BALF Lymphocyte and Cytokine Profiling as Biomarkers of Acute Rejection After Lung Transplantation
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CLAD | Chronic lung allograft dysfunction |
| ACR | Acute cellular rejection |
| ISHLT | International Society for Heart and Lung Transplantation |
| TBB | Transbronchial biopsy |
| EOS | Peripheral blood eosinophil |
| BALF | Bronchoalveolar lavage |
| IFN-γ | Interferon-gamma |
| TNF-α | Tumor necrosis factor alpha |
| LTx | Lung transplant |
| FBC | Fiber-optic bronchoscopy |
| FEV1 | Forced expiratory volume in 1 s |
| ELISA | Enzyme-linked immunosorbent assay |
References
- International Society for Heart and Lung Transplantation (ISHLT) Registry. ISHLT Registry Quarterly Reports, January 1995–June 2018; ISHLT: Minneapolis, MN, USA, 2018. [Google Scholar]
- Martinu, T.; Pavlisko, E.N.; Chen, D.F.; Palmer, S.M. Acute allograft rejection: Cellular and humoral processes. Clin. Chest Med. 2011, 32, 295–310. [Google Scholar] [CrossRef] [PubMed]
- International Society for Heart and Lung Transplantation (ISHLT). ISHLT Registry slides. JHLT 2019, 38, 1015–1066. [Google Scholar]
- Koutsokera, A. Rethinking bronchoalveolar lavage in acute cellular rejection: How golden is the standard of transbronchial biopsies? J. Heart Lung Transplant. 2019, 38, 856–857. [Google Scholar] [CrossRef] [PubMed]
