1. Introduction
The human appendix, an anatomical structure characterized by its slender, tubular projection from the cecum situated in the lower right quadrant of the abdomen, has remained a focal point of scientific inquiry [
1]. Frequently categorized as a trivial organ, the appendix continues to captivate scientific interest due to its intriguing presence within human anatomy [
1,
2]. Morphologically, the appendix typically measures approximately 9 cm in length, exhibiting variations ranging from 5 to 35 cm, with a diameter averaging around 6 mm [
2,
3].
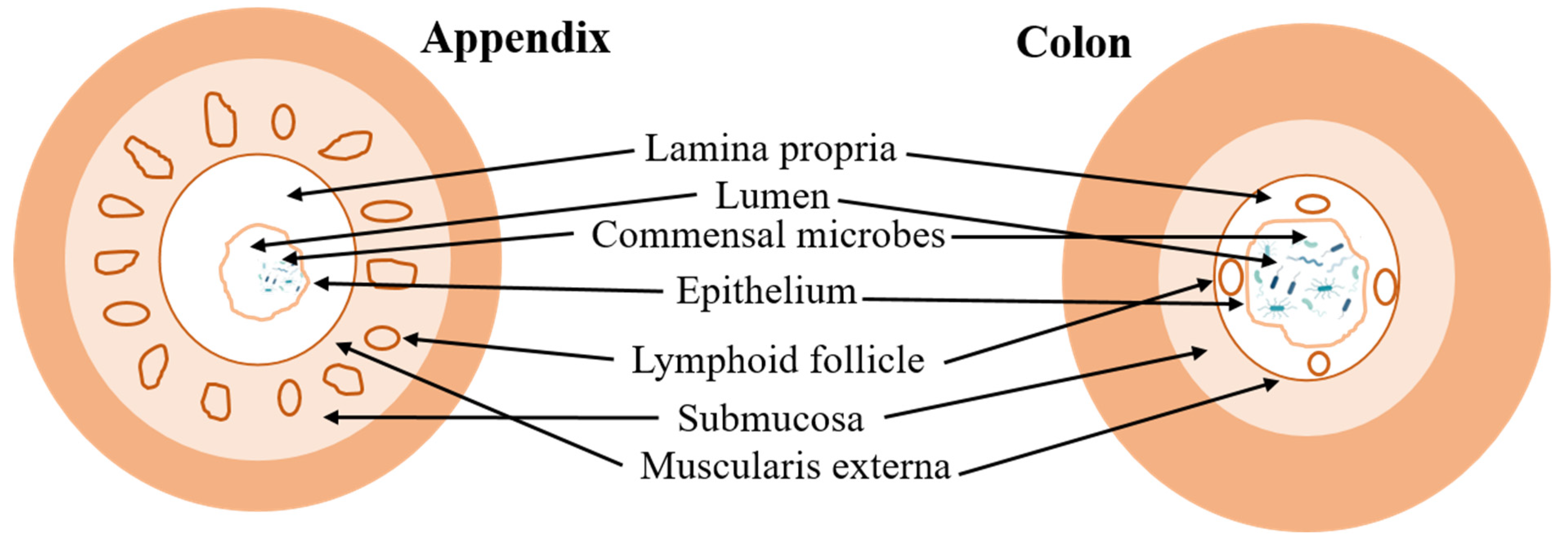
The anatomical positioning of the appendix is notably variable, impacting both diagnostic procedures and surgical intervention. The most common anatomical orientation of the appendix is retrocecal, located posterior to the cecum, accounting for approximately 65–70% of all cases. Alternatively, in about 25–30% of individuals, the appendix descends into the pelvic region, known as the pelvic position. Less frequently, the appendix may adopt other positions such as pre- or post-ileal (5%), subcecal, paracaecal (alongside the cecum), or near the sacral promontory [
4,
5,
6,
7]. These positional variations can influence the clinical presentation and diagnosis of appendicitis, as the location of pain may differ depending on the appendix’s position within the abdominal cavity [
7].
The evolutionary history of the appendix is fascinating and complex. Initially deemed an imperceptible organ with no apparent function, the appendix was thought to be a remnant of a larger cecal structure that aided in the digestion of cellulose when our ancestors had a more herbivorous diet [
8,
9]. However, this view has shifted dramatically in recent years. Research suggests that the appendix has grown autonomously in several mammalian lineages, indicating that it may serve an important purpose [
1,
2,
8,
10]. It appears that the appendix has been part of mammalian anatomy for at least 80 million years, much longer than Darwin’s theory would suggest [
1]. This longevity across evolutionary history implies that the appendix may have conferred a selective advantage.
