adrados, M.; Muñoz-Calleja, C.; Cuevas, C.; et al. CD13 as a new tumor target for antibody-drug conjugates: Validation with the conjugate MI130110. J. Hematol. Oncol. 2020, 13, 32. [Google Scholar] [CrossRef]
- Joshkon, A.; Heim, X.; Dubrou, C.; Bachelier, R.; Traboulsi, W.; Stalin, J.; Fayyad-Kazan, H.; Badran, B.; Foucault-Bertaud, A.; Leroyer, A.S.; et al. Role of CD146 (MCAM) in Physiological and Pathological Angiogenesis-Contribution of New Antibodies for Therapy. Biomedicines 2020, 8, 633. [Google Scholar] [CrossRef]
- Oladejo, M.; Nguyen, H.M.; Wood, L. CD105 in the progression and therapy of renal cell carcinoma. Cancer Lett. 2023, 570, 216327. [Google Scholar] [CrossRef]
- Li, L.; Zhong, L.; Tang, C.; Gan, L.; Mo, T.; Na, J.; He, J.; Huang, Y. CD105: Tumor diagnosis, prognostic marker and future tumor therapeutic target. Clin. Transl. Oncol. 2022, 24, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Sur, D.; Havasi, A.; Lungulescu, C.V.; Volovat, S.R.; Burz, C.; Irimie, A. Endoglin (CD105) as a putative prognostic biomarker for colorectal cancer: A systematic review. Med. Pharm. Rep. 2022, 95, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Hassn Mesrati, M.; Syafruddin, S.E.; Mohtar, M.A.; Syahir, A. CD44: A Multifunctional Mediator of Cancer Progression. Biomolecules 2021, 11, 1850. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Maxwell-Warburton, S.; Hasib, A.; Ma, L.; Kang, L. The membrane receptor CD44: Novel insights into metabolism. Trends Endocrinol. Metab. 2022, 33, 318–332. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Z.; Pan, F.; Li, P.; Yao, W.; Chen, Y.; Xiong, L.; Wang, T.; Li, Y.; Huang, G. Pericytes recruited by CCL28 promote vascular normalization after anti-angiogenesis therapy through RA/RXRA/ANGPT1 pathway in lung adenocarcinoma. J. Exp. Clin. Cancer Res. 2024, 43, 210. [Google Scholar] [CrossRef]
- Luo, J.; Lu, C.; Chen, Y.; Wu, X.; Zhu, C.; Cui, W.; Yu, S.; Li, N.; Pan, Y.; Zhao, W.; et al. Nuclear translocation of cGAS orchestrates VEGF-A-mediated angiogenesis. Cell Rep. 2023, 42, 112328. [Google Scholar] [CrossRef]
- Kang, Y.; Li, H.; Liu, Y.; Li, Z. Regulation of VEGF-A expression and VEGF-A-targeted therapy in malignant tumors. J. Cancer Res. Clin. Oncol. 2024, 150, 221. [Google Scholar] [CrossRef]
- Payne, L.B.; Hoque, M.; Houk, C.; Darden, J.; Chappell, J.C. Pericytes in Vascular Development. Curr. Tissue Microenviron. Rep. 2020, 1, 143–154. [Google Scholar] [CrossRef]
- Sun, Z.; Lin, P.K.; Yrigoin, K.; Kemp, S.S.; Davis, G.E. Increased Matrix Metalloproteinase-1 Activation Enhances Disruption and Regression of k-RasV12-Expressing Arteriovenous Malformation-Like Vessels. Am. J. Pathol. 2023, 193, 1319–1334. [Google Scholar] [CrossRef]
- Malik, A.; Chawla, S.; Tauseef, A.; Sohail, H.; Ijaz, F.; Malik, A.; Rahman, F.; Muhammad, G.; Khakwani, S. Association of Oxidative Stress and Production of Inflammatory Mediators Matrix Metalloproteinase-9 and Interleukin 6: Systemic Events in Radicular Cysts. Cureus 2020, 12, e7822. [Google Scholar] [CrossRef]
- Fan, L.J.; Kan, H.M.; Chen, X.T.; Sun, Y.Y.; Chen, L.P.; Shen, W. Vascular endothelial growth factor-A/vascular endothelial growth factor2 signaling in spinal neurons contributes to bone cancer pain. Mol. Pain 2022, 18, 17448069221075891. [Google Scholar] [CrossRef] [PubMed]
