Mineralocorticoid Receptor Antagonists and Cognitive Outcomes in Cardiovascular Disease and Beyond: A Systematic Review
Abstract
1. Introduction
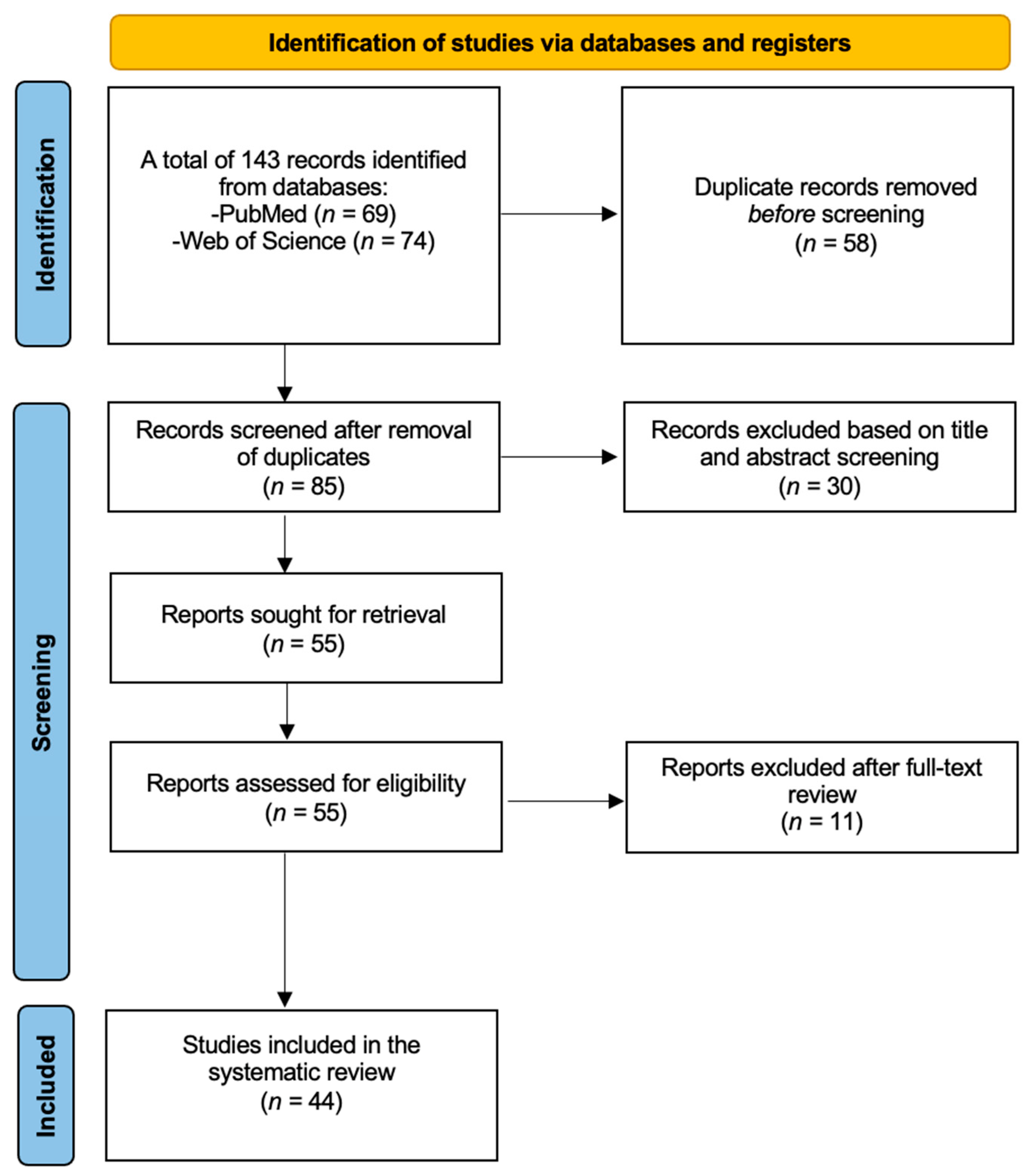
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection and Data Extraction
2.4. Data Synthesis
2.5. Risk of Bias
3. Results
3.1. Preclinical Studies
| Authors | Year | Study Type | Species | Experimental Model | Exposure of Interest | Outcome | Main Findings |
|---|---|---|---|---|---|---|---|
| Yau et al. [10] | 2011 | Preclinical study | Mouse/Rat | Aged 11βHSD1 deficient mice and control mice | MRA (spironolactone) and GR antagonist (RU486) | Spatial memory | Spironolactone did not improve cognitive decline. It impaired memory in mice with previously intact spatial memory (11βHSD1 deficient mice) and had no improvement in the control mice with already impaired memory. |
| Atucha et al. [11] | 2015 | Preclinical study | Mouse/Rat | 5 to 7 rats receiving C118335 and 5 to 7 rats as control receiving vector | Selective GR modulator and MRA (C118335); GR antagonist (RU486); MRA (spironolactone) | Memory consolidation | Spironolactone impaired memory consolidation when administered post-training. |
| Zhou et al. [12] | 2011 | Preclinical study | Mouse/Rat | Healthy mice undergoing fear conditioning | MRA spironolactone; GR antagonist RU486 | Effects on fear memory retrieval (contextual and tone-cued fear) | Spironolactone reduced the expression of contextual fear memory when administered prior to retrieval but had no effect on tone-cued fear memory. |
| Smythe et al. [13] | 1997 | Preclinical study | Mouse/Rat | 10 mice per group in experiment 1, 6 mice per group in experiment 2 | MRA spironolactone in rats with scopolamine-induced cognitive impairment | Cognitive performance in a water maze task (memory and spatial learning) | Systemic spironolactone improved cognitive performance in rats with scopolamine-induced cognitive impairment |
| Yau et al. [14] | 1999 | Preclinical study | Mouse/Rat | Male lister hooded rats divided in 4 groups: MRA, GR antagonist, acute swim stress, control | GR antagonist RU38486/mifepristone, MRA spironolactone, acute swim stress | Spatial learning and memory in water maze | Chronic MR blockade with spironolactone impaired spatial memory retention, decreasing time spent in the target quadrant, suggesting MR activation supports cognitive function. Acute swim stress elevated corticosterone levels, impairing spatial memory retention and reducing time spent in the target quadrant, suggesting that intense activation of stress pathways negatively affects cognitive function. |
| Avital et al. [15] | 2006 | Preclinical study | Mouse/Rat | Male Wistar rats, divided in 4 groups: MRA, GR antagonist, combined MR and GR antagonist, control | GR antagonist (RU38486/mifepristone), MRA (spironolactone), acute swim stress | Hippocampal plasticity (long term potentiation) | MR blockade impaired LTP, supporting that MR activation is essential for maintaining plasticity, particularly under stress. |
| Schwabe et al. [16] | 2010 | Preclinical study | Mouse/Rat | Male C57BL/6J mice, 12 weeks old | MRA (RU28318), restraint stress, corticosterone injection | Shift between spatial and stimulus–response (S-R) learning | Stress or corticosterone facilitated a switch from hippocampus-based spatial memory to caudate nucleus-based S-R memory, enhancing performance under stress. Spironolactone prevented the stress-induced shift to a habit-based memory system, leading to poorer spatial memory performance, suggesting that MR activation enables adaptive cognitive switching under stress, which supports cognitive resilience. |
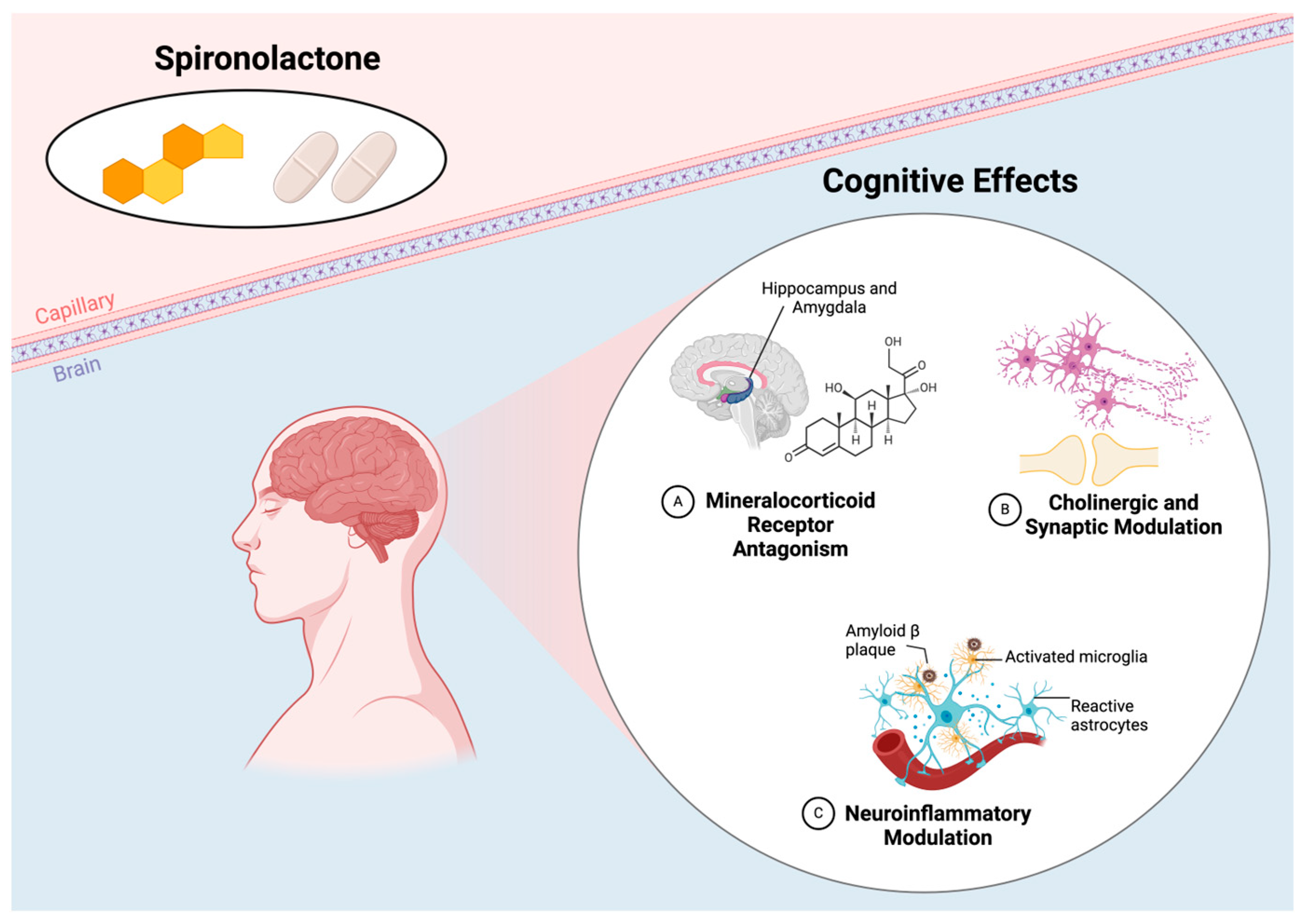
| Douma et al. [17] | 1999 | Preclinical study | Mouse/Rat | 42 male Wistar rats divided into 4 groups: sham group, ADX group, corticosterone replacement therapy, MRA group, GR antagonist group | MRA (RU28318), GR antagonist (RU38486), adrenalectomy | Muscarinic receptor expression in hippocampus | MRA increased muscarinic receptor expression in hippocampus regions CA1 and CA3 while GR antagonism had no effect, suggesting that blocking MR (as with spironolactone) disrupts cholinergic modulation in the hippocampus, potentially impairing cognitive function. |