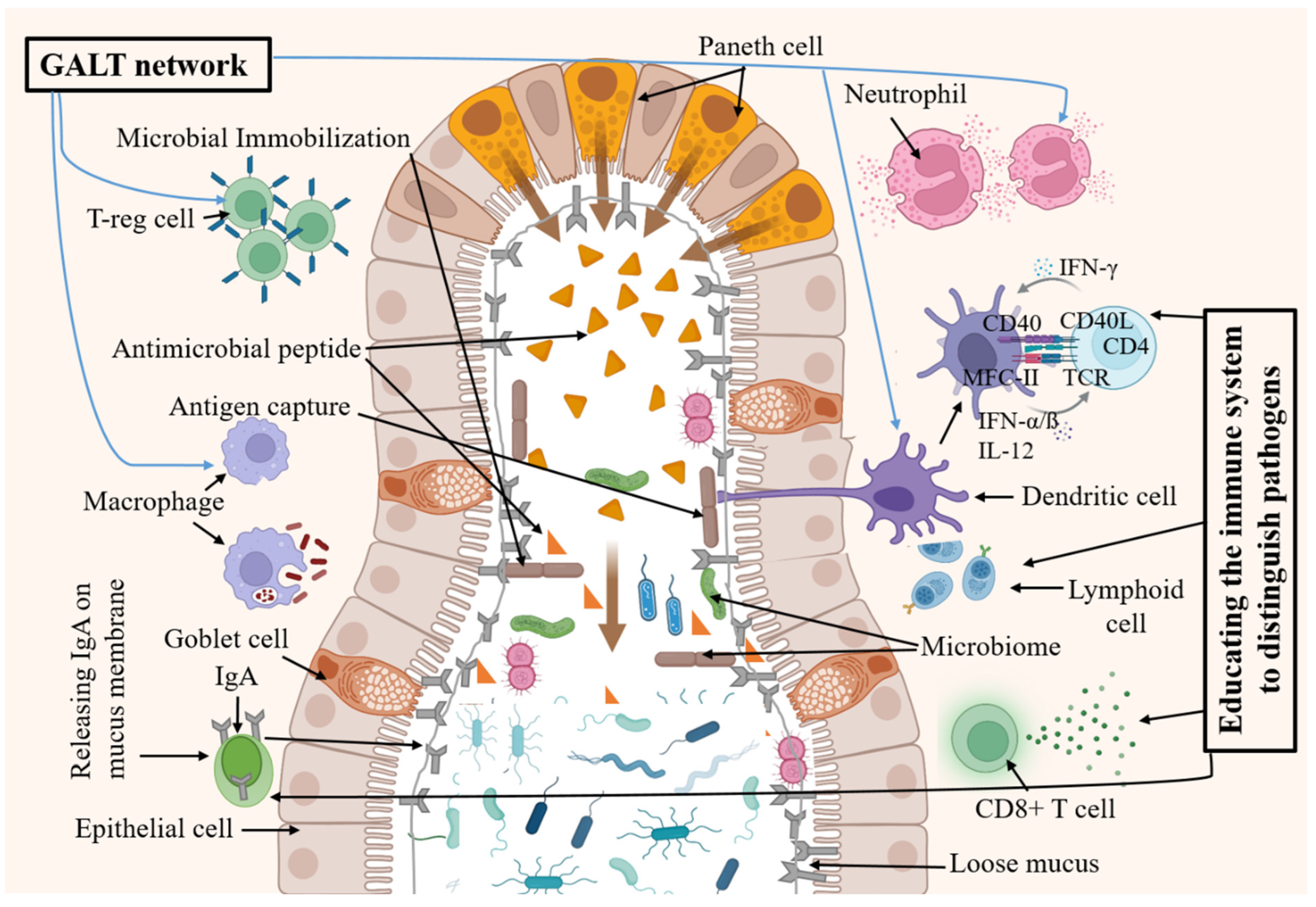
Emerging theories propose that the appendix’s microbiome and its interaction with the gut microbiome are crucial for maintaining a balanced immune response. One of the leading theories today is that the appendix acts as a reservoir for commensal bacteria (probiotics), playing a crucial role in repopulating the gut microbiome (GM) after episodes of gastrointestinal (GI) disease [
11,
12]. This function is supported by the existence of a dense concentration of lymphoid tissue within the appendix, which suggests an immunological role. This “backup” system underscores the appendix’s significance in gut health, suggesting that while we can live without it, its presence may offer subtle yet important benefits [
11,
12]. This lymphatic tissue can encourage the growth of useful gut bacteria and play a part in the body’s immune response. The appendix’s association with the immune system is further supported by its role in the development of B and T lymphocytes and the production of IgA antibodies. These functions are particularly active during the first few years of life, indicating that the appendix may play a role in the development of the immune system in young individuals [
2,
13,
14].
Despite these findings, the exact function of the appendix remains a topic of debate. Some scientists argue that if the appendix were truly vital, its removal would result in more significant health consequences [
14,
15,
16]. However, the lack of observable health problems following an appendectomy does not necessarily mean the appendix is useless; it may indicate that other parts of the body can compensate for its absence [
15,
16]. The appendix is an integral part of the immune system with a significant role in maintaining gut health. Its evolutionary persistence suggests that it provides a beneficial function, possibly related to the GM and immune responses [
2,
9,
10]. As research continues, our understanding of the appendix’s role in human physiology will likely become clearer, potentially leading to new insights into its contribution to our overall health and well-being.
2. Appendix Microbiome Composition in a Healthy Person
Studies investigating the microbial composition of the human appendix have revealed a rich and diverse ecosystem predominantly comprising four major phyla:
Firmicutes,
Bacteroidetes,
Actinobacteria, and
Proteobacteria [
17,
18]. This microbial consortium exhibits dynamic interactions, forming intricate networks that influence various aspects of host physiology, including nutrient metabolism, immune modulation, and pathogen resistance [
12,
19,
20].
Firmicutes are often the most abundant phylum within the AM. They are known for their role in fermenting indigestible carbohydrates, producing short-chain fatty acids (SCFAs) such as butyrate, propionate, and acetate [
21,
22]. Bacterial genera of this phylum include
Ruminiococcus,
Clostridium, and
Lactobacillus [
23]. These SCFAs serve as a primary energy source for colonocytes and exhibit anti-inflammatory properties, which are vital for maintaining intestinal barrier integrity and preventing the invasion of pathogens [
21,
24]. Moreover,
Firmicutes contribute to the regulation of the host’s immune system. They influence the balance of pro- and anti-inflammatory cytokines, which are key mediators in immune response [
24]. This modulation is essential for maintaining gut homeostasis and can impact the development and progression of various infectious and inflammatory diseases [
24]. The
Firmicutes/
Bacteroidetes ratio is also a significant marker of gut health, changing throughout different life stages and reflecting the dynamic nature of the GM [
25,
26].
Firmicutes and
Bacteroidetes account for 90% of human bacterial species [
27]. Alterations in this ratio have been associated with various health conditions, including obesity and metabolic syndrome [
28].
Firmicutes interact with the immune system by impacting the proliferation, growth, and differentiation of epithelial and immune cells [
21,
25]. This interaction is crucial for the proper functioning of the gut’s immune defense mechanisms. Both diversity and functionality in
Firmicutes are essential for optimal gut health. Their ability to produce butyrate supports both immune regulation and the proper functioning of the intestinal lining, highlighting their importance in the overall health of the host [
24,
25,
26,
28].
Bacteroidetes are another significant group within the AM. Bacterial genera of this phylum include
Bacteroides,
Prevotella, and
Porphyromonas, in which
Bacteroides alone account for around 30% of all gut bacteria [
27]. Bacteroidetes are renowned for their ability to degrade complex polysaccharides and proteins, facilitating digestion and nutrient absorption [
12]. Their enzymatic machinery is specialized for breaking down dietary fibers, which are otherwise indigestible by human enzymes, thus playing a pivotal role in the host’s energy harvest [
29]. Beyond digestion,
Bacteroidetes are integral to gut health and immunity. They engage in colonization resistance, a process where they outcompete pathogenic bacteria for adhesion sites and nutrients, and produce bacteriocins to inhibit the growth of potential pathogens [
30]. This competitive exclusion is crucial for maintaining a balanced GM and preventing infections. Moreover,
Bacteroidetes influence the immune system by modulating the production of anti-inflammatory molecules and interacting with the gut’s mucosal immune system [
31]. They help maintain the integrity of the intestinal epithelium, which serves as the first line of defense against external pathogens [
32]. The mode of action of
Bacteroidetes includes the stimulation of immune responses through the production of signaling molecules that can affect immune cell differentiation and function. This interaction is essential for the maturation of a strong immune system capable of responding to a variety of challenges [
33,
34].
