Phenotype-Driven Variability in Longitudinal Body Composition Changes After a Very Low-Calorie Ketogenic Intervention: A Machine Learning Cluster Approach
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Study Design
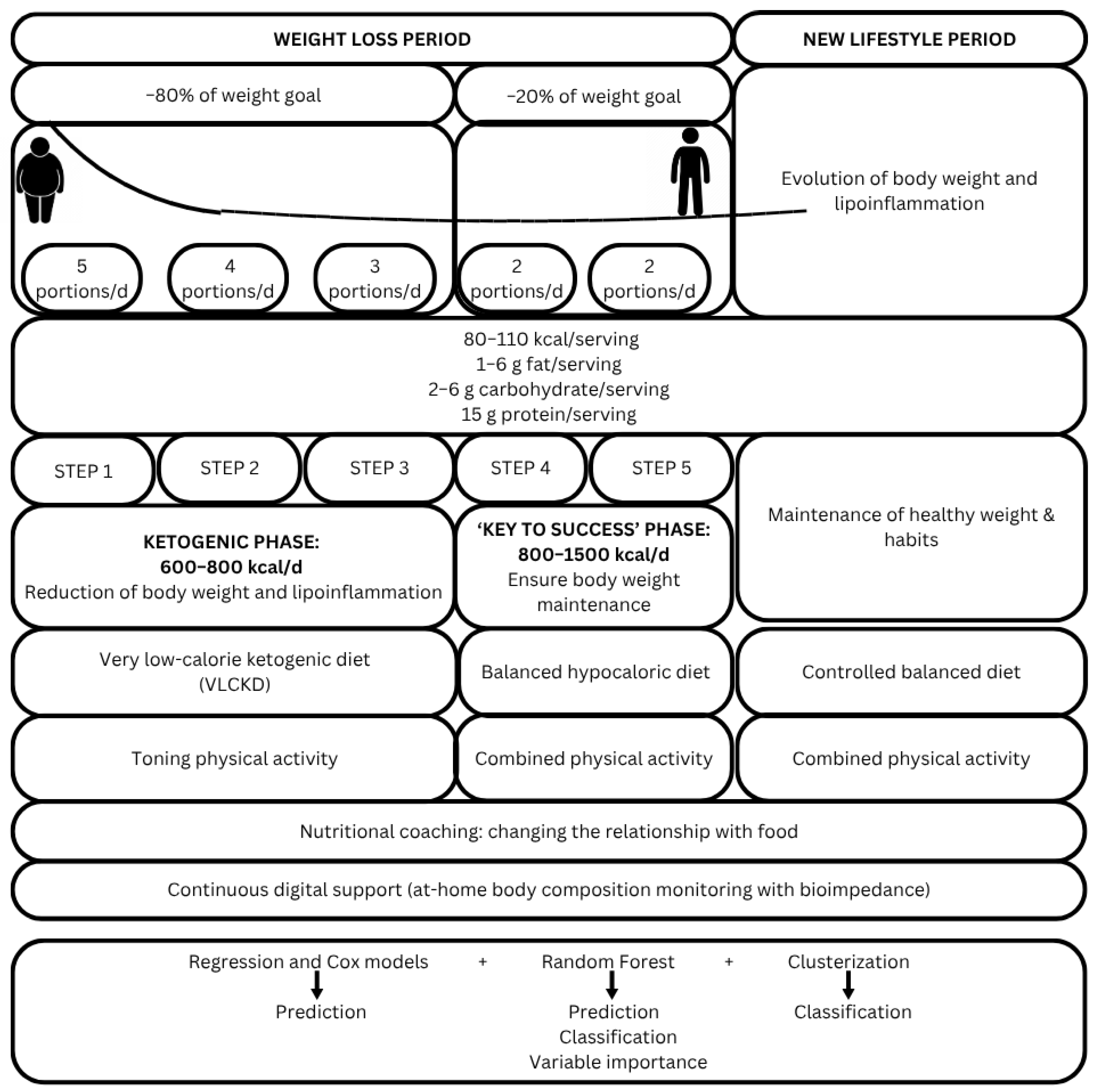
2.3. Multidisciplinary Nutritional Intervention
2.4. Foods Suitable for Ketosis
2.5. Nutritional Intervention Programming
2.6. Duration of Intervention and Visits Schedule
2.7. Lifestyle Assessments and Anthropometric Measurements
2.8. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Prediction Models: Multiple Regression Models
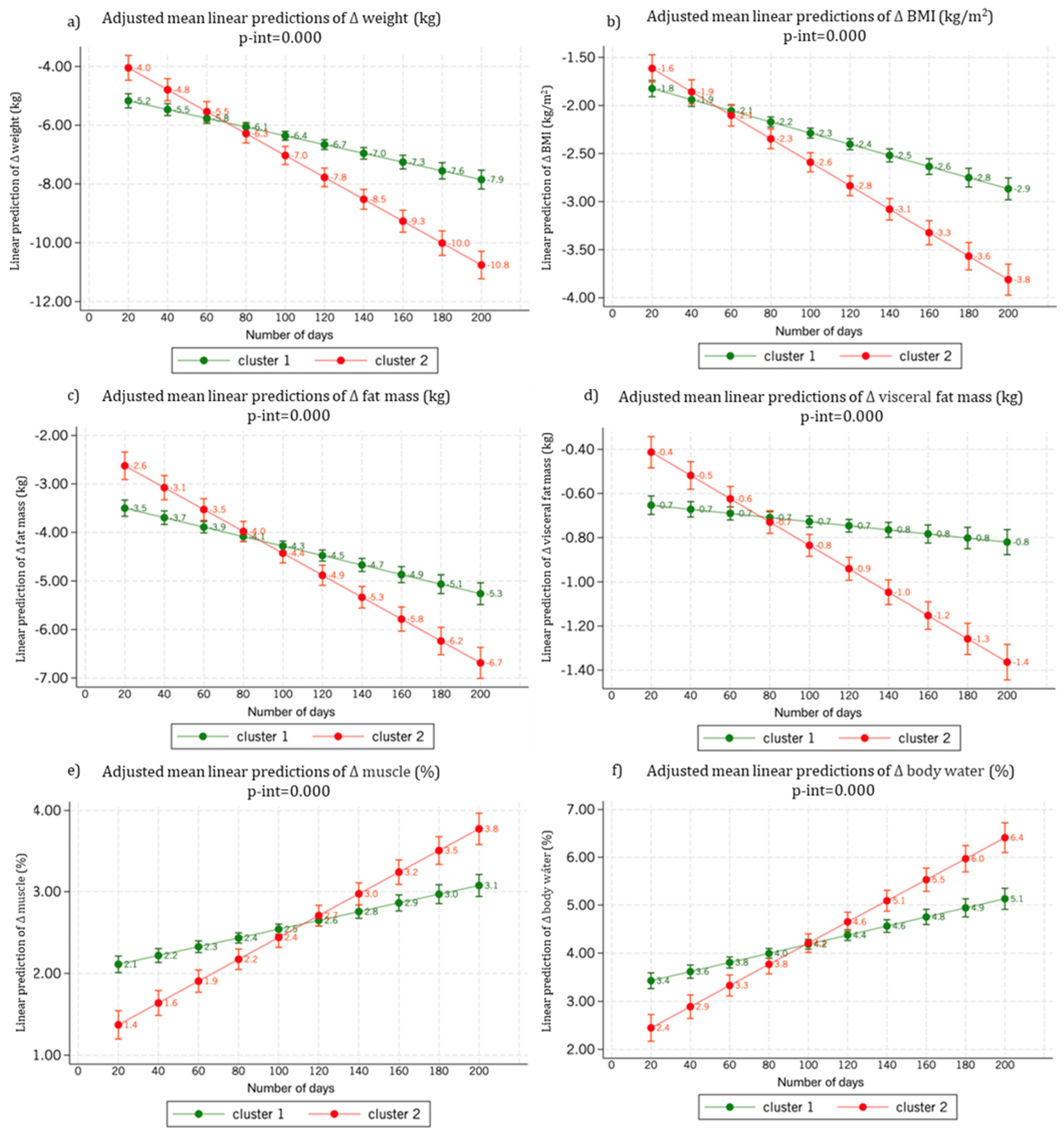
3.3. Body Weight and Body Composition Changes by Cluster
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koliaki, C.; Dalamaga, M.; Liatis, S. Update on the Obesity Epidemic: After the Sudden Rise, Is the Upward Trajectory Beginning to Flatten? Curr. Obes. Rep. 2023, 12, 514. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Hashimoto, Y.; Hamaguchi, M.; Obora, A.; Kojima, T.; Fukui, M. Ectopic fat obesity presents the greatest risk for incident type 2 diabetes: A population-based longitudinal study. Int. J. Obes. 2019, 43, 139–148. [Google Scholar] [CrossRef]
- Gilad, T.; Gal, Y.; Hagai, L.; Adi, L.; Nehama, G.; Estela, D.; Twig, G.; Yaniv, G.; Levine, H.; Leiba, A.; et al. Body-Mass Index in 2.3 Million Adolescents and Cardiovascular Death in Adulthood. N. Engl. J. Med. 2016, 374, 2430–2440. [Google Scholar] [CrossRef]
- De Pergola, G.; Silvestris, F. Obesity as a Major Risk Factor for Cancer. J. Obes. 2013, 1, 291546. [Google Scholar] [CrossRef]
- Bhaskaran, K.; dos-Santos-Silva, I.; Leon, D.A.; Douglas, I.J.; Smeeth, L. Association of BMI with overall and cause-specific mortality: A population-based cohort study of 3.6 million adults in the UK. Lancet Diabetes Endocrinol. 2018, 6, 944–953. [Google Scholar] [CrossRef]
- Kovač Blaz, M.; Švab, I. A Multidisciplinary Approach to Treating Obesity in a Community Health Centre. Slov. J. Public Health 2015, 54, 252. [Google Scholar] [CrossRef]
- Institute of Medicine (US), Subcommittee on Military Weight. Weight-Loss and Maintenance Strategies. In Weight Management: State of the Science and Opportunities for Military Programs; National Academies Press: Washington, DC, USA, 2004. [Google Scholar]
- Wadden, T.A.; Tronieri, J.S.; Butryn, M.L. Lifestyle Modification Approaches for the Treatment of Obesity in Adults. Am. Psychol. 2020, 75, 235. [Google Scholar] [CrossRef] [PubMed]
- Kheniser, K.; Saxon, D.R.; Kashyap, S.R. Long-Term Weight Loss Strategies for Obesity. J. Clin. Endocrinol. Metab. 2021, 106, 1854. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.O.; Wyatt, H.R.; Peters, J.C. The Importance of Energy Balance. Eur. Endocrinol. 2013, 9, 111. [Google Scholar] [CrossRef]
