Developing a Risk Stratification Tool to Predict Patients with Gestational Diabetes Mellitus at Risk of Insulin Treatment: A Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Ethical Approval
2.3. Candidate Predictors and Outcomes
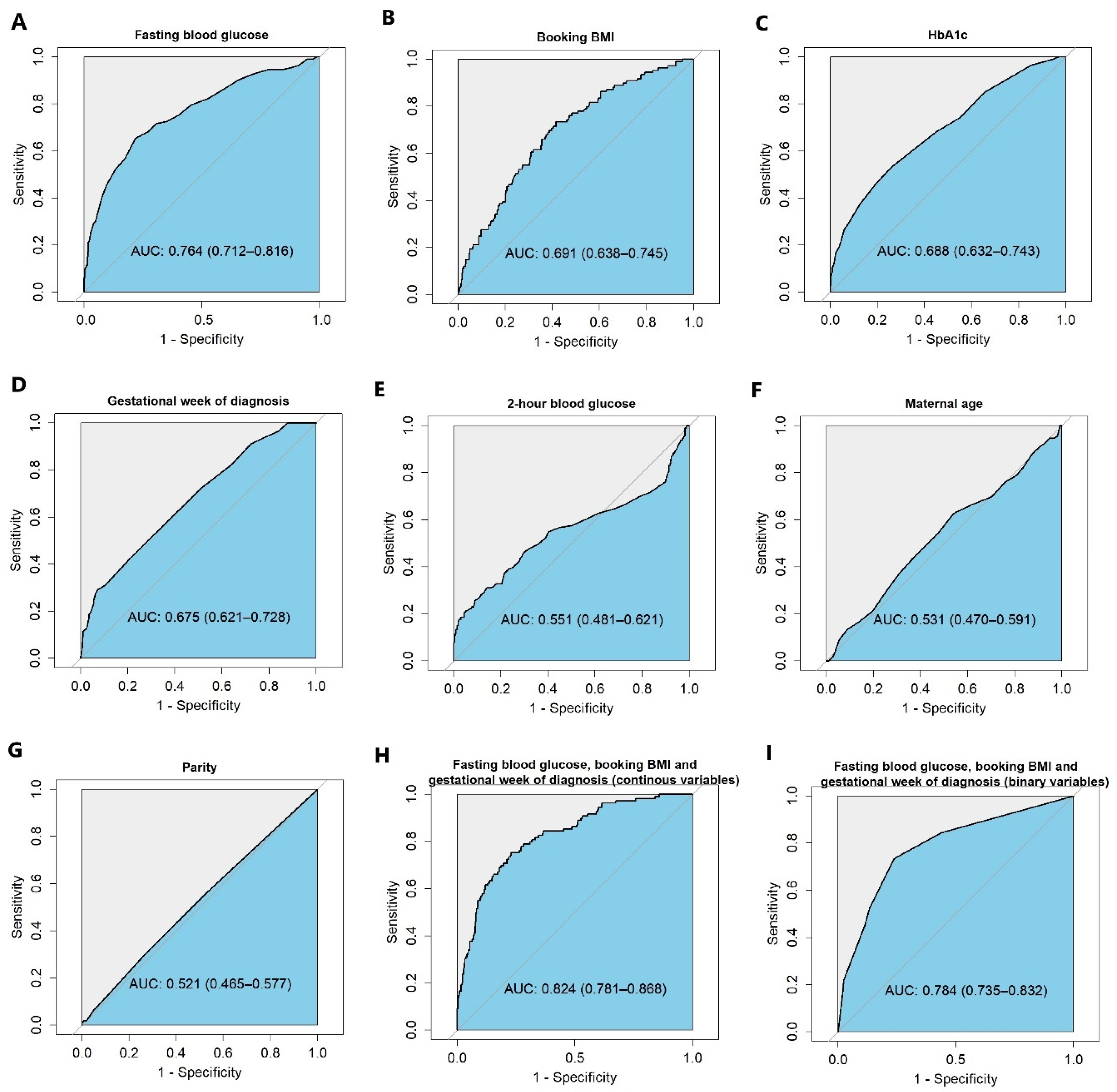
2.4. Statistical Analysis
2.5. Sample Size Calculation
3. Results
3.1. Variable Selection and Model Development
3.2. Developing a Simple Risk Stratification Tool to Stratify the Two Groups and the Performance of This Simple Risk Stratification Tool
3.3. Internal and External Validation
4. Discussion
5. Conclusions
6. Patients
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GDM | Gestational diabetes mellitus |
| OR | Odds ratio |
| NPV | Negative predictive value |
| OGTT | Oral glucose tolerance test |
| OASI | Obstetric anal sphincter injury |
| IQR | Interquartile range |
| AIC | Akaike Information Criterion |
| AUC | Area under the ROC curve |
| PPV | Positive predictive value |
| CI | Confidence interval |
| BMI | Body mass index |
References
- Biju, P.B.; Chaudhary, R.K.; Radhakrishnan, K.; Shetty, S.; Mateti, U.V.; Raju, B.N.; Sonkusare, S. Gestational Diabetes and Analysis of Maternal and Fetal Outcomes Among Pregnant Women: A Cross-Sectional Study. J. Obstet. Gynecol. India 2023, 74, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Cao, Y.; Fadl, H.; Gustafson, H.; Simmons, D. Increasing prevalence of gestational diabetes mellitus when implementing the IADPSG criteria: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2021, 172, 108642. [Google Scholar] [CrossRef] [PubMed]