Actinobacteria, while less abundant in the AM compared to
Firmicutes and
Bacteroidetes, play a significant role in human health [
35]. Bacterial genera of this phylum include both anaerobic and aerobic bacterial including
Bifidobacteria,
Propionibacteria,
Corynebacteria,
Streptomyces,
Nocardia,
Micromonospora,
Actinoplanes, and
Streptosporangium, of which
Bifidobacteria are most common in the human gut [
36]. They are adept at degrading complex sugars, which is crucial for the digestion of dietary fibers that human enzymes cannot process. This degradation process results in the production of bioactive compounds, including various vitamins and anti-inflammatory substances [
37].
Bifidobacteria, a well-known genus within the
Actinobacteria phylum, are widely recognized as probiotics. They contribute to gut health by enhancing the intestinal barrier, modulating the immune system, and producing lactic acid, which lowers the pH of the gut environment, inhibiting harmful bacteria [
38]. Furthermore,
Bifidobacteria can synthesize essential vitamins such as biotin, folate, and vitamin K, which are vital for the host’s metabolism [
35,
39]. The mode of action of
Actinobacteria involves not only the breakdown of complex carbohydrates but also the interaction with the host’s immune cells. They can influence the maturation and function of immune cells, thereby playing a role in both innate and adaptive immunity. This interaction is particularly important for the development of immune tolerance and the prevention of inflammatory diseases [
40]. Recent studies have also highlighted the role of
Actinobacteria in aging, showing that their abundance and diversity can change with age, impacting the overall health of the individual [
37].
Proteobacteria are a major group (phylum) of bacteria that includes different pathogens, for instance, Brucella, Rickettsia, Bordetella, Neisseria, Escherichia, Salmonella, Vibrio, and Helicobacter [
32,
41]. Proteobacteria form a minor portion [
42]. Desulfovibrio, Escherichia, Klebsiella, and Shigella are the major representative Proteobacteria in the intestinal microbiota. They form a minor portion of the GM, and are most unstable [
43]. While some members are opportunistic pathogens, many non-pathogenic Proteobacteria are beneficial, contributing to microbial diversity and performing essential metabolic functions [
44]. These beneficial Proteobacteria are involved in nitrogen fixation, an important process for recycling nitrogen within the gut ecosystem [
45]. They also participate in the breakdown of complex molecules, which can contribute to nutrient cycling and energy production within the gut [
37,
44]. Moreover, Proteobacteria can influence the immune system. They are involved in the production of molecules like lipopolysaccharides, which can modulate immune responses. Some Proteobacteria are known to stimulate the production of anti-inflammatory cytokines, helping to maintain a balanced immune response and protect against inflammatory diseases [
46]. The mode of action of Proteobacteria in the gut includes their interaction with other microbes and the host’s immune cells. They can compete with pathogenic bacteria, preventing their overgrowth and colonization. This competitive interaction is crucial for maintaining a healthy GM and preventing infections [
47].
Overall, the AM in a healthy person is a complex and dynamic community that plays a significant role in gut health and the immune system. The four major phyla, Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria, contribute to the functionality of the GM, with implications for the gut–brain axis and overall well-being. Understanding the composition and function of the AM is vital for the development of strategies to maintain gut health and prevent diseases related to microbial imbalances.
5. Appendectomy’s Link to Disease Outcome
While appendectomies are generally considered safe, some studies suggest they may be associated with an increased risk of certain conditions. Patients who have undergone appendectomy might have a greater risk of developing inflammatory bowel diseases (IBDs) like Crohn’s disease (CD) [
14]. Additionally, there is a noted higher risk of infections like
Clostridium difficile and sepsis following the procedure [
82,
83]. Though the evidence is inconclusive, some research has also explored a potential link between appendectomy and an increased risk of CRC [
84,
85].
Figure 3 shows the VOSviewer 1.6.20 network analysis combining insights from related research publications on appendectomies and related disease outcomes. Moreover, changes in the composition of the AM have been linked to specific GI disorders, underscoring its significance in human health [
17,
19]. Advancements in sequencing technologies and bioinformatics analyses have modernized our understanding of the diversity and roles of appendix-associated microbes [
17]. Metagenomics studies have unveiled their ability to metabolize diverse dietary compounds, synthesize vital vitamins, and modulate the host’s immune system, highlighting the multifaceted functions of the AM [
86,
87,
88,
89].
5.1. Gut Dysbiosis
The GM, a complex ecosystem of trillions of microorganisms including bacteria, viruses, fungi, and protozoa, primarily resides in the GI tract, with the highest concentration found in the large intestine [
11]. Recent research has shifted perspectives on the human appendix, highlighting its significant role in regulating the intestinal immune system and maintaining a healthy gut microbiome [
19]. This function is particularly important in the context of the gut–brain axis, where the GM has been shown to influence brain function and behavior, potentially impacting neurodevelopmental and psychiatric disorders [
37,
42,
90,
91].
