Digital Twin Models in Atrial Fibrillation: Charting the Future of Precision Therapy?
Abstract
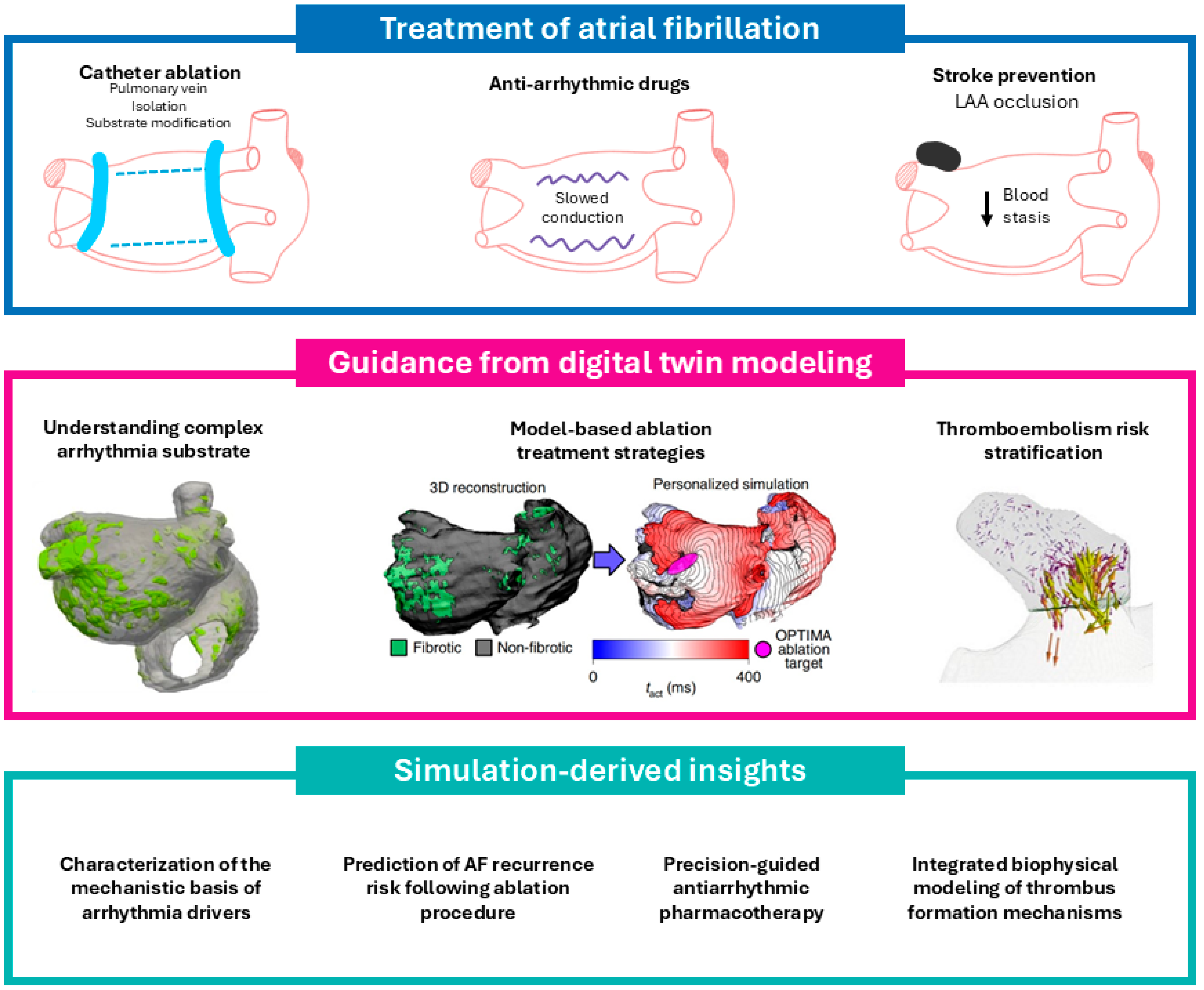
1. Introduction
2. Defining Digital Twins in Atrial Fibrillation: Principles and Potential
- •
- Mechanistically related models replicate general electrophysiological behavior through first-principle biophysical simulations, but lack direct calibration to patient-specific data;
- •
- Functionally similar digital twins are constrained by cohort-specific observations and aim to reflect interpatient variability across defined populations, often serving as the basis for in silico trials or population-level prediction;
- •
- Functionally equivalent digital twins represent the highest level of fidelity, being quantitatively calibrated to mirror the structure and function of a single patient’s atria, and thus capable of supporting individual clinical decision-making through predictive simulation.
- Anatomical twinning, wherein imaging data (e.g., LGE-MRI or contrast CT) are segmented and meshed to reconstruct the atrial geometry, including structural heterogeneities like fibrosis or scarring.
- Functional twinning, wherein model parameters such as conduction velocity, action potential duration, and ion channel kinetics are iteratively adjusted to replicate patient-specific electrical behavior, often using ECGs, electrograms, or pacing responses for calibration.
3. Digital Twin Models for Stroke Risk Prediction in Atrial Fibrillation
4. Digital Twin Models in Pharmacologic Therapy for Atrial Fibrillation: Mechanistic Evaluation and Personalized Prediction
4.1. Ion Channel Targeting and Mechanistic Screening in Virtual Atria
4.2. Genotype-Specific Drug Response: The Case of PITX2 Deficiency
4.3. Spatial Electrophysiologic Remodeling Under AADs
4.4. Clinical Translation: The Virtual Amiodarone Test
4.5. Toward a Digital Pharmacology Paradigm in AF
- •
- Drug screening: High-throughput testing of candidate compounds across heterogeneous substrates (e.g., fibrotic vs. non-fibrotic, atrial-selective vs. non-selective) using large in silico populations;
- •
- Genotype-informed therapy: Tailoring AAD selection based on the electrophysiological consequences of common AF risk alleles (e.g., PITX2, SCN5A);
- •
- Toxicity and proarrhythmia prediction: Classifying compounds by proarrhythmic risk profiles using virtual phenotyping (e.g., as shown by Sanchez de la Nava et al. via Ik1 weighting in random forest models);
- •
- Adaptive therapy planning: Iteratively updating drug models in real time using patient response data and integrating with ablation strategies for hybrid digital twin-guided management.
5. Digital Twin-Guided Approaches to AF Ablation: Mechanistic Insight and Clinical Translation
5.1. Mechanistic Foundations and Driver Mapping
5.2. Strategy Testing and Computational Ablation Selection
5.3. Real-Time Implementation and Restitution-Guided Targeting
5.4. Iterative Elimination and Personalized Substrate Neutralization
5.5. Artificial Intelligence and Simulation-Efficient Learning
5.6. Summary and Perspective
- Personalized lesion planning: Digital twins consistently outperform empirical strategies by tailoring lesion sets to each patient’s anatomy and substrate;
- Mechanism-targeted ablation: Models identify patient-specific RDs, rotors, or macro-reentrant circuits that may not be visible during clinical mapping;
- Functional phenotyping: Integration of restitution dynamics (e.g., Smax) enhances stratification and target validation;
- Dynamic simulation: Iterative non-inducibility protocols predict residual substrates and emergent arrhythmias, helping to avoid under-treatment or pro-arrhythmia;
- Translational feasibility: Several frameworks (CUVIA-AF, OPTIMA) demonstrate procedural integration without increasing duration or complication rates.