- Westerterp, K.R. Exercise, energy balance and body composition. Eur. J. Clin. Nutr. 2018, 72, 1246. [Google Scholar] [CrossRef]
- Ard, J.; Fitch, A.; Fruh, S.; Herman, L. Weight Loss and Maintenance Related to the Mechanism of Action of Glucagon-Like Peptide 1 Receptor Agonists. Adv. Ther. 2021, 38, 2821. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Bachiller, B.; López-Gómez, J.J.; García-Calvo, S.; Torres-Torres, B.; Primo-Martín, D.; Pinto-Fuentes, P.; Pacheco-Sánchez, D.; De Cegama, F.U.-L.; de Luis, D.A. Quality of Life and Psychological Changes in Bariatric Surgery: An Observational Study. Ann. Nutr. Metab. 2024, 81, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Gentil, P.; Neri, M.; Paoli, A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 2016, 14, 290. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Guo, J. Obesity Energetics: Body Weight Regulation and the Effects of Diet Composition. Gastroenterology 2017, 152, 1718–1727. [Google Scholar] [CrossRef]
- Thomas, D.M.; Bouchard, C.; Church, T.; Slentz, C.; Kraus, W.E.; Redman, L.M.; Martin, C.K.; Silva, A.M.; Vossen, M.; Westerterp, K.; et al. Why do individuals not lose more weight from an exercise intervention at a defined dose? An energy balance analysis. Obes. Rev. 2012, 13, 835. [Google Scholar] [CrossRef]
- Ross, R.; Chaput, J.P.; Giangregorio, L.M.; Janssen, I.; Saunders, T.J.; Kho, M.E.; Poitras, V.J.; Tomasone, J.R.; El-Kotob, R.; McLaughlin, E.C.; et al. Canadian 24-Hour Movement Guidelines for Adults aged 18–64 years and Adults aged 65 years or older: An integration of physical activity, sedentary behaviour, and sleep. Appl. Physiol. Nutr. Metab. 2020, 45 (Suppl. S2), S57–S102. [Google Scholar] [CrossRef]
- Freire, R. Scientific evidence of diets for weight loss: Different macronutrient composition, intermittent fasting, and popular diets. Nutrition 2020, 69, 110549. [Google Scholar] [CrossRef]
- Castellana, M.; Conte, E.; Cignarelli, A.; Perrini, S.; Giustina, A.; Giovanella, L.; Giorgino, F.; Trimboli, P. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2020, 21, 5–16. [Google Scholar] [CrossRef]
- de Luis, D.; Domingo, J.C.; Izaola, O.; Casanueva, F.F.; Bellido, D.; Sajoux, I. Effect of DHA supplementation in a very low-calorie ketogenic diet in the treatment of obesity: A randomized clinical trial. Endocrine 2016, 54, 111–122. [Google Scholar] [CrossRef]
- Martínez, J.A.; Dolores Parra, M. Life-threatening complications of the Atkins diet? Lancet 2006, 368, 23. [Google Scholar] [CrossRef]
