Accuracy of Lower Extremity Alignment Correction Using Patient-Specific Cutting Guides and Anatomically Contoured Plates
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Surgical Correction
2.3. Radiographic Analysis
The Alignment Parameters Assessed Were
2.4. Data Analysis
3. Results
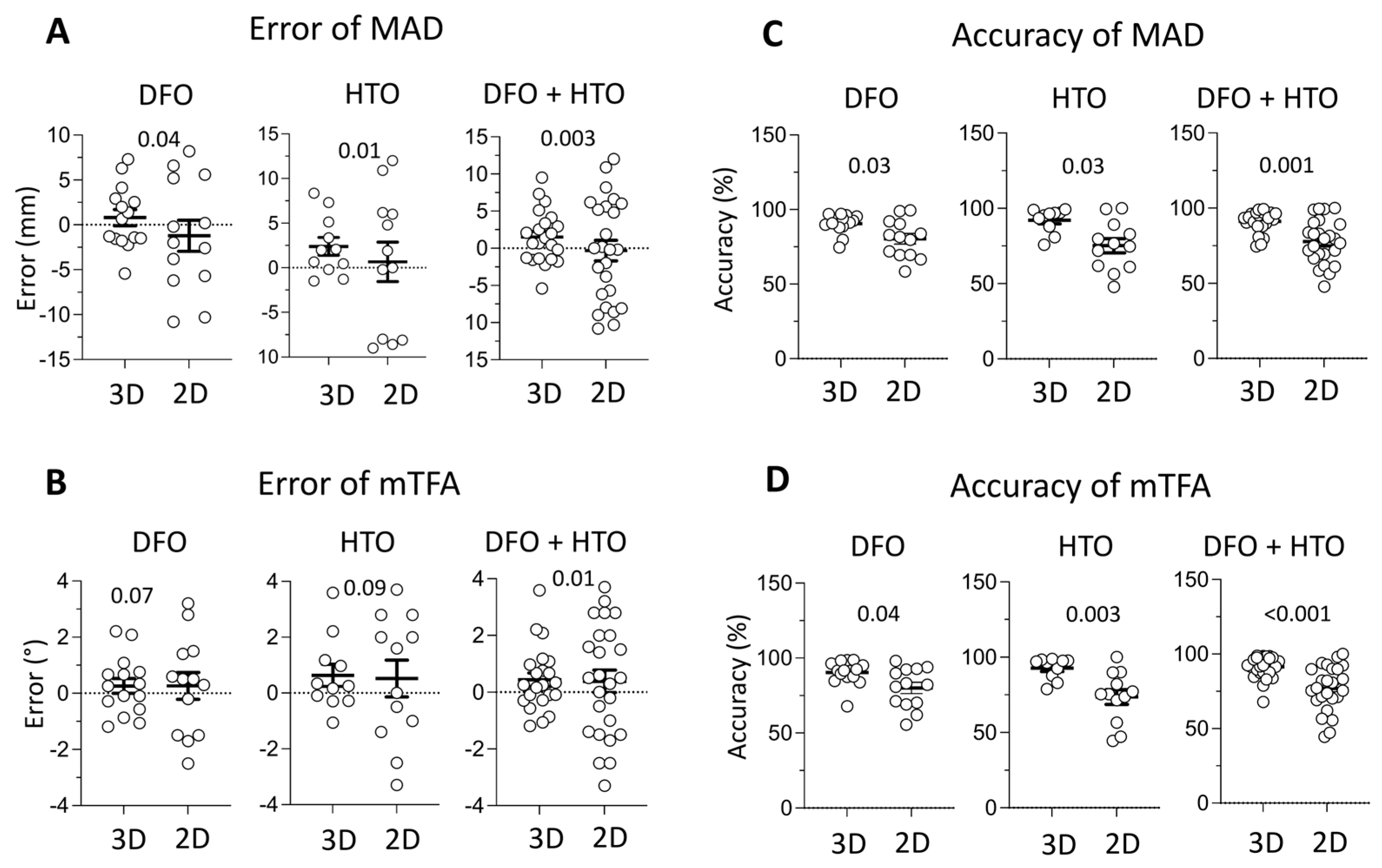
3.1. MAD and mTFA Error Analysis
3.2. Accuracy Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DFO | Distal femoral osteotomy |
| HTO | High tibial osteotomy |
| MAD | Mechanical axis deviation |
| mTFA | Mechanical tibiofemoral angle |
References
- Pullen, W.M.; Slone, H.; Abrams, G.; Sherman, S.L. High Tibial Osteotomy in Knee Reconstruction and Joint Preservation. J. Am. Acad. Orthop. Surg. 2024, 32, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Bertha, N.; Manfre, M.G.; Chin, G.; Peszek, A.; Batiste, A.J.; Maak, T.G.; Frank, R.M. Osteotomies of the Knee for Valgus Malalignment. JBJS Rev. 2025, 13, e.24.00189. [Google Scholar] [CrossRef] [PubMed]
- Strecker, W. Planning analysis of knee-adjacent deformities. I. Frontal plane deformities. Oper. Orthop. Traumatol. 2006, 18, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.C. 3D-printed patient-specific applications in orthopedics. Orthop. Res. Rev. 2016, 8, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Watrinet, J.; Schlaich, J.; Vieider, R.; Rupp, M.-C.; Mehl, J.; Siebenlist, S.; Runer, A. Measuring osteotomy wedge angle is more important than measuring wedge height in open wedge osteotomies around the knee in preoperative planning. Knee Surg. Sports Traumatol. Arthrosc. 2025, 33, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.M.; Lee, J.H.; Cho, I.Y.; Park, B.K.; Han, S.B. Intraoperative Fluoroscopic Assessment of Limb Alignment is a Reliable Predictor for Postoperative Limb Alignment in Biplanar Medial Opening-Wedge High Tibial Osteotomy. J. Arthroplast. 2017, 32, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, Y.; Nejima, S.; Tsuji, M.; Kobayashi, H.; Muramatsu, S. Open-wedge high tibial osteotomy using intraoperative control of joint line convergence angle with reference to preoperative supine radiograph. Arch. Orthop. Trauma Surg. 2021, 141, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Oldhoff, M.G.E.; Alvarez, C.P.; Ten Duis, K.; Doornberg, J.N.; Assink, N.; FFA, I.J. Patient-specific implants combined with 3D-printed drilling guides for corrective osteotomies of multiplanar tibial and femoral shaft malunions leads to more accurate corrections. Eur. J. Trauma Emerg. Surg. 2025, 51, 53. [Google Scholar] [CrossRef]
