An Assessment of Real-World Evidence and Other Sources Supporting Payer Coverage Decisions for Pharmacogenomic Testing in Psychiatry
Abstract
1. Introduction
2. Methods
2.1. Payer Policy Search and Selection
2.2. Policy Content Analysis
- ▪
- Policy type (general or psychiatry specific)
- ▪
- Payer type
- For-profit or mutual fund: entity with a tax filing status based on individual shareholder investments or an investment fund.
- Non-profit or government: entity with a tax filing status based on public benefit, charity, or social cause.
- ▪
- Coverage determination
- No coverage
- Coverage
- ○
- Specified (i.e., coverage for a specific test/subpopulation).
- ○
- Conditional (i.e., coverage based on meeting clinical or prior authorization criteria).
- ▪
- Active company subsidiaries (when applicable and when information was freely available online) to exclude non-parent or subsidiary policies with redundant or boilerplate language seen in parent company policies (commercial payers only).
- ▪
- References cited
- For policies specific to PGx testing for mental health or psychiatric purposes, all references were quantified and catalogued.
- For policies non-specific to PGx testing for mental health or psychiatric purposes, only references relevant to mental health or psychiatry were quantified and catalogued.
2.3. Evaluation of Payer Types and Coverage Decisions
2.4. Assessment of Sources Cited in Payer Policies
2.5. Ethics Statement
3. Results
3.1. Payer Assessment and Coverage Analysis
3.2. Statistical Assessment of Sources Cited
3.3. Assessment of Sources Cited Across Payer Types
3.4. Assessment of Peer-Reviewed Literature Cited Across Payer Types
3.5. Assessment of RWE in Peer-Reviewed Literature Cited Across Payer Types
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saadullah Khani, N.; Hudson, G.; Mills, G.; Ramesh, S.; Varney, L.; Cotic, M.; Abidoph, R.; Richards-Belle, A.; Carrascal-Laso, L.; Franco-Martin, M.; et al. A Systematic Review of Pharmacogenetic Testing to Guide Antipsychotic Treatment. Nat. Ment. Health 2024, 2, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, J.P.; Peter, A.P.; Keogh, M.; Baldasare, V.; Beanland, G.M.; Wilkerson, Z.T.; Kradel, S.; Shaman, J.A. Real-World Impact of a Pharmacogenomics-Enriched Comprehensive Medication Management Program. J. Pers. Med. 2022, 12, 421. [Google Scholar] [CrossRef] [PubMed]
- ELSIconversations—Pharmacogenomic Testing (PGx) in Psychiatric Care: Exploring Ethical and Regulatory Frontiers, Session 1|ELSIhub. Available online: https://elsihub.org/video/elsiconversations-pharmacogenomic-testing-pgx-psychiatric-care-exploring-ethical-and (accessed on 3 November 2024).
- Lu, C.Y.; Loomer, S.; Ceccarelli, R.; Mazor, K.M.; Sabin, J.; Clayton, E.W.; Ginsburg, G.S.; Wu, A.C. Insurance Coverage Policies for Pharmacogenomic and Multi-Gene Testing for Cancer. J. Pers. Med. 2018, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Groessl, E.J.; Tally, S.R.; Hillery, N.; Maciel, A.; Garces, J.A. Cost-Effectiveness of a Pharmacogenetic Test to Guide Treatment for Major Depressive Disorder. JMCP 2018, 24, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Wiedower, J.; Smith, H.S.; Farrell, C.L.; Parker, V.; Rebek, L.; Davis, S.C. Payer Perspectives on Genomic Testing in the United States: A Systematic Literature Review. Genet. Med. 2025, 27, 101329. [Google Scholar] [CrossRef] [PubMed]
- Center for Drug Evaluation and Research, US Food and Drug Administration Table of Pharmacogenomic Biomarkers in Drug Labeling. Available online: https://www.fda.gov/drugs/science-and-research-drugs/table-pharmacogenomic-biomarkers-drug-labeling (accessed on 12 February 2025).
- OCEBM Levels of Evidence. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence (accessed on 25 February 2025).
