Surgical Management of Mediastinal Ectopic Parathyroids
Abstract
1. Introduction
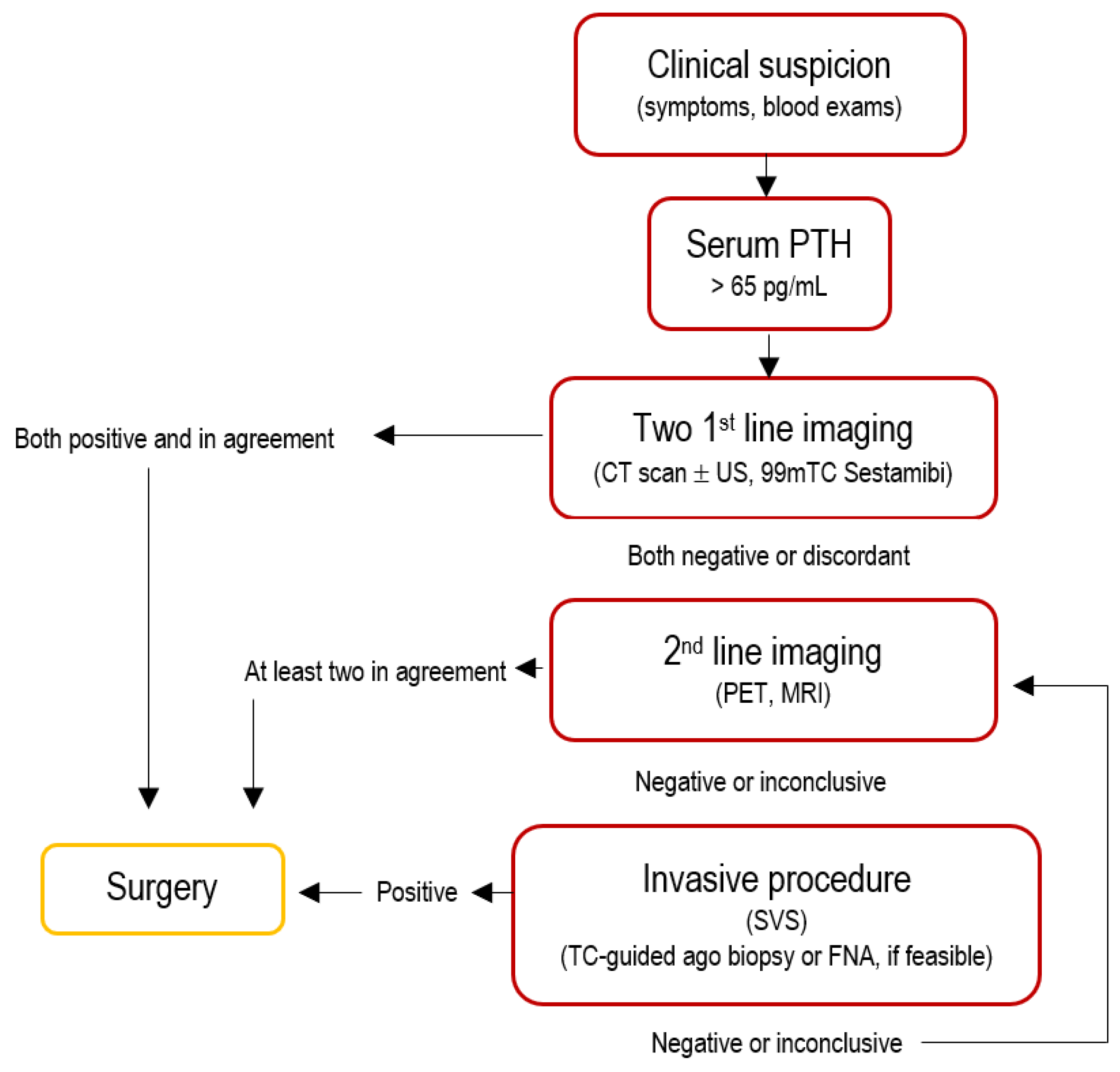
2. Preoperative Identification Strategies
2.1. Computed Tomography
2.2. Tc-99m Sestamibi Scintigraphy
2.3. Single Photon Emission Computed Tomography
2.4. Neck Ultrasound
2.5. Magnetic Resonance Imaging (MRI)
2.6. Positron Emission Tomography (PET/CT)
2.7. Pre-Operative Diagnosis
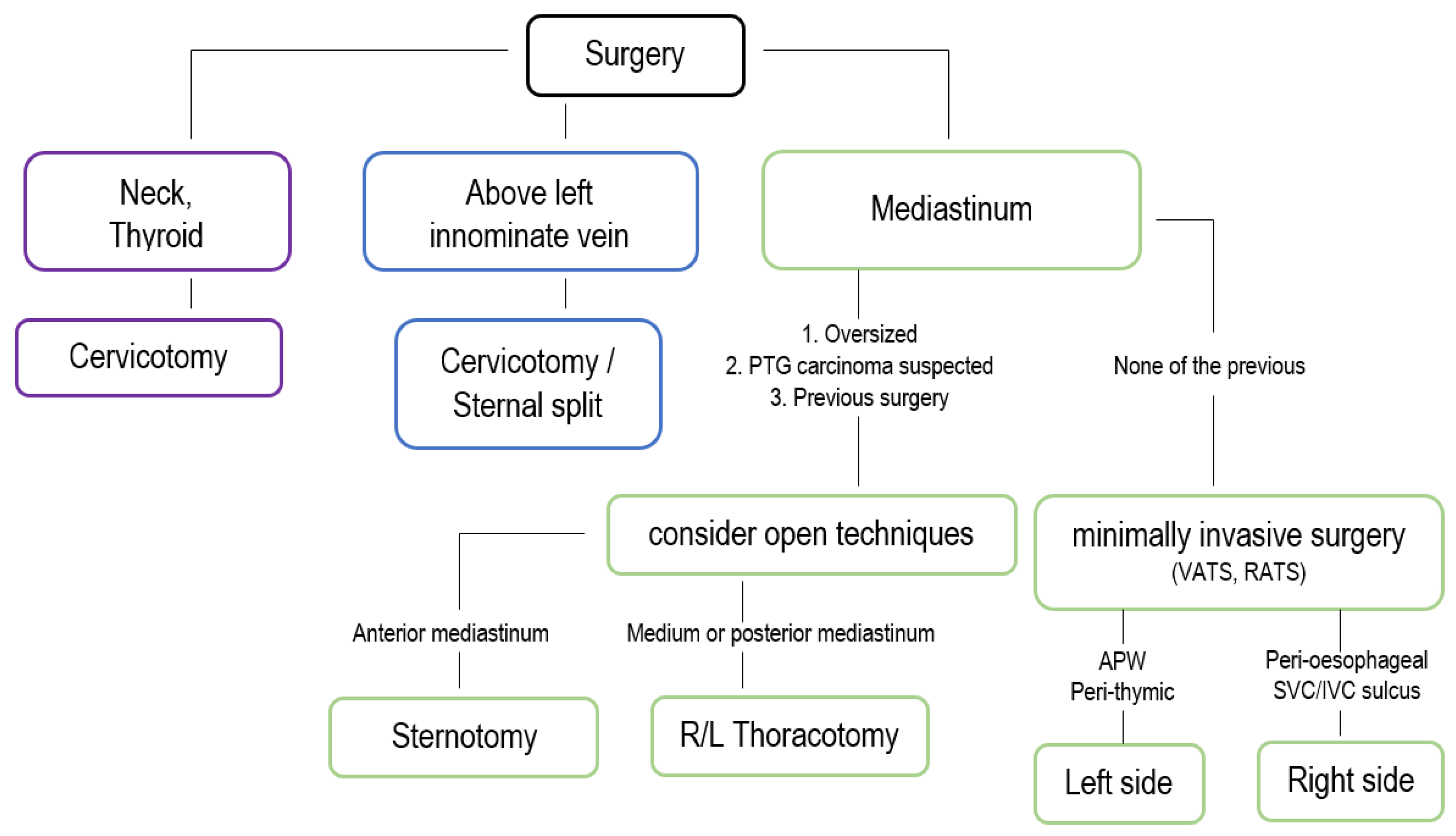
3. Surgical Treatment
3.1. Open Approaches
3.2. Minimally Invasive Approaches
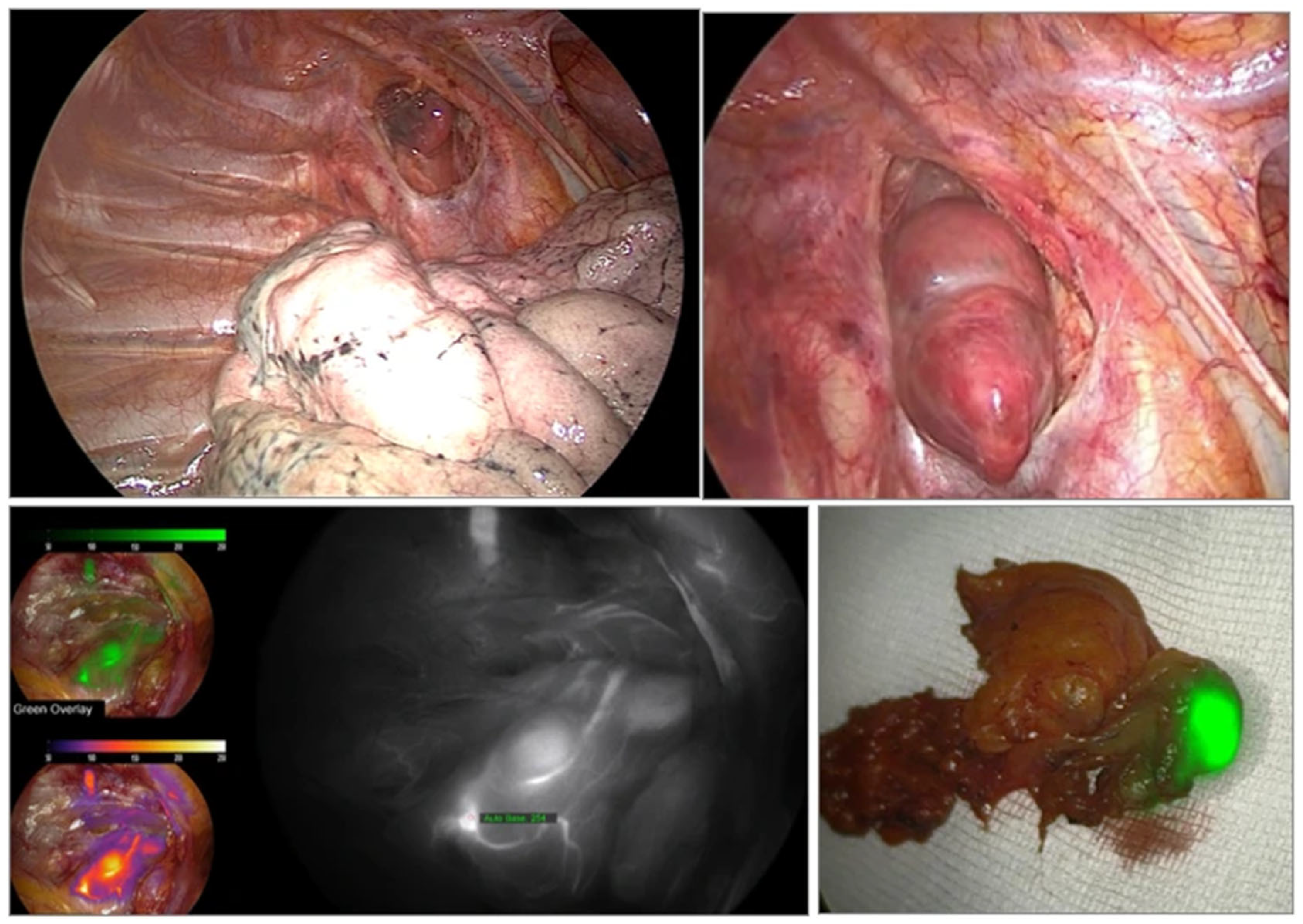
3.3. Intraoperative Evaluation
- •
- Confirmation of the removal of all hyperfunctioning parathyroid tissue without visualizing all glands
- •
- Identification of inadequate PTH decline indicating the presence of additional hyperfunctioning glands
- •
- Determination of the need for further exploration
- •
- Differentiation between parathyroid and non-parathyroid tissues
- •
- Bilateral internal jugular vein sampling to lateralize hyperfunctioning glands [60]
4. Surgical Outcomes
Redo Surgery
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APW | Aortopulmonary Window |
| BNE | Bilateral Neck Exploration |
| CT | Computed Tomography |
| DOCI | Dynamic Optical Contrast Imaging |
| EANM | European Association of Nuclear Medicine |
| ESRD | End-Stage Renal Disease |
| FNA | Fine-Needle Aspiration |
| ICG | Indocyanine Green |
| ioPTH | Intraoperative Parathyroid Hormone |
| IVC | Inferior Vena Cava |
| LSCI | Laser Speckle Contrast Imaging |
| MEN1 | Multiple Endocrine Neoplasia Type 1 |
| MIS | Minimally Invasive Surgery |
| MRI | Magnetic Resonance Imaging |
| MIP | Minimally Invasive Parathyroidectomy |
| OCT | Optical Coherence Tomography |
| PET/CT | Positron Emission Tomography/Computed Tomography |
| PHPT | Primary Hyperparathyroidism |
| PTH | Parathyroid Hormone |
| PTG | Parathyroid Gland |
| R/L | Right/Left |
| RATS | Robot-Assisted Thoracoscopic Surgery |
| SCa | Serum Calcium |
| SHPT | Secondary Hyperparathyroidism |
| SPECT | Single Photon Emission Computed Tomography |
| SVS | Selective Venous Sampling |
| 99mTc | Technetium-99m |
| THPT | Tertiary Hyperparathyroidism |
| US | Ultrasound/Ultrasonography |
| VATS | Video-Assisted Thoracoscopic Surgery |
References
- Van Udelsman, B.; Udelsman, R. Surgery in primary hyperparathyroidism: Extensive personal experience. J. Clin. Densitom. 2013, 16, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Said, S.M.; Cassivi, S.D.; Allen, M.S.; Deschamps, C.; Nichols, F.C.; Shen, K.R.; Wigle, D.A. Minimally Invasive Resection for Mediastinal Ectopic Parathyroid Glands. Ann. Thorac. Surg. 2013, 96, 1229–1233. [Google Scholar] [CrossRef]
- Randone, B.; Costi, R.; Scatton, O.; Fulla, Y.; Bertagna, X.; Soubrane, O. Bonnichon PThoracoscopic removal of mediastinal parathyroid glands: A critical appraisal of an emerging technique. Ann. Surg. 2010, 251, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Akin, R.D.; Pinheiro, A.D. Hypercalcemic crisis secondary to a superior mediastinal parathyroid adenoma: A case report. Ear Nose Throat J. 2022, 101, NP1–NP3. [Google Scholar] [CrossRef]
- Cheung, K.; Wang, T.S.; Farrokhyar, F.; Roman, S.A.; Sosa, J.A. A Meta-analysis of Preoperative Localization Techniques for Patients with Primary Hyperparathyroidism. Ann. Surg. Oncol. 2011, 19, 577–583. [Google Scholar] [CrossRef]
