The Search for Predictive Biomarkers in Response to Immune Checkpoint Inhibitors and Associated Adverse Events
Abstract
1. Introduction
The Emerging Role of Spatial Multi-Omics
2. Discussion
2.1. Observations Regarding Researched Immune Checkpoints (Inhibitory Immunoreceptors)
2.2. Some Approved Predictive Biomarkers
2.2.1. Cell Death Ligand-L1 (PD-L1)
2.2.2. Tumor Mutational Burden (TMB)
2.3. The Search for New Predictive Biomarkers
2.3.1. B7 Homolog 3 Protein (B7-H3, Also Known as CD276)
2.3.2. B7-H3 as a Therapeutic Target
2.3.3. B and T Lymphocyte Attenuator (BTLA)
2.3.4. BTL Including Its Soluble Form as a Therapeutic Target
2.4. The Search for Predictive Biomarkers for IrAEs
3. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ICIs | Immune checkpoint inhibitors. |
| IrAES | Immune-related adverse events. |
| CTLA-4 | Cytotoxic T lymphocyte-associated antigen 4. |
| PD-1 | Programmed death cell protein. |
| PD-L1 | Programmed death cell protein legend-1. |
| MSI/Dmmr | Microsatellite instability/defective mismatch repair. |
| TMB | Tumor mutational burden. |
| LAG-3 | Lymphocyte activation gene-3. |
| TIGIT | T cell immunoglobulin and ITIM domain. |
| TIM-3 | T cell immunoglobulin and mucin-domain containing-3. |
| VISTA | V-domain immunoglobulin suppressor of T cell activation. |
| B7-H3 | B7 homolog 3 protein. |
| ICOS | Inducible T cell costimulatory. |
| BTLA | BTLA B and T lymphocyte attenuator. |
| PTMs | Post-translational modifications. |
| LC-MS/MS | Liquid chromatography/tandem mass spectrometry. |
| MALDI-TOF-MS | Matrix-assisted laser desorption ionization-mass spectrometry. |
| aNSCLC | Advanced non-small cell lung cancer. |
| rwOS | Real world overall survival. |
| IgV | Immunoglobulin variable-like domain. |
| IgC | Immunoglobulin constant-like domain. |
| NK | Natural killer cells. |
| qRT-PCR | Quantitative real-time polymerase chain reaction. |
| WB | Western blotting. |
| ELISA | Enzyme-linked immunosorbent assay. |
| HVEM | Herpes virus entry mediator. |
| EOC | Epithelial ovarian carcinomas. |
| ITIM | Immunoreceptor tyrosine inhibitory motif. |
| ITSM | ITSM Immunoreceptor tyrosine-based switch motif. |
| Grb2 | Growth-factor receptor-bound protein. |
| cfDNA | Cell-free DNA. |
References
- Peeraphatdit, T.B.; Wang, J.; Odenwald, M.A.; Hu, S.; Hart, J.; Charlton, M.R. Hepatotoxicity from Immune Checkpoint Inhibitors: A Systematic Review and Management Recommendation. Hepatology 2020, 72, 315–329. [Google Scholar] [CrossRef]
- España, S.; Pérez Montes de Oca, A.; Marques-Pamies, M.; Cucurull, M.; Domenech, M.; Velarde, J.M.; Salinas, I.; Moran, T.; Etxaniz, O. Endocrine adverse events related to immune-oncology agents: Retrospective experience of a single institution. Transl. Lung Cancer Res. 2020, 9, 103–110. [Google Scholar] [CrossRef]
- Wang, E.; Kraehenbuehl, L.; Ketosugbo, K.; Kern, J.A.; Lacouture, M.E.; Leung, D.Y.M. Immune-related cutaneous mmadverse events due to checkpoint inhibitors. Ann. Allergy Asthma Immunol. 2021, 126, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Duong, S.L.; Barbiero, F.J.; Nowak, R.J.; Baehring, J.M. Neurotoxicities associated with immune checkpoint inhibitor therapy. J. Neurooncol. 2021, 152, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Walian, S.; Lee, D.; Witteles, R.M.; Neal, J.W.; Nguyen, P.; Davis, M.M.; Salem, J.E.; Wu, S.M.; Moslehi, J.J.; Zhu, H. Immune Checkpoint Inhibitor Cardiotoxicity: Understanding Basic Mechanisms and Clinical Characteristics and Finding a Cure. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 113–134. [Google Scholar] [CrossRef]
- Wang, H.; Guo, X.; Zhou, J.; Li, Y.; Duan, L.; Si, X.; Zhang, L.; Liu, X.; Wang, M.; Shi, J.; et al. Clinical diagnosis and treatment of immune checkpoint inhibitor-associated pneumonitis. Thorac. Cancer 2020, 11, 191–197. [Google Scholar]