- Rahman, M.; Dal Pan, G.; Stein, P.; Levenson, M.; Kraus, S.; Chakravarty, A.; Rivera, D.R.; Forshee, R.; Concato, J. When Can Real-World Data Generate Real-World Evidence? Pharmacoepidemiol. Drug Saf. 2024, 33, e5715. [Google Scholar] [CrossRef] [PubMed]
- Baumfeld Andre, E.; Carrington, N.; Siami, F.S.; Hiatt, J.C.; McWilliams, C.; Hiller, C.; Surinach, A.; Zamorano, A.; Pashos, C.L.; Schulz, W.L. The Current Landscape and Emerging Applications for Real-World Data in Diagnostics and Clinical Decision Support and Its Impact on Regulatory Decision Making. Clin. Pharmacol. Ther. 2022, 112, 1172–1182. [Google Scholar] [CrossRef] [PubMed]
- L Rogers, S.; Keeling, N.J.; Giri, J.; Gonzaludo, N.; Jones, J.S.; Glogowski, E.; Formea, C.M. PARC Report: A Health-Systems Focus on Reimbursement and Patient Access to Pharmacogenomics Testing. Pharmacogenomics 2020, 21, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Deverka, P.A.; Douglas, M.P.; Phillips, K.A. Use of Real-World Evidence in US Payer Coverage Decision-Making for Next-Generation Sequencing–Based Tests: Challenges, Opportunities, and Potential Solutions. Value Health 2020, 23, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Jürgens, G.; Rasmussen, H.B.; Werge, T.; Dalhoff, K.; Nordentoft, M.; Andersen, S.E. Does the Medication Pattern Reflect the CYP2D6 Genotype in Patients with Diagnoses within the Schizophrenic Spectrum? J. Clin. Psychopharmacol. 2012, 32, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Hall-Flavin, D.K.; Winner, J.G.; Allen, J.D.; Jordan, J.J.; Nesheim, R.S.; Snyder, K.A.; Drews, M.S.; Eisterhold, L.L.; Biernacka, J.M.; Mrazek, D.A. Using a Pharmacogenomic Algorithm to Guide the Treatment of Depression. Transl. Psychiatry 2012, 2, e172. [Google Scholar] [CrossRef] [PubMed]
- Hall-Flavin, D.K.; Winner, J.G.; Allen, J.D.; Carhart, J.M.; Proctor, B.; Snyder, K.A.; Drews, M.S.; Eisterhold, L.L.; Geske, J.; Mrazek, D.A. Utility of Integrated Pharmacogenomic Testing to Support the Treatment of Major Depressive Disorder in a Psychiatric Outpatient Setting. Pharmacogenet. Genom. 2013, 23, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Brennan, F.X.; Gardner, K.R.; Lombard, J.; Perlis, R.H.; Fava, M.; Harris, H.W.; Scott, R. A Naturalistic Study of the Effectiveness of Pharmacogenetic Testing to Guide Treatment in Psychiatric Patients With Mood and Anxiety Disorders. Prim. Care Companion CNS Disord. 2015, 17, 25610. [Google Scholar] [CrossRef] [PubMed]
- Zeier, Z.; Carpenter, L.L.; Kalin, N.H.; Rodriguez, C.I.; McDonald, W.M.; Widge, A.S.; Nemeroff, C.B. Clinical Implementation of Pharmacogenetic Decision Support Tools for Antidepressant Drug Prescribing. Am. J. Psychiatry 2018, 175, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Saldarriaga, E.M.; Hauber, B.; Carlson, J.J.; Barthold, D.; Veenstra, D.L.; Devine, B. Assessing Payers’ Preferences for Real-World Evidence in the United States: A Discrete Choice Experiment. Value Health 2022, 25, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.Y.; Williams, M.S.; Ginsburg, G.S.; Toh, S.; Brown, J.S.; Khoury, M.J. A Proposed Approach to Accelerate Evidence Generation for Genomic-Based Technologies in the Context of a Learning Health System. Genet. Med. 2018, 20, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Silver, J. Real World Evidence (RWE) Initiative|AMCP.Org. Available online: https://www.amcp.org/real-world-evidence-rwe-initiative (accessed on 25 February 2025).
- Center for Medicare and Medicaid Services. Study Protocols That Use Real-World Data; Center for Medicare and Medicaid Services: Baltimore, MD, USA, 2025.
- Institute for Clinical and Economic Review (ICER) ICER and Aetion Partner to Develop Real-World Evidence for Value Assessment of Treatments. Available online: https://icer.org/news-insights/press-releases/icer-and-aetion-rwe/ (accessed on 15 May 2025).