The impact of appendectomy (surgical removal of the appendix) on the GM has been a focus of recent studies. A cross-sectional study comparing individuals with and without a history of appendectomy (HwA and HwoA, respectively) revealed key differences in their gut microbial compositions [
92,
93]. HwA individuals exhibited reduced microbial diversity compared to their HwoA counterparts [
19]. Specifically, beneficial microbes such as
Roseburia,
Barnesiella,
Butyricicoccus,
Odoribacter, and
Butyricimonas were less abundant in HwA samples, which means the appendix has some special microbial content [
19]. These microbes are known for producing SCFAs, which are crucial for gut health. This shift in microbial populations can have far-reaching effects on gut health, potentially increasing susceptibility to GI diseases and affecting the body’s immune response [
19]. Although there is a tendency for the GM to gradually restore its diversity over time post-appendectomy, significant differences remain [
19,
52]. Notably, HwA individuals showed higher gut fungal diversity and more complex fungal–bacterial interactions even five years after appendectomy [
52,
94,
95]. Moreover, the appendix is thought to have a higher concentration of certain beneficial bacteria, such as
Bacteroidetes and
Firmicutes [
95,
96,
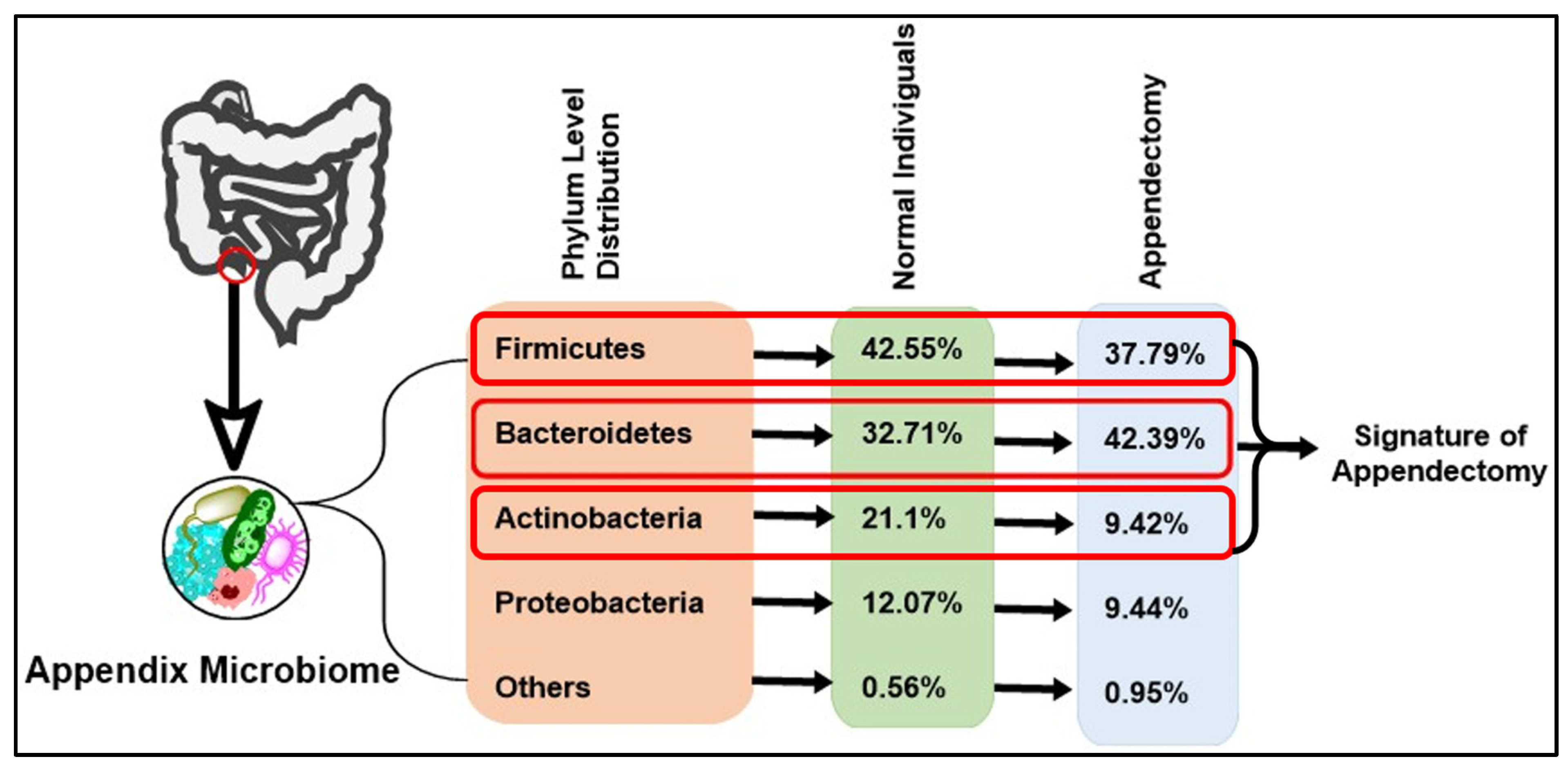
97] (
Figure 4). These bacteria are essential for various functions, including the fermentation of indigestible fibers and the production of SCFAs, which are vital for colon health [
2]. Beneficial bacteria, such as
Lactobacillus and
Bifidobacterium, are essential for maintaining a healthy GM. These “backup” probiotics are then released from the appendix to restore balance within the large intestine, aiding in recovery and maintaining gut health [
19,
66,
95].
5.2. Risk of Colorectal Cancer (CRC)
Emerging research has established a link between appendectomy and an elevated risk of CRC, believed to be influenced by changes in the GM following the procedure [
52,
98,
99]. After an appendectomy, there is a notable increase in CRC-promoting bacteria such as
Bacteroides vulgatus,
Bacteroides fragilis,
Veillonella dispar,
Prevotella ruminicola,
Prevotella fucsa,
Prevotella dentalis, and
Prevotella denticola. These bacteria are associated with promoting colorectal carcinogenesis [
52,
100]. Conversely, beneficial commensals like
Blautia sp. YL58,
Enterococcus hirae,
L. bacterium Choco86,
C. aerofaciens, and
Blautia sp. SC05B48 are found to be depleted in individuals who have undergone an appendectomy [
52,
100]. These commensals are generally associated with maintaining gut health, and their reduction can disrupt the GM balance.
This shift in the GM, marked by an increase in CRC-promoting bacteria and a decrease in beneficial commensals, suggests that GM may play a vital role in the development of CRC caused by appendectomy [
99,
101]. The appendix contains substantial lymphatic tissue associated with immune function, and some studies suggest it may have a protective effect against colorectal carcinoma due to its immunological role [
98,
102]. However, an appendectomy remains the most frequently performed emergency surgical procedure, and recent evidence highlights its potential link to increased CRC risk [
103,
104,
105]. Researchers continue to explore the impact of appendectomy on GM, which is crucial for maintaining intestinal health [
98,
99,
102,
103,
104,
106,
107,
108].