- Kelly, H.R.; Hamberg, L.M.; Hunter, G.J. 4D-CT for preoperative localization of abnormal parathyroid glands in patients with hyperparathyroidism: Accuracy and ability to stratify patients by unilateral versus bilateral disease in surgery-naïve and re-exploration patients. AJNR Am. J. Neuroradiol. 2014, 35, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Noussios, G.; Anagnostis, P.; Natsis, K. Ectopic parathyroid glands and their anatomical, clinical and surgical implications. Exp. Clin. Endocrinol. Diabetes 2012, 120, 604–610. [Google Scholar] [CrossRef]
- Manzil, F.F.P.; Eichhorn, J.; Vattoth, S. Synchronous Ectopic Thyroid Gland and Ectopic Parathyroid Adenoma on 99mTc-Sestamibi Scintigraphy and Correlative Imaging. J. Nucl. Med. Technol. 2023, 51, 263–264. [Google Scholar] [CrossRef]
- Fang, S.; Zhu, Q.L.; Liu, Y.M.; Zhang, Z.H.; Wang, O.; Xing, X.P.; Hu, Y.; Liao, Q.; Liu, H.; Li, J.C.; et al. Localization of Ectopic Hyperparathyroidism: Ultrasound Versus 99mTc-Sestamibi, 4-Dimensional Computed Tomography, and 11C-Choline Positron Emission Tomography/Computed Tomography. Endocr. Pract. 2024, 30, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Memon, S.S.; Goroshi, M.; Goroshi, S.; Patil, V.; Badhe, P.V.; Thakkar, H.; Sarathi, V.; Phadte, A.; Channaiah, C.Y.; et al. The polar vessel sign: Insights from CT imaging analysis in Asian Indian primary hyperparathyroidism. Endocrine 2025, 87, 800–809. [Google Scholar] [CrossRef]
- Hsieh, M.-C.P.; Nemer, J.S.; Beylergil, V.; Yeh, R. Ectopic Parathyroid Adenoma of the Piriform Sinus on Parathyroid 4d-CT and 99mtc-MIBI SPECT/Ct. Clin. Nucl. Med. 2020, 45, e358–e359. [Google Scholar] [CrossRef] [PubMed]
- Lebastchi, A.H.; Aruny, J.E.; Donovan, P.I.; Quinn, C.E.; Callender, G.G.; Carling, T.; Udelsman, R. Real-Time Super Selective Venous Sampling in Remedial Parathyroid Surgery. J. Am. Coll. Surg. 2015, 220, 994–1000. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Li, N.; Xu, K.; Zhang, W. Quantitative application of dual-phase 99mTc-sestamibi SPECT/CT imaging of parathyroid lesions: Identification of optimal timing in secondary hyperparathyroidism. EJNMMI Phys. 2023, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mohebati, A.; Shaha, A.R. Imaging techniques in parathyroid surgery for primary hyperparathyroidism. Am. J. Otolaryngol. 2012, 33, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Iihara, M.; Suzuki, R.; Kawamata, A.; Horiuchi, K.; Okamoto, T. Thoracoscopic Removal of Mediastinal Parathyroid Lesions: Selection of Surgical Approach and Pitfalls of Preoperative and Intraoperative Localization. World J. Surg. 2011, 36, 1327–1334. [Google Scholar] [CrossRef]
- Fiorelli, A.; Vicidomini, G.; Laperuta, P.; Rambaldi, P.; Mansi, L.; Rotondo, A.; Santini, M. The role of Tc-99m-2-Methoxy-Isobutyl-Isonitrile Single Photon Emission Computed Tomography in visualizing anterior mediastinal tumor and differentiating histologic type of thymoma. Eur. J. Cardio-Thoracic Surg. 2011, 40, 136–142. [Google Scholar] [CrossRef][Green Version]