- Institute for Clinical and Economic Review (ICER) Evidence Rating Matrix. Available online: https://icer.org/evidence-rating-matrix/ (accessed on 15 May 2025).
- Wang, S.V.; Pottegård, A.; Crown, W.; Arlett, P.; Ashcroft, D.M.; Benchimol, E.I.; Berger, M.L.; Crane, G.; Goettsch, W.; Hua, W.; et al. HARmonized Protocol Template to Enhance Reproducibility of Hypothesis Evaluating Real-World Evidence Studies on Treatment Effects: A Good Practices Report of a Joint ISPE/ISPOR Task Force. Value Health 2022, 25, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Bridging the Credibility Gap: Establishing Competent and Reliable Scientific Evidence (CARSE) to Support Productive Healthcare Economic Information (HCEI) Discussions With US Payer Audiences. Available online: https://www.ispor.org/conferences-education/conferences/upcoming-conferences/ispor-2025/program/program/session/ispor-2025/bridging-the-credibility-gap-establishing-competent-and-reliable-scientific-evidence-carse-to-support-productive-healthcare-economic-information-hcei-discussions-with-us-payer-audiences (accessed on 15 May 2025).
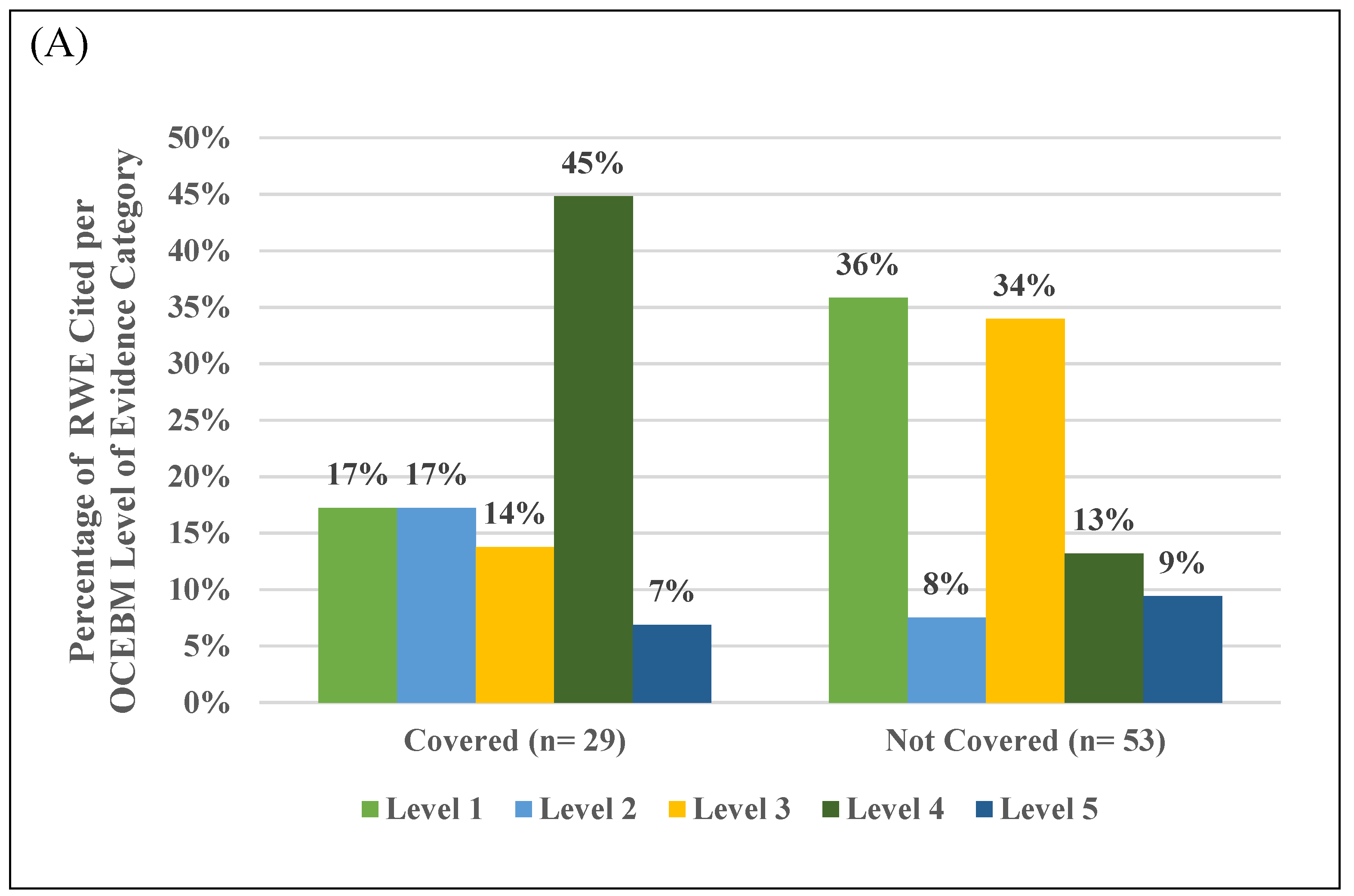
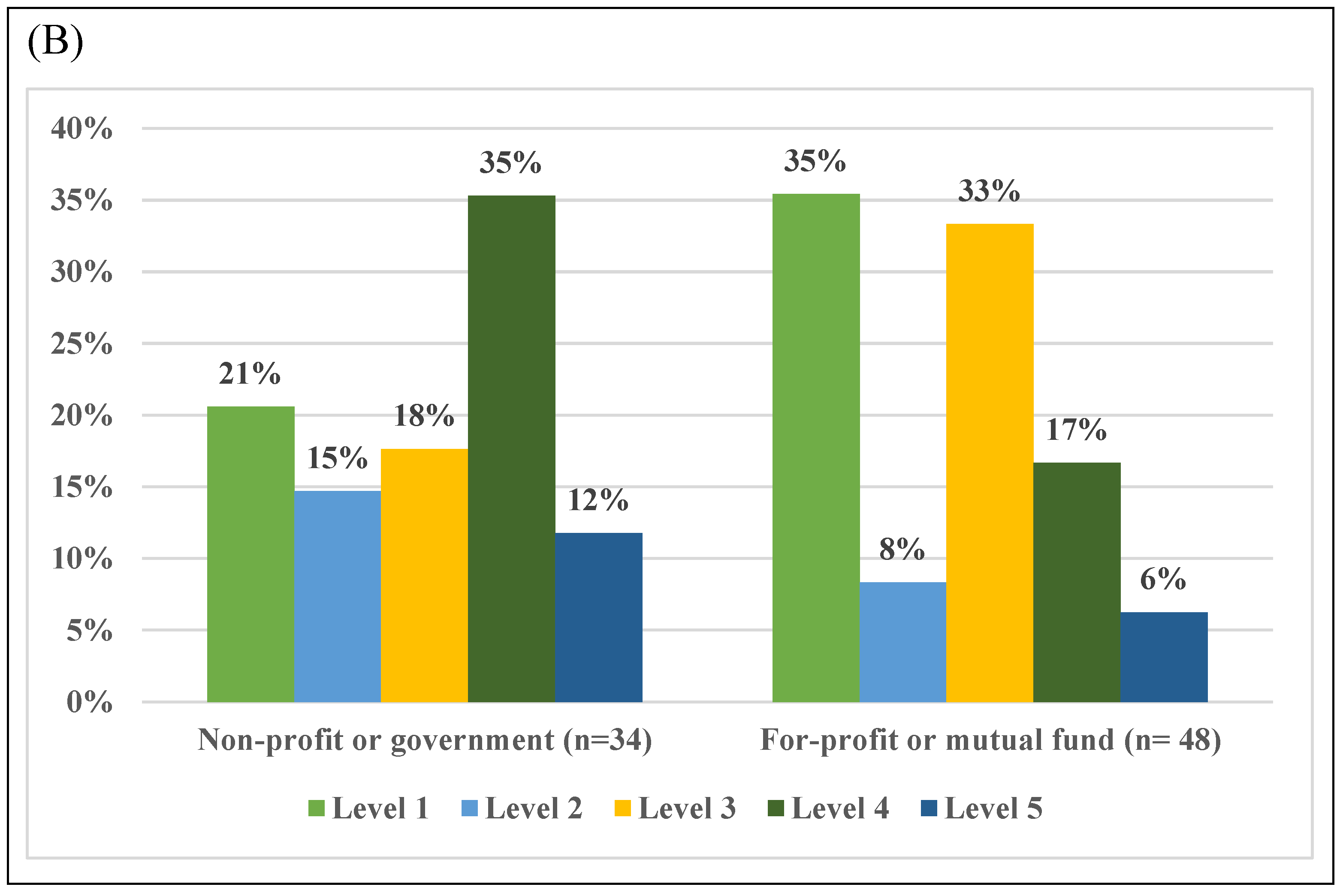
| Number of References per Payer Type (For-Profit or Mutual Fund [n = 7] and Non-Profit or Government [n = 7]) and per Coverage Decision (Covered [n = 7] and no Coverage [n = 7]) | ||||
|---|---|---|---|---|
| Source Cited (Total of 32) | For-Profit or Mutual Fund (215 Total References) | Non-Profit or Government (131 Total References) | Covered (Specified or Partial; 146 Total References) | Not Covered (200 Total References) |
| Centers for Medicare and Medicaid Services (CMS; 16 total citations) | 4 (2%) | 12 (9%) | 11 (8%) | 5 (3%) |
| Centers for Disease Control and Prevention (CDC; 8 total citations) | 3 (1%) | 5 (4%) | 6 (4%) | 2 (1%) |
| United States (US) Food and Drug Administration (FDA; 14 total citations) | 10 (5%) | 4 (3%) | 5 (3%) | 9 (5%) |
| UptoDate (4 total citations) | 3 (1%) | 1 (1%) | 1 (1%) | 3 (2%) |
| National Institute of Health (NIH; 9 total citations) | 9 (4%) | 0 (0%) | 3 (2%) | 6 (3%) |