- Quach, H.T.; Johnson, D.B.; LeBoeuf, N.R.; Zwerner, J.P.; Dewan, A.K. Cutaneous adverse events caused by immune checkpoint inhibitors. J. Am. Acad. Dermatol. 2021, 85, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Short, S.A.P.; Sise, M.E.; Prosek, J.M.; Madhavan, S.M.; Soler, M.J.; Ostermann, M.; Herrmann, M.; Abudayyeh, A.; Shuchi Anand, S.; et al. Acute kidney injury in patients treated with immune checkpoint inhibitors. J. Immunother. Cancer 2021, 9, e003467. [Google Scholar] [CrossRef]
- Conroy, M.; Naidoo, J. Immune-related adverse events and the balancing act of immunotherapy. Nat. Commun. 2022, 13, 392. [Google Scholar] [CrossRef]
- Michailidou, D.; Khaki, A.R.; Morelli, M.P.; Diamantopoulos, L.; Singh, N.; Grivas, P. Association of Blood Biomarkers and Autoimmunity with Immune Related Adverse Events in Patients with Cancer Treated with Immune Checkpoint Inhibitors. Sci. Rep. 2021, 11, 9029. [Google Scholar] [CrossRef]
- Marković, F.; Stjepanović, M.; Samardžić, N.; Kontić, M. The Association of Immune-Related Adverse Events with the Efficacy of Atezolizumab in Previously Treated Advanced Non-Small-Cell Lung Cancer Patients: A Single-Center Experience. Cancers 2024, 16, 2995. [Google Scholar] [CrossRef]
- Reid, P.D.; Cifu, A.S.; Bass, A.R. Management of immunotherapy-related toxicities in patients treated with immune checkpoint inhibitor therapy. JAMA 2021, 325, 482–483. [Google Scholar] [CrossRef]
- Si, X.; He, C.; Zhang, L.; Liu, X.; Li, Y.; Wang, H.; Guo, X.; Zhou, J.; Duan, L.; Wang, M.; et al. Management of immune checkpoint inhibitor-related dermatologic adverse events. Thorac. Cancer 2020, 11, 488–492. [Google Scholar] [CrossRef]
- Gowen, M.F.; Giles, K.M.; Simpson, D.; Tchack, J.; Zhou, H.; Moran, U.; Dawood, Z.; Pavlick, A.C.; Hu, S.; Wilson, M.A.; et al. Baseline Antibody Profiles Predict Toxicity in Melanoma Patients Treated with Immune Checkpoint Inhibitors. J. Transl. Med. 2018, 16, 82. [Google Scholar] [CrossRef] [PubMed]
- Willsmore, Z.N.; Booth, L.; Patel, A.; Di Meo, A.; Prassas, I.; Chauhan, J.; Wu, Y.; Fitzpartick, A.; Stoker, K.; Kapiris, M.; et al. Circulating Immunoregulatory B Cell and Autoreactive Antibody Profiles Predict Lack of Toxicity to Anti-PD-1 Checkpoint Inhibitor Treatment in Advanced Melanoma. J. Immunother. Cancer 2025, 13, e011682. [Google Scholar] [CrossRef]
- Guo, A.-J.; Deng, Q.-Y.; Dong, P.; Zhou, L.; Shi, L. Biomarkers Associated with Immune-related Adverse Events Induced by Immune Checkpoint Inhibitors. World J. Clin. Oncol. 2024, 15, 1002–1020. [Google Scholar] [CrossRef]
- Budczies, J.; Kazdal, D.; Menzel, M.; Beck, S.; Kluck, K.; Altbürger, C.; Schwab, C.; Allgäuer, M.; Ahadova, A.; Kloor, M. Tumormutational burden: Clinical utility, challenges and emerging improvements. Nat. Rev. Clin. Oncol. 2024, 21, 725–742. [Google Scholar] [CrossRef]
- Williams, C.J.M.; Peddle, A.M.; Kasi, P.M.; Seligmann, J.; Roxburgh, C.S.; Middleton, G.W.; Tejpar, S. Neoadjuvant immunotherapy for dMMR and pMMR colorectal cancers: Therapeutic strategies and putative biomarkers of response. Nat. Rev. Clin. Oncol. 2024, 21, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Wang, X.; Hu, Y. The opportunities and challenges in immunotherapy: Insights from the regulation of PD-L1 in cancer cells. Cancer Lett. 2023, 569, 216318. [Google Scholar] [CrossRef] [PubMed]
- Bachtella, L.; Chunsheng, J.; Fentker, K.; Ertürk, G.R.; Safferthal, M.; Polewski, L.; Götze, M.; Graeber, S.Y.; Vos, G.M.; Struwe, W.B.; et al. Ion mobility-tandem mass spectrometry of mucin type O glycans. Nat. Commun. 2024, 15, 2611. [Google Scholar] [CrossRef]
- Fröhlich, F.; Fahrner, M.; Brombacher, E.; Seredynska, A.; Maldacker, M.; Schmidt1, A.; Schilling, O. Data-independent acquisition: A Milestone and prospect in clinical mass spectrometry–based proteomics. Mol. Cell Proteom. 2024, 23, 100800. [Google Scholar] [CrossRef]
