Ultrasonographic Assessment of Calcium Pyrophosphate Deposition Disease: A Comprehensive Review
Abstract
1. Introduction
2. Pathogenesis and Etiology
3. Clinical Aspects
4. Classification Criteria (ACR/EULAR 2023)
5. Sonographic Findings of CPPD
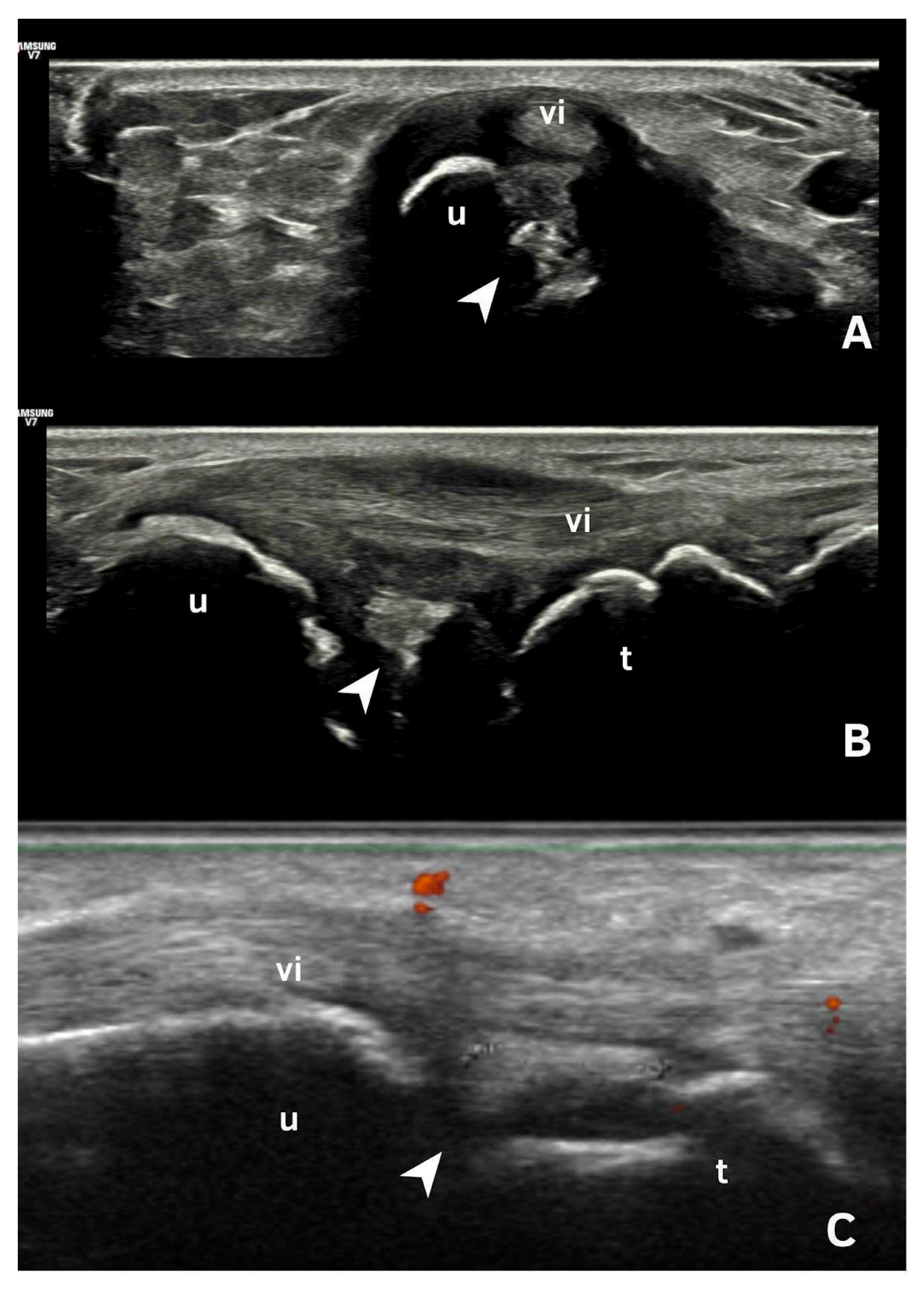
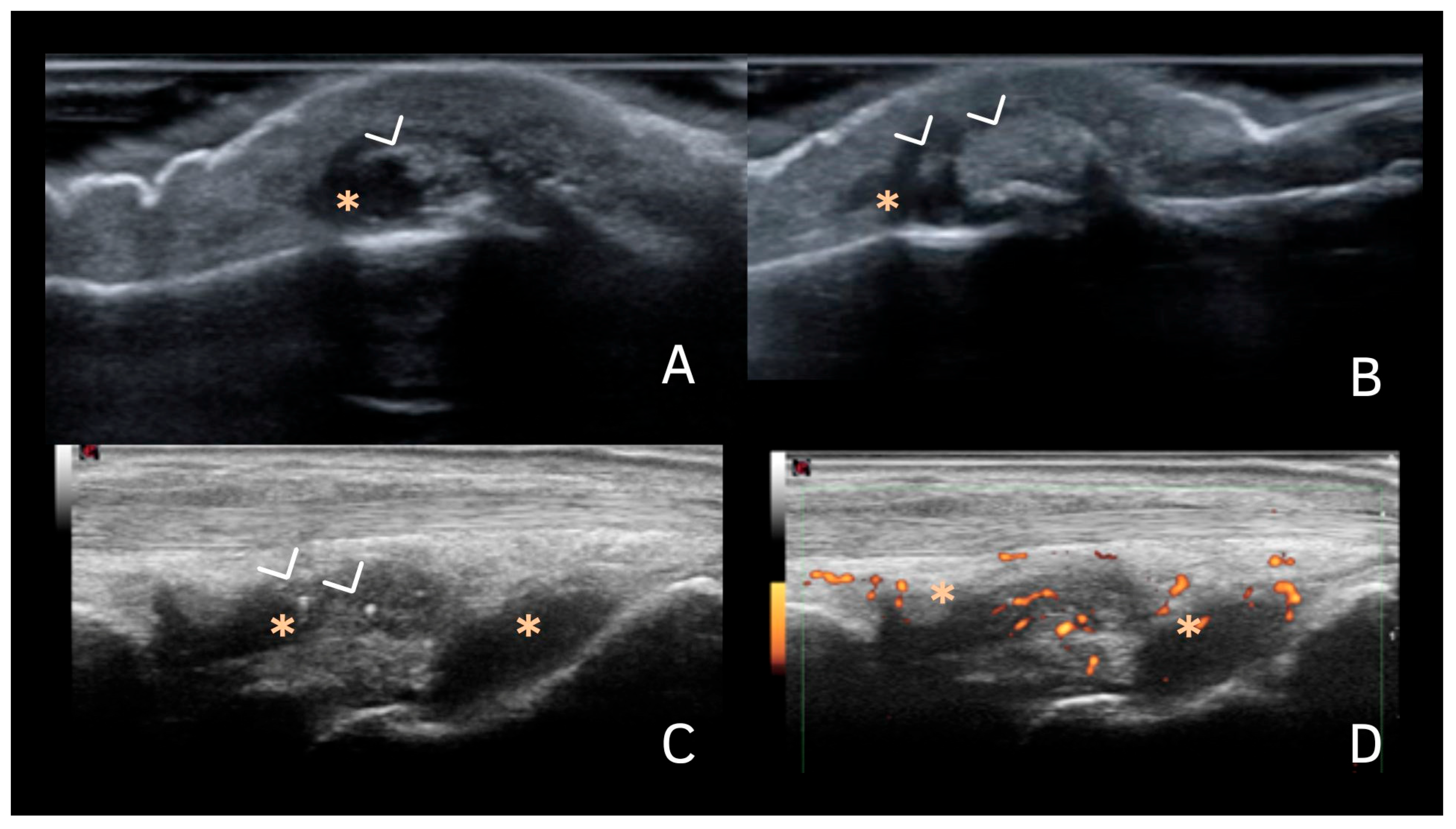
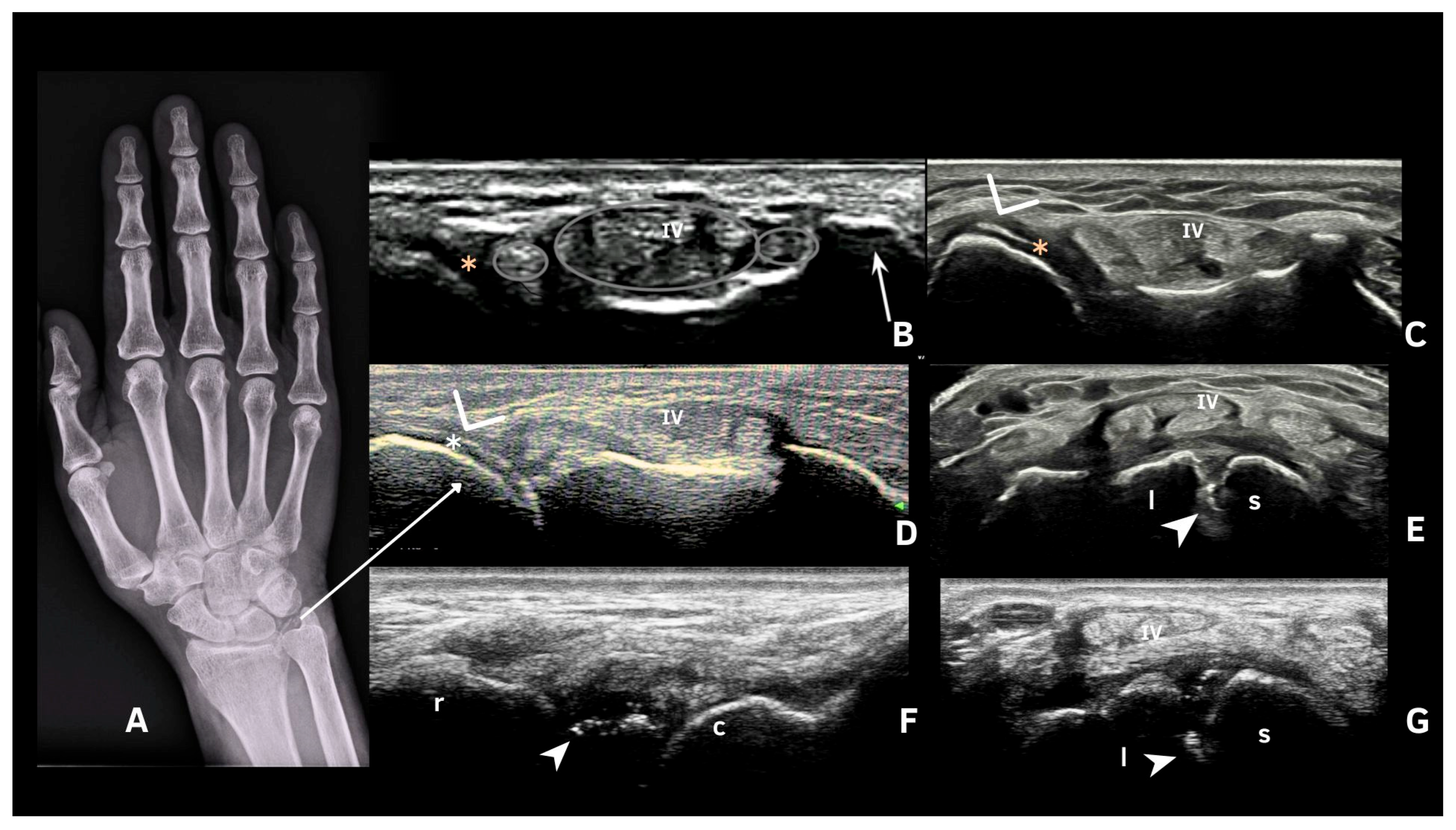
6. Deposits in Fibrocartilage
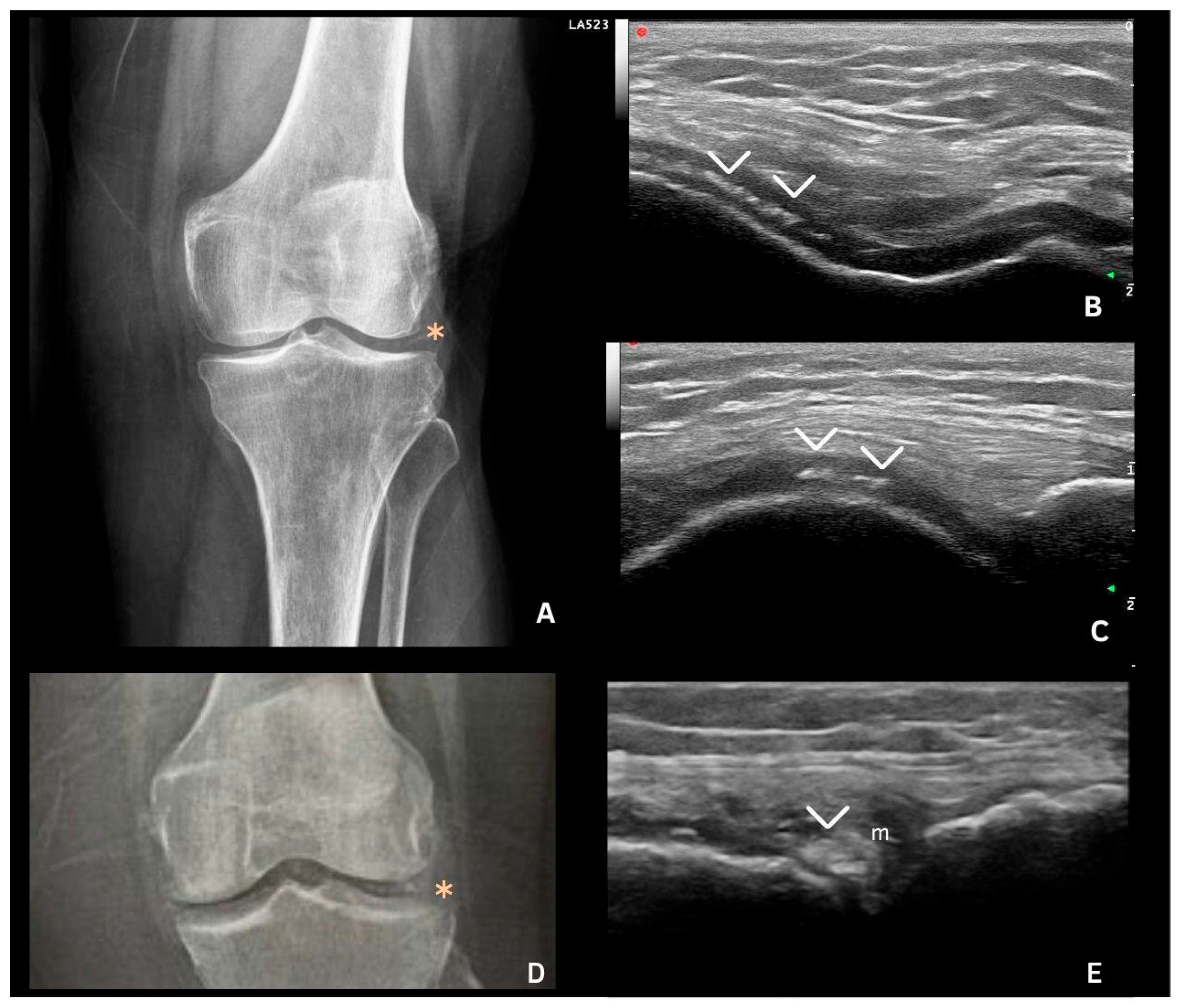
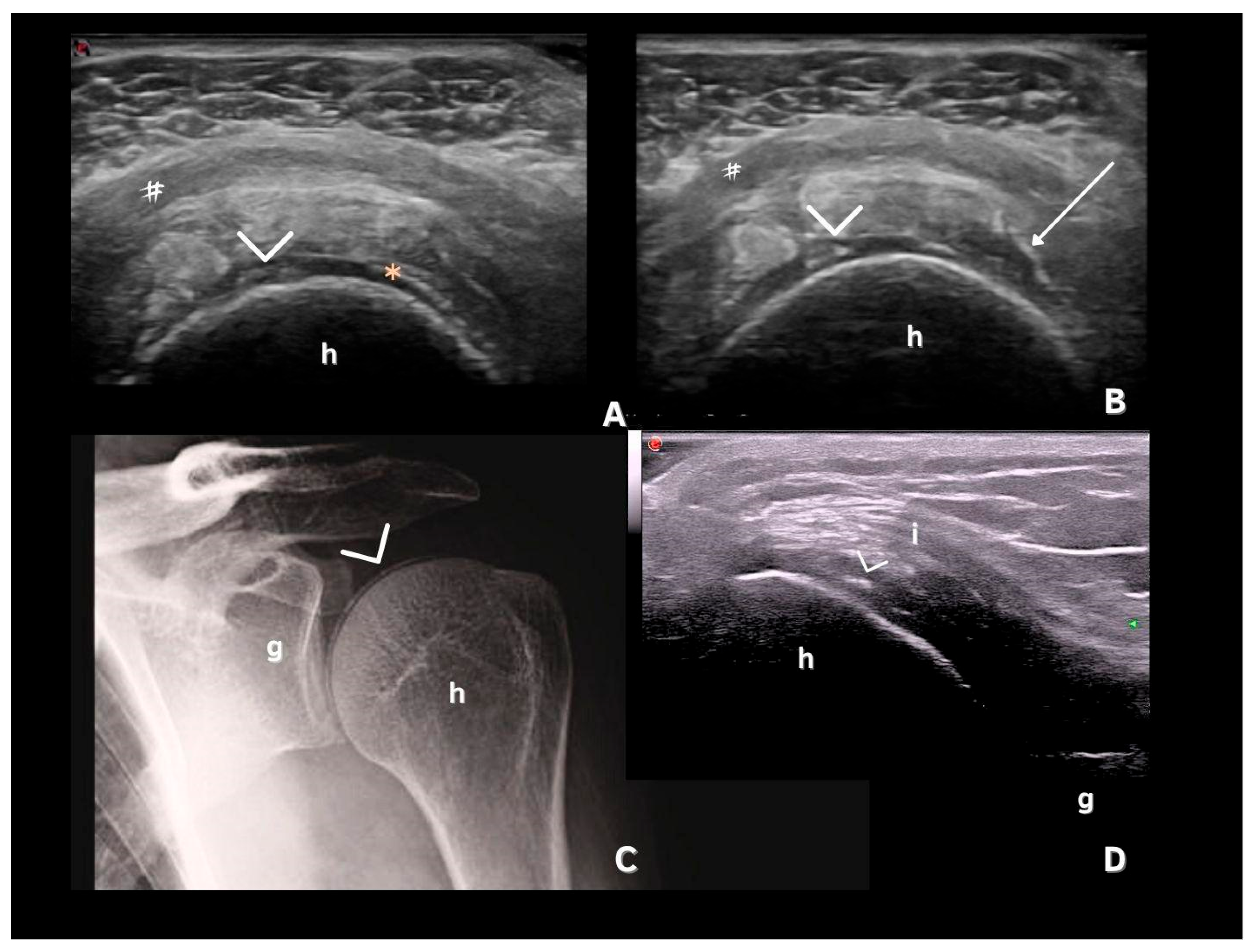
7. Deposits in Hyaline Cartilage
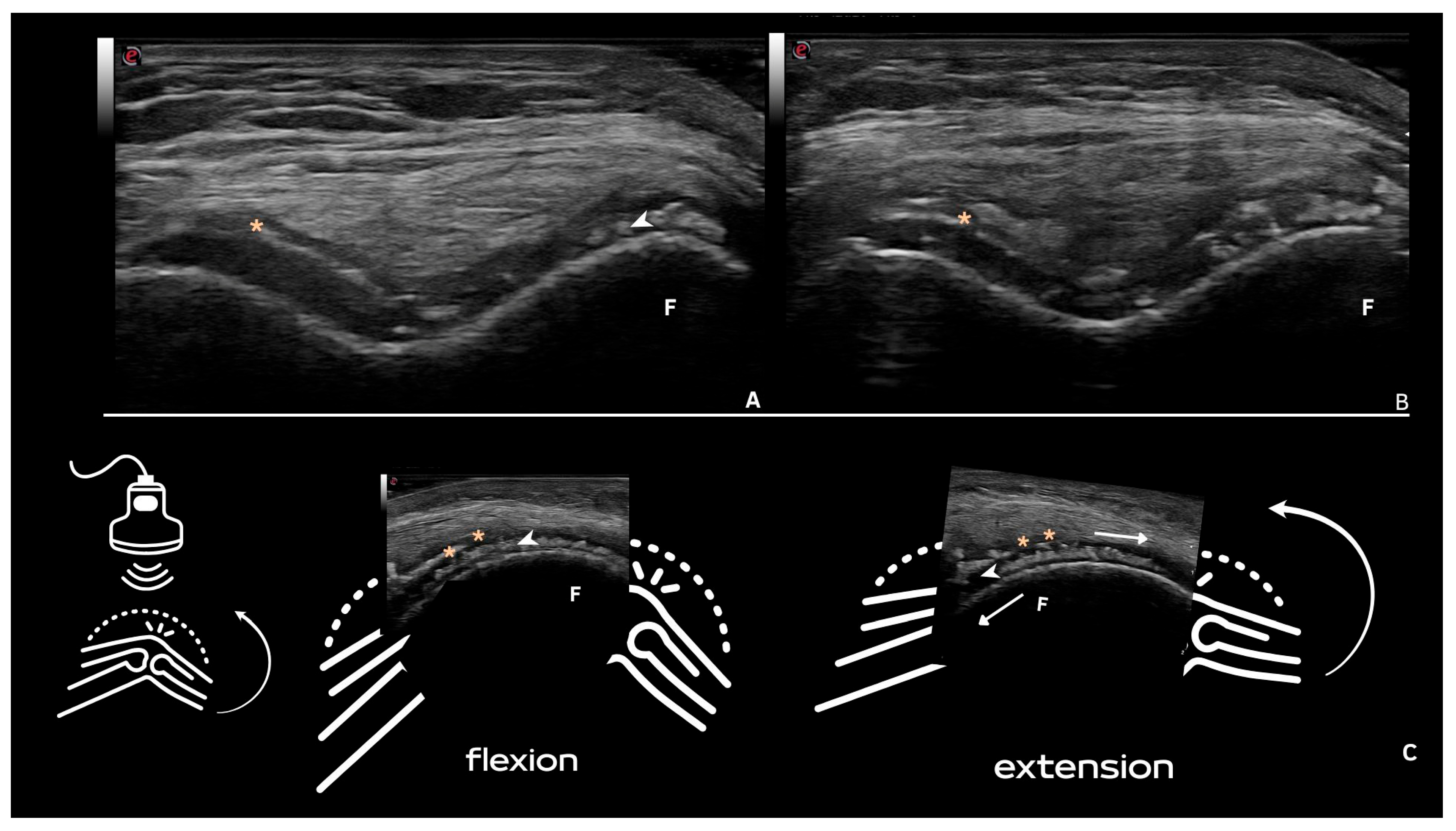
8. Deposits in Tendons
9. Deposits in Synovial Fluid or Tissue
10. Distribution of Deposits
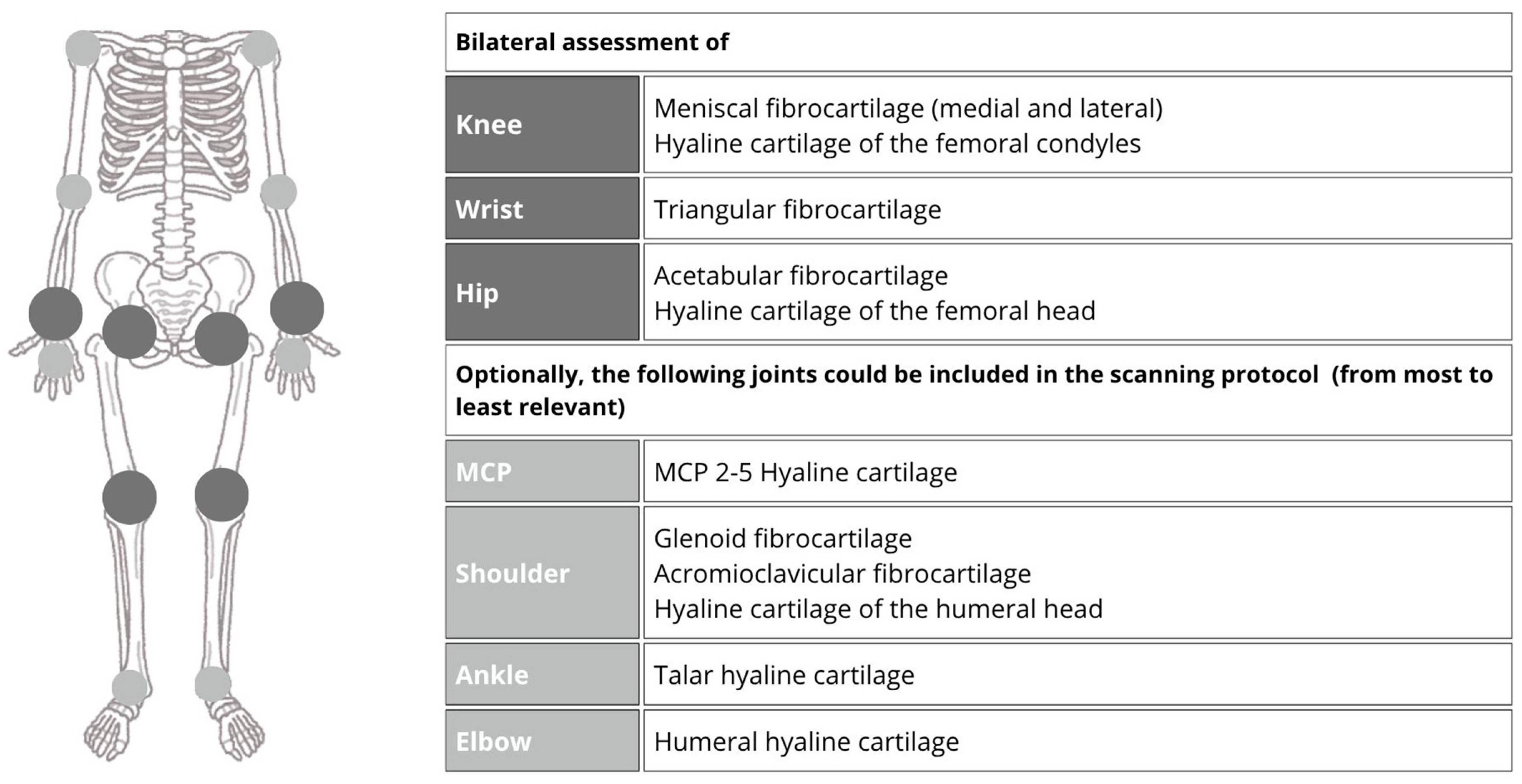
11. Ultrasound Technique for CPPD Evaluation
12. Limitations
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACR | American College of Rheumatology |
| CMCJ | Carpometacarpal Joint |
| CPPD | Calcium Pyrophosphate Deposition Disease |
| CT | Computed Tomography |
| DECT | Dual-Energy Computed Tomography |
| EULAR | European Alliance of Associations for Rheumatology |
| MCP | Metacarpophalangeal |
| NTPPPH | Nucleoside Triphosphate Pyrophosphohydrolase |
| OMERACT | Outcome Measures in Rheumatology |
| PPi | Inorganic pyrophosphate |
| Pi | Phosphate |
| US | Ultrasonography |
References
- Pascart, T.; Filippou, G.; Lioté, F.; Sirotti, S.; Jauffret, C.; Abhishek, A. Calcium pyrophosphate deposition disease. Lancet Rheumatol. 2024, 6, e791–e804. [Google Scholar] [CrossRef] [PubMed]
- Abhishek, A. Calcium pyrophosphate deposition disease. Curr. Opin. Rheumatol. 2016, 28, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, A.K.; Ryan, L.M. Calcium Pyrophosphate Deposition Disease. N. Engl. J. Med. 2016, 374, 2575–2584. [Google Scholar] [CrossRef]
- Abhishek, A.; Doherty, M. Update on calcium pyrophosphate deposition. Clin. Exp. Rheumatol. 2016, 34 (Suppl. 98), 32–38. [Google Scholar] [PubMed]
- Stücker, S.; Bollmann, M.; Garbers, C.; Bertrand, J. The role of calcium crystals and their effect on osteoarthritis pathogenesis. Best. Pract. Res. Clin. Rheumatol. 2021, 35, 101722. [Google Scholar] [CrossRef]