- Deitch, J.; Yates, C.J.; Hamblin, P.S.; Kevat, D.; Shahid, I.; Teale, G.; Lee, I. Prevalence of gestational diabetes mellitus, maternal obesity and associated perinatal outcomes over 10 years in an Australian tertiary maternity provider. Diabetes Res. Clin. Pract. 2023, 203, 110793. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Dainelli, L.; Yu, K.; Ma, L.; Silva Zolezzi, I.; Detzel, P.; Fang, H. The short-term health and economic burden of gestational diabetes mellitus in China: A modelling study. BMJ Open 2017, 7, e018893. [Google Scholar] [CrossRef]
- Yu, Y.H.; Platt, R.W.; Reynier, P.; Yu, O.H.Y.; Filion, K.B. Use of metformin and insulin among pregnant women with gestation diabetes in the United Kingdom: A population-based cohort study. Diabet. Med. J. Br. Diabet. Assoc. 2023, 40, e15108. [Google Scholar] [CrossRef]
- Bogdanet, D.; Egan, A.; Reddin, C.; Kirwan, B.; Carmody, L.; Dunne, F. ATLANTIC DIP: Despite insulin therapy in women with IADPSG diagnosed GDM, desired pregnancy outcomes are still not achieved. What are we missing? Diabetes Res. Clin. Pract. 2018, 136, 116–123. [Google Scholar] [CrossRef]
- Sheng, B.; Ni, J.; Lv, B.; Jiang, G.; Lin, X.; Li, H. Short-term neonatal outcomes in women with gestational diabetes treated using metformin versus insulin: A systematic review and meta-analysis of randomized controlled trials. Acta Diabetol. 2023, 60, 595–608. [Google Scholar] [CrossRef]
- Wu, R.; Li, Z. Cost-effectiveness analysis of metformin versus insulin in the treatment of gestational diabetes mellitus. Arch. Gynecol. Obstet. 2024, 310, 135–144. [Google Scholar] [CrossRef]
- Yeung, R.O.; Retnakaran, R.; Savu, A.; Butalia, S.; Kaul, P. Gestational diabetes: One size does not fit all-an observational study of maternal and neonatal outcomes by maternal glucose profile. Diabet. Med. J. Br. Diabet. Assoc. 2024, 41, e15205. [Google Scholar] [CrossRef]
- NICE Diabetes in Pregnancy: Management from Preconception to the Postnatal Period. 2015. Available online: https://www.nice.org.uk/guidance/ng3 (accessed on 15 May 2025).