- Wei, D.; Horton, K.L.; Chen, J.; Dong, L.; Chen, S.; Abdul-Hadi, K.; Zhang, T.T.; Casson, C.N.; Shaw, M.; Shiraishi, T.; et al. Development of a highly sensitive hybrid LC/MS assay for the quantitative measurement of CTLA-4 in human T Cells. Molecules 2023, 28, 3311. [Google Scholar] [CrossRef]
- Wenk, D.; Zuo, C.; Kislinger, T.; Sepiashvili, L. Recent developments in mass-spectrometry-based targeted proteomics of clinical cancer biomarkers. Clin. Proteom. 2024, 21, 6. [Google Scholar] [CrossRef]
- Smyrnakis, A.; Levin, N.; Kosmopoulou, M.; Jha, A.; Fort, K.; Makarov, A.; Papanastasiou, D.; Mohammed, S. Characterization of an omnitrap-orbitrap platform equipped with Infrared multiphoton dissociation, ultraviolet photodissociation, and electron capture dissociation for the analysis of peptides and proteins. Anal. Chem. 2023, 95, 12039–12046. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.L.; Frei, A.L.; Koessler, T.; Berger, M.D.; Dawson, H.; Michielin, O.; Zlobec, I. The current landscape of spatial biomarkers for prediction of response to immune checkpoint inhibition. Precis. Oncol. 2024, 8, 178. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Kim, J.; Lewy, T.; Rice, C.M.; Elemento, O.; Rendeiro, A.F.; Mason, C.E. Spatial omics technologies at multimodal and single cell/subcellular level. Genome Biol. 2022, 23, 256. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Peng, T.; Xu, M.; Lin, S.; Hu, B.; Chu, T.; Liu, B.; Xu, Y.; Ding, W.; Li, L.; et al. Spatial multi-omics: Deciphering technological landscape of integration of multi-omics and its applications. J. Hematol. Oncol. 2024, 17, 72. [Google Scholar] [CrossRef]
- Bernard, D.; Hansen, J.; Dupasquier, L.; LeFranc, M.; Benmansour, A.; Boudinot, P. Costimulatory Receptors in Jawed Vertebrates: Conserved CD28, Odd CTLA4 and Multiple BTLAs. Dev. Comp. Immunol. 2007, 31, 255–271. [Google Scholar] [CrossRef]
- Rezazadeh-Gavgani, E.; Majidazar, R.; Lotfinejad, P.; Kazemi, T.; Shamekh, A. Immune Checkpoint Molecules: A Review on Pathways and Immunotherapy Implications. Immun. Inflamm. Dis. 2025, 13, e70196. [Google Scholar] [CrossRef]
- Quan, Z.; Yang, Y.; Zheng, H.; Zhan, Y.; Luo, J.; Ning, Y.; Fan, S. Clinical implications of the interaction between PD-1/PD-L1 and PI3K/AKT/mTOR pathway in progression and treatment of non-small cell lung cancer. J. Cancer 2022, 13, 3434–3443. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Huang, Y.; Gu, W.; Gan, J.; Wang, W.; Zhang, S.; Wang, K.; Zhan, J.; Yang, Y.; Huang, Y.; et al. PI3K-AKT-mTOR pathway alterations in advanced NSCLC patients after progression on EGFR-TKI and clinical response to EGFR-TKI plus everolimus combination therapy. Transl. Lung Cancer Res. 2020, 9, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Patsoukis, N.; Li, L.; Sari, D.; Petkova, V.; Boussiotis, V.A. PD-1 Increases PTEN Phosphatase Activity While Decreasing Pten Protein Stability by Inhibiting Casein Kinase 2. Mol. Cell. Biol. 2013, 33, 3091–3098. [Google Scholar] [CrossRef]
- Gascón, M.; Isla, D.; Cruellas, M.; Gálvez, E.M.; Lastra, R.; Ocáriz, M.; Paño, J.R.; Ramírez, A.; Sesma, A.; Torres-Ramón, I.; et al. Intratumoral Versus Circulating Lymphoid Cells as Predictive Biomarkers in Lung Cancer Patients Treated with Immune Checkpoint Inhibitors: Is the Easiest Path the Best One? Cells 2020, 9, 1525–1541. [Google Scholar] [CrossRef]
- Riemann, D.; Turzer, S.; Ganchev, G.; Schütte, W.; Seliger, B.; Möller, M. Monitoring Blood Immune Cells in Patients with Advanced Small Cell Lung Cancer Undergoing a Combined Immune Checkpoint Inhibitor/Chemotherapy. Biomolecules 2023, 13, 190. [Google Scholar] [CrossRef]