- Altomare, A.; Corrado, A.; Maruotti, N.; Cici, D.; Cantatore, F.P. The role of Interleukin-1 receptor antagonist as a treatment option in calcium pyrophosphate crystal deposition disease. Mol. Biol. Rep. 2021, 48, 4789–4796. [Google Scholar] [CrossRef]
- Terkeltaub, R.; Lotz, M.; Johnson, K.; Deng, D.; Hashimoto, S.; Goldring, M.B.; Burton, D.; Deftos, L.J. Parathyroid hormone-related protein is abundant in osteoarthritic cartilage, and the parathyroid hormone-related protein 1-173 isoform is selectively induced by transforming growth factor? in articular chondrocytes and suppresses generation of extracellular inorganic pyrophosphate. Arthritis Rheum. 1998, 41, 2152–2164. [Google Scholar]
- Sirotti, S.; Scanu, A.; Pascart, T.; Niessink, T.; Maroni, P.; Lombardi, G.; Filippou, G. Calcium Pyrophosphate Crystal Formation and Deposition: Where Do we Stand and What Does the Future hold? Curr. Rheumatol. Rep. 2024, 26, 354–365. [Google Scholar] [CrossRef]
- Zimmer, V. Crowned dens syndrome. Clin. Case Rep. 2020, 8, 2088–2089. [Google Scholar] [CrossRef]
- Lee, J.S.; Hong, S.; Kwon, O.C.; Ghang, B.; Kim, Y.G.; Lee, C.K.; Yoo, B. Clinical features and risk of recurrence of acute calcium pyrophosphate crystal arthritis. Clin. Exp. Rheumatol. 2019, 37, 254–259. [Google Scholar]
- Vele, P.; Simon, S.P.; Damian, L.; Felea, I.; Muntean, L.; Filipescu, I.; Muntean, L.; Filipescu, I.; Rednic, S. Clinical and ultrasound findings in patients with calcium pyrophosphate dihydrate deposition disease. Med. Ultrason. 2018, 20, 159–163. [Google Scholar] [CrossRef] [PubMed]
- McCarty, D. Arthritis and Allied Conditions: A Textbook of Rheumatology, 7th ed.; Lea & Febiger: Philadelphia, PA, USA, 1966; pp. 947–964. [Google Scholar]
- Sirotti, S.; Becce, F.; Sconfienza, L.M.; Terslev, L.; Naredo, E.; Zufferey, P.; Pineda, C.; Gutierrez, M.; Adinolfi, A.; Serban, T. Reliability and Diagnostic Accuracy of Radiography for the Diagnosis of Calcium Pyrophosphate Deposition: Performance of the Novel Definitions Developed by an International Multidisciplinary Working Group. Arthritis Rheumatol. 