- Lee, S.H.; Yu, J.; Han, K.; Lee, S.W.; You, S.Y.; Kim, H.S.; Cho, J.-H.; Yoon, K.-H.; Kim, M.K. Predicting the Risk of Insulin-Requiring Gestational Diabetes before Pregnancy: A Model Generated from a Nationwide Population-Based Cohort Study in Korea. Endocrinol. Metab. 2023, 38, 129–138. [Google Scholar] [CrossRef]
- Liao, L.D.; Ferrara, A.; Greenberg, M.B.; Ngo, A.L.; Feng, J.; Zhang, Z.; Bradshaw, P.T.; Hubbard, A.E.; Zhu, Y. Development and validation of prediction models for gestational diabetes treatment modality using supervised machine learning: A population-based cohort study. BMC Med. 2022, 20, 307. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Silvares, E.; Bermúdez-González, M.; Vilouta-Romero, M.; García-Lavandeira, S.; Seoane-Pillado, T. Prediction of insulin therapy in women with gestational diabetes: A systematic review and meta-analysis of observational studies. J. Perinat. Med. 2022, 50, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.; Costa, R.A.; Paganoti, C.F.; Rodrigues, A.S.; Zugaib, M.; Hadar, E.; Hod, M.; Francisco, R.P.V. Can we stratify the risk for insulin need in women diagnosed early with gestational diabetes by fasting blood glucose? J. Matern.-Fetal Neonatal Med. 2019, 32, 2036–2041. [Google Scholar] [CrossRef]
- Nicolaides, K.H.; Wright, D.; Syngelaki, A.; Wright, A.; Akolekar, R. Fetal Medicine Foundation fetal and neonatal population weight charts. Ultrasound Obstet. Gynecol. 2018, 52, 44–51. [Google Scholar] [CrossRef]
- Chowdhury, M.Z.I.; Turin, T.C. Variable selection strategies and its importance in clinical prediction modelling. Fam. Med. Community Health 2020, 8, e000262. [Google Scholar] [CrossRef]
- Schomaker, M.; Heumann, C. Model selection and model averaging after multiple imputation. Comput. Stat. Data Anal. 2014, 71, 758–770. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org (accessed on 5 May 2025).
- van Smeden, M.; de Groot, J.A.; Moons, K.G.; Collins, G.S.; Altman, D.G.; Eijkemans, M.J.; Reitsma, J.B. No rationale for 1 variable per 10 events criterion for binary logistic regression analysis. BMC Med. Res. Methodol. 2016, 16, 163. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, J.; Li, F.; Xu, X. Factors in Gestational Diabetes Mellitus Predicting the Needs for Insulin Therapy. Int. J. Endocrinol. 2016, 2016, 4858976. [Google Scholar] [CrossRef]
- Benham, J.L.; Gingras, V.; McLennan, N.M.; Most, J.; Yamamoto, J.M.; Aiken, C.E.; Ozanne, S.E.; Reynolds, R.M. Precision gestational diabetes treatment: A systematic review and meta-analyses. Commun. Med. 2023, 3, 135. [Google Scholar] [CrossRef]
- Eleftheriades, M.; Chatzakis, C.; Papachatzopoulou, E.; Papadopoulos, V.; Lambrinoudaki, I.; Dinas, K.; Chrousos, G.; Sotiriadis, A. Prediction of insulin treatment in women with gestational diabetes mellitus. Nutr. Diabetes 2021, 11, 30. [Google Scholar] [CrossRef]
- Koning, S.H.; Scheuneman, K.A.; Lutgers, H.L.; Korteweg, F.J.; van den Berg, G.; Sollie, K.M.; Roos, A.; van Loon, A.J.; Links, T.P.; van Tol, K.M.; et al. Risk stratification for healthcare planning in women with gestational diabetes mellitus. Neth. J. Med. 2016, 74, 262–269. [Google Scholar] [PubMed]
- Ford, H.L.; Champion, I.; Wan, A.; Reddy, M.; Mol, B.W.; Rolnik, D.L. Predictors for insulin use in gestational diabetes mellitus. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 272, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Akinci, B.; Celtik, A.; Yener, S.; Yesil, S. Is fasting glucose level during oral glucose tolerance test an indicator of the insulin need in gestational diabetes? Diabetes Res. Clin. Pract. 2008, 82, 219–225. [Google Scholar] [CrossRef]