- Trulock, E.P.; Ettinger, N.A.; Brunt, E.M.; Pasque, M.K.; Kaiser, L.R.; Cooper, J.D. The role of transbronchial lung biopsy in the treatment of lung transplant recipients. An analysis of 200 consecutive procedures. Chest 1992, 102, 1049–1054. [Google Scholar] [CrossRef]
- Silva, T.; Voisey, J.; Hopkins, P.; Apte, S.; Chambers, D.; O’Sullivan, B. Markers of rejection of a lung allograft: State of the art. Biomark. Med. 2022, 16, 483–498. [Google Scholar] [CrossRef] [PubMed]
- De Vlaminck, I.; Martin, L.; Kertesz, M.; Patel, K.; Kowarsky, M.; Strehl, C.; Cohen, G.; Luikart, H.; Neff, N.F.; Okamoto, J.; et al. Noninvasive monitoring of infection and rejection after lung transplantation. Proc. Natl. Acad. Sci. USA 2015, 112, 13336–13341. [Google Scholar] [CrossRef]
- Tissot, A.; Danger, R.; Claustre, J.; Magnan, A.; Brouard, S. Early Identification of Chronic Lung Allograft Dysfunction: The Need of Biomarkers. Front. Immunol. 2019, 10, 1681. [Google Scholar] [CrossRef]
- Shtraichman, O.; Diamond, J.M. Emerging biomarkers in chronic lung allograft dysfunction. Expert Rev. Mol. Diagn. 2020, 20, 467–475. [Google Scholar] [CrossRef]
- Goldman, M.; Le Moine, A.; Braun, M.; Flamand, V.; Abramowicz, D. A role for eosinophils in transplant rejection. Trends Immunol. 2001, 22, 247–251. [Google Scholar] [CrossRef]
- Ibáñez, S.A.; Aguilar, M.P.; Vicente, A.R.; García-Gallo, C.L.; Nuevo, G.D.; Antón, C.S.; Gil, M.P.U. Peripheral blood eosinophilia as a marker of acute cellular rejection in lung transplant recipients. J. Heart Lung Transplant. 2022, 41, 501–507. [Google Scholar] [CrossRef]