| Maggio et al. [18] | 2009 | Preclinical study | Mouse/Rat | Male Wistar rats, 2–3 weeks old | Spironolactone (MRA), corticosterone, aldosterone, dexamethasone | Inhibitory synaptic currents in hippocampus | Spironolactone blocked corticosterone’s MR-mediated effects in the ventral hippocampus, reducing inhibitory signaling, likely increasing excitability in that region. Therefore, it disrupted the hippocampal balance necessary for optimal cognitive function under stress, suggesting it could potentially worsen cognitive decline. |
| Thai et al. [19] | 2013 | Preclinical study | Mouse/Rat | Male Sprague Dawley rats; groups: control, acute stress on set-shifting, acute stress on reversal | MRA (spironolactone) and GR antagonist (RU38486) with stress | Behavioral flexibility in learning | Spironolactone did not impact the facilitation of reversal learning by acute stress, suggesting that MR activation or blockade did not play a role in the observed cognitive effects. |
| Wang et al. [20] | 2020 | Preclinical study | Mouse/Rat | SHRs treated with eplerenone (n = 10), untreated SHRs (n = 10), control Wistar–Kyoto rats (n = 10) | MRA (eplerenone, 50 mg/kg/day) | Brain tissue changes in the hippocampus | Eplerenone reduced aldosterone levels, prevented cortical thinning, and reduced apoptosis in SHRs, suggesting that MR antagonism can protect against aldosterone-induced brain damage in hypertension. |
| Sakata et al. [5] | 2012 | Preclinical study | Mouse/Rat | Female KKAy (T2DM model) mice, WT controls | MRA (spironolactone, 50 mg/kg/day) | Cognitive function (Morris Water Maze, shuttle avoidance test) | Spironolactone improved cognitive function in female diabetic mice, suggesting MR antagonism mitigates cognitive decline associated with type 2 diabetes, especially in females, possibly through MR-mediated mechanisms. |
| Hira et al. [21] | 2020 | Preclinical study | Mouse/Rat | STZ-induced Alzheimer’s model, different treatment doses | MRA (eplerinone) | Cognitive performance, AChE inhibition | Eplerenone treatment improved memory and reduced AChE activity and neuroinflammation in an STZ-induced Alzheimer’s model, indicating potential cognitive protection through MR antagonism in Alzheimer’s disease models. |
| Martisova et al. [22] | 2012 | Preclinical study | Mouse/Rat, Cell Culture | Early-life stressed rats (maternal separation), SHSY-5Y neuroblastoma cells | MRA (spironolactone), corticosterone, JNK inhibitor | BACE expression, amyloid pathology | Spironolactone blocked corticosterone-induced increases in BACE and pJNK expression, suggesting MR antagonism may mitigate amyloidogenic processes, although direct effects on cognitive decline were not assessed. |
| Dorey et al. [23] | 2011 | Preclinical study | Mouse/Rat | Male C57BL/6 mice, intra-hippocampal injections | MRA (RU-28318), corticosterone | Memory retrieval in delayed alternation task | MR antagonism with RU-28318 blocked corticosterone-induced memory retrieval impairment, suggesting that MR activation in the dorsal hippocampus contributes to stress-related cognitive decline. |
| Solas et al. [24] | 2013 | Preclinical study | Mouse/Rat | Male C57BL/6 mice, 3 months old | MRA (spironolactone), chronic corticosterone treatment | Cognitive performance (novel object recognition) | Spironolactone reversed corticosterone-induced cognitive impairments and insulin resistance, suggesting MR antagonism may mitigate stress-related cognitive deficits. |
| Diaz-Otero et al. [25] | 2018 | Preclinical study | Mouse/Rat | Male C57Bl/6 mice with angiotensin II-induced hypertension | MRA (eplerenone) | Cognitive function (Barnes maze, novel object recognition) | Eplerenone prevented cognitive dysfunction in hypertensive mice by improving arteriole dilation and reducing microglia density, indicating MR antagonism may protect cognition by enhancing cerebrovascular function. |
| Chambers et al. [26] | 2022 | Preclinical study | Mouse/Rat | Male SHRSP rats with hypertension, Sprague Dawley controls | MRA (eplerenone) | Cognitive function (Y-maze), neuroinflammation markers | Eplerenone improved spatial memory and reduced neuroinflammation, indicating that MR antagonism may protect against cognitive decline and vascular damage in hypertension. |
| Pires et al. [27] | 2018 | Preclinical study | Mouse/Rat | Sprague Dawley rats, high-fat diet model | MRA (canrenoic acid, active spironolactone metabolite) | Cerebral artery remodeling, white matter injury | MR antagonism prevented artery remodeling and reduced white matter injury in obesity, suggesting a protective effect of MRAs like spironolactone against obesity-related cerebrovascular and cognitive decline risks. |