- Narita, T.; Kondo, M.; Oishi, Y. Macroscopic Banding Pattern of Collagen Gel Formed by a Diffusion-Reaction Process. ACS Omega 2022, 7, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.E.; Kim, D.J.; Meng, C.X.; Norden, P.R.; Speichinger, K.R.; Davis, M.T.; Smith, A.O.; Bowers, S.L.; Stratman, A.N. Control of vascular tube morphogenesis and maturation in 3D extracellular matrices by endothelial cells and pericytes. Methods Mol. Biol. 2013, 1066, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, L.E.; Golijanin, D.; Itin, A.; Pode, D.; Keshet, E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J. Clin. Investig. 1999, 103, 159–165. [Google Scholar] [CrossRef]
- Chang, Y.T.; Chu, L.J.; Liu, Y.C.; Chen, C.J.; Wu, S.F.; Chen, C.H.; Chang, I.Y.; Wang, J.S.; Wu, T.Y.; Dash, S.; et al. Verification of Saliva Matrix Metalloproteinase-1 as a Strong Diagnostic Marker of Oral Cavity Cancer. Cancers 2020, 12, 2273. [Google Scholar] [CrossRef]
- Saunders, W.B.; Bayless, K.J.; Davis, G.E. MMP-1 activation by serine proteases and MMP-10 induces human capillary tubular network collapse and regression in 3D collagen matrices. J. Cell Sci. 2005, 118, 2325–2340. [Google Scholar] [CrossRef]
- Nirwane, A.; Kang, M.; Adithan, A.; Maharaj, V.; Nguyen, F.; Santaella Aguilar, E.; Nasrollahi, A.; Yao, Y. Endothelial and mural laminin-α5 contributes to neurovascular integrity maintenance. Fluids Barriers CNS 2024, 21, 18. [Google Scholar] [CrossRef]
- Lin, P.K.; Salvador, J.; Xie, J.; Aguera, K.N.; Koller, G.M.; Kemp, S.S.; Griffin, C.T.; Davis, G.E. Selective and Marked Blockade of Endothelial Sprouting Behavior Using Paclitaxel and Related Pharmacologic Agents. Am. J. Pathol. 2021, 191, 2245–2264. [Google Scholar] [CrossRef]
- Aplin, A.C.; Fogel, E.; Zorzi, P.; Nicosia, R.F. The aortic ring model of angiogenesis. Methods Enzym. 2008, 443, 119–136. [Google Scholar] [CrossRef]
- Ucar, B.; Yusufogullari, S.; Humpel, C. Collagen hydrogels loaded with fibroblast growth factor-2 as a bridge to repair brain vessels in organotypic brain slices. Exp. Brain Res. 2020, 238, 2521–2529. [Google Scholar] [CrossRef]
- Sacharidou, A.; Koh, W.; Stratman, A.N.; Mayo, A.M.; Fisher, K.E.; Davis, G.E. Endothelial lumen signaling complexes control 3D matrix-specific tubulogenesis through interdependent Cdc42- and MT1-MMP-mediated events. Blood 2010, 115, 5259–5269. [Google Scholar] [CrossRef] [PubMed]
- Hînganu, M.V.; Hînganu, D.; Frâncu, L.L. Microanatomic aspects of arterial blood supply in rectal carcinomas—Predictive models. Rom. J. Morphol. Embryol. 2013, 54, 561–565. [Google Scholar] [PubMed]
- Stratman, A.N.; Malotte, K.M.; Mahan, R.D.; Davis, M.J.; Davis, G.E. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood 2009, 114, 5091–5101. [Google Scholar] [CrossRef]
- Dima-Cozma, L.C.; Cozma, S.; Hinganu, D.; Ghiciuc, C.M.; Mitu, F. Targeting Matrix Metalloproteinases in Atherosclerosis and Cardiovascular Dysfunction. Rev. Chim. 2019, 70, 718–720. [Google Scholar] [CrossRef]
- Kemp, S.S.; Lin, P.K.; Sun, Z.; Castaño, M.A.; Yrigoin, K.; Penn, M.R.; Davis, G.E. Molecular basis for pericyte-induced capillary tube network assembly and maturation. Front. Cell Dev. Biol. 2022, 10, 943533. [Google Scholar] [CrossRef]