- Ma, W.; Wei, S.; Long, S.; Tian, E.C.; McLaughlin, B.; Jaimes, M.; Montoya, D.J.; Viswanath, V.R.; Chien, J.; Zhang, Q.; et al. Dynamic evaluation of blood immune cells predictive of response to immune checkpoint inhibitors in NSCLC by multicolor spectrum flow cytometry. Front. Immunol. 2023, 14, 1206631. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tong, Z.; Zhang, W.; Zhang, W.; Buzdin, A.; Mu, X.; Yan, Q.; Zhao, X.; Chang, H.-H.; Duhon, M.; et al. FDA-Approved and Emerging Next Generation Predictive Biomarkers for Immune Checkpoint Inhibitors in Cancer Patients. Front. Oncol. 2021, 11, 683419. [Google Scholar] [CrossRef] [PubMed]
- Govers, T.; van Well, E.; De Wijn, R.; van den Heuvel, M. Predicting response to immunotherapy in lung cancer: An early HTA of predictive tests. Int. J. Technol. Assess. Health Care 2025, 41, e57. [Google Scholar] [CrossRef]
- Jorgensen, J.T. An update on companion and complementary diagnostic assays for PD-1/PD-L1 checkpoint inhibitors in NSCLC. Expert Rev. Mol. Diagn. 2021, 21, 445–454. [Google Scholar]
- Niu, M.; Yi, M.; Li, N.; Luo, S.; Wu, K. Predictive biomarkers of anti-PD-1/PD-L1 therapy in NSCLC. Exp. Hematol. Oncol. 2021, 10, 18. [Google Scholar] [CrossRef]
- Paver, E.C.; Cooper, W.A.; Colebatch, A.J.; Ferguson, P.M.; Hill, S.K.; Lum, T.; Shin, J.S.; O’Toole, S.; Anderson, L.; Scolyer, R.A.; et al. Programmed death ligand-1 (PD-L1) as a predictive marker for immunotherapy in solid tumours: A guide to immuno-histochemistry implementation and interpretation. Pathology 2021, 53, 141–156. [Google Scholar] [CrossRef]
- Chebib, I.; MinoKenudson, M. PD-L1 immunohistochemistry: Clones, cutoffs, and controversies. APMIS 2022, 130, 295–313. [Google Scholar]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Costa, E.C.; Park, K.; Alexandruz, A.; Lorena Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Ciuleanu, T.E.; Pluzanski, A.; Lee, L.S.; Otterson, G.A.; Audigier-Valette, C.; Minenza, E.; Helena Linardou, H.; Sjaak Burgers, S.; Salman, P.; et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N. Engl. J. Med. 2018, 378, 2093–2104. [Google Scholar] [PubMed]
- Aggarwal, C.; Ben-Shachar, R.; Gao, Y.; Hyun, S.W.; Rivers, Z.; Epstein, C.; Kaneva, K.; Sangli, C.; Nimeiri, H.; Jyoti Pate, J. Assessment of Tumor Mutational Burden and Outcomes in Patients with Diverse Advanced Cancers Treated with Immunotherapy. JAMA Netw. Open 2023, 6, e2311181. [Google Scholar] [CrossRef]
- Marabelle, A.; Fakih, M.; Lopez, J.; Manisha Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Association of tumours mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Association of Tumor Mutational Burden with Outcomes in Patients with Select Advanced Solid Tumors Treated with Pembrolizumab in KEYNOTE-158. Ann. Oncol. 2019, 30, v477–v478. [Google Scholar]
- Gandara, D.R.; Agarwal, N.; Gupta, S.; Klempner, S.J.; Andrews, M.C.; Mahipal, A.; Subbiah, V.; Eskander, R.N.; Carbone, D.P.; Riess, J.W.; et al. Tumor mutational burden and survival on immune checkpoint inhibition in >8000 patients across 24 cancer types. J. Immunother. Cancer 2025, 13, e01031. [Google Scholar]
- Samstein, R.M.; Lee, C.H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef]
- Santhanam, B.; Oikonomou, P.; Tavazoie, S. Systematic assessment of prognostic molecular features across cancers. Cell Genom. 2023, 3, 100262. [Google Scholar] [CrossRef]
- Bu, J.; Zhu, T.; Zhu, X. Comment on ‘Tumor mutational burden and survival on immune checkpoint inhibition in >8000 patients across 24cancer types. J. Immunother. Cancer 2025, 13, e011943. [Google Scholar] [CrossRef]
- McNamara, M.G.; Jacobs, T.; Lamarca, A.; Hubner, R.A.; Valle, J.W.; Amir, E. Impact of high tumor mutational burden in solid tumors and challenges for biomarker application. Cancer Treat. Rev. 2020, 89, 102084. [Google Scholar] [CrossRef]