6. Limitations and Future Directions
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, S.; Zhou, J.; Veang, T.; Lin, Q.; Liu, Q. Global, Regional, and National Burden of Atrial Fibrillation and Atrial Flutter from 1990 to 2021: Sex Differences and Global Burden Projections to 2046—A Systematic Analysis of the Global Burden of Disease Study 2021. EP Europace 2025, 27, euaf027. [Google Scholar] [CrossRef] [PubMed]
- Kornej, J.; Börschel, C.S.; Benjamin, E.J.; Schnabel, R.B. Epidemiology of Atrial Fibrillation in the 21st Century: Novel Methods and New Insights. Circ. Res. 2020, 127, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Linz, D.; Gawalko, M.; Betz, K.; Hendriks, J.M.; Lip, G.Y.H.; Vinter, N.; Guo, Y.; Johnsen, S. Atrial Fibrillation: Epidemiology, Screening and Digital Health. Lancet Reg. Health Eur. 2024, 37, 100786. [Google Scholar] [CrossRef]
- Alonso, A.; Bengtson, L.G.S. A Rising Tide: The Global Epidemic of Atrial Fibrillation. Circulation 2014, 129, 829–830. [Google Scholar] [CrossRef]
- Linz, D.; Andrade, J.G.; Arbelo, E.; Boriani, G.; Breithardt, G.; Camm, A.J.; Caso, V.; Nielsen, J.C.; De Melis, M.; De Potter, T.; et al. Longer and Better Lives for Patients with Atrial Fibrillation: The 9th AFNET/EHRA Consensus Conference. EP Europace 2024, 26, euae070. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Pamporis, K.; Siontis, K.C.; Theofilis, P.; Samaras, A.; Patoulias, D.; Stachteas, P.; Karagiannidis, E.; Stavropoulos, G.; Tzikas, A.; et al. Major Clinical Outcomes in Symptomatic vs. Asymptomatic Atrial Fibrillation: A Meta-Analysis. Eur. Heart J. 2024, 46, 1189–1202. [Google Scholar] [CrossRef]
- Pamporis, K.; Karakasis, P.; Sagris, M.; Theofilis, P.; Milaras, N.; Pantelidaki, A.; Mourouzis, I.; Fragakis, N.; Vlachos, K.; Kordalis, A.; et al. Prevalence of Asymptomatic Atrial Fibrillation and Risk Factors Associated with Asymptomatic Status: A Systematic Review and Meta-Analysis. Eur. J. Prev. Cardiol. 2025. online ahead of print. [Google Scholar] [CrossRef]
- Karakasis, P.; Fragakis, N.; Patoulias, D.; Theofilis, P.; Kassimis, G.; Karamitsos, T.; El-Tanani, M.; Rizzo, M. Effects of Glucagon-Like Peptide 1 Receptor Agonists on Atrial Fibrillation Recurrence After Catheter Ablation: A Systematic Review and Meta-Analysis. Adv. Ther. 2024, 41, 3749–3756. [Google Scholar] [CrossRef]
- Goette, A.; Corradi, D.; Dobrev, D.; Aguinaga, L.; Cabrera, J.-A.; Chugh, S.S.; de Groot, J.R.; Soulat-Dufour, L.; Fenelon, G.; Hatem, S.N.; et al. Atrial Cardiomyopathy Revisited-Evolution of a Concept: A Clinical Consensus Statement of the European Heart Rhythm Association (EHRA) of the ESC, the Heart Rhythm Society (HRS), the Asian Pacific Heart Rhythm Society (APHRS), and the Latin American Hear. EP Europace 2024, 26, euae204. [Google Scholar] [CrossRef]
- Shen, M.J.; Arora, R.; Jalife, J. Atrial Myopathy. JACC Basic Transl. Sci. 2019, 4, 640–654. [Google Scholar] [CrossRef]
- Boriani, G.; Gerra, L.; Mantovani, M.; Tartaglia, E.; Mei, D.A.; Imberti, J.F.; Vitolo, M.; Bonini, N. Atrial Cardiomyopathy: An Entity of Emerging Interest in the Clinical Setting. Eur. J. Intern. Med. 2023, 118, 14–21. [Google Scholar] [CrossRef]
- Karakasis, P.; Patoulias, D.; Popovic, D.S.; Pamporis, K.; Theofilis, P.; Nasoufidou, A.; Stachteas, P.; Samaras, A.; Tzikas, A.; Giannakoulas, G.; et al. Effects of Mineralocorticoid Receptor Antagonists on New-Onset or Recurrent Atrial Fibrillation: A Bayesian and Frequentist Network Meta-Analysis of Randomized Trials. Curr. Probl. Cardiol. 2024, 49, 102742. [Google Scholar] [CrossRef] [PubMed]
- Wilber, D.J.; Pappone, C.; Neuzil, P.; De Paola, A.; Marchlinski, F.; Natale, A.; Macle, L.; Daoud, E.G.; Calkins, H.; Hall, B.; et al. Comparison of Antiarrhythmic Drug Therapy and Radiofrequency Catheter Ablation in Patients with Paroxysmal Atrial Fibrillation: A Randomized Controlled Trial. JAMA 2010, 303, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Razzack, A.A.; Lak, H.M.; Pothuru, S.; Rahman, S.; Hassan, S.A.; Hussain, N.; Najeeb, H.; Reddy, K.T.; Syeda, H.; Yasmin, F.; et al. Efficacy and Safety of Catheter Ablation vs Antiarrhythmic Drugs as Initial Therapy for Management of Symptomatic Paroxysmal Atrial Fibrillation: A Meta-Analysis. Rev. Cardiovasc. Med. 2022, 23, 112. [Google Scholar] [CrossRef]
- Sanchez-Somonte, P.; Kittichamroen, N.; Gao-Kang, J.; Azizi, Z.; Alipour, P.; Kahykin, Y.; Pantano, A.; Verma, A. Incremental Efficacy for Repeat Ablation Procedures for Catheter Ablation of Atrial Fibrillation: 5-Year Follow-Up. JACC Adv. 2024, 3, 101200. [Google Scholar] [CrossRef] [PubMed]
- Ngo, L.; Lee, X.W.; Elwashahy, M.; Arumugam, P.; Yang, I.A.; Denman, R.; Haqqani, H.; Ranasinghe, I. Freedom from Atrial Arrhythmia and Other Clinical Outcomes at 5 Years and beyond after Catheter Ablation of Atrial Fibrillation: A Systematic Review and Meta-Analysis. Eur. Heart J. Qual. Care Clin. Outcomes 2023, 9, 447–458. [Google Scholar] [CrossRef]
- Karakasis, P.; Tzeis, S.; Pamporis, K.; Schuermans, A.; Theofilis, P.; Milaras, N.; Tsiachris, D.; Efremidis, M.; Antoniadis, A.P.; Fragakis, N. Impact of Catheter Ablation Timing According to Duration of Atrial Fibrillation History on Arrhythmia Recurrences and Clinical Outcomes: A Meta-Analysis. EP Europace 2025. online ahead of print. [Google Scholar] [CrossRef]