- Kemp, S.S.; Aguera, K.N.; Cha, B.; Davis, G.E. Defining Endothelial Cell-Derived Factors That Promote Pericyte Recruitment and Capillary Network Assembly. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2632–2648. [Google Scholar] [CrossRef]
- Cerkevich, C.M.; Rathelot, J.A.; Strick, P.L. Cortical basis for skilled vocalization. Proc. Natl. Acad. Sci. USA 2022, 119, e2122345119. [Google Scholar] [CrossRef]
- Torralva, R.; Janowsky, A. Noradrenergic Mechanisms in Fentanyl-Mediated Rapid Death Explain Failure of Naloxone in the Opioid Crisis. J. Pharmacol. Exp. Ther. 2019, 371, 453–475. [Google Scholar] [CrossRef]
- González-García, M.; Carrillo-Franco, L.; Morales-Luque, C.; Ponce-Velasco, M.; Gago, B.; Dawid-Milner, M.S.; López-González, M.V. Uncovering the neural control of laryngeal activity and subglottic pressure in anaesthetized rats: Insights from mesencephalic regions. Pflug. Arch. 2024, 476, 1235–1247. [Google Scholar] [CrossRef]
- Mukudai, S.; Sugiyama, Y.; Hisa, Y.; Hisa, Y. Neuroanatomy and Neurophysiology of the Larynx; Springer: Tokyo, Japan, 2016. [Google Scholar]
- Comeras, L.B.; Hörmer, N.; Mohan Bethuraj, P.; Tasan, R.O. NPY Released From GABA Neurons of the Dentate Gyrus Specially Reduces Contextual Fear Without Affecting Cued or Trace Fear. Front. Synaptic Neurosci. 2021, 13, 635726. [Google Scholar] [CrossRef]
- Tatemoto, K. Neuropeptide Y: Complete amino acid sequence of the brain peptide. Proc. Natl. Acad. Sci. USA 1982, 79, 5485–5489. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liang, Z.; Yue, Q.; Wang, X.; Siu, S.W.I.; Pui-Man Hoi, M.; Lee, S.M. A Neuropeptide Y/F-like Polypeptide Derived from the Transcriptome of Turbinaria peltata Suppresses LPS-Induced Astrocytic Inflammation. J. Nat. Prod. 2022, 85, 1569–1580. [Google Scholar] [CrossRef]
- Shende, P.; Desai, D. Physiological and Therapeutic Roles of Neuropeptide Y on Biological Functions. Adv. Exp. Med. Biol. 2020, 1237, 37–47. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Q.; Huang, Y.; Xu, Z.; Chen, X.; Jiang, B.; Huang, Y.; Jian, J. Vasoactive Intestinal Peptide (VIP) Protects Nile Tilapia (Oreochromis niloticus) against Streptococcus agalatiae Infection. Int. J. Mol. Sci. 2022, 23, 14895. [Google Scholar] [CrossRef]
- Tellİ, G.; Tel, B.C.; GÜmÜŞel, B. The Cardiopulmonary Effects of the Calcitonin Gene-related Peptide Family. Turk. J. Pharm. Sci. 2020, 17, 349–356. [Google Scholar] [CrossRef]
- Tanaka, Y.; Yoshida, Y.; Hirano, M. Precise localization of VIP-, NPY-, and TH-immunoreactivities of cat laryngeal glands. Brain Res. Bull. 1995, 36, 219–224. [Google Scholar] [CrossRef]
| Marker | Associated Cell Types | References |
|---|---|---|
| PDGFR-β | Myofibroblasts, neurons and progenitors, mesenchymal cells, mesenchymal stem cells | [54,55,56,57] |
| PDGFR-α | Mesenchymal cells, neural stem cells/B cells | [58,59,60,61] |
| NG2 | vSMC, adipocytes, neuronal progenitors, glial cells, developing bone, muscle, skin | [62,63,64,65,66,67] |
| Desmin | Skeletal muscle cells, cardiac smooth muscle cells, mesangial cells | [55,68,69,70] |
| α-SMA | vSMC, myofibroblasts | [71,72,73,74] |
| RGS5 | vSMC | [75,76,77] |
| Endosialin | Myofibroblasts, fibroblasts, vSMC | [78,79,80] |
| CD73 | Mesenchymal stem cells | [55] |