- Caiti, G.; Dobbe, J.G.G.; Strijkers, G.J.; Strackee, S.D.; Streekstra, G.J. Positioning error of custom 3D-printed surgical guides for the radius: Influence of fitting location and guide design. Int. J. Comput. Assist. Radiol. Surg. 2018, 13, 507–518. [Google Scholar] [CrossRef]
- Fayard, J.M.; Saad, M.; Gomes, L.; Kacem, S.; Abid, H.; Vieira, T.D.; Lambrey, P.J.; Ollivier, M.; Thaunat, M. Patient-specific cutting guides increase accuracy of medial opening wedge high tibial osteotomy procedure: A retrospective case-control study. J. Exp. Orthop. 2024, 11, e12013. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, C.; Chan-Yu-Kin, J.; Sharma, A.; Argenson, J.N.; Parratte, S.; Ollivier, M. More accurate correction using “patient-specific” cutting guides in opening wedge distal femur varization osteotomies. Int. Orthop. 2019, 43, 2285–2291. [Google Scholar] [CrossRef] [PubMed]
- Mettler, F.A., Jr.; Huda, W.; Yoshizumi, T.T.; Mahesh, M. Effective doses in radiology and diagnostic nuclear medicine: A catalog. Radiology 2008, 248, 254–263. [Google Scholar] [CrossRef]
- Dasari, S.P.; Hevesi, M.; Mameri, E.; Ferrer-Rivero, R.; Fortier, L.M.; Jackson, G.R.; Warrier, A.A.; Maheshwer, B.; Jawanda, H.; Khan, Z.A.; et al. Patient-specific instrumentation for medial opening wedge high tibial osteotomies in the management of medial compartment osteoarthritis yields high accuracy and low complication rates: A systematic review. J. ISAKOS 2023, 8, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Aman, Z.S.; DePhillipo, N.N.; Peebles, L.A.; Familiari, F.; LaPrade, R.F.; Dekker, T.J. Improved Accuracy of Coronal Alignment Can Be Attained Using 3D-Printed Patient-Specific Instrumentation for Knee Osteotomies: A Systematic Review of Level III and IV Studies. Arthroscopy 2022, 38, 2741–2758. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Song, J.; Dunlop, D.; Felson, D.; Lewis, C.E.; Segal, N.; Torner, J.; Cooke, T.D.; Hietpas, J.; Lynch, J.; et al. Varus and valgus alignment and incident and progressive knee osteoarthritis. Ann. Rheum. Dis. 2010, 69, 1940–1945. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Patel, A.; Patrick, C.; Delhougne, G. Total Hospital Costs and Readmission Rate of Patient-Specific Instrument in Total Knee Arthroplasty Patients. J. Knee Surg. 2022, 35, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Beyer, F.; Lützner, C.; Stalp, M.; Köster, G.; Lützner, J. Does the use of patient-specific instrumentation improve resource use in the operating room and outcome after total knee arthroplasty?—A multicenter study. PLoS ONE 2022, 17, e0277464. [Google Scholar] [CrossRef] [PubMed]