- Barnes, R.A.; Wong, T.; Ross, G.P.; Jalaludin, B.B.; Wong, V.W.; Smart, C.E.; Collins, C.E.; MacDonald-Wicks, L.; Flack, J.R. A novel validated model for the prediction of insulin therapy initiation and adverse perinatal outcomes in women with gestational diabetes mellitus. Diabetologia 2016, 59, 2331–2338. [Google Scholar] [CrossRef]
- Aktun, L.H.; Yorgunlar, B.; Karaca, N.; Akpak, Y.K. Predictive Risk Factors in the Treatment of Gestational Diabetes Mellitus. Clin. Med. Insights Women’s Health 2015, 8, 25–28. [Google Scholar] [CrossRef]
- Greenland, S. The need for reorientation toward cost-effective prediction: Comments on ‘Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond’ by M. J. Pencina et al., Statistics in Medicine (DOI: 10.1002/sim.2929). Stat. Med. 2008, 27, 199–206. [Google Scholar] [CrossRef]
- Sapienza, A.D.; Francisco, R.P.; Trindade, T.C.; Zugaib, M. Factors predicting the need for insulin therapy in patients with gestational diabetes mellitus. Diabetes Res. Clin. Pract. 2010, 88, 81–86. [Google Scholar] [CrossRef]
- Thériault, S.; Giguère, Y.; Massé, J.; Girouard, J.; Forest, J.C. Early prediction of gestational diabetes: A practical model combining clinical and biochemical markers. Clin. Chem. Lab. Med. 2016, 54, 509–518. [Google Scholar] [CrossRef]
- Koefoed, A.S.; McIntyre, H.D.; Gibbons, K.S.; Poulsen, C.W.; Fuglsang, J.; Ovesen, P.G. Predicting the Need for Insulin Treatment: A Risk-Based Approach to the Management of Women with Gestational Diabetes Mellitus. Reprod. Med. 2023, 4, 133–144. [Google Scholar] [CrossRef]
- Kim, M.K.; Han, K.; You, S.Y.; Kwon, H.S.; Yoon, K.H.; Lee, S.H. Prepregnancy smoking and the risk of gestational diabetes requiring insulin therapy. Sci. Rep. 2020, 10, 13901. [Google Scholar] [CrossRef]
| No Insulin Treatment (n = 504) | Insulin Treatment (n = 113) | |
|---|---|---|
| Maternal characteristics | ||
| Maternal age (years), median (IQR) | 34 (31, 38) | 35 (31, 38) |
| Ethnicity (n, %) | ||
| Asian | 110 (21.8) | 21 (18.6) |
| Black | 120 (23.8) | 21 (18.6) |
| Mixed | 13 (2.6) | 7 (6.2) |
| White | 138 (27.4) | 38 (33.6) |
| Unknown | 83 (16.5) | 19 (16.8) |
| None of the above | 40 (7.9) | 7 (6.2) |
| Parity (n, %) | ||
| Primipara | 234 (46.4) | 49 (43.4) |
| Multipara | 270 (53.6) | 64 (56.6) |
| Booking BMI (kg/m2), median (IQR) | 26.1 (23.0, 31.0) | 31.2 (26.7, 35.5) |
| Fasting blood glucose (mmol/L), median (IQR) | 5.0 (4.6, 5.5) | 5.8 (5.2, 6.5) |
| 2 h blood glucose (mmol/L), median (IQR) | 8.4 (8.0, 9.3) | 8.8 (7.8, 10.6) |
| Gestational week of GDM diagnosis (weeks),median (IQR) | 26 (26, 29) | 26 (22, 27) |
| HbA1c (mmol/mol), median (IQR) | 46.4 (44.7, 49.0) | 49.0 (45.6, 52.5) |
| Pregnancy outcomes | ||
| Preterm birth (n, %) | ||
| No | 455 (90.6) | 105 (92.9) |
| Yes | 47 (9.4) | 8 (7.1) |
| Mode of birth (n, %) | ||
| Vaginal birth | 281 (56.0) | 51 (45.1) |
| Caesarean birth | 221 (44.0) | 62 (54.9) |
| Shoulder dystocia (n, %) | ||
| No | 498 (99.2) | 109 (96.5) |
| Yes | 4 (0.8) | 4 (3.5) |
| Neonatal unit admission (n, %) | ||
| No | 464 (92.4) | 103 (91.2) |
| Yes | 38 (7.6) | 10 (8.8) |
| Large for gestational age (n, %) | ||
| No | 467 (93.0) | 100 (88.5) |
| Yes | 35 (7.0) | 13 (11.5) |
| Obstetric anal sphincter injury (n, %) | ||