| Chen et al. [6] | 2020 | Preclinical study | Mouse/Rat | Swiss albino mice, amyloid-beta induced AD model | MRAs (spironolactone, eplerenone) | Cognitive performance (Morris water maze) | Spironolactone and eplerenone improved learning and memory in an Alzheimer’s model, likely by increasing BDNF, H2S, and Nrf2 levels, decreasing amyloid-beta, and reducing neuroinflammation in the brain. |
| Loscertales et al. [28] | 1998 | Preclinical study | Chick | Day-old chicks, passive avoidance task model | Nootropic (piracetam), MRA (RU28318), GR antagonist (RU38486) | Memory retention in passive avoidance task | The memory-enhancing effect of piracetam on long-term retention in a passive avoidance task was blocked when MR antagonist was administered. |
| Douma et al. [29] | 1998 | Preclinical study | Mouse/Rat | Adult male Wistar rats, divided into vehicle, MRA(RU28318), GR antagonist (RU38486), and combined MR + GR antagonist groups | MRA (RU28318), GR antagonist (RU38486) | Spatial learning in food-rewarded task | MR antagonism with RU28318 impaired reference memory and delayed working memory acquisition in spatial learning, suggesting MR activation is crucial for optimal memory function in spatial tasks. |
| Stephan et al. [30] | 2022 | Preclinical study | Mouse/Rat | Tcf4 transgenic mice with social defeat, treated with spironolactone, aripiprazole, or both | MRA (spironolactone) | Reversal learning, working memory | Spironolactone alone improved reversal learning in the schizophrenia model; however, co-treatment with aripiprazole reduced this benefit, highlighting spironolactone’s potential for specific cognitive improvements in schizophrenia. |
| Wang et al. [31] | 2013 | Preclinical study | Mouse/Rat | Male adult Wistar rats, early-life stress (maternal separation) | MRA (eplerenone) | LTP in hippocampus | MR antagonism blocked stress-induced LTP in the hippocampus, showing MR’s essential role in stress-related memory formation and indicating that MR activation supports cognitive resilience. |
| Dorey et al. [32] | 2012 | Preclinical study | Mouse/Rat | Male C57BL/6 mice, subjected to acute stress and treated with MRA (RU-28318) or GR antagonist (RU-38486) | MRA (RU-28318), GR antagonist (RU-38486) | Memory retrieval in delayed alternation task | MR antagonism prevented immediate (15 min post-stress) memory impairments, while GR involvement was delayed. Therefore, MR activation is critical for rapid memory retrieval under stress, with MR antagonism preventing early stress-induced memory deficits, while GR influences memory at later stages. |
| Mehdipour et al. [33] | 2022 | Preclinical study | Mouse/Rat | Male Sprague Dawley rats with Aβ injection to induce memory impairment | MRA (spironolactone at doses of 10, 25, and 50 mg/kg) | Memory performance, microglial activation (Iba1 protein) | Spironolactone reduced microglial activation (Iba1 levels) but did not improve memory impairment in β-amyloid-induced AD model, suggesting its anti-inflammatory effects without direct cognitive benefits. |
| Albernaz-Mariano et al. [34] | 2022 | Preclinical study | Mouse/Rat | Male Wistar rats, subjected to restraint stress and IL-mPFC spironolactone infusion | MRA (spironolactone) | Aversive memory extinction, corticosterone release | Infralimbic spironolactone blocked stress-induced corticosterone increase and prevented impairment in aversive memory extinction, suggesting MR antagonism supports adaptive extinction of stress-related memories. |
| Licata et al. [35] | 2022 | Preclinical study | Human cell lines, iPSC-derived motor neurons, Drosophila model | Cells and fly models expressing neurotoxic DPRs | MRA (spironolactone) | DPR levels and neuroprotection | Spironolactone decreased DPR levels by promoting autophagy-mediated degradation, suggesting potential protective effects against neurotoxicity in C9ALS/FTD, though its effects on cognitive decline specifically were not assessed. |
3.2. Clinical Studies
3.2.1. Healthy Volunteers
| Authors | Year | Study Type | Population | Exposure | Outcome | Main Findings |
|---|---|---|---|---|---|---|
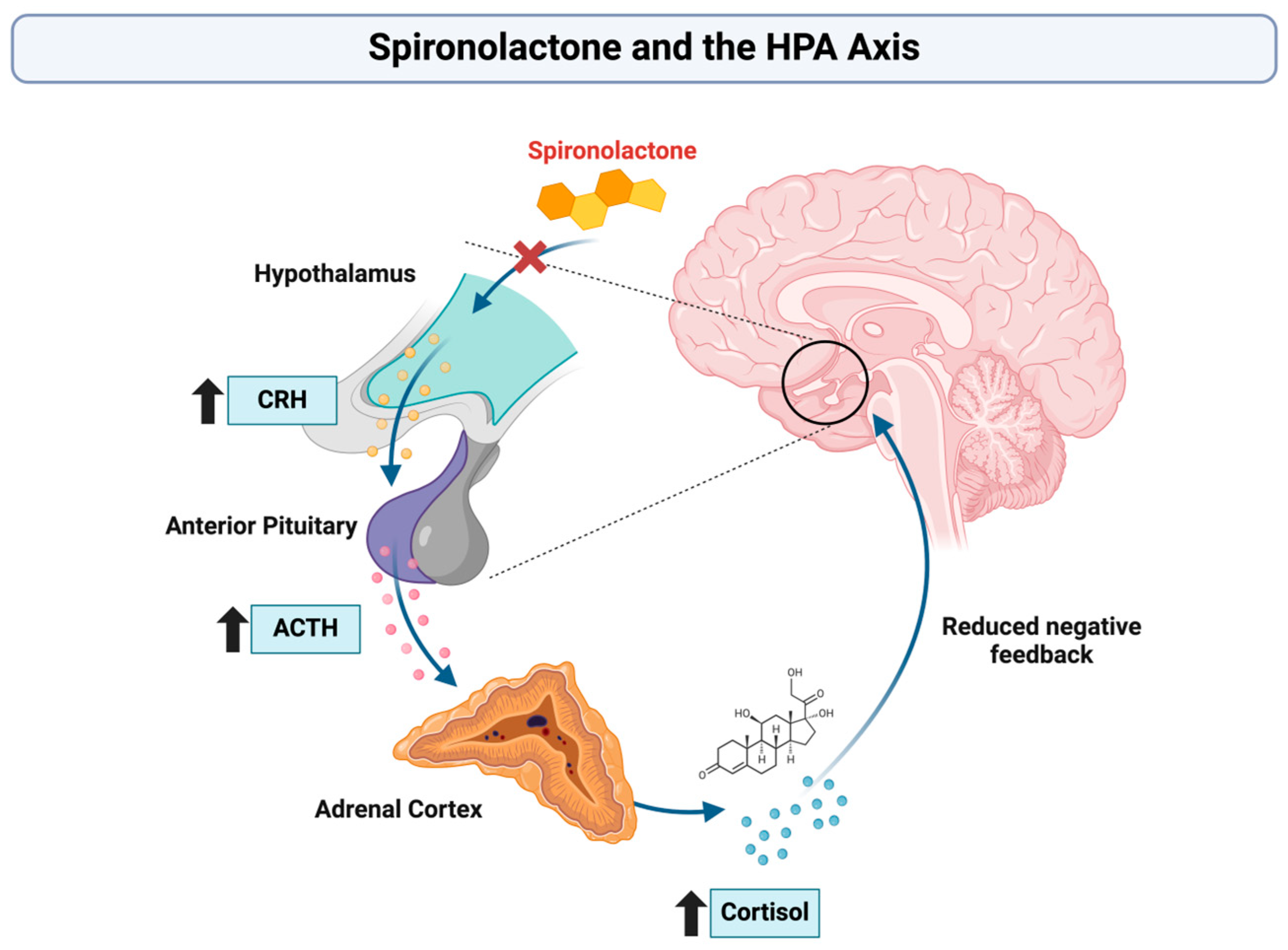
