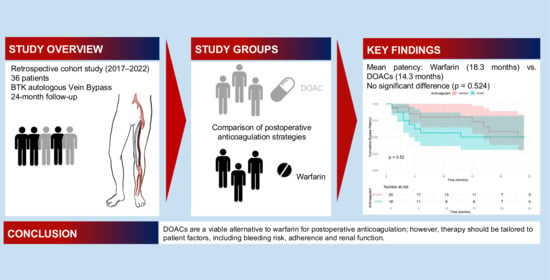
Comparing the Impact of DOACs and Warfarin on Below-the-Knee Autologous Vein Bypass Patency in Peripheral Artery Disease: A Retrospective Cohort Study with 2-Year Follow-Up
Abstract
1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Participants
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Participant Selection
2.4. Variables
2.5. Data Measurement
2.6. Bias
2.7. Study Size
2.8. Statistical Methods
3. Results
3.1. Descriptive Data
3.2. Outcome Data
3.3. Main Results
| −2 Log Likelihood | Chi-Square | Dif | Sig |
|---|---|---|---|
| 102.014 | 2.023 | 7 | 0.959 |
| 95% CI for Exp(B) | ||||||
|---|---|---|---|---|---|---|
| B | df | Sig | Exp(B) | Lower | Upper | |
| Anticoagulation | 0.145 | 1 | 0.857 | 1.156 | 0.238 | 5.618 |
| Bypass Type | −.343 | 1 | 0.651 | 0.710 | 0.161 | 3.131 |
| Sex | 0.154 | 1 | 0.809 | 1.167 | 0.333 | 4.090 |
| Antiplatelet Coadministration | −0.205 | 1 | 0.796 | 0.814 | 0.171 | 3.876 |
| Diabetes Mellitus | 0.160 | 1 | 0.824 | 1.173 | 0.288 | 4.787 |
| Arterial Hypertension | 0.368 | 1 | 0.755 | 1.444 | 0.144 | 14.482 |
| Age | 0.037 | 1 | 0.392 | 1.038 | 0.953 | 1.130 |
| Atrial Fibrillation | 0.281 | 1 | 0.741 | 1.324 | 0.252 | 6.967 |
| Current Smoking | −0.443 | 1 | 0.446 | 0.642 | 0.206 | 2.005 |
| Dyslipidemia | 0.792 | 1 | 0.257 | 2.208 | 0.562 | 8.681 |
| Kidney Failure | 0.019 | 1 | 0.889 | 1.097 | 0.297 | 4.055 |
| 95% CI for Exp(B) | ||||||
|---|---|---|---|---|---|---|
| B | df | Sig | Exp(B) | Lower | Upper | |
| Bypass Type | 0.065 | 1 | 0.898 | 1.067 | 0.397 | 2.869 |
| Sex | −0.167 | 1 | 0.773 | 0.846 | 0.272 | 2.632 |
| Antiplatelet Coadministration | −0.303 | 1 | 0.636 | 0.738 | 0.210 | 2.594 |
| Diabetes Mellitus | 0.057 | 1 | 0.912 | 1.059 | 0.384 | 2.917 |
| Arterial Hypertension | 0.506 | 1 | 0.625 | 1.658 | 0.218 | 12.631 |
| Age | 0.037 | 1 | 0.253 | 1.038 | 0.974 | 1.107 |
| Atrial Fibrillation | 0.493 | 1 | 0.362 | 1.638 | 0.568 | 4.725 |
| Current smoking | −0.215 | 1 | 0.677 | 0.806 | 0.292 | 2.224 |
| Dyslipidemia | 0.561 | 1 | 0.334 | 1.752 | 0.561 | 5.469 |
| Kidney failure | 0.202 | 1 | 0.708 | 1.224 | 0.424 | 3.532 |
| Variable | No Event (n = 20) | Event (n = 16) | p-Value | |
|---|---|---|---|---|
| Age | 67.95 (SD = 9.02) | 71.63 (SD = 7.65) | 0.203 | |
| Sex | Male | 14 | 12 | 1.0 |
| Female | 6 | 4 | ||
| Diabetes | No | 13 | 10 | 0.877 |
| Yes | 7 | 6 | ||
| Hypertension | No | 3 | 1 | 0.613 |
| Yes | 17 | 15 | ||
| Bypass Type | Femorocrural | 10 | 7 | 0.709 |
| Femoropopliteal | 10 | 9 | ||
| Antiplatelet Coadministration | No | 2 | 3 | 0.637 |
| Yes | 18 | 13 | ||