- Prop, J.; Wagenaar-Hilbers, J.P.; Petersen, A.H.; Wildevuur, C.R. Characteristics of cells lavaged from rejecting lung allografts in rats. Transplant. Proc. 1988, 20, 217–218. [Google Scholar] [PubMed]
- Vos, R.; Vanaudenaerde, B.M.; Verleden, S.E.; De Vleeschauwer, S.I.; Willems-Widyastuti, A.; Van Raemdonck, D.E.; Dupont, L.J.; Nawrot, T.S.; Verbeken, E.K.; Verleden, G.M. Bronchoalveolar lavage neutrophilia in acute lung allograft rejection and lymphocytic bronchiolitis. J. Heart Lung Transplant. 2010, 29, 1259–1269. [Google Scholar] [CrossRef]
- Zheng, L.; Orsida, B.; Whitford, H.; Levvey, B.; Ward, C.; Walters, E.H.; Williams, T.J.; Snell, G.I. Longitudinal comparisons of lymphocytes and subtypes between airway wall and bronchoalveolar lavage after human lung transplantation. Transplantation 2005, 80, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, S.D.; Burgess, J.K.; Verschuuren, E.; Slebos, D.J. The cellular composition of the lung lining fluid gradually changes from bronchus to alveolus. Respir. Res. 2021, 22, 285. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Slebos, D.J.; Postma, D.S.; Koëter, G.H.; Van Der Bij, W.; Boezen, M.; Kauffman, H.F. Bronchoalveolar lavage fluid characteristics in acute and chronic lung transplant rejection. J. Heart Lung Transplant. 2004, 23, 532–540. [Google Scholar] [CrossRef]