- Brun, V.H.; Håskjold, O.I.; Bogsrud, T.V. Use of choline PET and ultrasound for preoperative localization of hyperfunctioning parathyroid glands: A single-institution retrospective cohort study. J. Nucl. Med. Technol. 2025, 14, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Ovčariček, P.P.; Giovanella, L.; Gasset, I.C.; Hindié, E.; Huellner, M.W.; Luster, M.; Piccardo, A.; Weber, T.; Talbot, J.-N.; Verburg, F.A. The EANM practice guidelines for parathyroid imaging. Eur. J. Nucl. Med. 2021, 48, 2801–2822. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Xu, H.; Chen, J.; Yao, B.; Lin, Q.; Deng, H.; Xu, W. Selective venous sampling in primary hyperparathyroidism caused by ectopic parathyroid gland: A case report and literature review. BMC Endocr. Disord. 2023, 23, 141. [Google Scholar] [CrossRef]
- Frank, E.; Watson, W.; Fujimoto, S.; De Andrade Filho, P.; Inman, J.; Simental, A. Surgery Versus Imaging in Non-Localizing Primary Hyperparathyroidism: A Cost-Effectiveness Model. Laryngoscope 2020, 18, 127. [Google Scholar] [CrossRef]
- Rubello, D.; Pelizzo, M.R.; Boni, G.; Schiavo, R.; Vaggelli, L.; Villa, G.; Sandrucci, S.; Piotto, A.; Manca, G.; Marini, P.; et al. Radioguided surgery of primary hyperparathyroidism using the lowdose99mTc-sestamibi protocol: Multi-institutional experience from the Italian Study Group on Radioguided Surgery and Immunoscintigraphy (GISCRIS). J. Nucl. Med. 2005, 46, 220–226. [Google Scholar]
- Zhou, P.; Xu, J.; Guo, Y.; Chen, L.; Liu, Y.; Guo, H.; Shao, C.; He, Q. The role of anatomical and functional orientation in identification of parathyroid glands for patients with parathyroidectomy. Front. Endocrinol. 2024, 15, 1428669. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vitetta, G.M.; Ravera, A.; Mensa, G.; Fuso, L.; Neri, P.; Carriero, A.; Cirillo, S. Actual role of color-doppler high-resolution neck ultrasonography in primary hyperparathyroidism: A clinical review and an observational study with a comparison of 99mTc-sestamibi parathyroid scintigraphy. J. Ultrasound 2018, 22, 291–308. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.A.; Saboury, B.; Ahlman, M.; Malayeri, A.A.; Jones, E.C.; Chen, C.C.; Millo, C. Parathyroid Imaging: Past, Present, and Future. Front. Endocrinol. 2022, 12, 760419. [Google Scholar] [CrossRef] [PubMed]
- Huynh, K.A.; MacFarlane, J.; Newman, C.; Gillett, D.; Das, T.; Scoffings, D.; Cheow, H.K.; Moyle, P.; Koulouri, O.; Harper, I.; et al. Diagnostic utility of 11C-methionine PET/CT in primary hyperparathyroidism in a UK cohort: A single-centre experience and literature review. Clin. Endocrinol. 2023, 99, 233–245. [Google Scholar] [CrossRef]
- Treglia, G.; Piccardo, A.; Imperiale, A.; Strobel, K.; Kaufmann, P.A.; Prior, J.O.; Giovanella, L. Diagnostic performance of choline PET for detection of hyperfunctioning parathyroid glands in hyperparathyroidism: A systematic review and meta-analysis. Eur. J. Nucl. Med. 2018, 46, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Shim, S.R.; Jeong, S.Y.; Kim, S.J. Direct comparison of preoperative imaging modalities for localization of primary hyperparathyroidism: A systematic review and network meta-analysis. JAMA Otolaryngol. Head. Neck Surg. 2021, 147, 692–706. [Google Scholar] [CrossRef]
- Bossert, I.; Chytiris, S.; Hodolic, M.; Croce, L.; Mansi, L.; Chiovato, L.; Mariani, G.; Trifirò, G. PETC/CT with 18F-Choline localizes hyperfunctioning parathyroid adenomas equally well in normocalcemic hyperparathyroidism as in overt hyperparathyroidism. J. Endocrinol. Investig. 2018, 42, 419–426. [Google Scholar] [CrossRef]