| Department of Energy (2 total citations) | 0 (0%) | 1 (1%) | 1 (1%) | 0 (0%) |
| Federal Register (1 total citation) | 1 (0.5%) | 0 (0%) | 0 (0%) | 1 (0.5%) |
| PharmGKB (2 total citations) | 1 (0.5%) | 1 (1%) | 1 (1%) | 1 (0.5%) |
| American College of Medical Genetics and Genomics (ACMG; 10 total citations) | 10 (5%) | 0 (0%) | 9 (6%) | 1 (0.5%) |
| International Statements or Guidelines (1 total citation) | 0 (0%) | 1 (1%) | 1 (1%) | 0 (0%) |
| Peer-reviewed literature (207 total citations) | 132 (61%) | 75 (57%) | 78 (53%) | 129 (65%) |
| Industrial or market solution a (13 total citations) | 2 (1%) | 11 (8%) | 9 (6%) | 4 (2%) |
| Clinical Pharmacogenetics Implementation Consortium (CPIC; 12 total citations) | 6 (3%) | 6 (5%) | 9 (6%) | 3 (2%) |
| News article (1 total citation) | 0 (0%) | 1 (1%) | 1 (1%) | 0 (0%) |
| Canadian Agency for Drugs and Technologies in Health (CADTH; 2 total citations) | 1 (0.5%) | 1 (1%) | 1 (1%) | 1 (0.5%) |
| International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH; 1 total citation) | 0 (0%) | 1 (1%) | 1 (1%) | 0 (0%) |
| International Society of Psychiatric Genetics (1 total citation) | 0 (0%) | 1 (1%) | 1 (1%) | 0 (0%) |
| Agency for Healthcare Research and Quality (AHRQ; 1 total citation) | 1 (0.5%) | 0 (0%) | 0 (0%) | 1 (0.5%) |
| Payer Technology Evaluation Center (4 total citations) | 4 (2%) | 0 (0%) | 0 (0%) | 4 (2%) |
| PGx test provider webpage (3 total citations) | 3 (1%) | 0 (0%) | 0 (0%) | 3 (2%) |
| Academic resources b (1 total citation) | 1 (0.5%) | 0 (0%) | 0 (0%) | 1 (0.5%) |
| Association for Molecular Pathology and/or College of American Pathologists (6 total citations) | 4 (2%) | 2 (2%) | 2 (1%) | 4 (2%) |
| ClinKey (1 total citation) | 1 (0.5%) | 0 (0%) | 0 (0%) | 1 (0.5%) |
| Emergency Care Research Institute (ECRI) Institute (7 total citations) | 7 (3%) | 0 (0%) | 0 (0%) | 7 (4%) |
| Hayes Knowledge Center (6 total citations) | 6 (3%) | 0 (0%) | 0 (0%) | 6 (3%) |
| Payer health guidelines (4 total citations) | 3 (1%) | 1 (1%) | 2 (1%) | 2 (1%) |