- Ansari, A.; Ray, S.K.; Sharma, M.; Rawal, R.; Singh, P. Tumor Mutational Burden as a Biomarker of Immunotherapy Response: An Immunogram Approach in Onco-immunology. Curr. Mol. Med. 2024, 24, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shien, A.; Chmielecki, J.; et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017, 9, 34. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, X.; Ju, L.; Li, Y.; Li, W.; Pang, H. Establishing a predictive model for tumor mutation burden status based on 18F-FDG PET/CT and clinical features of non-small cell lung cancer patients. Transl. Lung Cancer Res. 2024, 13, 2269–2281. [Google Scholar] [CrossRef]
- McGrail, D.J.; Pilié, P.G.; Rashid, N.U.; Voorwerk, L.; Slagter, M.; Kok, M.; Jonasch, E.; Khusraw, M.; Heimberger, A.B.; Lim, B.; et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann. Oncol. 2021, 32, 661–672. [Google Scholar] [CrossRef]
- Ricciuti, B.; Wang, X.; Alessi, J.V.; Rizvi, H.; Mahadevan, N.R.; Li, Y.Y.; Polio, A.; Lindsay, J.; Umeton, R.; Sinha, R.; et al. Association of high tumor mutation burden in non–small cell lung cancers with increased immune infiltration and improved clinical outcomes of PD-L1 blockade across PD-L1 expression Levels. JAMA Oncol. 2022, 8, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Sha, D.; Jin, Z.; Budzcies, J.; Kluck, K.; Stenzinger, A.; Maincrop, F. Tumor Mutational Burden (TMB) as a Predictive Biomarker in Solid Tumors. Cancer Discov. 2020, 10, 1808–1825. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, R.; Aurora-Garg, D.; Albright, A.; Xu, L.; Liu, X.Q.; Loboda, A.; Lang, L.; Jin, F.; Rubin, E.H.; Snyder, A.; et al. Tumor mutational burden predicts the efficacy of pembrolizumab monotherapy: A pan-tumor retrospective analysis of participants with advanced solid tumors. J. Immunother. Cancer 2022, 10, e003091. [Google Scholar] [CrossRef]
- Strickler, J.H.; Hanks, B.A.; Khasraw, M. Tumor Mutational Burden as a Predictor of Immunotherapy Response: Is More Always Better? Clin. Cancer Res. 2021, 27, 1236–1241. [Google Scholar] [CrossRef]
- Yarchoan, M.; Albacker, L.A.; Hopkins, A.C.; Montesion, M.; Murugesan, K.; Vithayathil, T.T.; Zaidi, N.; Azad, N.S.; Laheru, D.A.; Frampton, G.M.; et al. PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers. JCI Insight 2019, 4, e126908. [Google Scholar] [CrossRef]
- Shi, S.; Wang, Y.; Wu, J.; Zha, B.; Li, P.; Liu, Y.; Yang, Y.; Kong, J.; Gao, S.; Cui, H.; et al. Predictive value of PD-L1 and TMB for short-term efficacy prog-nosis in non-small cell lung cancer and construction of prediction models. Front. Oncol. 2024, 14, 1342262. [Google Scholar] [CrossRef]
- Ling, V.; Wu, P.W.; Spaulding, V.; Kieleczawa, J.; Luxenberg, D.; Carreno, B.M.; Mary Collins, M. Duplication of primate and rodent B7-H3 immunoglobulin V- and C-like domains: Divergent history of functional redundancy and exon loss. Genomics 2003, 82, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Zhao, X. B7-H3 in acute myeloid leukemia: From prognostic biomarker to immunotherapeutic target. Chin. Med. J. 2024, 137, 2540–2551. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, H.; Shang, G. Research progress of B7-H3 in malignant tumors. Front. Immunol. 2025, 16, 1586759. [Google Scholar] [PubMed]
- Mielcarska, S.; Kot, A.; Dawidowicz, M.; Kula, A.; Sobków, P.; Kłaczka, D.; Waniczek, D.; Świętochowska, E. B7-H3 in Cancer Immunotherapy-Prospects and Challenges: A Review of the Literature. Cells 2025, 14, 1209. [Google Scholar] [CrossRef]
- Meng, F.; Yin, Z.; Lu, F.; Wang, W.; Zhang, H. Disruption of LPA-LPAR1 pathway results in lung tumor growth inhibition by downregulating B7-H3 expression in fibroblasts. Thorac. Cancer 2024, 15, 316–326. [Google Scholar] [CrossRef]
- Guo, X.; Chang, M.; Wang, Y.; Xing, B.; Wenbin, M. B7-H3 in Brain Malignancies: Immunology and Immunotherapy. Int. J. Biol. Sci. 2023, 19, 3762–3780. [Google Scholar] [CrossRef]
- Liu, J.; Deng, Y.; Pu, Z.; Miao, Y.; Hao, Z.; Wang, H.; Zhang, S.; Liu, H.; Wang, J.; Lv, Y.; et al. The AURKA inhibitor alters the immune microenvironment and enhances targeting B7-H3 immunotherapy in glioblastoma. JCI Insight 2025, 10, e173700. [Google Scholar] [CrossRef]