2023, 75, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Bernal, J.; Andrés, M.; López-Salguero, S.; Jovaní, V.; Vela-Casasempere, P.; Pascual, E. Agreement Among Multiple Observers on Crystal Identification by Synovial Fluid Microscopy. Arthritis Care Res. (Hoboken) 2023, 75, 682–688. [Google Scholar] [CrossRef]
- Filippou, G.; Adinolfi, A.; Cimmino, M.A.; Scirè, C.A.; Carta, S.; Lorenzini, S.; Santoro, P.; Sconfienza, L.M.; Bertoldi, I.; Picerno, V. Diagnostic accuracy of ultrasound, conventional radiography and synovial fluid analysis in the diagnosis of calcium pyrophosphate dihydrate crystal deposition disease. Clin. Exp. Rheumatol. 2016, 34, 254–260. [Google Scholar]
- Abhishek, A.; Tedeschi, S.K.; Pascart, T.; Latourte, A.; Dalbeth, N.; Neogi, T.; Fuller, A.; Rosenthal, A.; Becce, F.; Bardin, T. The 2023 ACR/EULAR classification criteria for calcium pyrophosphate deposition disease. Ann. Rheum. Dis. 2023, 82, 1248–1257. [Google Scholar] [CrossRef]
- Frediani, B.; Filippou, G.; Falsetti, P.; Lorenzini, S.; Baldi, F.; Acciai, C.; Siagkri, C.; Marotto, D.; Galeazzi, M.; Marcolong, R. o Diagnosis of calcium pyrophosphate dihydrate crystal deposition disease: Ultrasonographic criteria proposed. Ann. Rheum. Dis. 2005, 64, 638–640. [Google Scholar] [CrossRef]
- Filippou, G.; Scirè, C.A.; Adinolfi, A.; Damjanov, N.S.; Carrara, G.; Bruyn, G.A.W.; Cazenave, T.; D’Agostino, M.A.; Delle Sedie, A.; Di Sabatino, V. Identification of calcium pyrophosphate deposition disease (CPPD) by ultrasound: Reliability of the OMERACT definitions in an extended set of joints—An international multiobserver study by the OMERACT Calcium Pyrophosphate Deposition Disease Ultrasound Subtask Force. Ann. Rheum. Dis. 2018, 77, 1194–1199. [Google Scholar] [PubMed]
- Gamon, E.; Combe, B.; Barnetche, T.; Mouterde, G. Diagnostic value of ultrasound in calcium pyrophosphate deposition disease: A systematic review and meta-analysis. RMD Open 2015, 1, e000118. [Google Scholar] [CrossRef]