- Sun, Y.; Ling, Y.; Chen, Z.; Wang, Z.; Li, T.; Tong, Q.; Qian, Y. Finding Low CHA2DS2-VASc Scores Unreliable? Why Not Give Morphological and Hemodynamic Methods a Try? Front. Cardiovasc. Med. 2022, 9, 1032736. [Google Scholar] [CrossRef]
- Østergaard, L.; Olesen, J.B.; Petersen, J.K.; Nielsen, L.S.; Kristensen, S.L.; Schou, M.; Køber, L.; Fosbøl, E. Arterial Thromboembolism in Patients With Atrial Fibrillation and CHA(2)DS(2)-VASc Score 1: A Nationwide Study. Circulation 2024, 149, 764–773. [Google Scholar] [CrossRef]
- Neefs, J.; Krul, S.P.J.; de Groot, J.R. A CHA2DS2-VASc Score of 1 Is Not the Same for Every Patient with Atrial Fibrillation. Eur. Heart J. 2025, 46, 99. [Google Scholar] [CrossRef]
- Tortora, M.; Pacchiano, F.; Ferraciolli, S.F.; Criscuolo, S.; Gagliardo, C.; Jaber, K.; Angelicchio, M.; Briganti, F.; Caranci, F.; Tortora, F.; et al. Medical Digital Twin: A Review on Technical Principles and Clinical Applications. J. Clin. Med. 2025, 14, 324. [Google Scholar] [CrossRef]
- Sel, K.; Osman, D.; Zare, F.; Masoumi Shahrbabak, S.; Brattain, L.; Hahn, J.-O.; Inan, O.T.; Mukkamala, R.; Palmer, J.; Paydarfar, D.; et al. Building Digital Twins for Cardiovascular Health: From Principles to Clinical Impact. J. Am. Heart Assoc. 2024, 13, e031981. [Google Scholar] [CrossRef]
- Waight, M.C.; Prakosa, A.; Li, A.C.; Bunce, N.; Marciniak, A.; Trayanova, N.A.; Saba, M.M. Personalized Heart Digital Twins Detect Substrate Abnormalities in Scar-Dependent Ventricular Tachycardia. Circulation 2025, 151, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Trayanova, N.A.; Prakosa, A. Up Digital and Personal: How Heart Digital Twins Can Transform Heart Patient Care. Heart Rhythm 2024, 21, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Heijman, J.; Sutanto, H.; Crijns, H.J.G.M.; Nattel, S.; Trayanova, N.A. Computational Models of Atrial Fibrillation: Achievements, Challenges, and Perspectives for Improving Clinical Care. Cardiovasc. Res. 2021, 117, 1682–1699. [Google Scholar] [CrossRef] [PubMed]
- Gillette, K.; Gsell, M.A.F.; Prassl, A.J.; Karabelas, E.; Reiter, U.; Reiter, G.; Grandits, T.; Payer, C.; Štern, D.; Urschler, M.; et al. A Framework for the Generation of Digital Twins of Cardiac Electrophysiology from Clinical 12-Leads ECGs. Med. Image Anal. 2021, 71, 102080. [Google Scholar] [CrossRef]
- Cluitmans, M.J.M.; Plank, G.; Heijman, J. Digital Twins for Cardiac Electrophysiology: State of the Art and Future Challenges. Herzschrittmacherther. Elektrophysiol. 2024, 35, 118–123. [Google Scholar] [CrossRef]
- Trayanova, N.A.; Lyon, A.; Shade, J.; Heijman, J. Computational Modeling of Cardiac Electrophysiology and Arrhythmogenesis: Toward Clinical Translation. Physiol. Rev. 2024, 104, 1265–1333. [Google Scholar] [CrossRef]
- Falanga, M.; Cortesi, C.; Chiaravalloti, A.; Monte, A.D.; Tomasi, C.; Corsi, C. A Digital Twin Approach for Stroke Risk Assessment in Atrial Fibrillation Patients. Heliyon 2024, 10, e39527. [Google Scholar] [CrossRef]
- Hwang, T.; Lim, B.; Kwon, O.-S.; Kim, M.-H.; Kim, D.; Park, J.-W.; Yu, H.T.; Kim, T.-H.; Uhm, J.-S.; Joung, B.; et al. Clinical Usefulness of Digital Twin Guided Virtual Amiodarone Test in Patients with Atrial Fibrillation Ablation. NPJ Digit. Med. 2024, 7, 297. [Google Scholar] [CrossRef]
- Sakata, K.; Bradley, R.P.; Prakosa, A.; Yamamoto, C.A.P.; Ali, S.Y.; Loeffler, S.; Tice, B.M.; Boyle, P.M.; Kholmovski, E.G.; Yadav, R.; et al. Assessing the Arrhythmogenic Propensity of Fibrotic Substrate Using Digital Twins to Inform a Mechanisms-Based Atrial Fibrillation Ablation Strategy. Nat. Cardiovasc. Res. 2024, 3, 857–868. [Google Scholar] [CrossRef]
- Kamel Boulos, M.N.; Zhang, P. Digital Twins: From Personalised Medicine to Precision Public Health. J. Pers. Med. 2021, 11, 745. [Google Scholar] [CrossRef]
- Armeni, P.; Polat, I.; De Rossi, L.M.; Diaferia, L.; Meregalli, S.; Gatti, A. Digital Twins in Healthcare: Is It the Beginning of a New Era of Evidence-Based Medicine? A Critical Review. J. Pers. Med. 2022, 12, 1255. [Google Scholar] [CrossRef]
- Johnson, K.W.; Shameer, K.; Glicksberg, B.S.; Readhead, B.; Sengupta, P.P.; Björkegren, J.L.M.; Kovacic, J.C.; Dudley, J.T. Enabling Precision Cardiology Through Multiscale Biology and Systems Medicine. JACC Basic Transl. Sci. 2017, 2, 311–327. [Google Scholar] [CrossRef]
- Meijer, C.; Uh, H.-W.; El Bouhaddani, S. Digital Twins in Healthcare: Methodological Challenges and Opportunities. J. Pers. Med. 2023, 13, 1522. [Google Scholar] [CrossRef] [PubMed]
- Sarani Rad, F.; Hendawi, R.; Yang, X.; Li, J. Personalized Diabetes Management with Digital Twins: A Patient-Centric Knowledge Graph Approach. J. Pers. Med. 2024, 14, 359. [Google Scholar] [CrossRef]
- Kolokotroni, E.; Abler, D.; Ghosh, A.; Tzamali, E.; Grogan, J.; Georgiadi, E.; Büchler, P.; Radhakrishnan, R.; Byrne, H.; Sakkalis, V.; et al. A Multidisciplinary Hyper-Modeling Scheme in Personalized In Silico Oncology: Coupling Cell Kinetics with Metabolism, Signaling Networks, and Biomechanics as Plug-In Component Models of a Cancer Digital Twin. J. Pers. Med. 2024, 14, 475. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, I.; Campagnola, L.; Consales, G. Electrical Impedance Tomography, Artificial Intelligence, and Variable Ventilation: Transforming Respiratory Monitoring and Treatment in Critical Care. J. Pers. Med. 2024, 14, 677. [Google Scholar] [CrossRef]