| Cornelisse et al. [37] | 2011 | Randomized controlled trial | 64 healthy young men divided into 4 groups: spironolactone-stress, spironolactone-no stress, no spironolactone-stress, no spironolactone-no stress | 400 mg of spironolactone (MRA) versus placebo, combined with TSST or control task | Cognitive performance (selective attention, working memory, long-term memory), cortisol response, stress response | Spironolactone increased cortisol, impaired selective attention and working memory under stress, but improved long-term memory. |
| Otte et al. [38] | 2007 | Randomized placebo-controlled cross-over study | 16 healthy young men divided into spironolactone and placebo groups, after one week washout inverted | 300 mg of spironolactone (MRA) versus placebo, combined with a panic-inducing compound (CCK-4) | Effects on panic symptoms, cortisol/ACTH levels, and cognitive function (selective attention, visuospatial memory, set shifting) | Spironolactone impaired selective attention, visuospatial memory, and flexibility; increased baseline cortisol but had no effect on panic symptoms. |
| Vogel et al. [41] | 2015 | Randomized controlled trial | 101 young healthy men, divided into 4 groups: stress-spironolactone, stress-placebo, control-spironolactone, control-placebo | Spironolactone (MRA) vs. placebo combined with stress induction | Effects on brain connectivity, specifically amygdala-striatal connectivity under stress | Spironolactone preserved cognitive control by preventing stress-induced amygdala–striatum connectivity shifts. |
| Schwabe et al. [42] | 2013 | Randomized controlled trial | Healthy participants, spironolactone vs. placebo | MRA (spironolactone, 300 mg) | Memory system engagement under stress | Spironolactone blocked stress-induced learning shifts to striatum, impairing cognitive flexibility. |
| Vogel et al. [39] | 2017 | Randomized controlled trial | Healthy male participants, divided into MR-blocked (spironolactone) and control groups with/without stress | MRA (spironolactone, 400 mg) | Spatial memory strategy (fMRI-based) | Spironolactone prevented stress-induced spatial memory shifts to striatum. |
| Schwabe et al. [43] | 2013 | Randomized controlled trial | Healthy adults, four groups: stress vs. control and spironolactone vs. placebo | MRA (spironolactone, 300 mg) | Response inhibition in stop-signal task | Spironolactone blocked stress-induced improvement in response inhibition. |
| Young et al. [44] | 2016 | Randomized, counter-balanced within-subjects design | 10 healthy male participants | MRA (spironolactone, 600 mg), GR antagonist (mifepristone, 600 mg) | Autobiographical memory recall, amygdala response to emotional faces | Spironolactone impaired memory specificity and increased amygdala response to sad faces. |
| Vogel et al. [40] | 2015 | Randomized controlled trial | 101 healthy men divided into 4 groups | 400 mg of spironolactone (MRA) versus placebo, combined with stress (cold pressor test) | Effects on fear learning, memory consolidation (trace vs. delay conditioning), and neural activation | Spironolactone prevented cognitive decline under stress and blocked the stress-induced shift to amygdala-based learning. |
3.2.2. Individuals with Psychiatric Disorders
| Authors | Year | Study Type | Population | Exposure | Outcome | Main Findings |
|---|---|---|---|---|---|---|
| Hasan et al. [46] | 2020 | Randomized controlled trial | 90 patients with schizophrenia | Spironolactone 100 mg, 200 mg, or placebo added to standard antipsychotic treatment | Changes in working memory, cognitive functions | Ongoing study evaluating spironolactone’s effect on working memory deficits in schizophrenia. |
| Hinklemann et al. [49] | 2012 | Longitudinal cohort study | 102 participants: 52 patients diagnosed with depression, 50 healthy control individuals | SSRI treatment and an add-on treatment modulating the MRA (spironolactone) | Cognitive improvement and changes in cortisol secretion (salivary cortisol levels) | Cortisol reduction improved cognitive functions, but spironolactone had no significant effect. |
| Sukhapure et al. [50] | 222 | Longitudinal cohort study | 73 participants with PCOS, 33 receiving drugs, 40 as control | MRA (spironolactone) and oral contraceptives | Changes in depression, anxiety symptoms, and cognitive function | Spironolactone improved depression and anxiety, however likely due to practice effects. |