- Grassi, W.; Meenagh, G.; Pascual, E.; Filippucci, E. “Crystal Clear”—Sonographic Assessment of Gout and Calcium Pyrophosphate Deposition Disease. Semin. Arthritis Rheum. 2006, 36, 197–202. [Google Scholar] [CrossRef]
- Ciapetti, A.; Filippucci, E.; Gutierrez, M.; Grassi, W. Calcium pyrophosphate dihydrate crystal deposition disease: Sonographic findings. Clin. Rheumatol. 2009, 28, 271–276. [Google Scholar] [CrossRef]
- Filippou, G.; Frediani, B.; Gallo, A.; Menza, L.; Falsetti, P.; Baldi, F.; Acciai, C.; Lorenzini, S.; Galeazzi, M.; Marcolongo, R. A “new” technique for the diagnosis of chondrocalcinosis of the knee: Sensitivity and specificity of high-frequency ultrasonography. Ann. Rheum. Dis. 2007, 66, 1126–1128. [Google Scholar] [CrossRef] [PubMed]
- Ellabban, A.S.; Kamel, S.R.; Omar, H.A.S.A.; El-Sherif, A.M.H.; Abdel-Magied, R.A. Ultrasonographic diagnosis of articular chondrocalcinosis. Rheumatol. Int. 2012, 32, 3863–3868. [Google Scholar] [CrossRef]
- Filippucci, E.; Scirè, C.A.; Delle Sedie, A.; Iagnocco, A.; Riente, L.; Meenagh, G.; Gutierrez, M.; Bombardieri, S.; Valesini, G.; Montecucco, C. Ultrasound imaging for the rheumatologist. XXV. Sonographic assessment of the knee in patients with gout and calcium pyrophosphate deposition disease. Clin. Exp. Rheumatol. 2010, 28, 2–5. [Google Scholar] [PubMed]
- Cipolletta, E.; Di Matteo, A.; Smerilli, G.; Di Carlo, M.; Di Battista, J.; Abhishek, A.; Grassi, W.; Filippucci, E. Ultrasound findings of calcium pyrophosphate deposition disease at metacarpophalangeal joints. Rheumatology 2022, 61, 3997–4005. [Google Scholar] [CrossRef]
- Filippucci, E.; Gutierrez, M.; Georgescu, D.; Salaffi, F.; Grassi, W. Hyaline cartilage involvement in patients with gout and calcium pyrophosphate deposition disease. An. ultrasound study. Osteoarthr. Cartil. 2009, 17, 178–181. [Google Scholar] [CrossRef]
- Ellabban, A.S.; Kamel, S.R.; Omar, H.A.A.; El-Sherif, A.M.; Abdel-Magied, R.A. Ultrasonographic findings of Achilles tendon and plantar fascia in patients with calcium pyrophosphate deposition disease. Clin. Rheumatol. 2012, 31, 697–704. [Google Scholar] [CrossRef]