- Heist, E.K.; Belalcazar, A.; Stahl, W.; Brouwer, T.F.; Knops, R.E. Determinants of Subcutaneous Implantable Cardioverter-Defibrillator Efficacy: A Computer Modeling Study. JACC Clin. Electrophysiol. 2017, 3, 405–414. [Google Scholar] [CrossRef]
- Swenson, D.J.; Taepke, R.T.; Blauer, J.J.E.; Kwan, E.; Ghafoori, E.; Plank, G.; Vigmond, E.; MacLeod, R.S.; DeGroot, P.; Ranjan, R. Direct Comparison of a Novel Antitachycardia Pacing Algorithm against Present Methods Using Virtual Patient Modeling. Heart Rhythm 2020, 17, 1602–1608. [Google Scholar] [CrossRef]
- Bhagirath, P.; Strocchi, M.; Bishop, M.J.; Boyle, P.M.; Plank, G. From Bits to Bedside: Entering the Age of Digital Twins in Cardiac Electrophysiology. EP Europace 2024, 26, euae295. [Google Scholar] [CrossRef] [PubMed]
- Trayanova, N.A. Mathematical Approaches to Understanding and Imaging Atrial Fibrillation: Significance for Mechanisms and Management. Circ. Res. 2014, 114, 1516–1531. [Google Scholar] [CrossRef]
- Hewage, S.; Jadamba, A.; Brain, D.; Parsonage, W.; McPhail, S.; Kularatna, S. Global and Regional Burden of Ischemic Stroke Associated with Atrial Fibrillation, 2009-2019. Prev. Med. 2023, 173, 107584. [Google Scholar] [CrossRef]
- Friberg, L.; Rosenqvist, M.; Lindgren, A.; Terént, A.; Norrving, B.; Asplund, K. High Prevalence of Atrial Fibrillation among Patients with Ischemic Stroke. Stroke 2014, 45, 2599–2605. [Google Scholar] [CrossRef]
- Piccini, J.P.S.; Fonarow, G.C. Preventing Stroke in Patients With Atrial Fibrillation-A Steep Climb Away From Achieving Peak Performance. JAMA Cardiol. 2016, 1, 63–64. [Google Scholar] [CrossRef]
- Putaala, J.; Teppo, K.; Halminen, O.; Haukka, J.; Tiili, P.; Jaakkola, J.; Karlsson, E.; Linna, M.; Mustonen, P.; Kinnunen, J.; et al. Ischemic Stroke Temporally Associated With New-Onset Atrial Fibrillation: A Population-Based Registry-Linkage Study. Stroke 2024, 55, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Ma, C.; Li, Y.; Lin, J.; Qian, X. Development and Validation of a Nomogram Superior to CHADS(2) and CHA(2)DS(2)-VASc Models for Predicting Left Atrial Appendage Dense Spontaneous Echo Contrast/Left Atrial Appendage Thrombus. J. Thorac. Dis. 2024, 16, 2102–2114. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, N.; Park, H.-C.; Mao, Y.; Hong, S.J.; Lee, Y.; Spragg, D.D.; Calkins, H.; Trayanova, N.A. Slow Blood-Flow in the Left Atrial Appendage Is Associated with Stroke in Atrial Fibrillation Patients. Heliyon 2024, 10, e26858. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, S.; Kamel, H. Stratifying Stroke Risk in Atrial Fibrillation: Beyond Clinical Risk Scores. Stroke 2017, 48, 2665–2670, Erratum in Stroke, 2017, 48, e368. https://doi.org/10.1161/STR.0000000000000155. [Google Scholar] [CrossRef]
- Kumar, D.R.; Hanlin, E.; Glurich, I.; Mazza, J.J.; Yale, S.H. Virchow’s Contribution to the Understanding of Thrombosis and Cellular Biology. Clin. Med. Res. 2010, 8, 168–172. [Google Scholar] [CrossRef]
- Chiaravalloti, A.; Tomasi, C.; Corsi, C.; Falanga, M. A Digital Twin Approach for Stroke Risk Assessment in Atrial Fibrillation Patients. In Proceedings of the 2023 IEEE International Conference on Metrology for eXtended Reality, Artificial Intelligence and Neural Engineering MetroXRAINE 2023, Milan, Italy, 25–27 October 2023; pp. 17–21. [Google Scholar] [CrossRef]
- Wazni, O.M.; Saliba, W.I.; Nair, D.G.; Marijon, E.; Schmidt, B.; Hounshell, T.; Ebelt, H.; Skurk, C.; Oza, S.; Patel, C.; et al. Left Atrial Appendage Closure after Ablation for Atrial Fibrillation. N. Engl. J. Med. 2025, 392, 1277–1287. [Google Scholar] [CrossRef]
- Karakasis, P.; Vlachakis, P.K.; Theofilis, P.; Ktenopoulos, N.; Patoulias, D.; Fyntanidou, B.; Antoniadis, A.P.; Fragakis, N. Atrial Cardiomyopathy in Atrial Fibrillation: A Multimodal Diagnostic Framework. Diagnostics 2025, 15, 1207. [Google Scholar] [CrossRef]
- Karakasis, P.; Theofilis, P.; Vlachakis, P.K.; Ktenopoulos, N.; Patoulias, D.; Antoniadis, A.P.; Fragakis, N. Atrial Cardiomyopathy in Atrial Fibrillation: Mechanistic Pathways and Emerging Treatment Concepts. J. Clin. Med. 2025, 14, 3250. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef]
- Tzeis, S.; Gerstenfeld, E.P.; Kalman, J.; Saad, E.B.; Sepehri Shamloo, A.; Andrade, J.G.; Barbhaiya, C.R.; Baykaner, T.; Boveda, S.; Calkins, H.; et al. 2024 European Heart Rhythm Association/Heart Rhythm Society/Asia Pacific Heart Rhythm Society/Latin American Heart Rhythm Society Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation. EP Europace 2024, 26, euae043. [Google Scholar] [CrossRef]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1–e156. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Sohrabi, C.; Providencia, R.; Ahsan, S.; Papageorgiou, N. Catheter Ablation for the Management of Atrial Fibrillation: An Update of the Literature. Life 2023, 13, 1784. [Google Scholar] [CrossRef]
- Merino, J.L.; Tamargo, J.; Blomström-Lundqvist, C.; Boriani, G.; Crijns, H.J.G.M.; Dobrev, D.; Goette, A.; Hohnloser, S.H.; Naccarelli, G.V.; Reiffel, J.A.; et al. Practical Compendium of Antiarrhythmic Drugs: A Clinical Consensus Statement of the European Heart Rhythm Association of the ESC. EP Europace 2025. online ahead of print. [Google Scholar] [CrossRef]