| Wingenfeld et al. [47] | 2016 | Randomized controlled trial | MDD patients and healthy individuals treated with spironolactone or placebo | MRA (spironolactone, 300 mg) | Cognitive and emotional empathy levels | Spironolactone reduced cognitive empathy in MDD but did not affect emotional empathy. |
| Zandifar et al. [48] | 2023 | Randomized controlled trial | Bipolar I disorder patients in manic episode (n = 60) | MRA (spironolactone, 50 mg/day) | Cognitive performance (MMSE), mania severity, sleep quality | Spironolactone improved cognitive performance and mania severity. |
3.2.3. Individuals with Cardiovascular Risk Factors
| Authors | Year | Study Type | Population | Exposure | Outcome | Main Findings |
|---|---|---|---|---|---|---|
| Hong et al. [52] | 2023 | Observational Cohort Study | Patients with PA treated with MRA ADX | MRA vs. ADX | Dementia incidence in PA patients | MRA-treated PA patients had a higher risk of vascular dementia, compared to no increased risk for ADX patients. |
| Rotenstein et al. [51] | 2015 | Randomized Controlled Trial | Obese adults (BMI 30–45), randomized to spironolactone or placebo | MRA (spironolactone, 50 mg/day) | Paired-associate learning task | Spironolactone improved hippocampal memory in obese adults |
| Yagi et al. [7] | 2011 | Observational Study with MR Blocker Intervention | Hypertensive patients with high aldosterone levels | MR blockers (spironolactone, eplerenone) | MMSE | High aldosterone levels were linked to cognitive impairment. MR blockers improved MMSE scores. |
4. Discussion
4.1. Cognitive Effects in Healthy Individuals: Indirect Evidence for Cardiovascular Impact
4.2. Psychiatric Populations: Bridging Cognitive and Cardiovascular Outcomes
4.3. Cardiovascular Populations: Direct Evidence of Cognitive Benefits
4.4. Study Limitations
4.5. Implications for Future Research and Practice
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, M.; Sun, D.; Wang, Y.; Yan, M.; Zheng, J.; Ren, J. Cognitive Impairment in Heart Failure: Landscape, Challenges, and Future Directions. Front. Cardiovasc. Med. 2021, 8, 831734. [Google Scholar] [CrossRef]
- Langa, K.M.; Levine, D.A. The diagnosis and management of mild cognitive impairment: A clinical review. J. Am. Med. Assoc. 2014, 312, 2551–2561. [Google Scholar] [CrossRef]
- Liori, S.; Arfaras-Melainis, A.; Bistola, V.; Polyzogopoulou, E.; Parissis, J. Cognitive impairment in heart failure: Clinical implications, tools of assessment, and therapeutic considerations. Heart Fail. Rev. 2022, 27, 993–999. [Google Scholar] [CrossRef] [PubMed]
- van Nieuwkerk, A.C.; Delewi, R.; Wolters, F.J.; Muller, M.; Daemen, M.; Biessels, G.J.; Heart-Brain Connection Consortium. Cognitive Impairment in Patients With Cardiac Disease: Implications for Clinical Practice. Stroke 2023, 54, 2181–2191. [Google Scholar] [CrossRef]
- Sakata, A.; Mogi, M.; Iwanami, J.; Tsukuda, K.; Min, L.J.; Jing, F.; Ohshima, K.; Ito, M.; Horiuchi, M. Improvement of cognitive impairment in female type 2 diabetes mellitus mice by spironolactone. J. Renin Angiotensin Aldosterone Syst. 2012, 13, 84–90. [Google Scholar] [CrossRef]
- Chen, L.; Shi, R.; She, X.; Gu, C.; Chong, L.; Zhang, L.; Li, R. Mineralocorticoid receptor antagonist-mediated cognitive improvement in a mouse model of Alzheimer’s type: Possible involvement of BDNF-H2S-Nrf2 signaling. Fundam. Clin. Pharmacol. 2020, 34, 697–707. [Google Scholar] [CrossRef]
- Yagi, S.; Akaike, M.; Aihara, K.; Iwase, T.; Yoshida, S.; Sumitomo-Ueda, Y.; Ikeda, Y.; Ishikawa, K.; Matsumoto, T.; Sata, M. High plasma aldosterone concentration is a novel risk factor of cognitive impairment in patients with hypertension. Hypertens. Res. 2011, 34, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.L.; Noble, J.; Seckl, J.R. 11β-hydroxysteroid dehydrogenase type 1 deficiency prevents memory deficits with aging by switching from glucocorticoid receptor to mineralocorticoid receptor-mediated cognitive control. J. Neurosci. 2011, 31, 4188–4193. [Google Scholar] [CrossRef] [PubMed]
- Atucha, E.; Zalachoras, I.; van den Heuvel, J.K.; van Weert, L.T.; Melchers, D.; Mol, I.M.; Belanoff, J.K.; Houtman, R.; Hunt, H.; Roozendaal, B.; et al. A Mixed Glucocorticoid/Mineralocorticoid Selective Modulator With Dominant Antagonism in the Male Rat Brain. Endocrinology 2015, 156, 4105–4114. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Kindt, M.; Joels, M.; Krugers, H.J. Blocking mineralocorticoid receptors prior to retrieval reduces contextual fear memory in mice. PLoS ONE 2011, 6, e26220. [Google Scholar] [CrossRef] [PubMed]