- Pogosian, K.; Karonova, T.; Ryzhkova, D.; Yanevskaya, L.; Tsoy, U.; Yudina, O.; Berkovich, G.; Dalmatova, A.; Grineva, E. 11C-methionine PET/CT and conventional imaging techniques in the diagnosis of primary hyperparathyroidism. Quant. Imaging Med. Surg. 2023, 13, 2352–2363. [Google Scholar] [CrossRef]
- Durma, A.D.; Saracyn, M.; Kołodziej, M.; Jóźwik-Plebanek, K.; Kamiński, G. The Use of [11C]C-Methionine in Diagnostics of Endocrine Disorders with Focus on Pituitary and Parathyroid Glands. Pharmaceuticals 2025, 18, 229. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Makey, I.A.; Geldmaker, L.E.; Casler, J.D.; El-Sayed Ahmed, M.M.; Jacob, S.; Thomas, M. Localization and surgical approach to mediastinal parathyroid glands. J. Cardiothorac. Surg. 2022, 17, 299. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Camenzuli, C.; DiMarco, A.; Isaacs, K.; Grant, Y.; Jackson, J.; Alsafi, A.; Harvey, C.; Barwick, T.; Tolley, N.; Palazzo, F. The changing face of reoperative parathyroidectomy: A single-centre comparison of 147 parathyroid reoperations. Ind. Mark. Manag. 2021, 103, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.J.; Brunaud, L.; Dowd, C.F.; Duh, Q.-Y.; Morita, E.; Clark, O.H. Accuracy of selective venous sampling for intact parathyroid hormone in difficult patients with recurrent or persistent hyperparathyroidism. Surgery 2002, 132, 944–951. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Brandi, M.L.; Eastell, R.; Silverberg, S.J.; Udelsman, R.; Marcocci, C.; Potts, J.T., Jr. Guidelines for the management of asymptomatic primary hyperparathyroidism: Summary statementfrom the fourth international workshop. J. Clin. Endocrinol. Metab. 2014, 99, 3561–3569. [Google Scholar] [CrossRef] [PubMed]
- Pappachan, J.M.; Lahart, I.M.; Viswanath, A.K.; Borumandi, F.; Sodi, R.; Metzendorf, M.-I.; Bongaerts, B. Parathyroidectomy for adults with primary hyperparathyroidism. Cochrane Database Syst. Rev. 2023, 2023, CD013035. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Wang, T.S.; Ruan, D.T.; Lee, J.A.; Asa, S.L.; Duh, Q.Y.; Doherty, G.M.; Herrera, M.F.; Pasieka, J.L.; Perrier, N.D.; et al. The American Association of Endocrine Surgeons. Guidelines for definitive management of primary hyperparathy- roidism. JAMA Surg. 2016, 151, 959. [Google Scholar] [CrossRef]
- Doherty, G.M.; Doppman, J.L.; Miller, D.L.; Gee, M.S.; Marx, S.J.; Spiegel, A.M.; Aurbach, G.D.; Pass, H.I.; Brennan, M.F.; Norton, J.A. Results of multidisciplinary strategy for management of mediastinal parathyroid adenoma as a cause of persistent primary hyper- parathyroidism. Ann. Surg. 1992, 215, 101–106. [Google Scholar] [CrossRef]
- Schlinkert, R.T.; Whitaker, M.D.; Argueta, R. Resction of selected mediastinal parathyroid adenomas through an anterior mediastinotomy. Mayo Clin. Proc. 1991, 66, 1110–1113. [Google Scholar] [CrossRef]
- Singh Ospina, N.; Thompson, G.B.; Lee, R.A.; Reading, C.C.; Young, W.F., Jr. Safety and efficacy of percutaneous parathyroid ethanol ablation in patients with recurrent primary hyperparathyroidism and multiple endocrine neoplasia type 1. J. Clin. Endocrinol. Metab. 2015, 100, 87–90. [Google Scholar] [CrossRef]
- Anderson, K.; Ruel, E.; Adam, M.A.; Thomas, S.; Youngwirth, L.; Stang, M.T.; Scheri, R.P.; Roman, S.A.; Sosa, J.A. Subtotal vs. total parathyroidectomy with autotransplantation for patients with renal hyperparathyroidism have similar outcomes. Am. J. Surg. 2017, 214, 914–919. [Google Scholar] [CrossRef]