| Atrial Fibrillation | No | 16 | 11 | 0.470 |
| Yes | 4 | 5 | ||
| Current Smoking | No | 6 | 6 | 0.635 |
| Yes | 14 | 10 | ||
| Dyslipidemia | No | 17 | 12 | 0.675 |
| Yes | 3 | 4 | ||
| Kidney Failure | No | 15 | 11 | 0.722 |
| Yes | 5 | 5 |
4. Discussion
4.1. Key Results
4.2. Limitations
4.3. Interpretation and Generalisability
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PAD | Peripheral artery disease |
| CLTI | Chronic limb-threatening ischemia |
| VKAs | Vitamin K antagonists |
| CVD | Cardiovascular disease |
| MACEs | Major adverse cardiovascular events |
| DAPT | Dual antiplatelet therapy |
| MALEs | Major adverse limb events |
| SFA | Superficial femoral artery |
| DOACs | Direct oral anticoagulants |
| CAD | Coronary artery disease |
| HBR | High bleeding risk |
| SD | Standard deviation |
| CIs | Confidence intervals |
References
- Criqui, M.; Aboyans, V. Epidemiology of Peripheral Artery Disease. Circ. Res. 2015, 116, 1509–1526. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Rudan, D.; Zhu, Y.; Fowkes, F.J.I.; Rahimi, K.; Fowkes, F.G.R.; Rudan, I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: An updated systematic review and analysis. Lancet Glob. Health 2019, 7, e1020–e1030. [Google Scholar] [CrossRef] [PubMed]
- Shamaki, G.R.; Markson, F.; Soji-Ayoade, D.; Agwuegbo, C.C.; Bamgbose, M.O.; Tamunoinemi, B.-M. Peripheral Artery Disease: A Comprehensive Updated Review. Curr. Probl. Cardiol. 2022, 47, 100947. [Google Scholar] [CrossRef]
- Shu, J.; Santulli, G. Update on peripheral artery disease: Epidemiology and evidence-based facts. Atherosclerosis 2018, 275, 379–381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sigvant, B.; Lundin, F.; Wahlberg, E. The Risk of Disease Progression in Peripheral Arterial Disease is Higher than Expected: A Meta-Analysis of Mortality and Disease Progression in Peripheral Arterial Disease. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 395–403. [Google Scholar] [CrossRef]
- Fridh, E.B.; Andersson, M.; Thuresson, M.; Sigvant, B.; Kragsterman, B.; Johansson, S.; Hasvold, P.; Falkenberg, M.; Nordanstig, J. Amputation Rates, Mortality, and Pre-operative Comorbidities in Patients Revascularised for Intermittent Claudication or Critical Limb Ischaemia: A Population Based Study. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Squizzato, A.; Bellesini, M.; Takeda, A.; Middeldorp, S.; Donadini, M.P. Cochrane Heart Group Clopidogrel plus aspirin versus aspirin alone for preventing cardiovascular events. Cochrane Database Syst. Rev. 2017, 2017, CD005158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baigent, C.; Blackwell, L.; Collins, R.; Emberson, J.; Godwin, J.; Peto, R.; Buring, J.; Hennekens, C.; Kearney, P.; Meade, T.; et al. Antithrombotic Trialists’ (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009, 373, 1849–1860. [Google Scholar]