- Musatova, O.; Kumar, V.; Vinogradov, K.; Rubtsov, Y. Immune checkpoints in immune response to glioma: Two sides of the same coin. Front. Immunol. 2025, 16, 1639521. [Google Scholar] [CrossRef]
- Digregorio, M.; Coppieters, N.; Lombard, A.; Paul Noel Lumapat, P.N.; Felix Scholtes, F.; Bernard Rogister, B. The expression of B7-H3 isoforms in newly diagnosed glioblastoma and recurrence and their functional role. Acta Neuropathol. Commun. 2021, 9, 59. [Google Scholar] [CrossRef]
- Acheampong, E.; Allsopp, R.C.; Page, K.; Wadsley, M.K.; Beasley, A.B.; Coombes, R.C.; Shaw, J.A.; Gray, E.S. Meta-Analysis o circulating Tumor Cell PD-L1 Expression and the Association with Clinical Outcomes in Non-Small Cell Lung Cancer. Clin. Chem. 2024, 70, 234–249. [Google Scholar] [CrossRef]
- Kong, D.; Zhang, W.; Yang, Z.; Li, G.; Cheng, S.; Zhang, K.; Feng, L. Correlation between PD-L1 expression ON CTCs and prognosis of patients with cancer: A systematic review and meta-analysis. Oncoimmunology 2021, 10, 1938476. [Google Scholar] [CrossRef]
- Agostini, M.; Traldi, P.; Hamdan, M. Programmed Cell Death Ligand as a Biomarker for Response to Immunotherapy: Contribution of Mass Spectrometry-Based Analysis. Cancers 2025, 17, 1001. [Google Scholar] [CrossRef]
- Bostanci, O.; Sayin, P.; Kiziltan, R.; Algul, S.; Aydin, M.A.; Kemik, O. B7-H3: A Useful Emerging Diagnostic Marker for Colon Cancer, Biomed. Res. Int. BioMed Res. Int. 2022, 2022, 1523338. [Google Scholar] [CrossRef]
- Steinberger, P.; Majdic, O.; Derdak, S.V.; Pfistershammer, K.; Kirchberger, S.; Klauser, C.; Zlabinger, G.; Pickl, W.F.; Stöckl, J.; Knapp, W. Molecular characterization of human 4Ig-B7-H3, a member of the B7 family with four Ig-like domains. J. Immunol. 2004, 172, 2352–2359. [Google Scholar] [CrossRef]
- Fernandes, B.; Olkowski, C.P.; Ghaemi, B.; Basuli, F.; Shi, J.; Kiesewetter, D.O.; Lang, L.; Elijah, E.; Rolf Swenson, R.; Choyke, P.L.; et al. Unraveling the dynamics of B7-H3-targeting therapeutic antibodies in cancer through PET imaging and antibody pharmacokinetics. J. Control. Release 2025, 37, 478–488. [Google Scholar] [CrossRef]
- Huang, Y.; Zhong, W.-Q.; Yang, X.-Y.; Shan, J.-L.; Zhou, L.; Li, Z.-L.; Guo, Y.-Q.; Zhang, K.-M.; Du, T.; Zhang, H.-L.; et al. Targeting site-specific N-glycosylated B7H3 induces potent antitumor immunity. Nat. Commun. 2025, 16, 3546. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, H.-L.; Li, Z.-L.; Du, T.; Chen, Y.-H.; Wang, Y.; Ni, H.-H.; Zhang, K.-M.; Mai, J.; Hu, B.-X.; et al. FUT8-mediated aberrant N-glycosylation of B7H3 suppresses the immune response in triple-negative breast cancer. Nat. Commun. 2021, 12, 2672. [Google Scholar] [CrossRef]
- Chongsaritsinsuk, J.; Steigmeyer, A.D.; Mahoney, K.E.; Rosenfeld, M.A.; Lucas, T.M.; Smith, C.M.; Li, A.; Ince, D.; Kearns, F.L.; Battison, A.S.; et al. Glycoproteomic landscape and structural dynamics of TIM family immune checkpoints enabled by mucinase SmE. Nat. Commun. 2023, 14, 6169. [Google Scholar] [CrossRef]
- Yamato, M.; Hasegawa, J.; Maejima, T.; Hattori, C.; Kumagai, K.; Watanabe, A.; Nishiya, Y.; Shibutani, T.; Aida, T.; Hayakawa, I.; et al. DS-7300a, a DNA topoisomerase I inhibitor, DXd-based antibody-drug conjugate targeting B7-H3, exerts potent antitumor activities in preclinical models. Mol. Cancer Ther. 2022, 21, 635–646. [Google Scholar] [CrossRef]
- Malapelle, U.; Parente, P.; Pepe, F.; Di Micco, M.C.; Russo, A.; Clemente, C.; Graziano, P.; Rossi, A. B7-H3/CD276 inhibitors: Is there room for the treatment of metastatic non-small cell lung cancer? Int. J. Mol. Sci. 2022, 23, 16077. [Google Scholar] [CrossRef]
- Joshi, V.; Beecher, K.; Lim, M.; Stacey, A.; Feng, Y.; Jat, P.S.; Duijf, P.H.G.; Simpson, P.T.; Lakhani, S.R.; McCart Reed, A.E. B7-H3 expression in breast cancer and brain metastasis. Int. J. Mol. Sci. 2024, 25, 3976. [Google Scholar] [CrossRef]