- Heijman, J.; Hohnloser, S.H.; Camm, A.J. Antiarrhythmic Drugs for Atrial Fibrillation: Lessons from the Past and Opportunities for the Future. EP Europace 2021, 23, ii14–ii22. [Google Scholar] [CrossRef]
- Darbar, D.; Roden, D.M. Genetic Mechanisms of Atrial Fibrillation: Impact on Response to Treatment. Nat. Rev. Cardiol. 2013, 10, 317–329. [Google Scholar] [CrossRef]
- Liberos, A.; Bueno-Orovio, A.; Rodrigo, M.; Ravens, U.; Hernandez-Romero, I.; Fernandez-Aviles, F.; Guillem, M.S.; Rodriguez, B.; Climent, A.M. Balance between Sodium and Calcium Currents Underlying Chronic Atrial Fibrillation Termination: An in Silico Intersubject Variability Study. Heart Rhythm 2016, 13, 2358–2365. [Google Scholar] [CrossRef] [PubMed]
- Scholz, E.P.; Carrillo-Bustamante, P.; Fischer, F.; Wilhelms, M.; Zitron, E.; Dössel, O.; Katus, H.A.; Seemann, G. Rotor Termination Is Critically Dependent on Kinetic Properties of I Kur Inhibitors in an in Silico Model of Chronic Atrial Fibrillation. PLoS ONE 2013, 8, e83179. [Google Scholar] [CrossRef][Green Version]
- Schmidt, C.; Wiedmann, F.; Beyersdorf, C.; Zhao, Z.; El-Battrawy, I.; Lan, H.; Szabo, G.; Li, X.; Lang, S.; Korkmaz-Icöz, S.; et al. Genetic Ablation of TASK-1 (Tandem of P Domains in a Weak Inward Rectifying K(+) Channel-Related Acid-Sensitive K(+) Channel-1) (K(2P)3.1) K(+) Channels Suppresses Atrial Fibrillation and Prevents Electrical Remodeling. Circ. Arrhythm. Electrophysiol. 2019, 12, e007465. [Google Scholar] [CrossRef]
- Sánchez, C.; Bueno-Orovio, A.; Pueyo, E.; Rodríguez, B. Atrial Fibrillation Dynamics and Ionic Block Effects in Six Heterogeneous Human 3D Virtual Atria with Distinct Repolarization Dynamics. Front. Bioeng. Biotechnol. 2017, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Fogli Iseppe, A.; Giles, W.R.; Narayan, S.M.; Zhang, H.; Edwards, A.G.; Morotti, S.; Grandi, E. Populations of in Silico Myocytes and Tissues Reveal Synergy of Multiatrial-Predominant K(+)-Current Block in Atrial Fibrillation. Br. J. Pharmacol. 2020, 177, 4497–4515. [Google Scholar] [CrossRef]
- Hwang, I.; Jin, Z.; Park, J.-W.; Kwon, O.-S.; Lim, B.; Hong, M.; Kim, M.; Yu, H.-T.; Kim, T.-H.; Uhm, J.-S.; et al. Computational Modeling for Antiarrhythmic Drugs for Atrial Fibrillation According to Genotype. Front. Physiol. 2021, 12, 650449. [Google Scholar] [CrossRef]
- Jin, Z.; Hwang, I.; Lim, B.; Kwon, O.-S.; Park, J.-W.; Yu, H.-T.; Kim, T.-H.; Joung, B.; Lee, M.-H.; Pak, H.-N. Ablation and Antiarrhythmic Drug Effects on PITX2 (+/-) Deficient Atrial Fibrillation: A Computational Modeling Study. Front. Cardiovasc. Med. 2022, 9, 942998. [Google Scholar] [CrossRef]
- Hwang, I.; Park, J.-W.; Kwon, O.-S.; Lim, B.; Lee, J.; Jin, Z.; Yu, H.-T.; Kim, T.-H.; Joung, B.; Pak, H.-N. Spatial Changes in the Atrial Fibrillation Wave-Dynamics After Using Antiarrhythmic Drugs: A Computational Modeling Study. Front. Physiol. 2021, 12, 733543. [Google Scholar] [CrossRef]
- Hwang, T.; Kwon, O.; Lim, B.; Jin, Z.; Yang, S.; Kim, D.; Park, J.; Yu, H.; Kim, T.; Uhm, J.; et al. Clinical Application of Virtual Antiarrhythmic Drug Test Using Digital Twins in Patients Who Recurred Atrial Fibrillation after Catheter Ablation. EP Eur. 2023, 25. [Google Scholar] [CrossRef]
- McDowell, K.S.; Zahid, S.; Vadakkumpadan, F.; Blauer, J.; MacLeod, R.S.; Trayanova, N.A. Virtual Electrophysiological Study of Atrial Fibrillation in Fibrotic Remodeling. PLoS ONE 2015, 10, e0117110. [Google Scholar] [CrossRef]
- Deng, D.; Murphy, M.J.; Hakim, J.B.; Franceschi, W.H.; Zahid, S.; Pashakhanloo, F.; Trayanova, N.A.; Boyle, P.M. Sensitivity of Reentrant Driver Localization to Electrophysiological Parameter Variability in Image-Based Computational Models of Persistent Atrial Fibrillation Sustained by a Fibrotic Substrate. Chaos 2017, 27, 93932. [Google Scholar] [CrossRef] [PubMed]
- Corrado, C.; Williams, S.; Roney, C.; Plank, G.; O’Neill, M.; Niederer, S. Using Machine Learning to Identify Local Cellular Properties That Support Re-Entrant Activation in Patient-Specific Models of Atrial Fibrillation. EP Europace 2021, 23, i12–i20. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-S.; Lim, B.; Shim, J.; Hwang, M.; Yu, H.T.; Kim, T.-H.; Uhm, J.-S.; Kim, S.-H.; Joung, B.; On, Y.K.; et al. Clinical Usefulness of Computational Modeling-Guided Persistent Atrial Fibrillation Ablation: Updated Outcome of Multicenter Randomized Study. Front. Physiol. 2019, 10, 1512. [Google Scholar] [CrossRef]
- Roney, C.H.; Sim, I.; Yu, J.; Beach, M.; Mehta, A.; Alonso Solis-Lemus, J.; Kotadia, I.; Whitaker, J.; Corrado, C.; Razeghi, O.; et al. Predicting Atrial Fibrillation Recurrence by Combining Population Data and Virtual Cohorts of Patient-Specific Left Atrial Models. Circ. Arrhythm. Electrophysiol. 2022, 15, e010253. [Google Scholar] [CrossRef] [PubMed]
- Seno, H.; Yamazaki, M.; Shibata, N.; Sakuma, I.; Tomii, N. In-Silico Deep Reinforcement Learning for Effective Cardiac Ablation Strategy. J. Med. Biol. Eng. 2021, 41, 953–965. [Google Scholar] [CrossRef]
- Shim, J.; Hwang, M.; Song, J.-S.; Lim, B.; Kim, T.-H.; Joung, B.; Kim, S.-H.; Oh, Y.-S.; Nam, G.-B.; On, Y.K.; et al. Virtual In-Silico Modeling Guided Catheter Ablation Predicts Effective Linear Ablation Lesion Set for Longstanding Persistent Atrial Fibrillation: Multicenter Prospective Randomized Study. Front. Physiol. 2017, 8, 792. [Google Scholar] [CrossRef]