- Whayne, T.F. A Review of the Role of Anticoagulation in the Treatment of Peripheral Arterial Disease. Int. J. Angiol. 2012, 21, 187–194. [Google Scholar] [CrossRef]
- Anand, S.S.; Caron, F.; Eikelboom, J.W.; Bosch, J.; Dyal, L.; Aboyans, V.; Abola, M.T.; Branch, K.R.; Keltai, K.; Bhatt, D.L.; et al. Major Adverse Limb Events and Mortality in Patients with Peripheral Artery Disease: The COMPASS Trial. J. Am. Coll. Cardiol. 2018, 71, 2306–2315. [Google Scholar] [CrossRef]
- Biscetti, F.; Nardella, E.; Rando, M.M.; Cecchini, A.L.; Gasbarrini, A.; Massetti, M.; Flex, A. Outcomes of Lower Extremity Endovascular Revascularization: Potential Predictors and Prevention Strategies. Int. J. Mol. Sci. 2021, 22, 2002. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abu Dabrh, A.M.; Steffen, M.W.; Undavalli, C.; Asi, N.; Wang, Z.; Elamin, M.B.; Conte, M.S.; Murad, M.H. The natural history of untreated severe or critical limb ischemia. J. Vasc. Surg. 2015, 62, 1642–1651.e3. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.L., Sr.; Conte, M.S.; Armstrong, D.G.; Pomposelli, F.B.; Schanzer, A.; Sidawy, A.N.; Andros, G. Society for Vascular Surgery Lower Extremity Guidelines Committee. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: Risk stratification based on Wound, Ischemia, and foot Infection (WIfI). J. Vasc. Surg. 2014, 59, 220–234.e2. [Google Scholar] [CrossRef] [PubMed]
- Cull, D.L.; Manos, G.; Hartley, M.C.; Taylor, S.M.; Langan, E.M.; Eidt, J.F.; Johnson, B.L. An early validation of the Society for Vascular Surgery Lower Extremity Threatened Limb Classification System. J. Vasc. Surg. 2014, 60, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Abu Dabrh, A.M.; Steffen, M.W.; Asi, N.; Undavalli, C.; Wang, Z.; Elamin, M.B.; Conte, M.S.; Murad, M.H. Bypass surgery versus endovascular interventions in severe or critical limb ischemia. J. Vasc. Surg. 2016, 63, 244–253.e11. [Google Scholar] [CrossRef]
- Bradbury, A.W.; Adam, D.J.; Bell, J.; Forbes, J.F.; Fowkes, F.G.R.; Gillespie, I.; Ruckley, C.V.; Raab, G.M. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: An intention-to-treat analysis of amputation-free and overall survival in patients randomized to a bypass surgery-first or a balloon angioplasty-first revascularization strategy. J. Vasc. Surg. 2010, 51, 5S–17S, Erratum in: J. Vasc. Surg. 2010, 52, 1751. Bhattachary, V [corrected to Bhattacharya, V]. [Google Scholar] [CrossRef] [PubMed]