| Subject Matter Panel and Advisory Committee c (4 total citations) | 2 (1%) | 2 (2%) | 3 (2%) | 1 (0.5%) |
| American Association for Clinical Chemistry (1 total citation) | 0 (0%) | 1 (1%) | 0 (0%) | 1 (0.5%) |
| American Psychiatric Association (1 total citation) | 0 (0%) | 1 (1%) | 0 (0%) | 1 (0.5%) |
| International Society of Psychiatric Genetics (1 total citation) | 0 (0%) | 1 (1%) | 0 (0%) | 1 (0.5%) |
| Government agency health technology assessment (2 total citations) | 0 (0%) | 2 (2%) | 0 (0%) | 2 (1%) |
| National Society of Genetic Counselors (1 total citation) | 1 (0.5%) | 0 (0%) | 1 (1%) | 0 (0%) |
| Study PMID (OCEBM Evidence Level) | Publication Year | Payer Coverage Decision(s) | Key Findings or Conclusions |
|---|---|---|---|
| 22198443 (Level 3) | 2012 | Not Covered (2 policies) | Poor metabolizers and ultra-rapid metabolizers received significantly higher chlorpromazine equivalent doses than extensive metabolizers and intermediate metabolizers. There was a tendency that the increase primarily was caused by CYP2D6-dependent antipsychotics and not as expected by CYP2D6-independent antipsychotics. |
| 23047243 (Level 3) | 2012 | Both Covered (1 policy) and Not Covered (3 policies) | A greater reduction in overall Quick Inventory of Depressive Symptomatology, Clinician Rated (QIDS-C16), and Hamilton Rating Scale for Depression (HAM-D17) scores were achieved with PGx-guided treatment. |
| 24018772 (Level 3) | 2013 | Both Covered (1 policy) and Not Covered (1 policy) | Study replicated the magnitude of effect previously observed in a prior smaller prospective pilot study (23047243). Reduction in depression scores from the baseline to the 8-week visit was greater in the PGx-guided group than in the PGx-unguided group. |
| 25686762 (Level 1) | 2015 | Both Covered (1 policy) and Not Covered (1 policy) | 8-week improvement in depressive symptoms in the three studies assessed displayed the same trend, with clinical outcomes differing overall as a function of the most severely categorized medication patients was prescribed at the study baseline. |