- Conn, J.M.; Goncalves, M.A.; Mansour, K.A.; McGarity, W.C. The mediastinal parathyroid. Am. Surg. 1991, 57, 62–66. [Google Scholar] [PubMed]
- Downey, N.J.; McGuigan, J.A.; Dolan, S.J.; Russell, C.F. Median sternotomy for parathyroid adenoma. Ir. J. Med. Sci. 1999, 168, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Obara, T.; Fujimoto, Y.; Tanaka, R.; Ito, Y.; Kodama, T.; Yashiro, T.; Kanaji, Y.; Yamashita, T.; Fukuuchi, A. Mid-mediastinal parathyroid lesions: Preoperative localization and surgical approach in two cases. Surg. Today 1990, 20, 481–486. [Google Scholar] [CrossRef]
- Wei, B.; Inabnet, W.; Lee, J.A.; Sonett, J.R. Optimizing the Minimally Invasive Approach to Mediastinal Parathyroid Adenomas. Ann. Thorac. Surg. 2011, 92, 1012–1017. [Google Scholar] [CrossRef]
- Titu, I.M.; Silaghi, C.A.; Ciulic, S.A.; Teterea, F.; Mlesnite, M.; Palade, E. Progress in the Management of Mediastinal Ectopic Parathyroid Adenomas: The Role of Minimally Invasive Surgery. J. Clin. Med. 2025, 14, 3020. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.F.; Edis, A.J.; Scholz, D.A.; Sheedy, P.F.; van Heerden, J.A. Mediastinal parathyroid tumors: Experience with 38 tumors requiring mediastinotomy for removal. Ann. Surg. 1981, 193, 805–880. [Google Scholar] [CrossRef]
- Sachdeva, U.M.; Wright, C.D.; Mathisen, D.J. 7—Approach to the Mediastinum: Transcervical, Transsternal, and Video-Assisted. In Surgery of the Thyroid and Parathyroid Glands, 3rd ed.; Randolph, G.W., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 70–78.e1. [Google Scholar]
- Bondje, S.; Kaplan, F.; Palazzo, F.; Barwick, T. Resection of an elusive parathyroid adenoma in the aortopulmonary window. BMJ Case Rep. 2023, 16, e250380. [Google Scholar] [CrossRef]
- Grozavu, C.; Pantile, D. Primary hyperparathyroidism through an ectopic parathyroid adenoma. Chirurgia 2016, 111, 156–160. [Google Scholar]
- Duman, S.; Sarıgül, A.; Erdoğdu, E.; Özkan, B.; Demir, A.; Kara, M.; Toker, S.A. Video-Assisted Thoracoscopic Surgery Is a Safe and Feasible Technique for Mediastinal Parathyroid Lesions. J. Laparoendosc. Adv. Surg. Tech. 2024, 34, 677–681. [Google Scholar] [CrossRef]
- Khanom, S.; Singh, K.; Blinkhorn, L.S.; Ravendran, K.; Blinkhorn, L.S., Sr. Robotic Resection of an Ectopic Parathyroid Gland: A Systematic Review. Cureus 2024, 16, e75096. [Google Scholar] [CrossRef]
- Anemoulis, M.; Kachtsidis, V.; Geropoulos, G.; Panagiotopoulos, N. Robot-Assisted Thoracoscopic Resection of Ectopic Parathyroid Tissue in Mediastinum: A Scoping Review. Innovations 2024, 19, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, K.E.; Belete, S.; Miller, B.J.; Di Marco, A.N.; Kirby, S.; Barwick, T.; Tolley, N.S.; Anderson, J.R.; Palazzo, F.F. Video-assisted thoracoscopic surgery for ectopic mediastinal parathyroid adenoma. BJS Open 2019, 3, 743–749. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Du, H.; Shi, M.; Zhu, L.; Che, J.; Hang, J.; Chen, Z.; Li, H. Comparison of video-assisted thoracic surgery with open surgery in the treatment of ectopic mediastinal parathyroid tumors. J. Thorac. Dis. 2017, 9, 5171–5175. [Google Scholar] [CrossRef] [PubMed]
- Nagano, H.; Suda, T.; Ishizawa, H.; Negi, T.; Kawai, H.; Kawakami, T.; Tochii, D.; Tochii, S.; Hoshikawa, Y. Video-assisted thoracoscopic surgery for ectopic mediastinal parathyroid tumor: Subxiphoid and lateral thoracic approach. J. Thorac. Dis. 2019, 11, 2932–2938. [Google Scholar] [CrossRef]