- Sin, A.; Hollabaugh, W.; Porras, L. Narrative review and call to action on reporting and representation in orthobiologics research for knee osteoarthritis. PM&R 2025, 17, 88–95. [Google Scholar] [CrossRef]
- Helmick, C.G.; Felson, D.T.; Lawrence, R.C.; Gabriel, S.; Hirsch, R.; Kwoh, C.K.; Liang, M.H.; Kremers, H.M.; Mayes, M.D.; Merkel, P.A.; et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part I. Arthritis Rheum. 2008, 58, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Karamitros, G.; Grant, M.P.; Lamaris, G.A. Associations in Medical Research Can Be Misleading: A Clinician’s Guide to Causal Inference. J. Surg. Res. 2025, 310, 145–154. [Google Scholar] [CrossRef] [PubMed]
| A | Distal Femoral Osteotomy | 2D Planning | 3D Planning | p-Value |
| Age at surgery (±SD) | 34.18 (±8.33) | 33.93 (±16.46) | 0.66 | |
| % female patients (% female femurs) | 67 (69) | 70 (67) | ||
| N patients (N femurs) | 9 (13) | 10 (15) | ||
| B | High tibial osteotomy | 2D planning | 3D planning | p-value |
| Age at surgery (±SD) | 44.67 (±16.52) | 38 (±10.49) | 0.29 | |
| % female patients (% female tibiae) | 22 (16.7) | 30 (36.4) | ||
| N patients (N tibiae) | 9 (12) | 10 (11) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matthias, J.; Rozbruch, S.R.; Fragomen, A.T.; Ranawat, A.S.; Reif, T.J. Accuracy of Lower Extremity Alignment Correction Using Patient-Specific Cutting Guides and Anatomically Contoured Plates. J. Pers. Med. 2025, 15, 289. https://doi.org/10.3390/jpm15070289
Matthias J, Rozbruch SR, Fragomen AT, Ranawat AS, Reif TJ. Accuracy of Lower Extremity Alignment Correction Using Patient-Specific Cutting Guides and Anatomically Contoured Plates. Journal of Personalized Medicine. 2025; 15(7):289. https://doi.org/10.3390/jpm15070289
Chicago/Turabian StyleMatthias, Julia, S Robert Rozbruch, Austin T. Fragomen, Anil S. Ranawat, and Taylor J. Reif. 2025. "Accuracy of Lower Extremity Alignment Correction Using Patient-Specific Cutting Guides and Anatomically Contoured Plates" Journal of Personalized Medicine 15, no. 7: 289. https://doi.org/10.3390/jpm15070289
APA StyleMatthias, J., Rozbruch, S. R., Fragomen, A. T., Ranawat, A. S., & Reif, T. J. (2025). Accuracy of Lower Extremity Alignment Correction Using Patient-Specific Cutting Guides and Anatomically Contoured Plates. Journal of Personalized Medicine, 15(7), 289. https://doi.org/10.3390/jpm15070289