| CD13 | vSMC, epithelial cells in the kidneys, tumor endothelial cells | [81,82,83] |
| CD146 | Mesenchymal stem cells | [84] |
| CD105 | Mesenchymal stem cells, endothelial cells, hematopoietic stem cells | [84,85,86,87] |
| CD44 | Mesenchymal stem cells, lymphocytes, hematopoietic stem cells | [88,89] |
| ANGPT1 | Hematopoietic progenitor cells, glioblastoma tumor cells, mast cells | [31,90] |
| VEGF-A | Tumor cells, macrophages | [91,92] |
| Functional Property | Endothelial Cells (ECs) | Pericytes |
|---|---|---|
| Tube Formation in 3D Collagen/Fibrin Matrices | ECs have the ability to form tubes in 3D collagen or fibrin matrices. | Pericytes do not form tubes in 3D matrices but invade as single cells. |
| Vascular Guidance Tunnel Creation | ECs create vascular guidance tunnels during tubulogenesis in 3D matrices, aided by ECM proteolysis, particularly through MT1-MMP activity. | Pericytes invade in response to ECs in a manner dependent on PDGF-BB; recruitment results in more elongated and narrow EC tubes. |
| Migration and Tube Assembly | ECs dramatically migrate within 3D matrices and co-assemble into tubes within vascular guidance tunnels. | Pericytes express very high levels of PDGFRβ and use this receptor to invade EC tubes and migrate along the tube abluminal surface. |
| Proliferation during Tube Formation | ECs exhibit minimal to no proliferation during tube formation in 3D matrices. | Pericytes proliferate in response to ECs in a manner dependent on PDGF-BB and HB-EGF in 3D matrices. |
| Co-assembly with Pericytes and Basement Membrane Formation | ECs co-assemble with pericytes to form capillary vessels and generate the vascular basement membrane. | Pericytes migrate along the EC tube abluminal surface within vascular guidance tunnels to facilitate basement membrane formation. |
| Response to Hematopoietic Cytokines | Human ECs form tubes and sprout in response to stem cell cytokines (SCF, IL-3, SDF-1α) and FGF-2 under serum-free, defined conditions. | Human EC–pericyte tube co-assembly with accompanying basement membrane formation occurs in 3D matrices in response to hematopoietic cytokines. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, R.-A.; Popa, C.-G.; Hînganu, D.; Hînganu, M.V. Microvascularization of the Vocal Folds: Molecular Architecture, Functional Insights, and Personalized Research Perspectives. J. Pers. Med. 2025, 15, 293. https://doi.org/10.3390/jpm15070293
Popa R-A, Popa C-G, Hînganu D, Hînganu MV. Microvascularization of the Vocal Folds: Molecular Architecture, Functional Insights, and Personalized Research Perspectives. Journal of Personalized Medicine. 2025; 15(7):293. https://doi.org/10.3390/jpm15070293
Chicago/Turabian StylePopa, Roxana-Andreea, Cosmin-Gabriel Popa, Delia Hînganu, and Marius Valeriu Hînganu. 2025. "Microvascularization of the Vocal Folds: Molecular Architecture, Functional Insights, and Personalized Research Perspectives" Journal of Personalized Medicine 15, no. 7: 293. https://doi.org/10.3390/jpm15070293
APA StylePopa, R.-A., Popa, C.-G., Hînganu, D., & Hînganu, M. V. (2025). Microvascularization of the Vocal Folds: Molecular Architecture, Functional Insights, and Personalized Research Perspectives. Journal of Personalized Medicine, 15(7), 293. https://doi.org/10.3390/jpm15070293