- Ismail, M.; Maza, S.; Swierzy, M.; Tsilimparis, N.; Rogalla, P.; Sandrock, D.; Rückert, R.I.; Müller, J.M.; Rückert, J.C. Resection of ectopic mediasti- nal parathyroid glands with the da Vinci robotic system. Br. J. Surg. 2010, 97, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Mansour, D.E.; Lee, M.E.; D’Souza, D.M.; Merritt, R.E.; Kneuertz, P.J. Robotic resection of ectopic parathyroid glands in the superior posterior mediastinum. J. Laparoendosc. Adv. Surg. Tech. A 2019, 29, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.F.; Lee, T.; Ogilvie, J.B.; Patel, K.N.; Hiotis, K.; Bizekis, C.; Zervos, M. Robot-assisted complete thymectomy for mediastinal ectopic parathyroid adenomas in primary hyperparathyroidism. J. Robot. Surg. 2016, 11, 163–169. [Google Scholar] [CrossRef]
- Nussbaum, S.R.; Thompson, A.R.; Hutcheson, K.A.; Gaz, R.D.; Wang, C.A. Intraoperative measurement of parathyroid hormone in the surgical management of hyperparathyroidism. Surgery 1988, 104, 1121–1127. [Google Scholar] [PubMed]
- Patel, K.N.; Caso, R. Intraoperative Parathyroid Hormone Monitoring: Optimal Utilization. Surg. Oncol. Clin. N. Am. 2016, 25, 91–101. [Google Scholar] [CrossRef]
- Mallick, R.; Chen, H. Diagnosis and Management of Hyperparathyroidism. Adv. Surg. 2018, 52, 137–153. [Google Scholar] [CrossRef]
- Carr, A.A.; Yen, T.W.; Wilson, S.D.; Evans, D.B.; Wang, T.S. Using parathyroid hormone spikes during parathyroidectomy to guide intraoperativedecision-making. J. Surg. Res. 2017, 209, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Irvin, G.L., 3rd; Solorzano, C.C.; Carneiro, D.M. Quick intraoperative parathyroid hormone assay:surgical adjunct to allow limited parathyroidectomy, improve success rate, and predict outcome. World J. Surg. 2004, 28, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Shawky, M.; Aziz, T.A.; Morley, S.; Beale, T.; Bomanji, J.; Soromani, C.; Lam, F.; Philips, I.; Matias, M.; Honour, J.; et al. Impact of intraoperative parathyroid hormone monitoring on the management of patients with primary hyperparathyroidism. Clin. Endocrinol. 2018, 90, 277–284. [Google Scholar] [CrossRef]
- Conrad, D.N.; Olson, J.E.; Hartwig, H.M.; Mack, E.; Chen, H. A prospective evaluation of novel methods to intraoperatively distinguish parathyroid tissue utilizing a parathyroid hormone assay. J. Surg. Res. 2006, 133, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Bergenfelz, A.; van Slycke, S.; Makay, Ö.; Brunaud, L. European multicentre study on outcome of surgery for sporadic primary hyperparathyroidism. Br. J. Surg. 2021, 108, 675–683. [Google Scholar] [CrossRef]
- Gasparri, G. Updates in primary hyperparathyroidism. Updates Surg. 2017, 69, 217–223. [Google Scholar] [CrossRef]
- Ramonell, K.M.; Fazendin, J.; Lindeman, B.; Chen, H. My surgical practice: Radioguided parathyroid surgery, how and why we use it. Am. J. Surg. 2022, 223, 203–205. [Google Scholar] [CrossRef]
- Desiato, V.; Melis, M.; Amato, B.; Bianco, T.; Rocca, A.; Amato, M.; Quarto, G.; Benassai, G. Minimally invasive radioguided parathyroid surgery: A literature review. Int. J. Surg. 2016, 28, S84–S93. [Google Scholar] [CrossRef]
- Chen, H.; Mack, E.; Starling, J.R. Radioguided parathyroidectomy is equally effective for both adenomatous and hyperplastic glands. Ann. Surg. 2003, 238, 332–337. [Google Scholar] [CrossRef]