| No | 491 (97.8) | 112 (99.1) |
| Yes | 11 (2.2) | 1 (0.9) |
| Apgar < 7 at 5 min, (n, %) | ||
| No | 483 (98.4) | 109 (97.3) |
| Yes | 8 (1.6) | 3 (2.7) |
| Foetal birth outcome (n, %) | ||
| Live birth | 498 (99.2) | 112 (99.1) |
| Stillbirth | 3 (0.6) | 1 (0.9) |
| Neonatal death | 1 (0.2) | 0 (0.0) |
| Univariate * | Multivariate † | Model Selection † | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Maternal age (years) | 1.10 (0.84–1.44) | 0.49 | – | – | – | – |
| Parity (n%) | ||||||
| Primipara | Ref | |||||
| Multipara | 0.88 (0.59–1.33) | 0.55 | – | – | – | – |
| Booking BMI (kg/m2) | 2.35 (1.79–3.09) | <0.001 | 1.55 (1.11–2.17) | 0.011 | 1.48 (1.07–2.03) | 0.017 |
| Fasting blood glucose (mmol/L) | 2.64 (2.06–3.38) | <0.001 | 2.35 (1.67–3.30) | <0.001 | 2.41 (1.84–3.15) | <0.001 |
| 2 h blood glucose (mmol/L) | 1.37 (1.19–1.56) | <0.001 | 1.10 (0.91–1.34) | 0.32 | – | – |
| Gestation of GDM diagnosis (weeks) | 0.66 (0.58–0.74) | <0.001 | 0.70 (0.61–0.81) | <0.001 | 0.71 (0.62–0.81) | <0.001 |
| HbA1c (mmol/mol) | 1.95 (1.57–2.42) | <0.001 | 0.94 (0.68–1.30) | 0.71 | – | – |
| Actual Treatment | |||
|---|---|---|---|
| Tool Predicted Insulin Need | Insulin | No Insulin | Total |
| High-risk group | 92 | 215 | 307 |
| Low-risk group | 17 | 274 | 291 |
| Total | 109 | 489 | |
| Internal Validation | External Validation | |||||
|---|---|---|---|---|---|---|
| Actual Treatment | Actual Treatment | |||||
| Tool Predicted Insulin Need | Insulin | No Insulin | Total | Insulin | No Insulin | Total |
| High-risk group | 59 | 174 | 233 | 36 | 65 | 101 |
| Low-risk group | 18 | 235 | 253 | 10 | 94 | 104 |
| Total | 77 | 409 | 46 | 159 | ||
| Sensitivity = 76.6% Specificity = 57.5% Positive predictive value (PPV) = 25.3% Negative predictive value (NPV) = 92.9% | Sensitivity = 78.3% Specificity = 59.1% Positive predictive value (PPV) = 35.6% Negative predictive value (NPV) = 90.4% | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Nathan, H.L.; Oyekan, E.E.; Korevaar, T.I.M.; Ahmed, D.; Pacifico, K.; Hameed, A.; Chandiramani, M.; Banerjee, A.; Ovadia, C. Developing a Risk Stratification Tool to Predict Patients with Gestational Diabetes Mellitus at Risk of Insulin Treatment: A Cohort Study. J. Pers. Med. 2025, 15, 223. https://doi.org/10.3390/jpm15060223
Yang X, Nathan HL, Oyekan EE, Korevaar TIM, Ahmed D, Pacifico K, Hameed A, Chandiramani M, Banerjee A, Ovadia C. Developing a Risk Stratification Tool to Predict Patients with Gestational Diabetes Mellitus at Risk of Insulin Treatment: A Cohort Study. Journal of Personalized Medicine. 2025; 15(6):223. https://doi.org/10.3390/jpm15060223
Chicago/Turabian StyleYang, Xi, Hannah L. Nathan, Ebruba E. Oyekan, Tim I. M. Korevaar, Doaa Ahmed, Katherine Pacifico, Aisha Hameed, Manju Chandiramani, Anita Banerjee, and Caroline Ovadia. 2025. "Developing a Risk Stratification Tool to Predict Patients with Gestational Diabetes Mellitus at Risk of Insulin Treatment: A Cohort Study" Journal of Personalized Medicine 15, no. 6: 223. https://doi.org/10.3390/jpm15060223
APA StyleYang, X., Nathan, H. L., Oyekan, E. E., Korevaar, T. I. M., Ahmed, D., Pacifico, K., Hameed, A., Chandiramani, M., Banerjee, A., & Ovadia, C. (2025). Developing a Risk Stratification Tool to Predict Patients with Gestational Diabetes Mellitus at Risk of Insulin Treatment: A Cohort Study. Journal of Personalized Medicine, 15(6), 223. https://doi.org/10.3390/jpm15060223