- Gutierrez, M.; Di Geso, L.; Salaffi, F.; Carotti, M.; Girolimetti, R.; De Angelis, R.; Filippucci, E.; Grassi, W. Ultrasound Detection of Cartilage Calcification at Knee Level in Calcium Pyrophosphate Deposition Disease. Arthritis Care Res. (Hoboken) 2014, 66, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Falsetti, P.; Frediani, B.; Acciai, C.; Baldi, F.; Filippou, G.; Prada, E.P.; Sabadini, L.; Marcolongo, R. Ultrasonographic study of Achilles tendon and plantar fascia in chondrocalcinosis. J. Rheumatol. 2004, 31, 2242–2250. [Google Scholar]
- Filippou, G.; Adinolfi, A.; Iagnocco, A.; Filippucci, E.; Cimmino, M.A.; Bertoldi, I.; Di Sabatino, V.; Picerno, V.; Delle Sedie, A.; Sconfienza, L.M. Ultrasound in the diagnosis of calcium pyrophosphate dihydrate deposition disease. A systematic literature review and a meta-analysis. Osteoarthr. Cartil. 2016, 24, 973–981. [Google Scholar] [CrossRef]
- Filippucci, E.; Delle Sedie, A.; Riente, L.; Di Geso, L.; Carli, L.; Ceccarelli, F.; Sakellariou, G.; Iagnocco, A.; Grassi, W. Ultrasound imaging for the rheumatologist. XLVII. Ultrasound of the shoulder in patients with gout and calcium pyrophosphate deposition disease. Clin. Exp. Rheumatol. 2013, 31, 659–664. [Google Scholar]
- Filippou, G.; Filippucci, E.; Tardella, M.; Bertoldi, I.; Di Carlo, M.; Adinolfi, A.; Grassi, W.; Frediani, B. Extent and distribution of CPP deposits in patients affected by calcium pyrophosphate dihydrate deposition disease: An ultrasonographic study. Ann. Rheum. Dis. 2013, 72, 1836–1839. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, A.; Sirotti, S.; Sakellariou, G.; Cipolletta, E.; Filippucci, E.; Porta, F.; Zanetti, A.; Ughi, N.; Sarzi-Puttini, P.; Scirè, C.A. Which are the most frequently involved peripheral joints in calcium pyrophosphate crystal deposition at imaging? A systematic literature review and meta-analysis by the OMERACT ultrasound—CPPD subgroup. Front. Med. (Lausanne) 2023, 10, 1131362. [Google Scholar] [CrossRef] [PubMed]
- Ruta, S.; Catay, E.; Marin, J.; Rosa, J.; García-Monaco, R.; Soriano, E.R. Knee effusion: Ultrasound as a useful tool for the detection of calcium pyrophosphate crystals. Clin. Rheumatol. 2016, 35, 1087–1091. [Google Scholar] [CrossRef]