- Greenland, J.R.; Jewell, N.P.; Gottschall, M.; Trivedi, N.N.; Kukreja, J.; Hays, S.R.; Singer, J.P.; Golden, J.A.; Caughey, G.H. Bronchoalveolar lavage cell immunophenotyping facilitates diagnosis of lung allograft rejection. Am J. Transplant. 2014, 14, 831–840. [Google Scholar] [CrossRef]
- Aguado Ibáñez, S.; Laporta Hernández, R.; Aguilar Pérez, M.; García Fadul, C.; López García Gallo, C.; Díaz Nuevo, G.; Salinas Castillo, S.; Castejón Diaz, R.; Salas Anton, C.; Royuela Vicente, A.; et al. A Combination of Cytological Biomarkers as a Guide in the Diagnosis of Acute Rejection in Lung Transplant Recipients. Transplantology 2023, 4, 102–110. [Google Scholar] [CrossRef]
- Speck, N.E.; Schuurmans, M.M.; Benden, C.; Robinson, C.A.; Huber, L.C. Plasma and bronchoalveolar lavage samples in acute lung allograft rejection: The potential role of cytokines as diagnostic markers. Respir. Res. 2017, 18, 151. [Google Scholar] [CrossRef]
- Weigt, S.S.; Wang, X.; Palchevskiy, V.; Li, X.; Patel, N.; Ross, D.J.; Reynolds, J.; Shah, P.D.; Danziger-Isakov, L.A.; Sweet, S.C.; et al. Usefulness of gene expression profiling of bronchoalveolar lavage cells in acute lung allograft rejection. J. Heart Lung Transplant. 2019, 38, 845–855. [Google Scholar] [CrossRef]
- Speck, N.E.; Probst-Müller, E.; Haile, S.R.; Benden, C.; Kohler, M.; Huber, L.C.; Robinson, C.A. Bronchoalveolar lavage cytokines are of minor value to diagnose complications following lung transplantation. Cytokine 2020, 125, 154794. [Google Scholar] [CrossRef]