Dysbiosis may also contribute to CRC risk by promoting carcinogenesis through various mechanisms. Direct DNA damage is one of them. Dysbiotic changes in the GM can indeed lead to DNA damage in colonic cells. This process occurs during DNA replication when unrepaired DNA damage in the template strand arrests replication fork progression, ultimately resulting in fork collapse, double-strand break formation, and genome instability [
109,
110,
111,
112,
113]. Dysbiosis may cause inflammation, which later induces immune cells to release Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS), and cause DNA damage. Moreover, GM converts many metabolites from the diet into DNA-damaging agents or oncometabolites. These types of metabolites include p-cresol, secondary bile acids, or N-nitrosamines, which favor mutagenesis and CRC development [
110]. Moreover, genotoxic metabolites produced by some GM promote CRC. For instance, colibactins, hybrid polyketide-nonribosomal peptides, synthesized by
E. coli,
Klebsiella pneumoniae, and other
Enterobacteriaceae harboring the
pks genomic island are genotoxic metabolites that have DNA damaging capability and promote CRC progression [
112]. Altered GM may increase oxidative stress, which is linked to cancer development. While oxidative stress is a natural consequence of cellular metabolism, dysbiosis can exacerbate it, potentially contributing to carcinogenesis [
105,
109,
114,
115,
116].
A recent study reviewed medical records of 455 patients diagnosed with colorectal adenocarcinoma (CRC) over a five-year period [
98]. The results showed the following: (1) Right colon adenocarcinoma (CA): appendectomy has been identified as the second-highest risk factor for right colon adenocarcinoma. According to the study, the risk of developing right colon adenocarcinoma was found to be significantly higher for individuals who had undergone an appendectomy [
98]. (2) Left CA: The same study reported that appendectomy increased the risk by 2.537 times. This suggests a strong association between the surgical removal of the appendix and the development of adenocarcinoma in the left colon [
98]. With regard to (3) rectum adenocarcinoma, the following was found: The risk of rectum adenocarcinoma was increased by 3.232 times following an appendectomy [
98]. This indicates that appendectomy is a notable risk factor for adenocarcinoma in the rectum as well [
98].
Recent research has challenged the conventional view that appendectomy increases the risk of cancer, particularly CRC. A prospective population-based cohort study found that a history of appendectomy was linked to a lower risk of cancer, including GI and colon cancers [
117,
118]. However, not all studies agree on this association; some suggest no overall increase in CRC risk or its subtypes among those who underwent appendectomy (
Table 2) [
102,
119,
120]. Additionally, appendectomy has been linked to improved clinical outcomes in UC but may intensify the risk of advanced colorectal neoplasia [
121].
The topic remains complex, with researchers actively exploring the underlying mechanisms and considering various individual factors. It is crucial to evaluate both sides of the argument and stay informed as new findings emerge. Although mechanisms are not yet clear, current evidence underscores the need for further investigation to clarify the underlying biology and challenge conventional views.
5.3. Risk of Inflammatory Bowel Diseases (IBDs)
The relationship between appendectomies and IBDs has been a subject of interest within the medical community [
127]. Appendectomy has been observed to have a protective effect against the development of UC, a finding supported by multiple studies. However, conflicting evidence exists, as some research suggests that appendectomy may also be associated with an increased risk of IBD development or flares post-diagnosis [
66,
104]. This complexity highlights the ongoing debate regarding whether it is the appendectomy procedure itself or underlying appendicitis that influences IBD susceptibility and progression. Several studies suggest that following an appendectomy, the prevalence of UC was lower, whereas the prevalence of CD was higher than in controls [
102].
Regardless of appendicitis incidence, some studies reported that appendectomies might increase the risk of UC and CD [
128,
129,
130,
131]. Compared to the comparator cohort, the appendectomy cohort showed a 2.23- and 3.48-fold increased risk of UC and CD (adjusted HR = 3.48, 95% CI = 2.42–4.99), respectively [
128]. Notably, the relationship between appendectomies and CD appears to be time-dependent, with an increased risk observed particularly within the initial years following surgery, after which the risk declines over time [
132]. A similar trend has been reported in other studies [
104,
133]. For instance, an appendectomy being performed between the ages of 18 and 29 has been especially linked to a higher incidence of CD (HR = 2.02; 95% CI: 1.66 to 2.44), reinforcing the need to consider the age at surgery when evaluating CD risk [
104]. Furthermore, long-term analyses show that the risk of developing CD remains elevated even five years post-appendectomy (RR = 1.24, 95% CI: 1.12–1.36) [
133]. The relationship between appendicitis, appendectomy, and CD remains controversial [
134]. Some studies suggest that patients who underwent appendectomy due to appendicitis had a modestly increased risk of CD (RR = 1.64, 95% CI: 1.17–2.31), whereas those who had an appendectomy without appendicitis exhibited an even higher risk (RR = 2.77, 95% CI: 1.84–4.16) [
130]. This distinction is crucial, as it suggests that the inflammatory process of appendicitis itself, rather than the surgical removal of the appendix, may contribute to disease development.
Scientific evidence supports the potential role of the appendix in UC pathogenesis and suggests that its removal might influence disease progression [
66,
104]. In contrast to age at appendectomy, the risk of UC appears to decline with increasing time after surgery (HR = 0.21; 95% CI: 0.06 to 0.72, comparing ≥5 with 0–4 years after appendectomy) [
104]. A similar trend has been noted in other studies. Importantly, appendectomy has been found to exert a protective effect against UC, with some reports indicating that the more time elapses post-surgery, the stronger the protective effect [
104]. Moreover, in patients already diagnosed with UC, appendectomy has been associated with a reduction in disease severity, suggesting a potential therapeutic benefit in certain cases [
135]. This interplay between the appendix, gut microbiota (GM), and the immune system further complicates our understanding of IBD risk. Emerging research suggests that appendectomy may influence immune modulation and microbial composition, which could subsequently alter IBD pathophysiology. Furthermore, studies investigating the risk of advanced colorectal neoplasia in IBD patients who have undergone appendectomy provide valuable insights into the long-term consequences of this surgical intervention [
121]. The impact of appendectomy on IBDs is multifaceted and varies depending on the type of IBD, patient age at surgery, underlying appendicitis status, and time elapsed post-appendectomy (
Table 3). While appendectomy appears to be protective against UC, its relationship with CD is more complex and requires further investigation to clarify whether it is appendectomy itself or the inflammatory process of appendicitis that influences CD risk.
5.4. Recurrent Clostridium difficile Associated Colitis
An appendectomy may increase susceptibility to
Clostridium difficile (
C. difficile) colitis due to changes in the GM [
143].