- Baek, Y.-S.; Kwon, O.-S.; Lim, B.; Yang, S.-Y.; Park, J.-W.; Yu, H.T.; Kim, T.-H.; Uhm, J.-S.; Joung, B.; Kim, D.-H.; et al. Clinical Outcomes of Computational Virtual Mapping-Guided Catheter Ablation in Patients With Persistent Atrial Fibrillation: A Multicenter Prospective Randomized Clinical Trial. Front. Cardiovasc. Med. 2021, 8, 772665. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-W.; Lim, B.; Hwang, I.; Kwon, O.-S.; Yu, H.T.; Kim, T.-H.; Uhm, J.-S.; Joung, B.; Lee, M.-H.; Pak, H.-N. Restitution Slope Affects the Outcome of Dominant Frequency Ablation in Persistent Atrial Fibrillation: CUVIA-AF2 Post-Hoc Analysis Based on Computational Modeling Study. Front. Cardiovasc. Med. 2022, 9, 838646. [Google Scholar] [CrossRef]
- Azzolin, L.; Eichenlaub, M.; Nagel, C.; Nairn, D.; Sanchez, J.; Unger, L.; Dössel, O.; Jadidi, A.; Loewe, A. Personalized Ablation vs. Conventional Ablation Strategies to Terminate Atrial Fibrillation and Prevent Recurrence. EP Europace 2023, 25, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.L.; Hakim, J.B.; Boyle, P.M.; Zahid, S.; Sivasambu, B.; Marine, J.E.; Calkins, H.; Trayanova, N.A.; Spragg, D.D. Arrhythmogenic Propensity of the Fibrotic Substrate after Atrial Fibrillation Ablation: A Longitudinal Study Using Magnetic Resonance Imaging-Based Atrial Models. Cardiovasc. Res. 2019, 115, 1757–1765. [Google Scholar] [CrossRef]
- Hakim, J.B.; Murphy, M.J.; Trayanova, N.A.; Boyle, P.M. Arrhythmia Dynamics in Computational Models of the Atria Following Virtual Ablation of Re-Entrant Drivers. EP Europace 2018, 20, iii45–iii54. [Google Scholar] [CrossRef]
- Boyle, P.M.; Zghaib, T.; Zahid, S.; Ali, R.L.; Deng, D.; Franceschi, W.H.; Hakim, J.B.; Murphy, M.J.; Prakosa, A.; Zimmerman, S.L.; et al. Computationally Guided Personalized Targeted Ablation of Persistent Atrial Fibrillation. Nat. Biomed. Eng. 2019, 3, 870–879. [Google Scholar] [CrossRef]
- Lim, B.; Park, J.-W.; Hwang, M.; Ryu, A.-J.; Kim, I.S.; Yu, H.T.; Joung, B.; Shim, E.B.; Pak, H.-N. Electrophysiological Significance of the Interatrial Conduction Including Cavo-Tricuspid Isthmus during Atrial Fibrillation. J. Physiol. 2020, 598, 3597–3612. [Google Scholar] [CrossRef]
- Boyle, P.M.; Hakim, J.B.; Zahid, S.; Franceschi, W.H.; Murphy, M.J.; Vigmond, E.J.; Dubois, R.; Haïssaguerre, M.; Hocini, M.; Jaïs, P.; et al. Comparing Reentrant Drivers Predicted by Image-Based Computational Modeling and Mapped by Electrocardiographic Imaging in Persistent Atrial Fibrillation. Front. Physiol. 2018, 9, 414. [Google Scholar] [CrossRef] [PubMed]
- Dasí, A.; Nagel, C.; Pope, M.T.B.; Wijesurendra, R.S.; Betts, T.R.; Sachetto, R.; Loewe, A.; Bueno-Orovio, A.; Rodriguez, B. In Silico TRials Guide Optimal Stratification of ATrIal FIbrillation Patients to Catheter Ablation and Pharmacological MedicaTION: The i-STRATIFICATION Study. EP Europace 2024, 26. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Theofilis, P.; Sagris, M.; Pamporis, K.; Stachteas, P.; Sidiropoulos, G.; Vlachakis, P.K.; Patoulias, D.; Antoniadis, A.P.; Fragakis, N. Artificial Intelligence in Atrial Fibrillation: From Early Detection to Precision Therapy. J. Clin. Med. 2025, 14, 2627. [Google Scholar] [CrossRef]
- Bai, J.; Lu, Y.; Wang, H.; Zhao, J. How Synergy between Mechanistic and Statistical Models Is Impacting Research in Atrial Fibrillation. Front. Physiol. 2022, 13, 957604. [Google Scholar] [CrossRef]
- Karakasis, P.; Theofilis, P.; Vlachakis, P.K.; Korantzopoulos, P.; Patoulias, D.; Antoniadis, A.P.; Fragakis, N. Atrial Fibrosis in Atrial Fibrillation: Mechanistic Insights, Diagnostic Challenges, and Emerging Therapeutic Targets. Int. J. Mol. Sci. 2024, 26, 209. [Google Scholar] [CrossRef]
- Niederer, S.; Mitchell, L.; Smith, N.; Plank, G. Simulating Human Cardiac Electrophysiology on Clinical Time-Scales. Front. Physiol. 2011, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Prassl, A.J.; Kickinger, F.; Ahammer, H.; Grau, V.; Schneider, J.E.; Hofer, E.; Vigmond, E.J.; Trayanova, N.A.; Plank, G. Automatically Generated, Anatomically Accurate Meshes for Cardiac Electrophysiology Problems. IEEE Trans. Biomed. Eng. 2009, 56, 1318–1330. [Google Scholar] [CrossRef] [PubMed]
- Nattel, S.; Sager, P.T.; Hüser, J.; Heijman, J.; Dobrev, D. Why Translation from Basic Discoveries to Clinical Applications Is so Difficult for Atrial Fibrillation and Possible Approaches to Improving It. Cardiovasc. Res. 2021, 117, 1616–1631. [Google Scholar] [CrossRef]
- Strocchi, M.; Augustin, C.M.; Gsell, M.A.F.; Karabelas, E.; Neic, A.; Gillette, K.; Razeghi, O.; Prassl, A.J.; Vigmond, E.J.; Behar, J.M.; et al. A Publicly Available Virtual Cohort of Four-Chamber Heart Meshes for Cardiac Electro-Mechanics Simulations. PLoS ONE 2020, 15, e0235145. [Google Scholar] [CrossRef]
- Crozier, A.; Augustin, C.M.; Neic, A.; Prassl, A.J.; Holler, M.; Fastl, T.E.; Hennemuth, A.; Bredies, K.; Kuehne, T.; Bishop, M.J.; et al. Image-Based Personalization of Cardiac Anatomy for Coupled Electromechanical Modeling. Ann. Biomed. Eng. 2016, 44, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Study Details|Comparing Pulmonary Vein Isolation to Pulmonary Vein Isolation + OPTIMA Ablation in Patients Undergoing Ablation for Atrial Fibrillation|ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT04101539 (accessed on 30 April 2025).