- Rubello, D.; Casara, D.; Giannini, S.; Piotto, A.; De Carlo, E.; Muzzio, P.C.; Pelizzo, M.R. Importance of radio-guided minimally invasive parathyroidectomy using hand-heldgamma probe and low (99m)Tc-MIBI dose. Technical considerations and long-termclinical results. Q. J. Nucl. Med. 2003, 47, 129–138. [Google Scholar]
- Murphy, C.; Norman, J. The 20% rule: A simple, instantaneous r dioactivity measurement defines cure and allows elimination of frozen sections and hormone assays during parathyroidectomy. Surgery 1999, 126, 1023–1028. [Google Scholar] [CrossRef]
- Glasgow, C.; Lau, E.Y.; Aloj, L.; Harper, I.; Cheow, H.; Das, T.; Casey, R.T. An approach to a patient with primary hyperparathyroidism and a suspected ectopic parathyroid adenoma. J. Clin. Endocrinol. Metab. 2022, 107, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Harrison, B.J.; Triponez, F. Intraoperative adjuncts in surgery for primary hyperparathyroidism. Langenbecks Arch. Surg. 2009, 394, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Mazeh, H.; Chen, H. Intraoperative adjuncts for parathyroid surgery. Expert. Rev. Endocrinol. Metab. 2011, 6, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Noureldine, S.I.; Gooi, Z.; Tufano, R.P. Minimally invasive parathyroid surgery. Gland. Surg. 2015, 4, 410–419. [Google Scholar] [CrossRef]
- Dudley, N.E. Methylene blue for rapid identification of the parathyroids. Br. Med. J. 1971, 3, 680–681. [Google Scholar] [CrossRef]
- Patel, H.P.; Chadwick, D.R.; Harrison, B.J.; Balasubramanian, S.P. Systematic review of intravenous methylene blue in parathyroid surgery. Br. J. Surg. 2012, 99, 1345–1351. [Google Scholar] [CrossRef]
- Anton, R.C.; Wheeler, T.M. Frozen section of thyroid and parathyroid specimens. Arch. Pathol. Lab. Med. 2005, 129, 1575–1584. [Google Scholar] [CrossRef]
- Egan, R.J.; Scott-Coombes, D.M. The surgical management of sporadic primary hyperparathyroidism. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 847–859. [Google Scholar] [CrossRef]
- Hiramitsu, T.; Hasegawa, Y.; Futamura, K.; Okada, M.; Goto, N.; Narumi, S.; Watarai, Y.; Tominaga, Y.; Ichimori, T. Intraoperative intact parathyroid hormone monitoring and frozen section diagnosis are essential for successful parathyroidectomy in secondary hyperparathyroidism. Front. Med. 2022, 9, 1007887. [Google Scholar] [CrossRef]
- Pace-Asciak, P.; Russell, J.; Solorzano, C.; Berber, E.; Singer, M.; Shaha, A.R.; Khafif, A.; Angelos, P.; Nixon, I.; Tufano, R.P. The utility of parathyroida utofluorescence as an adjunct in thyroid and parathyroid surgery 2023. Head Neck 2023, 45, 3157–3167. [Google Scholar] [CrossRef]
- Shinden, Y.; Nakajo, A.; Arima, H.; Tanoue, K.; Hirata, M.; Kijima, Y.; Maemura, K.; Natsugoe, S. Intraoperative Identification of the Parathyroid Gland with a Fluorescence Detection System. World J. Surg. 2017, 41, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Pannu, A.Y.; O’cOnnor-Manson, M.R.; Wyld, L.; Balasubramanian, S.P. Near-infrared fluorescent imaging for parathyroid identification and/or preservation in surgery for primary hyperparathyroidism. Front. Endocrinol. 2023, 14, 1240024. [Google Scholar] [CrossRef]