C. difficile colitis occurs due to the overgrowth of
C. difficile bacteria, typically following the disruption of normal gut flora by antibiotic use [
144]. Recurrent
C. difficile colitis is a significant issue in modern medicine, with hospital-acquired cases leading to increased mortality rates [
145,
146]. While it might be expected that the appendix would protect against
C. difficile overgrowth, it is uncertain if the appendix can shield beneficial bacteria from the broad-spectrum antibiotics that precede
C. difficile colitis. The appendix may have evolved to help recover from infections, but its protective role against modern antibiotics remains doubtful [
82].
However, strong evidence from a recent study indicates that the appendix may indeed play a protective role in recurrent
C. difficile colitis [
83]. A previous study found that patients without an appendix had a 2.5-fold increased risk of recurrent
C. difficile colitis compared to those with an appendix. This suggests that the appendix may protect normal gut flora from treatments like oral vancomycin, used for recurrent
C. difficile colitis [
82]. Clinical data indicate that while appendectomy increases the risk of recurrent
C. difficile colitis and potentially other microbiome-related diseases, it does not increase the risk of the initial onset of
C. difficile colitis (
Table 4) [
82]. These observations suggest that the appendix might not effectively protect the microbiome from broad-spectrum antibiotics that lead to the initial onset of
C. difficile colitis.
5.5. Risk of Parkinson’s Disease (PD)
The appendix has been implicated in the gut–brain axis, potentially influencing the pathogenesis of PD through its effect on GM and the immune system. There is conflicting evidence on the impact of GM on PD. For instance, research has suggested that appendectomy might reduce the risk of developing PD [
152]. A substantial study involving health records of around 1.7 million individuals found a 19.3% reduced cumulative incidence of PD among those who had undergone appendectomy [
153]. This could be attributed to the removal of the appendix, which harbors alpha-synuclein aggregates, a pathological hallmark of PD [
154]. The presence of these aggregates in the appendix, even in individuals without PD, implicates the organ as a potential source of neurodegenerative triggers [
152,
155]. Conversely, other studies have reported an increased risk of PD following an appendectomy. Analysis of health data revealed that individuals who had undergone an appendectomy were over three times more likely to develop PD later in life [
156]. This suggests that the appendix might have a protective role against PD, possibly through the regulation of GM and immune surveillance [
157]. Some studies reported that appendectomy may have no relation with PD. The debate over the role of appendectomy in PD is fueled by studies with conflicting outcomes. Some studies, like those conducted by Yilmaz et al. (2017) and Marras et al. (2016), did not find a significant correlation between appendectomy and the onset or clinical progression of PD [
158,
159]. Yilmaz et al. (2017) retrospectively analyzed 1625 patients and concluded that appendectomy did not influence the emergence or clinical characteristics of PD [
158]. Similarly, Marras et al. (2016) investigated a large cohort of nearly 43,000 individuals and also reported no association between appendectomies and decreased risk of developing PD [
159]. These findings suggest that the appendix’s removal does not necessarily alter the disease’s trajectory (
Table 5).
5.6. Risk of Amyotrophic Lateral Sclerosis (ALS)
The relationship between appendectomies and the risk of developing ALS has garnered significant interest in recent research [
168,
169]. Although studies in this area are still ongoing, several important observations have been made. Some research suggests a potential protective effect of appendectomies, indicating a reduced risk of ALS, while other studies find no significant association between the two [
169]. The mechanisms behind any potential link are not yet clear, but it is hypothesized that GM, which plays a crucial role in immune regulation, could be a contributing factor. ALS primarily affects individuals aged 55 and older, although it can occur at any age. Men are slightly more likely than women to develop ALS, though this gender difference decreases with age. Environmental factors, such as exposure to air pollution and certain toxins, may also play a role in increasing ALS risk [
170,
171]. While the connection between appendectomy and ALS risk is not fully understood, ongoing research aims to uncover potential links.
5.7. Risk of Auto-Immune Disease (AD)
Emerging epidemiological research found a potential link between appendectomies and the onset of AD. A pivotal study published in the Journal of the American Medical Directors Association (JAMDA) studies a comprehensive database from the Taiwan National Health Insurance Research Database to track individuals aged 45 and above who experienced acute appendicitis or underwent appendectomy [
164]. Spanning an impressive follow-up duration of over 15 years, this research uncovered a heightened occurrence of AD in patients with a history of appendicitis or appendectomy compared to a matched control group [
164]. Notably, the onset of dementia in these patients was significantly earlier, with a mean age of diagnosis at 70.18 years, roughly 5.84 years post-appendicitis event [
164]. These observations hint at a potential association between appendectomies and an elevated risk of AD.
Further bolstering this association are studies examining the gut–brain axis, which suggest that modifications in the GM may play a role in AD pathogenesis [
164]. Clinical evidence indicates that dysbiosis could influence brain health, potentially contributing to AD development [
172]. In animal models, the transfer of healthy microbiota has been shown to mitigate amyloid and tau pathology, which are hallmark features of AD, suggesting that restoring gut microbial balance could be a therapeutic strategy [
173]. Moreover, soluble alpha-synuclein, a protein implicated in neurodegenerative disorders, has been identified as a novel modulator of AD pathophysiology. Elevated levels of soluble alpha-synuclein may influence cognitive function and are associated with the aggregation of AD-related proteins [
106].
Beyond AD, the appendix has also been implicated in other autoimmune and chronic inflammatory conditions. The appendix plays a key role in immune regulation, acting as a reservoir for commensal gut microbiota and influencing systemic immune responses [
122]. Studies suggest that its removal may contribute to immune dysregulation, potentially increasing susceptibility to conditions such as rheumatoid arthritis, Crohn’s disease, and systemic lupus erythematosus (SLE) [
174,
175]. Moreover, its role in bacterial infections, particularly
Mycobacterium tuberculosis, has been noted, with research indicating that appendiceal tuberculosis, though rare, may be an underrecognized manifestation of extrapulmonary tuberculosis [
176].
The interplay between gut health, immune response, and neurodegeneration underscores the complexity of AD and highlights the need for comprehensive research. Emerging evidence suggests a possible link between appendectomy and an increased risk of AD, but these results should be approached with caution. The mechanisms underlying this association remain unclear, necessitating further investigation to determine causality and elucidate the biological pathways involved. The convergence of epidemiological data and preclinical insights offers a promising avenue for understanding the etiology of AD and exploring novel preventive and therapeutic approaches.