- Stewart, S.; Fishbein, M.C.; Snell, G.I.; Berry, G.J.; Boehler, A.; Burke, M.M.; Glanville, A.; Gould, F.K.; Magro, C.; Marboe, C.C.; et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J. Heart Lung Transplant. 2007, 26, 1229–1242. [Google Scholar] [CrossRef] [PubMed]
- Achterrath, U.; Blümcke, S.; Koerner, S.K.; Yipintsoi, T.; Siegelman, S.S.; Chandler, P.; Hagstrom, J.W.; Torres, M.; Cobbah, J.E.; Fujii, P.; et al. Alveolar lavage cytology in transplanted lungs. I. Staining methods and findings in dogs with autografts and allografts without immunosuppression. J. Thorac. Cardiovasc. Surg. 1975, 69, 510–520. [Google Scholar] [CrossRef] [PubMed]
- De Hoyos, A.; Chamberlain, D.; Schvartzman, R.; Ramirez, J.; Kesten, S.; Winton, T.L.; Maurer, J. Prospective assessment of a standardized pathologic grading system for acute rejection in lung transplantation. Chest 1993, 103, 1813–1818. [Google Scholar] [CrossRef]
- Elssner, A.; Jaumann, F.; Dobmann, S.; Behr, J.; Schwaiblmair, M.; Reichenspurner, H.; Fürst, H.; Briegel, J.; Vogelmeier, C. Elevated levels of interleukin-8 and transforming growth factor-beta in bronchoalveolar lavage fluid from patients with bronchiolitis obliterans syndrome: Proinflammatory role of bronchial epithelial cells. Munich Lung Transplant Group. Transplantation 2000, 70, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Reynaud-Gaubert, M.; Marin, V.; Thirion, X.; Farnarier, C.; Thomas, P.; Badier, M.; Bongrand, P.; Giudicelli, R.; Fuentes, P. Upregulation of chemokines in bronchoalveolar lavage fluid as a predictive marker of post-transplant airway obliteration. J. Heart Lung Transplant. 2002, 21, 721–730. [Google Scholar] [CrossRef]