| 26445691 (Level 3) | 2015 | Both Covered (1 policy) and Not Covered (1 policy) | Majority of patients showed clinically measurable improvement (rated as very much improved, much improved, or minimally improved), with most demonstrating clinically significant improvement. Among individuals with ≥ 2 prior treatment failures, the majority showed clinically measurable improvement. Patients also reported significant decreases in depression, anxiety, and medication side effects and increases in quality of life. |
| 29690793 (Level 1) | 2018 | Both Covered (1 policy) and Not Covered (2 policies) | At present, there are insufficient data to support the widespread use of combinatorial pharmacogenetic testing in clinical practice, although there are clinical situations in which the technology may be informative, particularly in predicting side effects. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yankah, S.E.; Nafie, M.; Hendricks-Sturrup, R.M.; Lu, C.Y. An Assessment of Real-World Evidence and Other Sources Supporting Payer Coverage Decisions for Pharmacogenomic Testing in Psychiatry. J. Pers. Med. 2025, 15, 232. https://doi.org/10.3390/jpm15060232
Yankah SE, Nafie M, Hendricks-Sturrup RM, Lu CY. An Assessment of Real-World Evidence and Other Sources Supporting Payer Coverage Decisions for Pharmacogenomic Testing in Psychiatry. Journal of Personalized Medicine. 2025; 15(6):232. https://doi.org/10.3390/jpm15060232
Chicago/Turabian StyleYankah, Sandra E., Maryam Nafie, Rachele M. Hendricks-Sturrup, and Christine Y. Lu. 2025. "An Assessment of Real-World Evidence and Other Sources Supporting Payer Coverage Decisions for Pharmacogenomic Testing in Psychiatry" Journal of Personalized Medicine 15, no. 6: 232. https://doi.org/10.3390/jpm15060232
APA StyleYankah, S. E., Nafie, M., Hendricks-Sturrup, R. M., & Lu, C. Y. (2025). An Assessment of Real-World Evidence and Other Sources Supporting Payer Coverage Decisions for Pharmacogenomic Testing in Psychiatry. Journal of Personalized Medicine, 15(6), 232. https://doi.org/10.3390/jpm15060232