- Smythe, J.W.; Murphy, D.; Timothy, C.; Gul, G.H.; Costall, B. Cognitive dysfunctions induced by scopolamine are reduced by systemic or intrahippocampal mineralocorticoid receptor blockade. Pharmacol. Biochem. Behav. 1997, 56, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.L.; Noble, J.; Seckl, J.R. Continuous blockade of brain mineralocorticoid receptors impairs spatial learning in rats. Neurosci. Lett. 1999, 277, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Avital, A.; Segal, M.; Richter-Levin, G. Contrasting roles of corticosteroid receptors in hippocampal plasticity. J. Neurosci. 2006, 26, 9130–9134. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, L.; Schachinger, H.; de Kloet, E.R.; Oitzl, M.S. Corticosteroids operate as a switch between memory systems. J. Cogn. Neurosci. 2010, 22, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Douma, B.R.; Jansen, K.; Korte, S.M.; Buwalda, B.; Van der Zee, E.A.; Luiten, P.G. Corticosterone modifies muscarinic receptor immunoreactivity in rat hippocampus. Neurosci. Lett. 1999, 268, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Maggio, N.; Segal, M. Differential corticosteroid modulation of inhibitory synaptic currents in the dorsal and ventral hippocampus. J. Neurosci. 2009, 29, 2857–2866. [Google Scholar] [CrossRef]
- Thai, C.A.; Zhang, Y.; Howland, J.G. Effects of acute restraint stress on set-shifting and reversal learning in male rats. Cogn. Affect. Behav. Neurosci. 2013, 13, 164–173. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Y.; Wang, S.; Wang, Z.; Sun, H.; He, Y.; Yao, W. Effects of eplerenone on cerebral aldosterone levels and brain lesions in spontaneously hypertensive rats. Clin. Exp. Hypertens. 2020, 42, 531–538. [Google Scholar] [CrossRef]
- Hira, S.; Saleem, U.; Anwar, F.; Raza, Z.; Rehman, A.U.; Ahmad, B. In Silico Study and Pharmacological Evaluation of Eplerinone as an Anti-Alzheimer’s Drug in STZ-Induced Alzheimer’s Disease Model. ACS Omega 2020, 5, 13973–13983. [Google Scholar] [CrossRef]
- Martisova, E.; Solas, M.; Gerenu, G.; Milagro, F.I.; Campion, J.; Ramirez, M.J. Mechanisms involved in BACE upregulation associated to stress. Curr. Alzheimer Res. 2012, 9, 822–829. [Google Scholar] [CrossRef]
- Dorey, R.; Pierard, C.; Shinkaruk, S.; Tronche, C.; Chauveau, F.; Baudonnat, M.; Beracochea, D. Membrane mineralocorticoid but not glucocorticoid receptors of the dorsal hippocampus mediate the rapid effects of corticosterone on memory retrieval. Neuropsychopharmacology 2011, 36, 2639–2649. [Google Scholar] [CrossRef] [PubMed]
- Solas, M.; Gerenu, G.; Gil-Bea, F.J.; Ramirez, M.J. Mineralocorticoid receptor activation induces insulin resistance through c-Jun N-terminal kinases in response to chronic corticosterone: Cognitive implications. J. Neuroendocrinol. 2013, 25, 350–356. [Google Scholar] [CrossRef]
- Diaz-Otero, J.M.; Yen, T.C.; Fisher, C.; Bota, D.; Jackson, W.F.; Dorrance, A.M. Mineralocorticoid receptor antagonism improves parenchymal arteriole dilation via a TRPV4-dependent mechanism and prevents cognitive dysfunction in hypertension. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1304–H1315. [Google Scholar] [CrossRef] [PubMed]
- Chambers, L.C.; Diaz-Otero, J.M.; Fisher, C.L.; Jackson, W.F.; Dorrance, A.M. Mineralocorticoid receptor antagonism improves transient receptor potential vanilloid 4-dependent dilation of cerebral parenchymal arterioles and cognition in a genetic model of hypertension. J. Hypertens. 2022, 40, 1722–1734. [Google Scholar] [CrossRef]
- Pires, P.W.; McClain, J.L.; Hayoz, S.F.; Dorrance, A.M. Mineralocorticoid receptor antagonism prevents obesity-induced cerebral artery remodeling and reduces white matter injury in rats. Microcirculation 2018, 25, e12460. [Google Scholar] [CrossRef] [PubMed]
- Loscertales, M.; Rose, S.P.; Daisley, J.N.; Sandi, C. Piracetam facilitates long-term memory for a passive avoidance task in chicks through a mechanism that requires a brain corticosteroid action. Eur. J. Neurosci. 1998, 10, 2238–2243. [Google Scholar] [CrossRef] [PubMed]
- Douma, B.R.; Korte, S.M.; Buwalda, B.; la Fleur, S.E.; Bohus, B.; Luiten, P.G. Repeated blockade of mineralocorticoid receptors, but not of glucocorticoid receptors impairs food rewarded spatial learning. Psychoneuroendocrinology 1998, 23, 33–44. [Google Scholar] [CrossRef]