5.8. Risk of Heart Disease
According to earlier research, genetically anticipated appendectomies may increase the chance of developing ischemic heart disease (IHD) and its varieties, angina pectoris (AP) and acute myocardial infarction (AMI) [
177,
178,
179]. Using Mendelian randomization (MR) research techniques and meta-analysis, the study demonstrated a strong positive causal link between appendectomy and IHD and its varieties, AMI and AP. IHD (OR: 1.128, 95% CI: 1.067–1.193,
p =2.459 × 10
−5), AMI (OR: 1.195, 95% CI: 1.095–1.305,
p = 6.898 × 10
−5), and AP (OR: 1.087, 95% CI: 1.016–1.164,
p = 1.598 × 10
−2) were all positively correlated with appendectomies, according to the paper [
177]. An elevated risk of recurrent IHD within three years following appendectomy was observed by another researcher [
178]. They revealed that having an appendectomy at the age of 18 or older was independently linked to a 1.54-fold higher risk of IHD throughout the course of the 3-year follow-up (95% CI = 1.29–1.84) [
178].
Childhood appendectomy and risk for premature acute myocardial infarction (AMI) was accessed in a cohort study. With adjusted HRs of 1.33 [95% CI: 1.05–1.70] for appendectomy, surgeries performed before the age of 20 were linked to a higher risk of AMI (417 occurrences as a result of the appendectomy). Additionally, the study indicated that there was no correlation between the incidence of AMI with an appendectomy performed at or after the age of 20 [
180]. According to a recent study, children with asthma are more likely to have appendicectomies than people of all ages. Compared to the control group, the appendectomy group had increased adjusted risks of asthma (adjusted OR 1.18, 95% CI 1.13–1.23,
p <.001) [
181].
5.9. Risk of Type 2 Diabetes
Appendectomy increases type 2 diabetes risk, but as an age-dependent consequence [
179]. A cohort study reported that individuals who had undergone appendectomy had a 7.9% greater overall incidence of type 2 diabetes than individuals who did not have the procedure. Compared to individuals who did not have an appendectomy, those under 30 years of age had an adjusted hazard ratio (HR) of 1.347 for type 2 diabetes (95% CI, 1.009–1.798). Compared to individuals who did not have an appendectomy, the incidence of type 2 diabetes was greater within three years of the follow-up (HR, 2.017; 95% CI, 1.07–3.802). In contrast, sex had no effect on the link between appendectomy and type 2 diabetes risk (
p < 0.88), while age had a significant influence (
p < 0.002) [
182].
Patients in the appendectomy group who had preoperative blood glucose (POBG) values of ≥123 mg/dL (adjusted relative risk [aRR] 1.19; 95% CI 1.06–1.33) were 19% more likely than those with POBG levels of <106 mg/dL to have a hospital length of stay (LOS) of more than three days. The study showed that among patients having an appendectomy, a higher POBG level was significantly linked to a longer LOS [
183].
5.10. Risk of Other Complications
Laparoscopic appendectomy (LA) is a new surgical procedure used to remove an infected appendix. Following a laparoscopic appendectomy, surgical site infections (SSI) are prevalent and can lengthen hospital stays. According to recent research, 45 patients (10.9%) experienced incisional SSI overall following LA. The probability of incisional SSI was independently correlated with the visceral fat area (HR 1.015, 95% CI 1.010–1.020,
p < 0.001) [
184].
According to certain research, the appendectomy groups had a higher pregnancy rate (first birth or first pregnancy) than the control groups [
185,
186,
187]. For the appendectomy groups, the adjusted HRs for first pregnancy events that showed higher pregnancy rates were 1.20 (95% CI: 1.10–1.31) [
186], 1.34 (95% CI: 1.32–1.35) [
187], and 1.54 (95% CI: 1.52–1.56) [
185]. Another study found that the pregnancy rate was higher following appendectomy for non-perforated appendicitis (HR = 1.11, 95% CI: 1.07–1.15) and after removing a normal appendix (HR = 1.48, 95% CI: 1.42–1.54), but not after perforated appendicitis (HR = 0.95, 95% CI: 0.88–1.04). In a recent study, open appendectomy was found to be safe for patients, but increased rates of cesarean delivery [
188].
Studies have previously been conducted to assess the short-term and long-term mortality rates after appendectomy undertaking. The median overall fatality rate in the studies comparing open and laparoscopic procedures was 1.7% (range: 0.6–7.6%). Using predicted survival estimates from the Swedish population, the five-year follow-up study computed standardized mortality ratios [
189]. The exposed cohort (appendectomy) saw fewer fatalities than anticipated when compared to the background population. With a standardized mortality ratio of 0.71 (95% CI: 0.67–0.75), the difference in mortality was particularly noticeable for non-perforated appendicitis. The median fatality rates for the open and laparoscopic procedures were 1.8% (range: 0.6–8.6%) and 0.9% (range: 0.3–3.6%), respectively. In two of the investigations, the laparoscopic group’s mortality rate was considerably lower than that of the control group [
189,
190].
Table 6 summarizes the research on appendectomy on heart disease, type 2 diabetes, and other complications.
6. Microbe-Based Therapies
Appendectomy leads to gut dysbiosis, resulting in IBDs, neurological disorders, ADs, and so on. Different methods aiming to adjust the gut microbial composition and restore dysbiosis have been explored as a means of managing the clinical conditions caused by gut dysbiosis. Examples of such methods include probiotics, prebiotics, postbiotics, symbiotics, fecal microbiota transplantation (FMT), phage-based therapeutics, and engineered microbes [
202].
Probiotics are good bacteria and yeast that are beneficial to the health. Prebiotics are high-fiber foods that help the growth of beneficial intestinal microbes. Postbiotics are beneficial metabolites or byproducts produced by gut microbes that have various beneficial health impacts [
202]. The use of prebiotics, probiotics, and postbiotics is of great interest to the scientific community for the treatment of complications associated with gut dysbiosis (
Table 7). Extensive use of antibiotics causes gut microbial dysbiosis and the emergence of multidrug-resistant pathogens [
203,
204,
205]. The use of antibiotics during pregnancy may also impact the GM of infants, resulting in different GM-associated complications. A recent study showed that prebiotics in mothers could be an amazing alternative for reducing the disease severity in infants [
206]. The use of multi-strain probiotics is one of the most-used microbe-based methods in clinical trials, which has a great impact on the functional diversity of the gut microbiota during pregnancy, reducing obesity and gastrointestinal complications in CRC patients, overcoming skin inflammation, and improving neurological disorders [
206,
207,
208,
209,
210] (
Table 7).