- Modak, S.; Zanzonico, P.; Milan Grkovski, M.; Slotkin, E.K.; Carrasquillo, J.A.; Lyashchenko, S.K.; Lewis, J.S.; Irene, Y.; Cheung, I.Y.; Heaton, T.; et al. B7H3-directed intraperitoneal radioimmuno- therapy with radio iodinated omburtamab for desmoplastic small round cell tumor and other peritoneal tumors: Results of a phase I study. J. Clin. Oncol. 2020, 38, 4283–4291. [Google Scholar] [CrossRef]
- Gorlick, R.; Kolb, E.A.; Wang, Y.; Houghton, P.; Kurmasheva, R.; Mosse, Y.; Maris, J.; Tsang, M.; Groff, D.; Krytska, K.; et al. Evaluation of the in vivo efficacy of the B7-H3 targeting antibody-drug conjugate (ADC) DS7300a: A report from the Pediatric Preclinical In Vivo Resting (PIVOT) program. Cancer Res. 2022, 82, LB061. [Google Scholar] [CrossRef]
- Gronbeck, C.; Hadfield, M.J.; Grant-Kels, J.M. Dermatologic of antibody-drug conjugates. J. Am. Acad. Dermatol. 2024, 91, 1177–1188. [Google Scholar] [CrossRef]
- He, J.; Zeng, X.; Wang, C.; Wang, E.; Li, Y. Antibody–drug conjugates in cancer therapy: Mechanisms and clinical studies. MedComm 2024, 5, e671. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, Y.; Huang, Y.; Fang, W.; Xue, J.; Meng, X.; Fan, Y.; Fu, S.; Wu, L.; Zheng, Y.; et al. A B7H3targeting antibody–drug conjugate in advanced solid tumors: A phase 1/1b trial. Nat. Med. 2025, 31, 1949–1957. [Google Scholar] [CrossRef]
- Patel, M.R.; Doi, T.; Koyama, T.; Falchook, G.S.; Friedman, C.F.; Piha-Paul, S.A.; Gutierrez, M.; Awad, M.M.; Mattour, A.H.; Satoh, T.; et al. 690P ifinatamab deruxtecan (I-DXd; DS-7300) in patients with advanced solid tumors: Updated clinical and biomarker results from a phase I/II study. Ann. Oncol. 2023, 34, S481–S482. [Google Scholar] [CrossRef]
- Wang, J.; Duan, J.; Sun, Y.; Xing, L.; Han, L.; Wang, Q.; Wu, L.; Chen, J.; Lu, P.; Guo, W.; et al. ARTEMIS-001: Data from a phase 1a/b study of HS- 20093 in patients with relapsed small cell lung cancer (SCLC). J. Clin. Oncol. 2024, 42, 8093. [Google Scholar] [CrossRef]
- Shen, L.; Zhou, C.; Meng, X.; Sun, Y.; Ji, Y.; Yang, H.; Ning, F.; Han, L.; Jiang, O.; Sun, M.; et al. Results of a phase 1/2 study of MHB088C: A novel B7H3 antibody-drug conjugate (ADC) incorporating a potent DNA topoisomerase I inhibitor in recurrent or metastatic solid tumors. J. Clin. Oncol. 2024, 42, 3012. [Google Scholar] [CrossRef]
- Carneiro, B.A.; Perets, R.; Dowlati, A.; LoRusso, P.; Yonemori, K.; He, L.; Munasinghe, W.; Noorani, B.; Johnson, E.F.; Zugazagoitia, J. Mirzotamab clezutoclax as monotherapy and in combination with taxane therapy in relapsed/refractory solid tumors: Dose expansion results. J. Clin. Oncol. 2023, 41, 3027. [Google Scholar] [CrossRef]
- Suzuki, H.; Nagase, S.; Saito, C.; Takatsuka, A.; Nagata, M.; Honda, K.; Kaneda, Y.; Nishiya, Y.; Honda, T.; Ishizaka, T.; et al. Raludotatug Deruxtecan, a CDH6-Targeting Antibody–Drug Conjugate with a DNA Topoisomerase I Inhibitor DXd, Is Efficacious in Human Ovarian and Kidney Cancer Models. Mol. Cancer Ther. 2024, 23, 257–271. [Google Scholar] [CrossRef]
- Zhong, C.; Tao, B.; Chen, Y.; Guo, Z.; Yang, X.; Peng, L.; Xia, X.; Chen, L. B7-H3 Regulates Glioma Growth and Cell Invasion Through a JAK2/STAT3/Slug-Dependent Signaling Pathway. OncoTargets Ther. 2020, 13, 2215–2224. [Google Scholar] [CrossRef]
- Nong, F.; Xing, S.; Cheng, W.; Deng, S. Zuojin Wan Suppresses the Progression of Colorectal Cancer by Inhibiting M2 Tumor-Associated Macrophages Through AK2/STAT3 Signaling Pathway and In Vivo and In Vitro. Integr. Cancer Ther. 2025, 24, 15347354251375951. [Google Scholar] [CrossRef]
- Andrzejczak, A.; Karabon, L. BTLA biology in cancer: From bench discoveries to clinical potentials. Biomark. Res. 2024, 12, 8. [Google Scholar] [CrossRef]