- Vanaudenaerde, B.M.; Dupont, L.J.; Wuyts, W.A.; Verbeken, E.K.; Meyts, I.; Bullens, D.M.; Dilissen, E.; Luyts, L.; Van Raemdonck, D.E.; Verleden, G.M. The role of interleukin-17 during acute rejection after lung transplantation. Eur. Respir. J. 2006, 27, 779–787. [Google Scholar] [CrossRef]

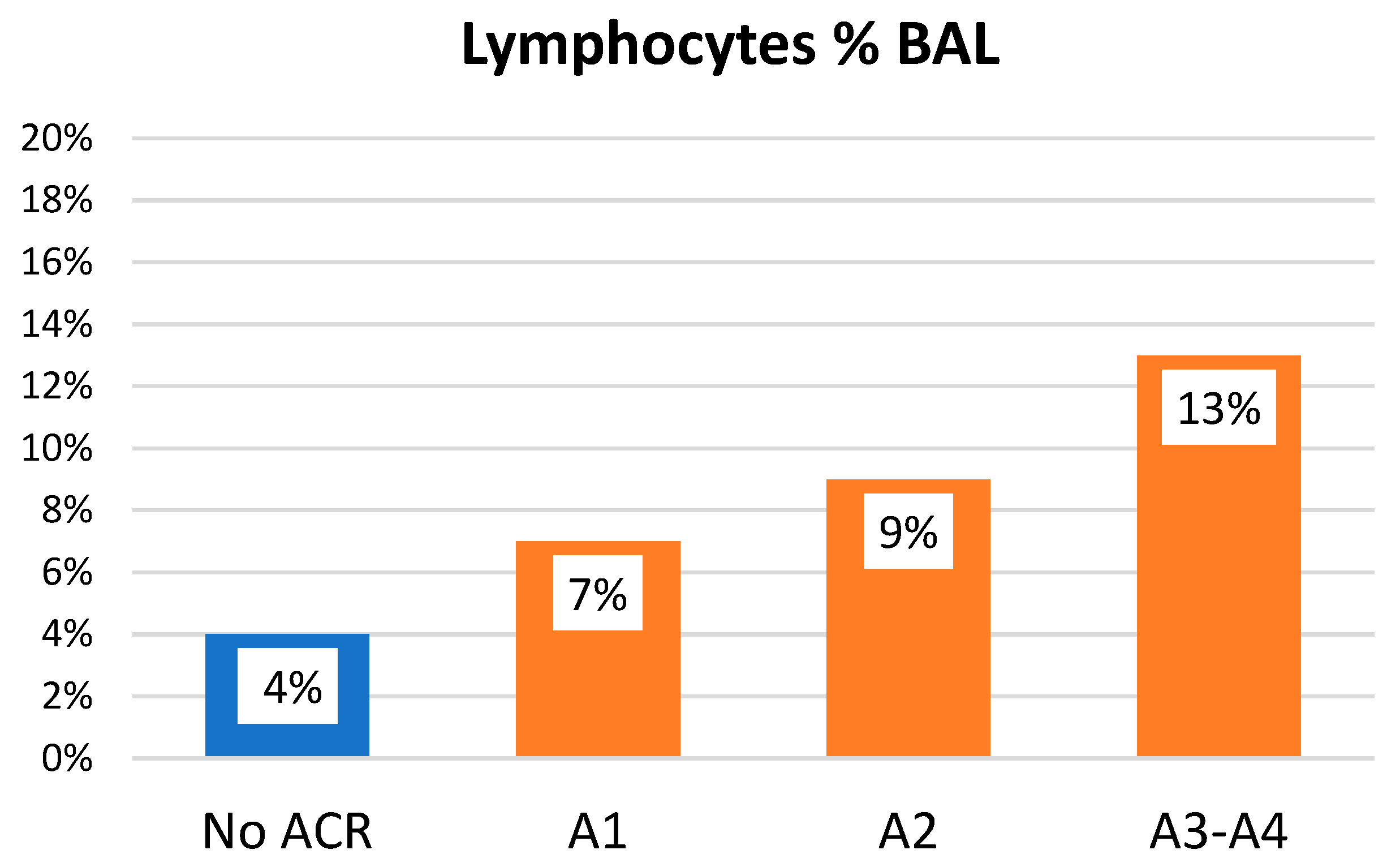
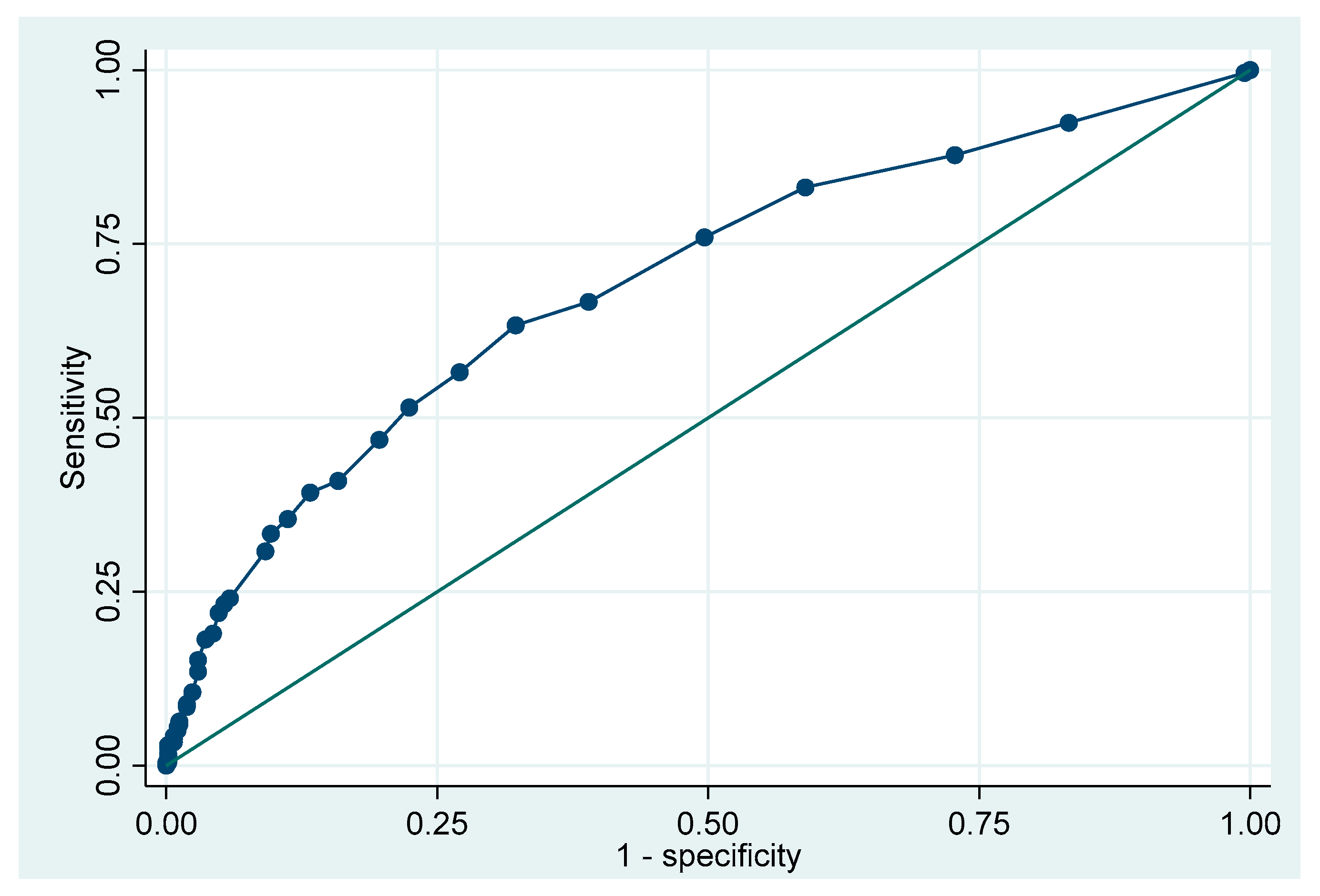
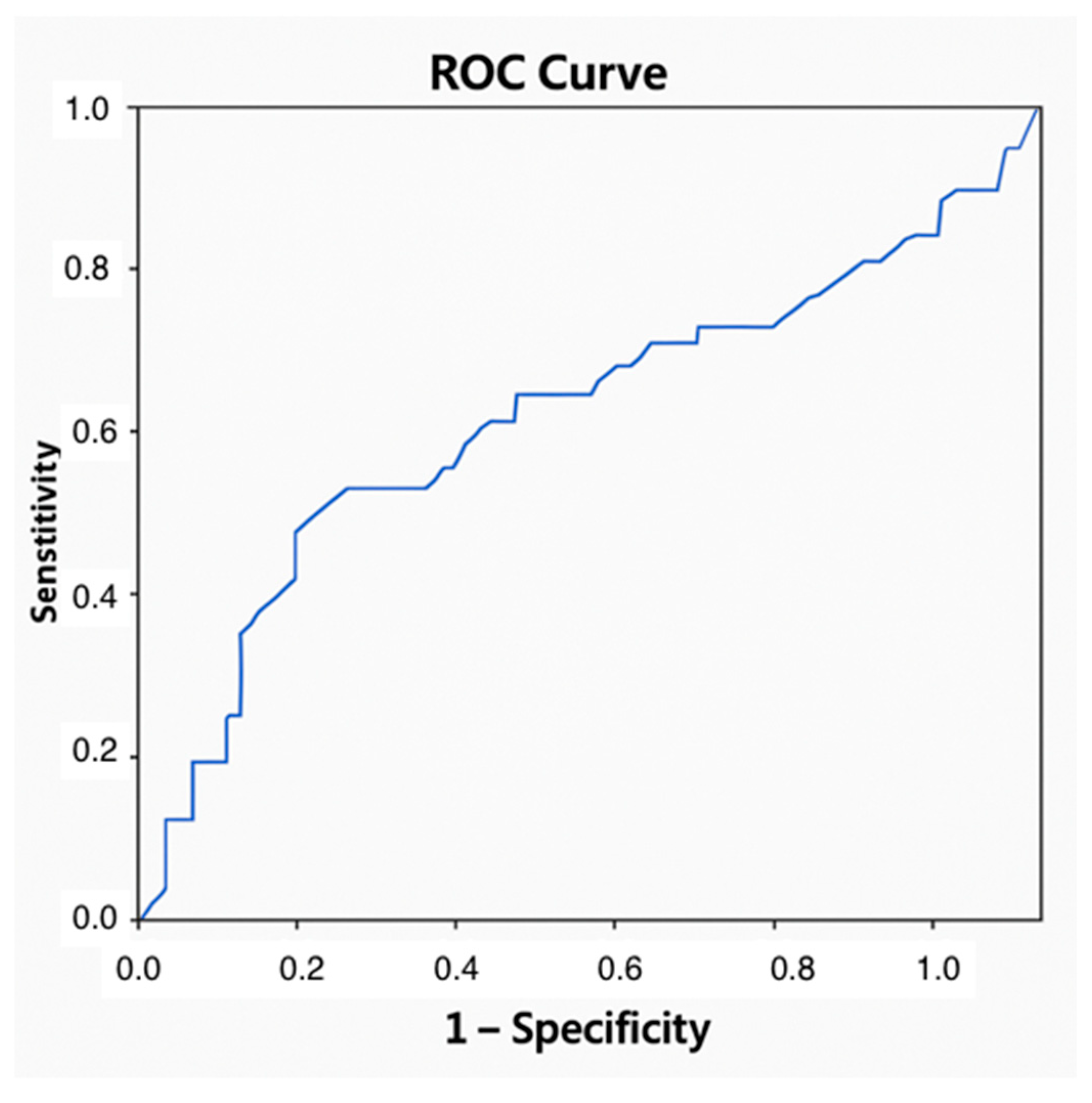
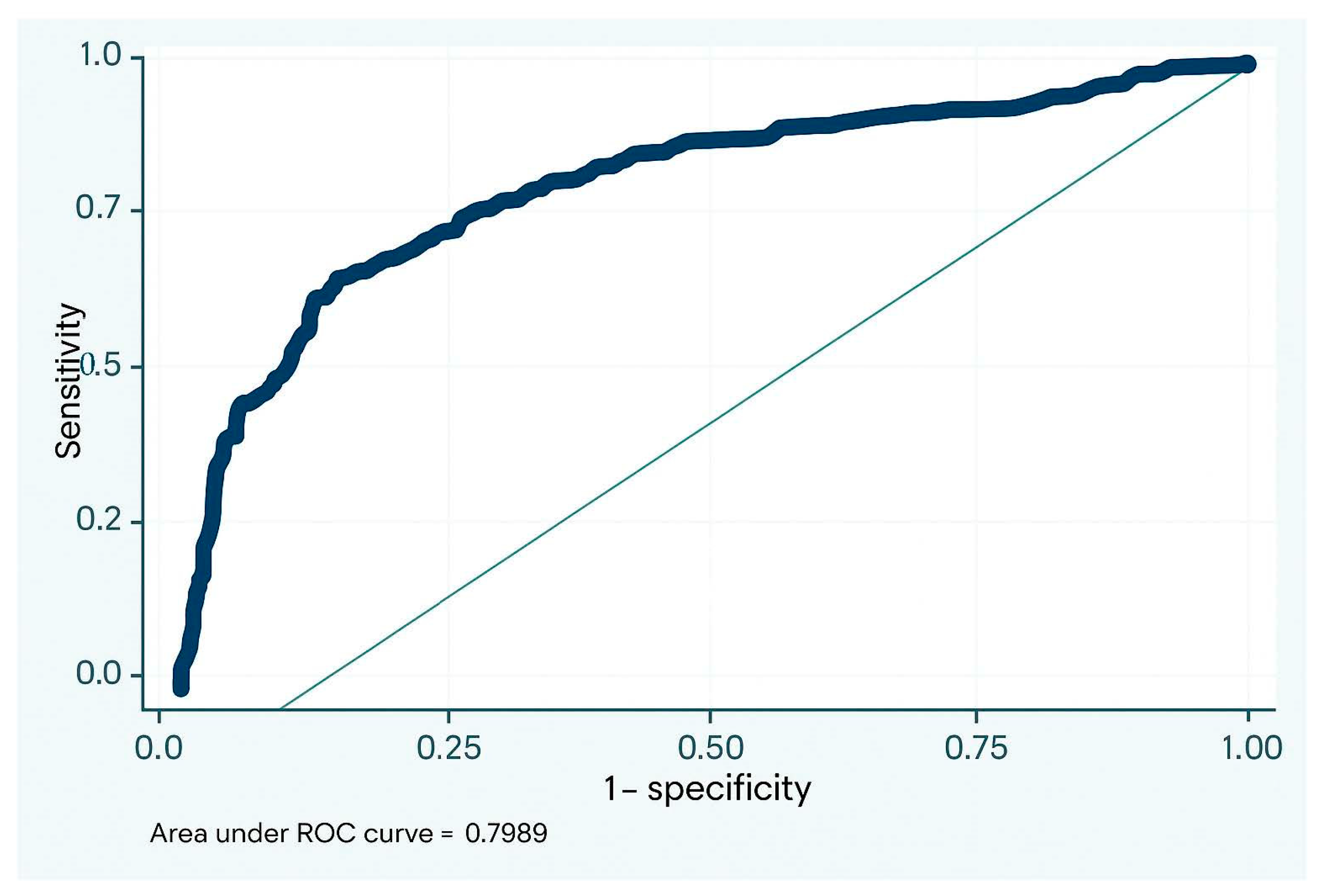
| Total N = 74 | No ACR n = 30; 40.5% | ACR n = 44; 59.5% | p | |
|---|---|---|---|---|
| Age at transplant (median *, years) | 53 (40; 65) | 53 (42; 63) | 53 (42; 65) | p = 0.812 |
| Male (n, %) | 47 (63.5%) | 11 (23.4%) | 36 (72.6%) | p = 0.291 |
| COPD (n, %) | 30 (40.5%) | 10 (33.3%) | 20 (45.5%) | p = 0.829 |
| IPF (n, %) | 16 (21.6%) | 7 (23.3%) | 9 (20.4%) | |
| DILD (n, %) | 16 (21.6%) | 5 (16.8%) | 11 (25.0%) | |
| CF (n, %) | 8 (10.9%) | 6 (20.0%) | 2 (4.5%) | |
| Bronchiectasis (n, %) | 2 (2.7%) | 1 (3.3%) | 1 (2.3%) | |
| Other causes (n, %) | 2 (2.7%) | 1 (3.3%) | 1 (2.3%) |
| BAL Cytokines | Total | No ACR | ACR | p |
|---|---|---|---|---|
| Human IL-4 (pg/mL) | 0 (0; 0) | 0 (0; 0) | 0 (0; 0) | p = 0.959 |
| Human IL-6 (pg/mL) | 2.4 (0; 13.16) | 0 (0; 7.3) | 3.1 (0; 19.0) | p = 0.257 |
| Human IL-10 (pg/mL) | 0.7 (0.5; 1.2) | 0.7 (0.4; 1.1) | 0.7 (0.5; 1.2) | p = 0.931 |