- Gomez-Arbelaez, D.; Bellido, D.; Castro, A.I.; Ordonez-Mayan, L.; Carreira, J.; Galban, C.; Martinez-Olmos, M.A.; Crujeiras, A.B.; Sajoux, I.; Casanueva, F.F. Body Composition Changes After Very-Low-Calorie Ketogenic Diet in Obesity Evaluated by 3 Standardized Methods. J. Clin. Endocrinol. Metab. 2017, 102, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Pombo, A.; Lorenzo, P.M.; Carreira, M.C.; Gomez-Arbelaez, D.; Castro, A.I.; Primo, D.; Rodriguez, J.; Sajoux, I.; Baltar, J.; de Luis, D.; et al. A very-low-calorie ketogenic diet normalises obesity-related enhanced levels of erythropoietin compared with a low-calorie diet or bariatric surgery. J. Endocrinol. Invest 2024, 47, 2701–2713. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.I.; Gomez-Arbelaez, D.; Crujeiras, A.B.; Granero, R.; Aguera, Z.; Jimenez-Murcia, S.; Sajoux, I.; Lopez-Jaramillo, P.; Fernandez-Aranda, F.; Casanueva, F.F. Effect of A Very Low-Calorie Ketogenic Diet on Food and Alcohol Cravings, Physical and Sexual Activity, Sleep Disturbances, and Quality of Life in Obese Patients. Nutrients 2018, 10, 1348. [Google Scholar] [CrossRef]
- Pronokal Ketogenic Diet Method Meal Products and Supplements [Internet]. Available online: https://www.pronokal.co.uk/meals-and-supplements (accessed on 12 February 2025).
- García-Perea, A.; Fernández-Cruz, E.; de la O-Pascual, V.; Gonzalez-Zorzano, E.; Moreno-Aliaga, M.J.; Tur, J.A.; Martinez, J.A. Nutritional and Lifestyle Features in a Mediterranean Cohort: An Epidemiological Instrument for Categorizing Metabotypes Based on a Computational Algorithm. Medicina 2024, 60, 610. [Google Scholar] [CrossRef]
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef]
- Cifuentes, L.; Hurtado, A.M.D.; Eckel-Passow, J.; Acosta, A. Precision Medicine for Obesity. Dig. Dis. Interv. 2021, 5, 239–248. [Google Scholar] [CrossRef]
- Magkos, F.; Fraterrigo, G.; Yoshino, J.; Luecking, C.; Kirbach, K.; Kelly, S.C.; De Las Fuentes, L.; He, S.; Okunade, A.L.; Patterson, B.W.; et al. Effects of Moderate and Subsequent Progressive Weight Loss on Metabolic Function and Adipose Tissue Biology in Humans with Obesity. Cell Metab. 2016, 23, 591–601. [Google Scholar] [CrossRef]
- Warkentin, L.M.; Majumdar, S.R.; Johnson, J.A.; Agborsangaya, C.B.; Rueda-Clausen, C.F.; Sharma, A.M.; Klarenbach, S.W.; Karmali, S.; Birch, D.W.; Padwal, R.S. Weight loss required by the severely obese to achieve clinically important differences in health-related quality of life: Two-year prospective cohort study. BMC Med. 2014, 12, 175. [Google Scholar] [CrossRef] [PubMed]
- Tahrani, A.A.; Morton, J. Benefits of weight loss of 10% or more in patients with overweight or obesity: A review. Obesity 2022, 30, 802–840. [Google Scholar] [CrossRef]
- Obesity [Internet]. Available online: https://www.who.int/health-topics/obesity#tab=tab_1 (accessed on 15 February 2025).