- Takeuchi, S.; Shimizu, K.; Shimizu, K., Jr.; Akasu, H.; Okamura, R. Identification of pathological and normal parathyroid tissue by fluorescent labeling with 5-aminolevulinic acid during endocrine neck surgery. J. Nippon. Med. Sch. 2014, 81, 84–93. [Google Scholar] [CrossRef]
- Sommerey, S.; Al Arabi, N.; Ladurner, R.; Chiapponi, C.; Stepp, H.; Hallfeldt, K.K.J.; Gallwas, J.K.S. Intraoperative optical coherence tomography imaging to identify parathyroid glands. Surg. Endosc. 2014, 29, 2698–2704. [Google Scholar] [CrossRef] [PubMed]
- Palermo, A.; Fosca, M.; Tabacco, G.; Marini, F.; Graziani, V.; Santarsia, M.C.; Longo, F.; Lauria, A.; Cesareo, R.; Giovannoni, I.; et al. Raman Spectroscopy Applied to Parathyroid Tissues: A New Diagnostic Tool to Discriminate Normal Tissue from Adenoma. Anal. Chem. 2017, 90, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Anastasopoulou, C.; Ngu, M.; Singh, S. Hypocalcemia; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Xu, J.; Kong, N.; Bai, N.; Zhang, Z.; Cui, A.; Tan, S.; Xu, Q. Identification of novel risk factors for postoperative severe hypocalcemia in patients with primary hyperparathyroidism undergoing parathyroidectomy: A case control study. BMC Endocr. Disord. 2024, 24, 88. [Google Scholar] [CrossRef]
- E Witteveen, J.; van Thiel, S.; A Romijn, J.; Hamdy, N.A.T. THERAPY OF ENDOCRINE DISEASE: Hungry bone syndrome: Still a challenge in the post-operative management of primary hyperparathyroidism: A systematic review of the literature. Eur. J. Endocrinol. 2013, 168, R45–R53. [Google Scholar] [CrossRef]
- English, K.A.; Pieterman, C.R.C.; Marini , F.; Lines, K.E.; Cuny, T.; Saulle, R.; Shariq, O.A.; Mitrova, Z.; Castinetti, F.; Waguespack, S.G.; et al. Treatments for MEN1-associated endocrine tumours: Three systematic reviews and a meta-analysis. Lancet Diabetes Endocrinol. 2025. [Google Scholar] [CrossRef]
- Başcı, Ö.K.; Özdemir, N.; Hekimsoy, Z. Spuriously High Levels in a Patient After Total Parathyroidectomy with Forearm Auto-Transplantation. Bosphorus Med. J. 2023, 10, 54. [Google Scholar]
- Aprile, V.; Korasidis, S.; Bacchin, D.; Ambrogi, M.C.; Lucchi, M. Extended surgery of antero-superior mediastinum. Curr. Challenges Thorac. Surg. 2019, 1, 21. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabazzi, G.; Elia, G.; Aprile, V.; Korasidis, S.; Mastromarino, M.G.; Bacchin, D.; Lenzini, A.; Ambrogi, M.C.; Alì, G.; Cetani, F.; et al. Surgical Management of Mediastinal Ectopic Parathyroids. J. Pers. Med. 2025, 15, 276. https://doi.org/10.3390/jpm15070276
Rabazzi G, Elia G, Aprile V, Korasidis S, Mastromarino MG, Bacchin D, Lenzini A, Ambrogi MC, Alì G, Cetani F, et al. Surgical Management of Mediastinal Ectopic Parathyroids. Journal of Personalized Medicine. 2025; 15(7):276. https://doi.org/10.3390/jpm15070276
Chicago/Turabian StyleRabazzi, Giacomo, Gianmarco Elia, Vittorio Aprile, Stylianos Korasidis, Maria Giovanna Mastromarino, Diana Bacchin, Alessandra Lenzini, Marcello Carlo Ambrogi, Greta Alì, Filomena Cetani, and et al. 2025. "Surgical Management of Mediastinal Ectopic Parathyroids" Journal of Personalized Medicine 15, no. 7: 276. https://doi.org/10.3390/jpm15070276
APA StyleRabazzi, G., Elia, G., Aprile, V., Korasidis, S., Mastromarino, M. G., Bacchin, D., Lenzini, A., Ambrogi, M. C., Alì, G., Cetani, F., Materazzi, G., & Lucchi, M. (2025). Surgical Management of Mediastinal Ectopic Parathyroids. Journal of Personalized Medicine, 15(7), 276. https://doi.org/10.3390/jpm15070276