- Forster, R.B.; Lethaby, A.; Maxwell, H.; Acosta, S.; Prins, M.H.; Cochrane Vascular Group; Bedenis, R. Antiplatelet agents for preventing thrombosis after peripheral arterial bypass surgery. Cochrane Database Syst. Rev. 2015, 2023, CD000535. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Geraghty, A.J.; Welch, K. Cochrane Vascular Group Antithrombotic agents for preventing thrombosis after infrainguinal arterial bypass surgery. Cochrane Database Syst. Rev. 2011, 2023, CD000536. [Google Scholar]
- Peppas, S.; Sagris, Μ.; Bikakis, I.; Giannopoulos, S.; Tzoumas, A.; Kokkinidis, D.G.; Ahmed, Z.; Korosoglou, G.; A Malgor, E.; Malgor, R.D. A Systematic Review and Meta-Analysis on the Efficacy and Safety of Direct Oral Anticoagulants in Patients with Peripheral Artery Disease. Ann. Vasc. Surg. 2022, 80, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pomozi, E.; Nagy, R.; Fehérvári, P.; Hegyi, P.; Kiss, B.; Dembrovszky, F.; Kosztin, A.; Nardai, S.; Zima, E.; Szeberin, Z. Direct Oral Anticoagulants as the First Choice of Anticoagulation for Patients with Peripheral Artery Disease to Prevent Adverse Vascular Events: A Systematic Review and Meta-Analysis. J. Cardiovasc. Dev. Dis. 2023, 10, 65. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nierlich, P.; Enzmann, F.K.; Metzger, P.; Dabernig, W.; Aspalter, M.; Akhavan, F.; Hitzl, W.; Hölzenbein, T. Alternative Venous Conduits for Below Knee Bypass in the Absence of Ipsilateral Great Saphenous Vein. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Suárez, C.; Zeymer, U.; Limbourg, T.; Baumgartner, I.; Cacoub, P.; Poldermans, D.; Röther, J.; Bhatt, D.L.; Steg, P.G.; Investigatorsa, R.R. Influence of polyvascular disease on cardiovascular event rates. Insights from the REACH Registry. Vasc. Med. 2010, 15, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; DeCarlo, C.S.; Patel, S.S.; McElroy, I.E.; Majumdar, M.; Jessula, S.; Lee, S.; Mohapatra, A.; Dua, A. Impact of anticoagulation/antiplatelet therapy on femoropopliteal bypass graft outcomes. J. Vasc. Surg. 2022, 76, 1045–1052.e1. [Google Scholar] [CrossRef] [PubMed]
- Magnani, G.; Denegri, A.; Gurgoglione, F.L.; Barocelli, F.; Indrigo, E.; Catellani, D.; Signoretta, G.; Bettella, A.; Tuttolomondo, D.; Solinas, E.; et al. Dual Antiplatelet Therapy or Antiplatelet Plus Anticoagulant Therapy in Patients with Peripheral and Chronic Coronary Artery Disease: An Updated Review. J. Clin. Med. 2023, 12, 5284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gaziano, J.M.; Brotons, C.; Coppolecchia, R.; Cricelli, C.; Darius, H.; Gorelick, P.B.; Howard, G.; A Pearson, T.; Rothwell, P.M.; Ruilope, L.M.; et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): A randomised, double-blind, placebo-controlled trial. Lancet 2018, 392, 1036–1046. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Variable | Warfin (n = 20) | DOAC (n = 16) | p-Value | |