- Fruh, S.M. Obesity: Risk factors, complications, and strategies for sustainable long-term weight management. J. Am. Assoc. Nurse Pract. 2017, 29 (Suppl. S1), S3–S14. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease A Scientific Statement From the American Heart Association. Circulation 2021, 143, E984–E1010. [Google Scholar] [CrossRef] [PubMed]
- Yashi, K.; Daley, S.F. Obesity and Type 2 Diabetes. In Handbook of Obesity–Volume 1: Epidemiology, Etiology, and Physiopathology, 4th ed.; StatPearls Publishing: Treasure Island, FL, USA, 2023; pp. 496–502. [Google Scholar]
- Shariq, O.A.; Mckenzie, T.J. Obesity-related hypertension: A review of pathophysiology, management, and the role of metabolic surgery. Gland. Surg. 2020, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Jehan, S.; Zizi, F.; Pandi-Perumal, S.R.; Wall, S.; Auguste, E.; Myers, A.K.; Jean-Louis, G.; McFarlane, S.I. Obstructive Sleep Apnea and Obesity: Implications for Public Health. Sleep. Med. Disord. 2017, 1, 00019. [Google Scholar]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K. Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management. Cancers 2023, 15, 485. [Google Scholar] [CrossRef]
- Rajan, T.M.; Menon, V. Psychiatric disorders and obesity: A review of association studies. J. Postgrad. Med. 2017, 63, 182. [Google Scholar] [CrossRef]
- Tiwari, A.; Balasundaram, P. Public Health Considerations Regarding Obesity. StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Abete, I.; Astrup, A.; Martínez, J.A.; Thorsdottir, I.; Zulet, M.A. Obesity and the metabolic syndrome: Role of different dietary macronutrient distribution patterns and specific nutritional components on weight loss and maintenance. Nutr. Rev. 2010, 68, 214–231. [Google Scholar] [CrossRef]
- Garvey, W.T.; Mechanick, J.I.; Brett, E.M.; Garber, A.J.; Hurley, D.L.; Jastreboff, A.M.; Nadolsky, K.; Pessah-Pollack, R.; Plodkowski, R.; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for Medical Care of Patients with Obesity. Endocr. Pract. 2016, 22 (Suppl. S3), 1–203. [Google Scholar] [CrossRef] [PubMed]
- Moreno, B.; Crujeiras, A.B.; Bellido, D.; Sajoux, I.; Casanueva, F.F. Obesity treatment by very low-calorie-ketogenic diet at two years: Reduction in visceral fat and on the burden of disease. Endocrine 2016, 54, 681–690. [Google Scholar] [CrossRef]
- Ashtary-Larky, D.; Bagheri, R.; Bavi, H.; Baker, J.S.; Moro, T.; Mancin, L.; Paoli, A. Ketogenic diets, physical activity and body composition: A review. Br. J. Nutr. 2022, 127, 1898. [Google Scholar] [CrossRef]
- Petersen, M.; Taylor, M.A.; Saris, W.H.M.; Verdich, C.; Toubro, S.; MacDonald, I.; Rössner, S.; Stich, V.; Guy-Grand, B.; Langin, D.; et al. Randomized, multi-center trial of two hypo-energetic diets in obese subjects: High- versus low-fat content. Int. J. Obes. 2006, 30, 552–560. [Google Scholar] [CrossRef][Green Version]
- Abete, I.; Parra, D.; Martinez, J.A. Energy-restricted diets based on a distinct food selection affecting the glycemic index induce different weight loss and oxidative response. Clin. Nutr. 2008, 27, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Labayen, I.; Diez, N.; Parra, M.D.; Gónzalez, A.; Martinez, J.A. Time-course changes in macronutrient metabolism induced by a nutritionally balanced low-calorie diet in obese women. Int. J. Food Sci. Nutr. 2004, 55, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Cenci, L.; Grimaldi, K.A. Effect of ketogenic mediterranean diet with phytoextracts and low carbohydrates/high-protein meals on weight, cardiovascular risk factors, body composition and diet compliance in Italian council employees. Nutr. J. 2011, 10, 112. [Google Scholar] [CrossRef]
- Di Rosa, C.; Lattanzi, G.; Spiezia, C.; Imperia, E.; Piccirilli, S.; Beato, I.; Gaspa, G.; Micheli, V.; De Joannon, F.; Vallecorsa, N.; et al. Mediterranean Diet versus Very Low-Calorie Ketogenic Diet: Effects of Reaching 5% Body Weight Loss on Body Composition in Subjects with Overweight and with Obesity—A Cohort Study. Int. J. Environ. Res. Public. Health 2022, 19, 13040. [Google Scholar] [CrossRef] [PubMed]