- Ortega-Martorell, S.; Olier, I.; Lip, G.Y.H. A European Network to Develop Virtual Twin Technology for Personalized Stroke Management in Atrial Fibrillation: The TARGET Consortium. Eur. Heart J. 2025, 46, 229–232. [Google Scholar] [CrossRef]
- Morrison, T.M.; Pathmanathan, P.; Adwan, M.; Margerrison, E. Advancing Regulatory Science With Computational Modeling for Medical Devices at the FDA’s Office of Science and Engineering Laboratories. Front. Med. 2018, 5, 241. [Google Scholar] [CrossRef]
| Author, Year | Study Type | Data Sources | Model Features | Primary Application | Key Findings |
|---|---|---|---|---|---|
| Liberos et al., 2016 [62] | In silico modeling study | AP recordings from 149 chronic AF patients | Population of 173 atrial tissue models with ionic remodeling variability | Mechanistic evaluation of ICaL, INa, and rotor dynamics | ICaL blockade terminated AF in 30% of models; efficacy modulated by INa availability; rotor destabilization promoted AF extinction |
| Scholz et al., 2013 [63] | In silico kinetic modeling study | Human CRN atrial model with AF remodeling | 2D tissue simulations with state- and voltage-dependent IKur blockade models | Mechanistic assessment of IKur inhibitors | Slow-recovery IKur blockers prolonged refractoriness and terminated rotors; kinetics critical for antiarrhythmic effects |
| Schmidt et al., 2019 [64] | Preclinical porcine study with in silico support | In vivo AF models, electrophysiology, simulations | Genetic suppression of TASK-1 (IK2P) via AAV9-siRNA; functional EP validation | Targeting TASK-1 for AF prevention | TASK-1 suppression prolonged APD, restored refractoriness, and reduced AF burden by 81.7% with no ventricular toxicity |
| Sánchez et al., 2017 [65] | In silico modeling study | 3D anatomical atrial models with AP population variability | Six heterogeneous models simulating parasympathetic AF remodeling | Evaluating ionic intervention strategies | IK1, INaK, and INa inhibition destabilized rotors and slowed fibrillation; early repolarization prolongation as anti-AF target |
| Ni et al., 2020 [66] | In silico population modeling study | Human atrial myocytes and tissue strands | Quantitative Systems Pharmacology framework testing IKur, IKCa, and IK2P blockade | Synergistic antiarrhythmic targeting | Combined K+ current block enhanced positive rate-dependent APD prolongation and reentry suppression |
| Hwang et al., 2024 [30] | Retrospective study with digital twin simulation | CT imaging and electroanatomical mapping | Patient-specific LA models simulating graded amiodarone dosing | Predicting clinical amiodarone efficacy | Virtual AF termination predicted 1-year recurrence (20.8% vs. 45.1%, HR 0.37); APD90 ↑ and dV/dt ↓ dose-dependently |
| Hwang et al., 2021 [67] | In silico modeling with PITX2 genotyping | CT imaging and electroanatomical mapping | LA models simulating PITX2+/– vs. WT genotypes and virtual AADs | Genotype-specific AAD response prediction | PITX2+/– models showed greater AF termination with class IC drugs (26% vs. 12%, p = 0.018); genotype modulated APD, DF, and PS dynamics |
| Hwang et al., 2021 [69] | In silico spatial analysis study | CT imaging and electroanatomical mapping (n = 25) | Patient-specific LA models simulating AAD effects spatially | Regional AF dynamics under drug therapy | AADs reduced DF especially in PV regions; higher DF heterogeneity associated with successful AF termination; DF inversely related to Smax |
| Jin et al., 2022 [68] | In silico modeling with clinical imaging and genotyping | CT imaging, bipolar electrograms, PITX2 genotyping | Patient-specific LA models integrating fibrosis and conduction maps; virtual CPVI and AADs | Evaluating genotype-specific efficacy of ablation vs. AADs | AADs more effective in PITX2+/– patients (defragmentation 49.3% vs. 34.7%, p = 0.014); V-CPVI efficacy was genotype-independent |
| Hwang et al., 2023 [70] | Retrospective single-center study with digital twin simulations | Cardiac CT and electroanatomical mapping (EAM) of 232 AF patients post AFCA | Patient-specific digital twins integrating anatomy, histology, and electrophysiology; virtual testing of 5 AADs (amiodarone, sotalol, dronedarone, flecainide, propafenone) at two doses each | Clinical reproducibility and utility of the virtual AAD test (V-AAD) | Patients treated with the most effective V-AAD had lower 1-year AF recurrence (40.9% vs. 54.1%, p = 0.046); recurrence trended lower with ≥2 effective drugs (42.4% vs. 59.3%, p = 0.056); supports feasibility of V-AAD for post-AFCA drug selection |
| Author, Year | Study Type | Data Sources | Model Features | Primary Application | Key Findings |
|---|---|---|---|---|---|
| Sakata et al., 2024 [31] | Prospective clinical study with digital twin modeling | LGE-MRI, electroanatomical mapping | Bi-atrial digital twins with fibrosis and rotor inducibility testing | Mechanism-based ablation targeting | Lesion-minimizing strategy reduced targets by 34% and mitigated AT risk |
| Roney et al., 2022 [75] | Retrospective modeling with machine learning | LGE-MRI, ECG follow-up | Patient-specific LA models with fibrosis, fiber orientation; stress tests; ML integration | AF recurrence prediction | Combined simulations and clinical data improved AF recurrence prediction (AUC 0.85) |
| Seno et al., 2021 [76] | In silico study with deep reinforcement learning | 2D cardiac tissue simulation | DAM trained on membrane potential movies to generate ablation patterns | Learning ablation strategies | DAM achieved 74.1% AF termination with minimal ablation compared to random or rotor-based strategies |
| Shim et al., 2017 [77] | Multicenter RCT with virtual modeling | CT + NavX mapping | Isotropic LA models tested with five lesion strategies | Strategy selection in PsAF | Simulation-guided ablation was feasible and not inferior; improved outcomes vs. empirical sets |