- Stephan, M.; Schoeller, J.; Raabe, F.J.; Schmitt, A.; Hasan, A.; Falkai, P.; Jensen, N.; Rossner, M.J. Spironolactone alleviates schizophrenia-related reversal learning in Tcf4 transgenic mice subjected to social defeat. Schizophrenia 2022, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Meyer, K.; Korz, V. Stress induced hippocampal mineralocorticoid and estrogen receptor β gene expression and long-term potentiation in male adult rats is sensitive to early-life stress experience. Psychoneuroendocrinology 2013, 38, 250–262. [Google Scholar] [CrossRef]
- Dorey, R.; Pierard, C.; Chauveau, F.; David, V.; Beracochea, D. Stress-induced memory retrieval impairments: Different time-course involvement of corticosterone and glucocorticoid receptors in dorsal and ventral hippocampus. Neuropsychopharmacology 2012, 37, 2870–2880. [Google Scholar] [CrossRef]
- Mehdipour, M.; Emamghoreishi, M.; Farrokhi, M.R.; Amirinezhadfard, E.; Keshavarz, M. The Effect of Spironolactone on β-amyloid-Induced Memory Impairment in Male Rats: The Role of Microglial Inhibition. Adv. Pharm. Bull. 2022, 12, 623–631. [Google Scholar] [CrossRef]
- Albernaz-Mariano, K.A.; Demarchi Munhoz, C. The infralimbic mineralocorticoid blockage prevents the stress-induced impairment of aversive memory extinction in rats. Transl. Psychiatry 2022, 12, 343. [Google Scholar] [CrossRef] [PubMed]
- Licata, N.V.; Cristofani, R.; Salomonsson, S.; Wilson, K.M.; Kempthorne, L.; Vaizoglu, D.; D’Agostino, V.G.; Pollini, D.; Loffredo, R.; Pancher, M.; et al. C9orf72 ALS/FTD dipeptide repeat protein levels are reduced by small molecules that inhibit PKA or enhance protein degradation. EMBO J. 2022, 41, e105026. [Google Scholar] [CrossRef]
- Ha, J.; Afana, D.; Moghaddam, K.N.; Nicholas, A. Using BioRender for Active Learning: Exploring Learning-Style Preference and Visual-Spatial Ability in Undergraduate Students. J. Undergrad. Neurosci. Educ. 2024, 22, A289–A295. [Google Scholar] [CrossRef] [PubMed]
- Cornelisse, S.; Joels, M.; Smeets, T. A randomized trial on mineralocorticoid receptor blockade in men: Effects on stress responses, selective attention, and memory. Neuropsychopharmacology 2011, 36, 2720–2728. [Google Scholar] [CrossRef] [PubMed]
- Otte, C.; Moritz, S.; Yassouridis, A.; Koop, M.; Madrischewski, A.M.; Wiedemann, K.; Kellner, M. Blockade of the mineralocorticoid receptor in healthy men: Effects on experimentally induced panic symptoms, stress hormones, and cognition. Neuropsychopharmacology 2007, 32, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.; Klumpers, F.; Schroder, T.N.; Oplaat, K.T.; Krugers, H.J.; Oitzl, M.S.; Joels, M.; Doeller, C.F.; Fernandez, G. Stress Induces a Shift Towards Striatum-Dependent Stimulus-Response Learning via the Mineralocorticoid Receptor. Neuropsychopharmacology 2017, 42, 1262–1271. [Google Scholar] [CrossRef]
- Vogel, S.; Klumpers, F.; Kroes, M.C.; Oplaat, K.T.; Krugers, H.J.; Oitzl, M.S.; Joels, M.; Fernandez, G. A Stress-Induced Shift From Trace to Delay Conditioning Depends on the Mineralocorticoid Receptor. Biol. Psychiatry 2015, 78, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.; Klumpers, F.; Krugers, H.J.; Fang, Z.; Oplaat, K.T.; Oitzl, M.S.; Joels, M.; Fernandez, G. Blocking the mineralocorticoid receptor in humans prevents the stress-induced enhancement of centromedial amygdala connectivity with the dorsal striatum. Neuropsychopharmacology 2015, 40, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, L.; Tegenthoff, M.; Hoffken, O.; Wolf, O.T. Mineralocorticoid receptor blockade prevents stress-induced modulation of multiple memory systems in the human brain. Biol. Psychiatry 2013, 74, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, L.; Hoffken, O.; Tegenthoff, M.; Wolf, O.T. Stress-induced enhancement of response inhibition depends on mineralocorticoid receptor activation. Psychoneuroendocrinology 2013, 38, 2319–2326. [Google Scholar] [CrossRef] [PubMed]
- Young, K.D.; Preskorn, S.H.; Victor, T.; Misaki, M.; Bodurka, J.; Drevets, W.C. The Effect of Mineralocorticoid and Glucocorticoid Receptor Antagonism on Autobiographical Memory Recall and Amygdala Response to Implicit Emotional Stimuli. Int. J. Neuropsychopharmacol. 2016, 19, pyw036. [Google Scholar] [CrossRef]