| Human TNF (pg/mL) | 1.0 (0.7; 1.5) | 0.9 (0.7; 1.6) | 1.1 (0.8; 1.4) | p = 0.300 |
| Human IFN (pg/mL) | 0.3 (0.2; 0.5) | 2.4 (0; 13.16) | 0.4 (0.3; 0.5) | p = 0.677 |
| IL-17A (pg/mL) | 12.7 (8.9; 18.1) | 11.5 (8.8; 15.0) | 15.5 (8.9; 20.7) | p = 0.032 ** |
| IL-13 (pg/mL) | 0 (0; 0) | 0 (0; 0) | 0 (0; 0) | p = 0.055 |
| IL-15 (fentogr/mL) | 104.8 (80.3; 134.6) | 98.3 (80.3; 0132.7 | 126.6 (70.03; 168.7) | p = 0.608 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguado Ibáñez, S.; Sanchez, C.A.; Ussetti Gil, P. BALF Lymphocyte and Cytokine Profiling as Biomarkers of Acute Rejection After Lung Transplantation. J. Pers. Med. 2025, 15, 267. https://doi.org/10.3390/jpm15070267
Aguado Ibáñez S, Sanchez CA, Ussetti Gil P. BALF Lymphocyte and Cytokine Profiling as Biomarkers of Acute Rejection After Lung Transplantation. Journal of Personalized Medicine. 2025; 15(7):267. https://doi.org/10.3390/jpm15070267
Chicago/Turabian StyleAguado Ibáñez, Silvia, Carlos Almonacid Sanchez, and Piedad Ussetti Gil. 2025. "BALF Lymphocyte and Cytokine Profiling as Biomarkers of Acute Rejection After Lung Transplantation" Journal of Personalized Medicine 15, no. 7: 267. https://doi.org/10.3390/jpm15070267
APA StyleAguado Ibáñez, S., Sanchez, C. A., & Ussetti Gil, P. (2025). BALF Lymphocyte and Cytokine Profiling as Biomarkers of Acute Rejection After Lung Transplantation. Journal of Personalized Medicine, 15(7), 267. https://doi.org/10.3390/jpm15070267