- Larsen, L.H.; Ängquist, L.; Vimaleswaran, K.S.; Hager, J.; Viguerie, N.; Loos, R.J.F.; Handjieva-Darlenska, T.; Jebb, S.A.; Kunešova, M.; Larsen, T.M.; et al. Analyses of single nucleotide polymorphisms in selected nutrient-sensitive genes in weight-regain prevention: The DIOGENES study. Am. J. Clin. Nutr. 2012, 95, 1254–1260. [Google Scholar] [CrossRef]
- de la Iglesia, R.; Lopez-Legarrea, P.; Celada, P.; Sánchez-Muniz, F.J.; Martinez, J.A.; Zulet, M.A. Beneficial Effects of the RESMENA Dietary Pattern on Oxidative Stress in Patients Suffering from Metabolic Syndrome with Hyperglycemia Are Associated to Dietary TAC and Fruit Consumption. Int. J. Mol. Sci. 2013, 14, 6903. [Google Scholar] [CrossRef]
- Thorsdottir, I.; Tomasson, H.; Gunnarsdottir, I.; Gisladottir, E.; Kiely, M.; Parra, M.D.; Bandarra, N.M.; Schaafsma, G.; A Martinéz, J.A. Randomized trial of weight-loss-diets for young adults varying in fish and fish oil content. Int. J. Obes. 2007, 31, 1560–1566. [Google Scholar] [CrossRef]
- Abete, I.; Parra, D.; Martinez, J.A. Legume-, fish-, or high-protein-based hypocaloric diets: Effects on weight loss and mitochondrial oxidation in obese men. J. Med. Food 2009, 12, 100–108. [Google Scholar] [CrossRef]
- Schübel, R.; Nattenmüller, J.; Sookthai, D.; Nonnenmacher, T.; Graf, M.E.; Riedl, L.; Schlett, C.L.; von Stackelberg, O.; Johnson, T.; Nabers, D.; et al. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 933–945. [Google Scholar] [CrossRef]
- Hoddy, K.K.; Kroeger, C.M.; Trepanowski, J.F.; Barnosky, A.; Bhutani, S.; Varady, K.A. Meal timing during alternate day fasting: Impact on body weight and cardiovascular disease risk in obese adults. Obesity 2014, 22, 2524–2531. [Google Scholar] [CrossRef]
- Chow, L.S.; Manoogian, E.N.C.; Alvear, A.; Fleischer, J.G.; Thor, H.; Dietsche, K.; Wang, Q.; Hodges, J.S.; Esch, N.; Malaeb, S.; et al. Time Restricted Eating Effects On Body Composition and Metabolic Measures in Humans who are Overweight: A Feasibility Study. Obesity 2020, 28, 860. [Google Scholar] [CrossRef]
- Alves, R.D.M.; De Oliveira, F.C.E.; Hermsdorff, H.H.M.; Abete, I.; Zulet, M.Á.; Martínez, J.A.; Bressan, J. Eating carbohydrate mostly at lunch and protein mostly at dinner within a covert hypocaloric diet influences morning glucose homeostasis in overweight/obese men. Eur. J. Nutr. 2014, 53, 49–60. [Google Scholar] [CrossRef]
- Han, K.; Kim, M.K. Factors Affecting High Body Weight Variability. J. Obes. Metab. Syndr. 2023, 32, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Susanto, A.; Burk, J.; Hocking, S.; Markovic, T.; Gill, T. Differences in weight loss outcomes for males and females on a low-carbohydrate diet: A systematic review. Obes. Res. Clin. Pract. 2022, 16, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, J.; Woźniak, K.; Wojciechowska, O.; Wrzosek, M.; Włodarek, D. Effect of Age and Gender on the Efficacy of a 12-Month Body Weight Reduction Program Conducted Online—A Prospective Cohort Study. Int. J. Environ. Res. Public. Health 2022, 19, 12009. [Google Scholar] [CrossRef] [PubMed]
- Nackers, L.M.; Ross, K.M.; Perri, M.G. The Association Between Rate of Initial Weight Loss and Long-Term Success in Obesity Treatment: Does Slow and Steady Win the Race? Int. J. Behav. Med. 2010, 17, 161. [Google Scholar] [CrossRef]
- Dorling, J.L.; Höchsmann, C.; Fearnbach, S.N.; Apolzan, J.W.; Hsia, D.S.; Johannsen, N.M.; Church, T.S.; Martin, C.K. Initial Weight Change and Long-Term Changes in Weight and Compensation during Supervised Exercise Training. Med. Sci. Sports Exerc. 2021, 53, 1675. [Google Scholar] [CrossRef]
- Kompaniyets, L.; Freedman, D.S.; Belay, B.; Pierce, S.L.; Kraus, E.M.; Blanck, H.M.; Goodman, A.B. Probability of 5% or Greater Weight Loss or BMI Reduction to Healthy Weight Among Adults with Overweight or Obesity. JAMA Netw. Open 2023, 6, e2327358. [Google Scholar] [CrossRef]
- Jensen, M.D. Role of Body Fat Distribution and the Metabolic Complications of Obesity. J. Clin. Endocrinol. Metab. 2008, 93 (Suppl. S1), S57. [Google Scholar] [CrossRef]
- Frank, A.P.; De Souza Santos, R.; Palmer, B.F.; Clegg, D.J. Determinants of body fat distribution in humans may provide insight about obesity-related health risks. J. Lipid Res. 2019, 60, 1710. [Google Scholar] [CrossRef]