- Foldes, K. Knee chondrocalcinosis. Clin. Imaging 2002, 26, 194–196. [Google Scholar] [CrossRef]
- Lee, K.A.; Lee, S.H.; Kim, H.R. Diagnostic value of ultrasound in calcium pyrophosphate deposition disease of the knee joint. Osteoarthr. Cartil. 2019, 27, 781–787. [Google Scholar] [CrossRef]
- Di Geso, L.; Filippucci, E.; Gutierrez, M.; Grassi, W. Calcium Pyrophosphate Deposition: Sonographic Features in a Familial Case: Figure 1. J. Rheumatol. 2012, 39, 1488–1490. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cipolletta, E.; Smerilli, G.; Mashadi Mirza, R.; Di Matteo, A.; Carotti, M.; Salaffi, F.; Grassi, W.; Filippucci, E. Sonographic assessment of calcium pyrophosphate deposition disease at wrist. A focus on the dorsal scapho-lunate ligament. Jt. Bone Spine 2020, 87, 611–617. [Google Scholar] [CrossRef]
- Di Matteo, A.; Filippucci, E.; Salaffi, F.; Carotti, M.; Carboni, D.; Di Donato, E.; Grassi, W. Diagnostic accuracy of musculoskeletal ultrasound and conventional radiography in the assessment of the wrist triangular fibrocartilage complex in patients with definite diagnosis of calcium pyrophosphate dihydrate deposition disease. Clin. Exp. Rheumatol. 2017, 35, 647–652. [Google Scholar]
- Forien, M.; Combier, A.; Gardette, A.; Palazzo, E.; Dieudé, P.; Ottaviani, S. Comparison of ultrasonography and radiography of the wrist for diagnosis of calcium pyrophosphate deposition. Jt. Bone Spine 2018, 85, 615–618. [Google Scholar] [CrossRef]
- Di Matteo, A.; Filippucci, E.; Cipolletta, E.; Musca, A.; Carotti, M.; Mashadi Mirza, R.; Jesus, D.; Martire, V.; Pierucci, D.; Di Carlo, M. Hip Involvement in Patients With Calcium Pyrophosphate Deposition Disease: Potential and Limits of Musculoskeletal Ultrasound. Arthritis Care Res. (Hoboken) 2019, 71, 1671–1677. [Google Scholar] [CrossRef]