|---|---|---|---|---|
| Age | 67.7 (SD = 8.05) | 71.94 (SD = 8.77) | 0.141 | |
| Sex | Male | 13 | 13 | 0.456 |
| Female | 7 | 3 | ||
| Diabetes | No | 15 | 8 | 0.121 |
| Yes | 5 | 8 | ||
| Hypertension | No | 4 | 0 | 0.113 |
| Yes | 16 | 16 | ||
| Bypass Type | Femorocrural | 11 | 6 | 0.296 |
| Femoropopliteal | 9 | 10 | ||
| Antiplatelet Coadministration | No | 4 | 1 | 0.355 |
| Yes | 16 | 15 | ||
| Atrial Fibrillation | No | 20 | 7 | 0.000 * |
| Yes | 0 | 9 | ||
| Current Smoking | No | 6 | 6 | 0.635 |
| Yes | 14 | 10 | ||
| Dyslipidemia | No | 15 | 14 | 0.426 |
| Yes | 5 | 2 | ||
| Kidney Failure | No | 16 | 10 | 0.285 |
| Yes | 4 | 6 |
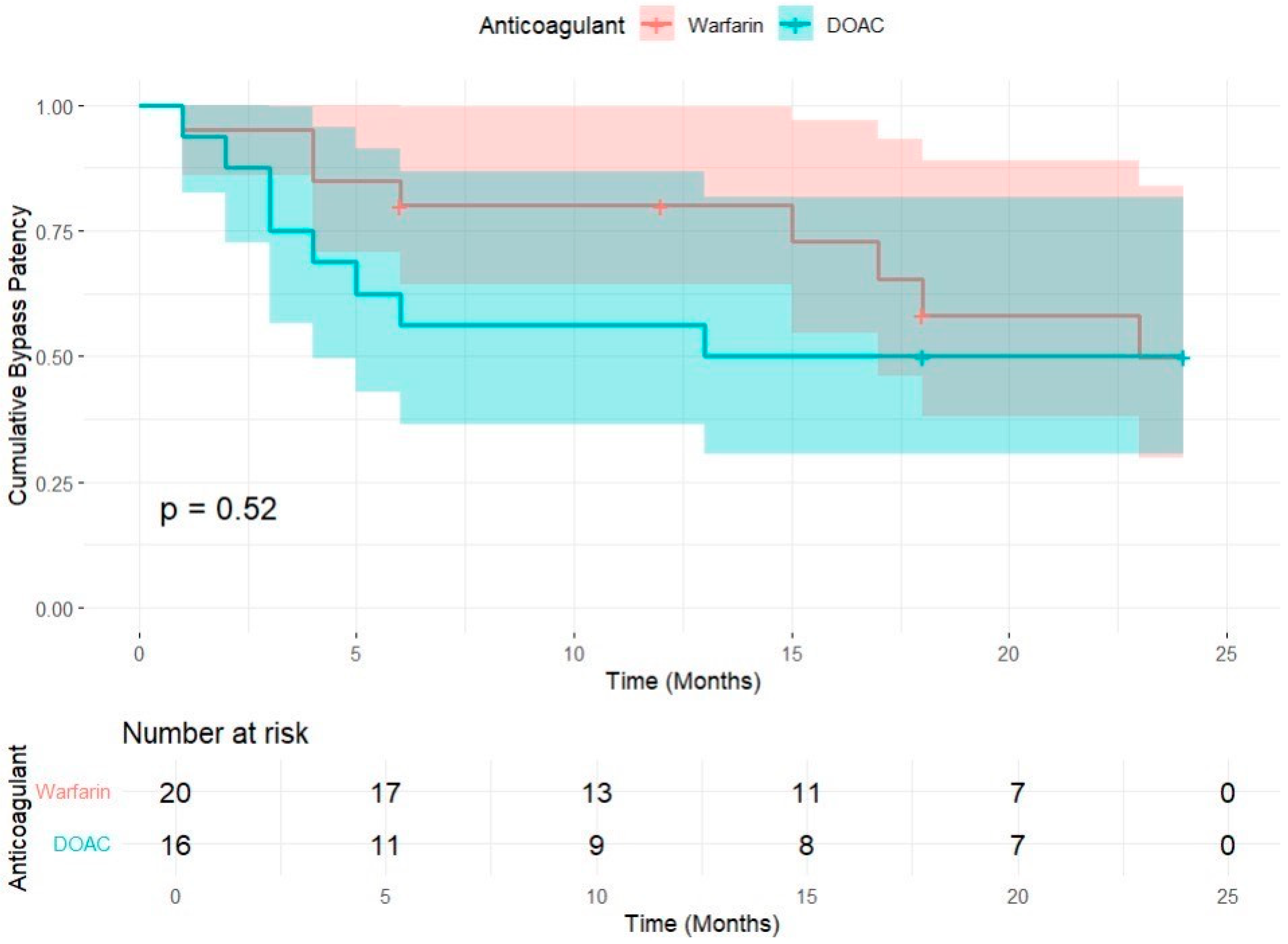
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Anticoagulation | Estimate | Std. Error | Lower Bound | Upper Bound |
| Warfin | 18.267 | 1.815 | 14.710 | 21.824 |
| DOAC | 14.313 | 2.500 | 9.413 | 19.212 |
| Overall | 16.564 | 1.540 | 13.545 | 19.584 |
| Chi-Square | Dif | Sig | |
|---|---|---|---|
| Log Rank (Mantel–Cox) | 0.406 | 1 | 0.524 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frias, F.; Kalender, G. Comparing the Impact of DOACs and Warfarin on Below-the-Knee Autologous Vein Bypass Patency in Peripheral Artery Disease: A Retrospective Cohort Study with 2-Year Follow-Up. J. Pers. Med. 2025, 15, 292. https://doi.org/10.3390/jpm15070292
Frias F, Kalender G. Comparing the Impact of DOACs and Warfarin on Below-the-Knee Autologous Vein Bypass Patency in Peripheral Artery Disease: A Retrospective Cohort Study with 2-Year Follow-Up. Journal of Personalized Medicine. 2025; 15(7):292. https://doi.org/10.3390/jpm15070292
Chicago/Turabian StyleFrias, Francisca, and Günay Kalender. 2025. "Comparing the Impact of DOACs and Warfarin on Below-the-Knee Autologous Vein Bypass Patency in Peripheral Artery Disease: A Retrospective Cohort Study with 2-Year Follow-Up" Journal of Personalized Medicine 15, no. 7: 292. https://doi.org/10.3390/jpm15070292
APA StyleFrias, F., & Kalender, G. (2025). Comparing the Impact of DOACs and Warfarin on Below-the-Knee Autologous Vein Bypass Patency in Peripheral Artery Disease: A Retrospective Cohort Study with 2-Year Follow-Up. Journal of Personalized Medicine, 15(7), 292. https://doi.org/10.3390/jpm15070292