- Gkouskou, K.K.; Grammatikopoulou, M.G.; Lazou, E.; Vasilogiannakopoulou, T.; Sanoudou, D.; Eliopoulos, A.G. A genomics perspective of personalized prevention and management of obesity. Human. Genomics 2024, 18, 4. [Google Scholar] [CrossRef]
- Dent, R.; McPherson, R.; Harper, M.E. Factors affecting weight loss variability in obesity. Metabolism 2020, 113, 154388. [Google Scholar] [CrossRef]
- Lamiquiz-Moneo, I.; Mateo-Gallego, R.; Bea, A.M.; Dehesa-García, B.; Pérez-Calahorra, S.; Marco-Benedí, V.; Baila-Rueda, L.; Laclaustra, M.; Civeira, F.; Cenarro, A. Genetic predictors of weight loss in overweight and obese subjects. Sci. Rep. 2019, 9, 10770. [Google Scholar] [CrossRef] [PubMed]
- Varkevisser, R.D.M.; van Stralen, M.M.; Kroeze, W.; Ket, J.C.F.; Steenhuis, I.H.M. Determinants of weight loss maintenance: A systematic review. Obes. Rev. 2019, 20, 171. [Google Scholar] [CrossRef]
- Ciobârcă, D.; Cătoi, A.F.; Copăescu, C.; Miere, D.; Crișan, G. Bariatric Surgery in Obesity: Effects on Gut Microbiota and Micronutrient Status. Nutrients 2020, 12, 235. [Google Scholar] [CrossRef] [PubMed]
- Perez-Guisado, J. Las dietas cetogénicas: Beneficios adicionales a la pérdida de peso y efectos secundarios infundados. Arch. Latinoam. Nutr. 2008, 58, 323–329. [Google Scholar] [PubMed]
- Yancy, W.S.; Olsen, M.K.; Guyton, J.R.; Bakst, R.P.; Westman, E.C. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: A randomized, controlled trial. Ann. Intern. Med. 2004, 140, 769–777. [Google Scholar] [CrossRef]
- Astrup, P.A.; Meinert Larsen, D.T.; Harper, A. Atkins and other low-carbohydrate diets: Hoax or an effective tool for weight loss? Lancet 2004, 364, 897–899. [Google Scholar] [CrossRef]
- Basciani, S.; Camajani, E.; Contini, S.; Persichetti, A.; Mariani, S.; Lubrano, C.; Watanabe, M.; Faro, M.; Di Bernardo, S.; Vari, M.; et al. Very Low-Calorie Ketogenic Diet Modifies Visceral Adipose Tissue Distribution and Taxonomic Composition of Gut Microbiota in Obese Patients with Insulin Resistance Depending on Protein Source. J. Endocr. Soc. 2020, 4 (Suppl. S1). [Google Scholar] [CrossRef]
- Burén, J.; Svensson, M.; Liv, P.; Sjödin, A. Effects of a Ketogenic Diet on Body Composition in Healthy, Young, Normal-Weight Women: A Randomized Controlled Feeding Trial. Nutrients 2024, 16, 2030. [Google Scholar] [CrossRef]
- Chung, N. Impact of the ketogenic diet on body fat, muscle mass, and exercise performance: A review. Phys. Act. Nutr. 2023, 27, 1. [Google Scholar] [CrossRef] [PubMed]
- Roekenes, J.; Martins, C. Ketogenic diets and appetite regulation. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Son, J.W.; Han, B.D.; Bennett, J.P.; Heymsfield, S.; Lim, S. Development and clinical application of bioelectrical impedance analysis method for body composition assessment. Obes. Rev. 2025, 26, e13844. [Google Scholar] [CrossRef] [PubMed]
- da Costa, R.F.; Nogueira, R.C.; Fisberg, M.; Ferrari, G. Editorial: Body composition assessment techniques in clinical and epidemiological settings: Development, validation and use in dietary programs, physical training and sports. Front. Nutr. 2023, 10, 1146553. [Google Scholar] [CrossRef]
- Hall, K.D.; Sacks, G.; Chandramohan, D.; Chow, C.C.; Wang, Y.C.; Gortmaker, S.L.; Swinburn, B.A. Quantification of the effect of energy imbalance on bodyweight. Lancet 2011, 378, 826–837. [Google Scholar] [CrossRef]
- Thomas, D.M.; Scioletti, M.; Heymsfield, S.B. Predictive Mathematical Models of Weight Loss. Curr. Diabetes Reports 2019, 19, 93. [Google Scholar] [CrossRef]
- Gutiérrez-Repiso, C.; Molina-Vega, M.; Bernal-López, M.R.; Garrido-Sánchez, L.; García-Almeida, J.M.; Sajoux, I.; Moreno-Indias, I.; Tinahones, F.J. Different Weight Loss Intervention Approaches Reveal a Lack of a Common Pattern of Gut Microbiota Changes. J. Pers. Med. 2021, 11, 109. [Google Scholar] [CrossRef]
- Sofia, M.; Agosta, M.; D’Amato, S.; Conti, G.N.; Mazzone, C.; Faletra, G.; La Greca, G.; Latteri, S. Postoperative Biochemical Outcomes in Metabolic Bariatric Surgery: Results from a High-Adherence Cohort. J. Pers. Med. 2024, 15, 7. [Google Scholar] [CrossRef]