- Filippou, G.; Scanu, A.; Adinolfi, A.; Toscano, C.; Gambera, D.; Largo, R.; Naredo, E.; Calvo, E.; Herrero-Beaumont, G.; Zufferey, P. Criterion validity of ultrasound in the identification of calcium pyrophosphate crystal deposits at the knee: An OMERACT ultrasound study. Ann. Rheum. Dis. 2021, 80, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Cipolletta, E.; Moscioni, E.; Sirotti, S.; Di Battista, J.; Abhishek, A.; Rozza, D.; Zanetti, A.; Carrara, G.; Scirè Carlo, A.; Grassi, W. Diagnosis of calcium pyrophosphate crystal deposition disease by ultrasonography: How many and which sites should be scanned? Rheumatology 2024, 63, 2205–2212. [Google Scholar] [CrossRef] [PubMed]

| Hand or Wrist Osteoarthritis (Kellgren and Lawrence Score ≥ 2) | |
| None or imaging not performed | 0 |
| Bilateral radiocarpal joint OA | +2 |
| Two or more specific OA features (STTJ OA without first CMCJ OA, second/third MCPJ OA) | +7 |
| Imaging evidence of CPPD in symptomatic joint (US, CT, DECT, or X-ray) | |
| None on any imaging | −4 |
| None on X-ray (no other imaging performed) | 0 |
| Present on any imaging modality | +16 |
| Number of peripheral joints with CPPD on imaging | |
| None | 0 |
| 1 joint | +16 |
| 2–3 joints | +23 |
| ≥4 joints | +25 |
| 1 | Hyperechoic bands, parallel to the surface of the hyaline cartilage |
| 2 | Thin hyperechoic spots in fibrocartilage |
| 3 | Homogeneous hyperechoic nodular or oval deposits in fibrocartilage |
| Fibrocartilage | Shape: Deposits of variable shape. Echogenicity: Hyperechoic (similar to the bone cortex echogenicity). Localization: Within the fibrocartilage structure. Dynamic scanning: Remain fixed and move together with the fibrocartilage during dynamic assessment. Examples: Menisci, TFC, hip labrum, and acromioclavicular joint. |
| Hyaline cartilage | Shape: Deposits of variable shape. Echogenicity: Hyperechoic, without posterior shadowing. Localization: Localized within the hyaline cartilage. Dynamic scanning: The deposits remain fixed and move together with the hyaline cartilage. Examples: Knee and MCP joint. |
| Pseudo-double contour sign | Shape: Deposits of variable shape. Echogenicity: Hyperechoic, without posterior shadowing. Localization: Localized at the chondro-synovial interface or in the joint capsule and adjacent ligaments. Dynamic scanning: Moves in the opposite direction of cartilage and adjacent bone. Examples: Knee, proximal, and distal radio-ulnar joint. |