FMT has garnered a lot of interest and investigation lately as a dysbiosis remedy, and different disease management strategies. FMT directly affects the gut microbiota by transferring the complete intestinal microbiota from a healthy donor to the recipient in the form of feces using colonoscopy, enema, nasogastric (NG) tube, or capsule form [
211,
212,
213]. Recent clinical trials showed that
L. acidophilus transplanted by FMT reduced the cholesterol level in patients by inhibiting hepatic Cholesterol 7α-hydroxylase, restoring ileum Fibroblast growth factor 15 and small heterodimer partner proteins [
214]. FMT of the mixed microbial population helped to reduce insulin resistance and interrupted CDI recurrence in the recipients [
211,
212].
Table 7.
Microbe-based therapies under clinical trial to treat diseases caused by gut dysbiosis.
Table 7.
Microbe-based therapies under clinical trial to treat diseases caused by gut dysbiosis.
| Therapy Method | Used Microbes | Disease Focus | Key Findings | References |
|---|
| Multi-strain probiotics | Streptococcus thermophilus NCIMB 30438, B. breve NCIMB 30441, B. lactis NCIMB 30435, B. lactis NCIMB 30436, L. acidophilus NCIMB 30442, L. plantarum NCIMB 30437, L. paracasei NCIMB 30439, and L. helveticus NCIMB 30440 | Obesity | Pregnant women treated with multi-strain probiotics gave birth to a child who had decreased obesity-associated genera, Collinsella. Vaginally delivered infants had an increased number of Bifidobacterium and Bacteroides, and a decreased number of Enterococcus compared to the control group. | [206] |
| Multi-strain probiotics | L. rhamnosus (CECT 30031) and the cyanobacterium Arthrospira platensis (BEA_IDA_0074B) | Skin inflammation | Of the patients using the Global Acne Grading System, 17/40 (42.50%) were in the probiotic group (p = 0.02), indicating that the probiotic used in this study was effective and well accepted. | [208] |
| FMT | L. acidophilus | Cholesterol level | Inhibited hepatic Cholesterol 7α-hydroxylase, restored ileum Fibroblast growth factor 15, and small heterodimer partner. | [214] |
| Probiotics | Liver function | L. acidophilus supplementation promoted the recovery of liver function. |
| Multi-strain probiotics | B. longum, L. delbrueckii bulgaricus, and S. thermophilus | Impact of probiotics on the functional diversity of the gut microbiota during pregnancy | Significantly increased the genera Blautia Ruminococcus, and Subdoligranulum (p < 0.05). The functional genes of the gut microbiota involved in ABC transporters, oxidative phosphorylation, folate biosynthesis, and biotin metabolism were significantly increased in subjects receiving the probiotics (p < 0.05). | [207] |
| Probiotics | B. longum CECT 7347 (ES1) | Irritable bowel syndrome | Responder rates, stool consistency, abdominal pain severity, and anxiety, when compared to placebo over an 84 d time period. | [215] |
| Postbiotics | Heat-treated B. longum CECT 7347 (HT-ES1) |
| Probiotics | B. longum NCC3001 | Irritable bowel syndrome (IBS) | Improvements in anxiety and depression scores and a decrease in amygdala activation were observed. Moreover, the levels of butyric acid, tryptophan, N-acetyl tryptophan, glycine-conjugated bile acids, and free fatty acids were increased. | [210] |
| Probiotics | B. breve M-16 V | Human mode | B. breve M-16 V improved mood and sleep scores, decreased the heart rate under stress, and increased levels of pipecolic acid in stool samples, and improved mood and sleep scores in participants with high anxiety levels. | [216] |
| Probiotics | Lacticaseibacillus rhamnosus LRa05 | Helicobacter pylori eradication | Did not improve H. pylori eradication significantly, but improved liver activity and reduced the levels of inflammatory markers like IL-6 and TNF-α. | [217] |
| Multi-strain probiotics | B. infants, L. acidophilus, E. faecalis, and Bacillus cereus | Gastrointestinal complications in CRC patients | Increased levels of Bifidobacterium, Streptococcus, and Blautia. Significantly Increased levels of SCFAs, mainly increasing acetate, butyrate, and propionate (p < 0.0001). | [209] |
| FMT | Mixed microbial population | Obesity | Significantly altered recipients’ phage and general microbial composition, suggesting that phages play an important role in changing the gut environment and thereby obesity. | [218] |
| Capsulized FMT | Mixed microbial population | UC | Remission induced in patients with UC by increasing the levels of Alistipes sp. and Odoribacter splanchnicus, and due to increased levels of indolelactic acid. Subjects without remission exhibited increased levels of E. coli and Klebsiella, and higher levels of 12,13-dihydroxy-9Z-octadecenoic acid and lipopolysaccharides. | [213] |
| FMT | Mixed microbial population | Insulin resistance | FMT with or without metformin significantly improved insulin resistance, body mass index, and gut microbial compositions of type 2 diabetes patients | [212] |
| FMT | Mixed microbial population | CDI | Recurrent CDI patients with sustained resolution after FMT had increased levels of Ruminococcaceae and Lachnospiraceae, and depletion of Enterobacteriaceae | [211] |
| Engineered bacteria | E. coli Nissle 1917 (EcN) | CRC | Oral administration of EcN as a probiotic increased their presence in the tumor cell the level of salicylate in the urine of adenoma-bearing mice was increased indicating that EcN could be used to treat CRC. | [219] |
There have been conflicting outcomes where probiotics, prebiotics, postbiotics, and FMT are used. For example, some probiotic strains have been shown to help manage UC in certain randomized controlled studies, while CD has not consistently shown the same advantages [
202]. It is difficult to draw firm conclusions on the efficacy of probiotics due to the wide variation in study designs, probiotic strains, doses, and patient demographics. Additionally, several studies have shown that FMT is useful in treating IBDs; however, the degree of success varies greatly. Furthermore, there is a continuous discussion about the long-term consequences and safety of microbiome manipulation, particularly when using aggressive techniques like FMT [
220]. The possibility of spreading harmful bacteria as well as the long-term effects on the recipient’s immune system and metabolic functions are causes for concern.