| Sex | Age | Baseline BMI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Men | Women | p-Value | <47 y.o. | ≥47 y.o. | p-Value | <30 kg/m2 | ≥30 kg/m2 | p-Value | |
| n | 1536 | 5700 | 3604 | 3632 | 3768 | 3468 | |||
| Age (years) | 47.2 (10.6) | 46.9 (10.4) | 0.297 | 38.7 (6.7) | 55.1 (6.3) | <0.001 | 46.8 (10.4) | 47.1 (10.5) | 0.297 |
| Women (%) | - | - | - | 79.4 | 78.2 | 0.205 | 85.7 | 72.4 | <0.001 |
| Total days of follow-up (days) | 99.6 (95.9) | 115.4 (112.3) | <0.001 | 100.4 (98.7) | 123.6 (117.6) | <0.001 | 95.2 (101.2) | 127.6 (113.9) | <0.001 |
| Number of visits (n) | 4.9 (4.0) | 5.7 (4.6) | <0.001 | 5.1 (4.1) | 6.0 (4.9) | <0.001 | 4.8 (4.0) | 6.2 (4.9) | <0.001 |
| Doses per day (n) | 4.95 (0.8) | 4.82 (0.7) | <0.001 | 4.9 (0.7) | 4.8 (0.8) | <0.001 | 4.8 (0.8) | 4.9 (0.7) | <0.001 |
| Total doses (n) | 86.4 (27.6) | 83.3 (24.3) | <0.001 | 84.6 (25.3) | 83.4 (24.9) | 0.036 | 82.2 (23.0) | 85.6 (26.7) | <0.001 |
| Accumulated expense (€) | 1859.2 (1489.5) | 2047.6 (1710.6) | <0.001 | 1840.4 (1486.5) | 2173.6 (1815.2) | <0.001 | 1669.7 (1387.5) | 2318.7 (1835.4) | 0.002 |
| Baseline weight (kg) | 98.2 (14.6) | 80.0 (9.8) | <0.001 | 84.5 (13.6) | 83.1 (12.9) | <0.001 | 80.0 (9.8) | 98.2 (14.6) | <0.001 |
| Δ weight (kg) | −9.2 (7.4) | −5.9 (5.5) | <0.001 | −6.7 (6.3) | −6.5 (6.0) | 0.372 | −4.0 (3.6) | −8.9 (7.0) | <0.001 |
| Baseline BMI (kg/m2) | 32.3 (4.4) | 30.0 (3.2) | <0.001 | 30.4 (3.8) | 30.5 (3.5) | 0.438 | 27.6 (1.4) | 33.1 (3.0) | <0.001 |
| Δ BMI (kg/m2) | −3.0 (2.5) | −2.2 (2.2) | <0.001 | −2.4 (2.4) | −2.4 (2.2) | 0.786 | −1.5 (1.3) | −3.3 (2.7) | <0.001 |
| Baseline fat mass (% of BW) | 36.7 (7.4) | 41.5 (5.2) | <0.001 | 40.3 (6.1) | 40.6 (6.1) | 0.022 | 36.4 (4.5) | 44.4 (4.7) | <0.001 |
| Δ fat mass (% of BW) | −5.9 (5.0) | −3.9 (3.9) | <0.001 | −4.4 (4.2) | −4.3 (4.2) | 0.389 | −2.7 (2.8) | −5.9 (4.8) | <0.001 |
| Baseline visceral fat (% of BW) | 12.4 (2.8) | 5.9 (1.3) | <0.001 | 7.3 (3.2) | 7.4 (3.2) | 0.695 | 6.1 (1.9) | 8.5 (3.7) | <0.001 |
| Δ visceral fat (% of BW) | −1.7 (1.6) | −0.5 (1.0) | <0.001 | −0.8 (1.3) | −0.8 (1.2) | 0.553 | −0.4 (0.6) | −1.1 (1.5) | <0.001 |
| Baseline muscle mass (% of BW) | 32.0 (5.1) | 29.4 (3.6) | <0.001 | 31.3 (3.9) | 28.6 (3.8) | <0.001 | 32.3 (3.4) | 27.7 (3.4) | <0.001 |
| Δ muscle (% of BW) | 3.7 (3.1) | 2.2 (2.3) | <0.001 | 2.5 (2.6) | 2.5 (2.5) | 0.365 | 1.6 (1.7) | 3.4 (2.9) | <0.001 |
| β | C.I. | p-Value | R2 | |
|---|---|---|---|---|
| Model 1 | Δ Body weight (kg) | 0.35 | ||
| Baseline body weight (kg) | −0.21 | −0.22; −0.19 | <0.001 | |
| Age (years) | 0.01 | −0.01; 0.02 | 0.268 | |
| Women | −0.16 | −0.51; 0 0.2 | 0.383 | |
| Accumulated expense (1000€) | −0.46 | −0.58; −0.33 | <0.001 | |
| Number of visits (n) | −0.28 | −0.33; −0.23 | <0.001 | |
| Model 2 | Δ BMI (kg/m2) | 0.36 | ||
| Baseline BMI (kg/m2) | −0.29 | −0.30; −0.28 | <0.001 | |
| Age (years) | 0.01 | 0.00; 0.01 | <0.001 | |
| Women | 0.24 | 0.14; 0.35 | <0.001 | |
| Accumulated expense (1000€) | −0.10 | −0.18; −0.09 | <0.001 | |
| Number of visits (n) | −0.10 | −0.12; −0.08 | <0.001 | |
| Model 3 | Δ Fat mass (%) | 0.35 | ||
| Baseline fat mass (% of BW) | −0.30 | −0.32; −0.29 | <0.001 | |
| Age (years) | 0.02 | 0.01; 0.03 | <0.001 | |
| Women | 3.65 | 3.45; 3.86 | <0.001 | |
| Accumulated expense (1000€) | −0.25 | −0.34; −0.16 | <0.001 | |
| Number of visits (n) | −0.17 | −0.20; −0.14 | <0.001 | |
| Model 4 | Δ Muscle (%) | 0.35 | ||
| Baseline muscle (% of BW) | −0.30 | −0.31; −0.28 | <0.001 | |
| Age (years) | −0.06 | −0.07; −0.06 | <0.001 | |
| Women | −2.42 | −2.55; −2.30 | <0.001 | |
| Accumulated expense (1000€) | 0.14 | 0.08; 0.19 | <0.001 | |
| Number of visits (n) | 0.10 | 0.08; 0.12 | <0.001 | |
| OR | C.I. | p-Value | R2 | |
|---|---|---|---|---|
| Model 1 | 10 kg of weight loss (kg) | 0.24 | ||
| Baseline body weight (kg) | 1.08 | 1.08; 1.09 | <0.001 | |
| Age (years) | 1.00 | 0.99; 1.01 | 0.619 | |
| Women | 1.19 | 0.98; 1.43 | 0.076 | |
| Accumulated expense (€) | 1.00 | 1.00; 1.00 | <0.001 | |
| Number of visits (n) | 1.13 | 1.10; 1.16 | <0.001 | |