| Tendon | Shape: Multiple, linear (parallel to the tendon fibrillar structure and not in continuity with the bone profile). Echogenicity: Hyperechoic, generally without posterior shadowing, maintaining hyperechogenicity even at very low levels of gain, not affected by anisotropy as the surrounding tendon. Localization: Within the tendon. Dynamic scanning: Remain fixed and move together with the tendon. |
| Synovial fluid | Shape: Deposits of variable shape and size. Echogenicity: Hyperechoic (similar to the bone cortex echogenicity), generally without posterior shadowing Localization: Within the synovial fluid. Dynamic scanning: Mobile according to joint movement and probe pressure. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guedes, L.K.N.; Figueiredo, L.Q.d.; Lopes, F.O.d.A.; Ferrari, L.F.F.; Bonfiglioli, K.R. Ultrasonographic Assessment of Calcium Pyrophosphate Deposition Disease: A Comprehensive Review. J. Pers. Med. 2025, 15, 280. https://doi.org/10.3390/jpm15070280
Guedes LKN, Figueiredo LQd, Lopes FOdA, Ferrari LFF, Bonfiglioli KR. Ultrasonographic Assessment of Calcium Pyrophosphate Deposition Disease: A Comprehensive Review. Journal of Personalized Medicine. 2025; 15(7):280. https://doi.org/10.3390/jpm15070280
Chicago/Turabian StyleGuedes, Lissiane Karine Noronha, Letícia Queiroga de Figueiredo, Fernanda Oliveira de Andrade Lopes, Luis Fernando Fernandes Ferrari, and Karina Rossi Bonfiglioli. 2025. "Ultrasonographic Assessment of Calcium Pyrophosphate Deposition Disease: A Comprehensive Review" Journal of Personalized Medicine 15, no. 7: 280. https://doi.org/10.3390/jpm15070280
APA StyleGuedes, L. K. N., Figueiredo, L. Q. d., Lopes, F. O. d. A., Ferrari, L. F. F., & Bonfiglioli, K. R. (2025). Ultrasonographic Assessment of Calcium Pyrophosphate Deposition Disease: A Comprehensive Review. Journal of Personalized Medicine, 15(7), 280. https://doi.org/10.3390/jpm15070280