| Baek et al., 2021 [78] | Multicenter RCT with real-time modeling (CUVIA-AF2) | CT + electroanatomical mapping | LA models with fibrosis, fiber orientation, DF analysis | DF-guided ablation during procedure | V-DF ablation lowered recurrence (HR 0.51, p = 0.016); completed in standard procedure time |
| Kim et al., 2019 [74] | Multicenter RCT with simulation (CUVIA-AF1) | CT-based 3D LA models | Monolayer models tested with five lesion sets for optimal choice | Guided lesion selection | Model-based strategy reduced recurrence (HR 0.29, p = 0.005); more effective in less remodeled LA |
| Park et al., 2022 [79] | Post hoc modeling study (CUVIA-AF2) | CT + mapping data | LA models with APD restitution (Smax) and DF integration | Assessing Smax-dependent ablation efficacy | DF ablation effective mainly in low-Smax patients; RD locations inversely related to Smax |
| Azzolin et al., 2023 [80] | In silico study with clinical mapping | LGE-MRI, electroanatomical mapping | Bilayer models; iterative PEERP induction; 13 ablation strategies tested | PersonAL ablation optimization | Iterative HDF targeting terminated AF in all models using 5–6% of atrial tissue |
| Corrado et al., 2021 [73] | In silico study with ML classifier | EAM-based LA models | 10 LA models with fitted CV/APD; ML prediction of PS tethering | Identifying reentry sites | Slow CV and short APD predicted PS tethering with 91–95% accuracy |
| Ali et al., 2019 [81] | Retrospective pre-post LGE-MRI study | Pre- and post-ablation LGE-MRI | 3D LA models with fibrosis; RD tracking pre/post ablation | Understanding ablation failure | Emergent RDs overlapped fibrosis entropy zones; explained AF recurrence |
| McDowell et al., 2015 [71] | Proof-of-concept in silico study | LGE-MRI-based LA models | 3D models with fibrosis, myofibroblasts, and rotor dynamics | Predicting RD zones | RDs anchored at fibrosis border zones; targeting them rendered models non-inducible |
| Deng et al., 2017 [72] | In silico sensitivity analysis | LGE-MRI-derived LA models | EP variability tested (±10% APD/CV); RD anchoring analyzed | Assessing EP sensitivity | 20–65% of RDs changed locations under varied EP; underlined need for robust modeling |
| Hakim et al., 2018 [82] | In silico post-ablation RD dynamics study | LGE-MRI-derived models | EPavg models with virtual RD ablation and pacing | Characterizing emergent RDs | Emergent RDs appeared post ablation in 75%; iterative targeting recommended |
| Boyle et al., 2019 [83] | Prospective feasibility study (OPTIMA) | LGE-MRI + MRA | Bi-atrial finite element models; RD/macro-AT elimination; targets imported into CARTO | Personalized non-inducibility via pre-planned ablation | OPTIMA-guided ablation eliminated inducibility in all PsAF cases; strong clinical feasibility |
| Lim et al., 2020 [84] | In silico modeling with clinical validation | CT imaging and electroanatomical mapping | 3D biatrial models integrating realistic anatomy, voltage maps, fiber orientation, fibrosis, and interatrial connections (BB, posterior/anterior septum, CTI) | Testing the impact of sequential interatrial conduction ablation post-CPVI | Virtual CTI ablation improved AF termination (80% vs. 30%, p < 0.001); in clinical cohort (n = 846), CTI ablation reduced 2-year recurrence (HR 0.60, p < 0.001) |
| Boyle et al., 2018 [85] | Retrospective comparative modeling and clinical mapping study | LGE-MRI, ECGI, CT imaging, electrograms in 12 PsAF patients | 3D patient-specific bi-atrial models from LGE-MRI; fibrosis distribution; Courtemanche-based membrane kinetics; 30 pacing sites; RD tracking algorithms | Comparison of RD locations predicted by modeling vs. ECGI | RDsim were found in 28 regions vs. 42 for RDECGI; modest spatial agreement (κ = 0.11); 19 regions had both RD types (ECGI+/Sim+); ECGI-driven ablation had higher success when targets overlapped RDsim sites (57% vs. 41%); simulations revealed latent fibrosis-mediated RD sites missed by ECGI; combined simulation–ECGI strategy proposed to improve ablation outcomes |
| Dasí et al., 2024 [86] | Large-scale in silico trial | Human CT, MRI, EAM data for anatomical/structural calibration; ionic current and ECG calibration | 800 virtual AF patients with heterogeneity in atrial anatomy, ionic currents (40 profiles), and low-voltage areas (LVAs); personalized 3D bi-atrial simulations with >7000 treatments tested | Patient stratification for optimal second-line AF therapy post-PVI (ablation and AADs) | Stratification based on LVA presence, atrial size, and ERP guided selection of PWI, MiLine, CTI, Marshall-PLAN, and AADs. LVA ablation in both atria + CTI block yielded 100% efficacy in LVA+ patients; pharmacologic success varied by INa/ICaL density (e.g., amiodarone success 57%). Decision algorithm proposed for individualized therapy based on electrophysiological and structural metrics |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakasis, P.; Antoniadis, A.P.; Theofilis, P.; Vlachakis, P.K.; Milaras, N.; Patoulias, D.; Karamitsos, T.; Fragakis, N. Digital Twin Models in Atrial Fibrillation: Charting the Future of Precision Therapy? J. Pers. Med. 2025, 15, 256. https://doi.org/10.3390/jpm15060256
Karakasis P, Antoniadis AP, Theofilis P, Vlachakis PK, Milaras N, Patoulias D, Karamitsos T, Fragakis N. Digital Twin Models in Atrial Fibrillation: Charting the Future of Precision Therapy? Journal of Personalized Medicine. 2025; 15(6):256. https://doi.org/10.3390/jpm15060256
Chicago/Turabian StyleKarakasis, Paschalis, Antonios P. Antoniadis, Panagiotis Theofilis, Panayotis K. Vlachakis, Nikias Milaras, Dimitrios Patoulias, Theodoros Karamitsos, and Nikolaos Fragakis. 2025. "Digital Twin Models in Atrial Fibrillation: Charting the Future of Precision Therapy?" Journal of Personalized Medicine 15, no. 6: 256. https://doi.org/10.3390/jpm15060256
APA StyleKarakasis, P., Antoniadis, A. P., Theofilis, P., Vlachakis, P. K., Milaras, N., Patoulias, D., Karamitsos, T., & Fragakis, N. (2025). Digital Twin Models in Atrial Fibrillation: Charting the Future of Precision Therapy? Journal of Personalized Medicine, 15(6), 256. https://doi.org/10.3390/jpm15060256