| Restart (yes) | 0.13 | 0.08; 0.21 | <0.001 | |
| Model 2 | 3 kg/m2 of BMI loss (kg/m2) | 0.24 | ||
| Baseline BMI (kg/m2) | 1.35 | 1.32; 1.38 | <0.001 | |
| Age (years) | 1.00 | 0.99; 1.00 | 0.267 | |
| Women | 0.78 | 0.67; 0.90 | 0.001 | |
| Accumulated expense (€) | 1.00 | 1.00; 1.00 | <0.001 | |
| Number of visits (n) | 1.13 | 1.10; 1.16 | <0.001 | |
| Restart (yes) | 0.14 | 0.10; 0.21 | <0.001 | |
| Model 3 | 5% of fat mass loss (%) | 0.11 | ||
| Baseline fat mass (% of BW) | 1.09 | 1.08; 1.10 | <0.001 | |
| Age (years) | 0.99 | 0.99; 1.00 | 0.006 | |
| Women | 0.25 | 0.21; 0.30 | <0.001 | |
| Accumulated expense (€) | 1.00 | 1.00; 1.00 | 0.004 | |
| Number of visits (n) | 1.08 | 1.06; 1.11 | <0.001 | |
| Restart (yes) | 0.27 | 0.22; 0.34 | <0.001 | |
| Model 4 | 2% of muscle gain (%) | 0.18 | ||
| Baseline muscle (% of BW) | 0.80 | 0.79; 0.82 | <0.001 | |
| Age (years) | 0.96 | 0.95; 0.97 | <0.001 | |
| Women | 0.18 | 0.15; 0.21 | <0.001 | |
| Accumulated expense (€) | 1.00 | 1.00; 1.00 | <0.001 | |
| Number of visits (n) | 1.10 | 1.07; 1.12 | <0.001 | |
| Restart (yes) | 0.20 | 0.15; 0.26 | <0.001 | |
| Cluster 1 | Cluster 2 | p-Value | Variable Contribution | |
|---|---|---|---|---|
| n | 5528 | 1885 | ||
| Age (years) | 47.5 (10.5) | 44.8 (10.0) | <0.001 | 1 |
| Women (%) | 5145 (93.1) | 663 (35.2) | <0.001 | 0.78 |
| Total days of follow-up (days) | 109.1 (113.3) | 111.2 (92.5) | 0.484 | 0.94 |
| Number of visits (n) | 5.3 (4.6) | 5.5 (4.2) | 0.026 | 0.94 |
| Doses per day (n) | 4.8 (0.8) | 5.0 (0.6) | <0.001 | 0.79 |
| Accumulated expense (€) | 2003.0 (1703.9) | 2139.3 (1541.3) | 0.002 | 0.86 |
| Baseline weight (kg) | 78.0 (8.1) | 98.7 (11.4) | <0.001 | 0.79 |
| Δ body weight (kg) | −5.0 (4.8) | −9.9 (7.3) | <0.001 | 0.70 |
| Δ body weight (%) | −6.3 (5.8) | −10.0 (7.1) | <0.001 | 0.58 |
| Baseline BMI (kg/m2) | 29.4 (2.8) | 32.5 (3.2) | <0.001 | 0.79 |
| Δ BMI (kg/m2) | −1.9 (1.9) | −3.3 (2.5) | <0.001 | 0.70 |
| Baseline fat mass (% of BW) | 40.2 (5.7) | 40.9 (7.1) | <0.001 | 0.67 |
| Δ fat mass (% of BW) | −3.5 (3.6) | −6.2 (5.0) | <0.001 | 0.61 |
| Δ fat mass (% of baseline value) | −8.2 (8.4) | −14.9 (11.5) | <0.001 | 0.53 |
| Baseline visceral fat (% of BW) | 6.1 (1.7) | 10.8 (3.8) | <0.001 | 0.69 |
| Δ visceral fat (% of BW) | −0.4 (0.8) | −1.6 (1.7) | <0.001 | 0.59 |
| Δ visceral fat (% of baseline value) | −6.5 (8.9) | −12.7 (28) | <0.001 | 0.50 |
| Baseline muscle mass (% of BW) | 30.1 (3.9) | 29.7 (4.6) | 0.001 | 0.76 |
| Δ muscle (% of BW) | 2.0 (2.1) | 3.7 (3.1) | <0.001 | 0.66 |
| Δ muscle (% of baseline value) | 6.9 (7.7) | 13.3 (11.8) | <0.001 | 0.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la O, V.; de Cuevillas, B.; Henkrich, M.; Vizmanos, B.; Nuñez-Garcia, M.; Sajoux, I.; de Luis, D.; Martínez, J.A. Phenotype-Driven Variability in Longitudinal Body Composition Changes After a Very Low-Calorie Ketogenic Intervention: A Machine Learning Cluster Approach. J. Pers. Med. 2025, 15, 251. https://doi.org/10.3390/jpm15060251
de la O V, de Cuevillas B, Henkrich M, Vizmanos B, Nuñez-Garcia M, Sajoux I, de Luis D, Martínez JA. Phenotype-Driven Variability in Longitudinal Body Composition Changes After a Very Low-Calorie Ketogenic Intervention: A Machine Learning Cluster Approach. Journal of Personalized Medicine. 2025; 15(6):251. https://doi.org/10.3390/jpm15060251
Chicago/Turabian Stylede la O, Victor, Begoña de Cuevillas, Miksa Henkrich, Barbara Vizmanos, Maitane Nuñez-Garcia, Ignacio Sajoux, Daniel de Luis, and J. Alfredo Martínez. 2025. "Phenotype-Driven Variability in Longitudinal Body Composition Changes After a Very Low-Calorie Ketogenic Intervention: A Machine Learning Cluster Approach" Journal of Personalized Medicine 15, no. 6: 251. https://doi.org/10.3390/jpm15060251
APA Stylede la O, V., de Cuevillas, B., Henkrich, M., Vizmanos, B., Nuñez-Garcia, M., Sajoux, I., de Luis, D., & Martínez, J. A. (2025). Phenotype-Driven Variability in Longitudinal Body Composition Changes After a Very Low-Calorie Ketogenic Intervention: A Machine Learning Cluster Approach. Journal of Personalized Medicine, 15(6), 251. https://doi.org/10.3390/jpm15060251