- Deuter, C.E.; Kaczmarczyk, M.; Hellmann-Regen, J.; Kuehl, L.K.; Wingenfeld, K.; Otte, C. The influence of pharmacological mineralocorticoid and glucocorticoid receptor blockade on the cortisol response to psychological stress. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 129, 110905. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Roeh, A.; Leucht, S.; Langguth, B.; Hansbauer, M.; Oviedo-Salcedo, T.; Kirchner, S.K.; Papazova, I.; Lohrs, L.; Wagner, E.; et al. Add-on spironolactone as antagonist of the NRG1-ERBB4 signaling pathway for the treatment of schizophrenia: Study design and methodology of a multicenter randomized, placebo-controlled trial. Contemp. Clin. Trials Commun. 2020, 17, 100537. [Google Scholar] [CrossRef] [PubMed]
- Wingenfeld, K.; Kuehl, L.K.; Dziobek, I.; Roepke, S.; Otte, C.; Hinkelmann, K. Effects of mineralocorticoid receptor blockade on empathy in patients with major depressive disorder. Cogn. Affect. Behav. Neurosci. 2016, 16, 902–910. [Google Scholar] [CrossRef]
- Zandifar, A.; Badrfam, R.; Gholamian, F.; Shafiee, A. Efficacy of spironolactone as adjunctive therapy to sodium valproate in bipolar-I disorder: A double-blind, randomized, placebo-controlled clinical trial. Brain Behav. 2023, 13, e3313. [Google Scholar] [CrossRef]
- Hinkelmann, K.; Moritz, S.; Botzenhardt, J.; Muhtz, C.; Wiedemann, K.; Kellner, M.; Otte, C. Changes in cortisol secretion during antidepressive treatment and cognitive improvement in patients with major depression: A longitudinal study. Psychoneuroendocrinology 2012, 37, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Sukhapure, M.; Eggleston, K.; Fenton, A.; Frampton, C.; Porter, R.J.; Douglas, K.M. Changes in Mood, Anxiety, and Cognition with Polycystic Ovary Syndrome Treatment: A Longitudinal, Naturalistic Study. Neuropsychiatr. Dis. Treat. 2022, 18, 2703–2712. [Google Scholar] [CrossRef]
- Rotenstein, L.S.; Sheridan, M.; Garg, R.; Adler, G.K. Effect of mineralocorticoid receptor blockade on hippocampal-dependent memory in adults with obesity. Obesity 2015, 23, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Hong, N.; Kim, K.J.; Yu, M.H.; Jeong, S.H.; Lee, S.; Lim, J.S.; Rhee, Y. Risk of dementia in primary aldosteronism compared with essential hypertension: A nationwide cohort study. Alzheimers Res. Ther. 2023, 15, 136. [Google Scholar] [CrossRef] [PubMed]
- Santisteban, M.M.; Iadecola, C.; Carnevale, D. Hypertension, Neurovascular Dysfunction, and Cognitive Impairment. Hypertension 2023, 80, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; MacDonald, T.M.; Morant, S.; Webb, D.J.; Sever, P.; McInnes, G.; Ford, I.; Cruickshank, J.K.; Caulfield, M.J.; Salsbury, J.; et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): A randomised, double-blind, crossover trial. Lancet 2015, 386, 2059–2068. [Google Scholar] [CrossRef]
- Grippo, A.J.; Johnson, A.K. Stress, depression and cardiovascular dysregulation: A review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress 2009, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.C. Hippocampal Vascular Supply and Its Role in Vascular Cognitive Impairment. Stroke 2023, 54, 673–685. [Google Scholar] [CrossRef]
- Yamaji, M.; Tsutamoto, T.; Kawahara, C.; Nishiyama, K.; Yamamoto, T.; Fujii, M.; Horie, M. Effect of eplerenone versus spironolactone on cortisol and hemoglobin A1c levels in patients with chronic heart failure. Am. Heart J. 2010, 160, 915–921. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastena, P.; Campagnoli, G.; Rahmani, A.R.; Kalogeropoulos, A.P. Mineralocorticoid Receptor Antagonists and Cognitive Outcomes in Cardiovascular Disease and Beyond: A Systematic Review. J. Pers. Med. 2025, 15, 57. https://doi.org/10.3390/jpm15020057
Pastena P, Campagnoli G, Rahmani AR, Kalogeropoulos AP. Mineralocorticoid Receptor Antagonists and Cognitive Outcomes in Cardiovascular Disease and Beyond: A Systematic Review. Journal of Personalized Medicine. 2025; 15(2):57. https://doi.org/10.3390/jpm15020057
Chicago/Turabian StylePastena, Paola, Gabriele Campagnoli, Ali Reza Rahmani, and Andreas P. Kalogeropoulos. 2025. "Mineralocorticoid Receptor Antagonists and Cognitive Outcomes in Cardiovascular Disease and Beyond: A Systematic Review" Journal of Personalized Medicine 15, no. 2: 57. https://doi.org/10.3390/jpm15020057
APA StylePastena, P., Campagnoli, G., Rahmani, A. R., & Kalogeropoulos, A. P. (2025). Mineralocorticoid Receptor Antagonists and Cognitive Outcomes in Cardiovascular Disease and Beyond: A Systematic Review. Journal of Personalized Medicine, 15(2), 57. https://doi.org/10.3390/jpm15020057