- Ning, Z.; Keyan Liu, K.; Xiong, H. Roles of BTLA in Immunity and Immune Disorders. Front. Immunol. 2021, 12, 654960. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Lin, H.-W.; Chien, C.-L.; Lai, Y.-L.; Sun, W.-Z.; Chen, C.-A.; Cheng, W.-F. BTLA blockade enhances Cancer therapy by inhibiting IL-6/IL-10-induced CD19 high B lymphocytes. J. Immunother. Cancer 2019, 7, 313. [Google Scholar] [CrossRef]
- Sun, L.; Li, C.W.; Chung, E.M.; Yang, R.; Kim, Y.S.; Park, A.H.; Lai, Y.-J.; Yang, Y.; Wang, Y.-H.; Liu, J.; et al. Targeting glycosylatedPD-1 induces potent Antitumor immunity. Cancer Res. 2020, 80, 2298–2310. [Google Scholar] [CrossRef]
- Duan, Z.; Shi, R.; Gao, B.; Cai, J. N-linked glycosylation of PD-L1/PD-1: An emerging target for cancer diagnosis and treatment. Transl. Med. 2024, 22, 705. [Google Scholar] [CrossRef]
- Ito, T.; Chiba, T.; Ozawa, R.; Yoshida, M.; Hattori, M.; Sakak, Y. A comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc. Natl. Acad. Sci. USA 2001, 98, 4569–4574. [Google Scholar] [CrossRef]
- Uetz, P.; Giot, L.; Cagney, G.; Mansfield, T.A.; Judson, R.S.; Knight, J.R.; Lockshon, D.; Narayan, V.; Srinivasan, M.; Pochart, P.; et al. A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature 2000, 403, 623–627. [Google Scholar] [CrossRef]
- Celis-Gutierrez, J.; Blattmann, P.; Zhai, Y.; Jarmuzynski, N.; Ruminski, K.; Grégoire, C.; Ounoughene, Y.; Fiore, F.; Aebersold, R.; Roncagalli, R.; et al. Quantitative Interactomics in Primary T Cells Provides a Rationale for Concomitant PD-1 and BTLA Coinhibitor Blockade in Cancer Immunotherapy. Cell Rep. 2019, 27, 3315–3330.e7. [Google Scholar] [CrossRef] [PubMed]
- Gavrieli, M.; Sedy, J.; Nelson, C.A.; Murphy, K.M. BTLA and HVEM cross talk regulates inhibition and costimulation. Adv. Immunol. 2006, 92, 157–185. [Google Scholar]
- Xu, X.; Hou, B.; Fulzele, A.; Masubuchi, T.; Zhao, Y.; Wu, Z.; Hu, Y.; Jiang, Y.; Ma, Y.; Wang, H.; et al. PD-1 and BTLA regulate T cell signaling differentially and only partially through SHP1 and SHP2. J. Cell Biol. 2020, 219, e201905085. [Google Scholar] [CrossRef]
- Monaghan, S.F.; Banerjee, D.; Chung, C.-S.; Lomas-Neira, J.; Cygan, K.J.; Rhine, C.L.; Fairbrother, W.G.; Heffernan, D.S.; Levy, M.M.; Cioffi, W.G.; et al. Changes in the process of alternative RNA splicing results in soluble B and T lymphocyte attenuator with biological and clinical implications in critical illness. Mol. Med. 2018, 24, 32. [Google Scholar] [CrossRef]
- Guo, C.; Figueired, I.; Gurel, B.; Neeb, A.; Seed, G.; Crespo, M.; Carreira, S.; Rekowski, J.; Burroni, L.; Welti, J.; et al. B7-H3 as a Therapeutic Target in Advanced Prostate Cancer. Eur. Urol. 2023, 83, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Fanale, D.; Dimino, A.; Pedone, E.; Brando, C.; Corsini, L.R.; Filorizzo, C.; Fiorino, A.; Lisanti, M.C.; Magrin, L.; Randazzo, U.; et al. Prognostic and Pre-dictive Role of Tumor-Infiltrating Lymphocytes (TILs) in Ovarian Cancer. Cancers 2022, 14, 4344. [Google Scholar] [CrossRef]
- Perovic, D.; Pjevic, M.D.; Perovic, V.; Grk, M.; Rasic, M.; Milickovic, M.; Mijovic, T.; Rasic, P. B7 homolog 3 in pancreatic cancer. World J. Gastroenterol. 2024, 30, 3654–3667. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, J.; Yu, Y.; Wang, Q.; Yang, R.; Xia, B.; Li, C.; Lv, D.; Yi, T.; Han, L.; et al. Phase I/II Study of Tifcemalimab, an Anti-B- and T-lymphocyte Attenuator Antibody, in Combination with Toripalimab in Previously Treated Advanced Lung Cancer. Clin. Cancer Res. 2025, 31, 2926–2934. [Google Scholar] [CrossRef]
- Song, Y.; Ma, J.; Zhang, H.; Xie, Y.; Peng, Z.; Shuang, Y.; Li, F.; Li, Y.; Yang, H.; Zou, L.; et al. Tifcemalimab as monotherapy or in combination with toripalimab in patients with relapsed/refractory lymphoma: A Phase I trial. Nat. Commun. 2025, 16, 4559. [Google Scholar] [CrossRef]
- Zulato, E.; Del Bianco, P.; Nardo, G.; Attili, I.; Pavan, A.; Boscolo Bragadin, A.; Marra, L.; Pasello, G.; Fassan, M.; Calabrese, F.; et al. Longitudinal liquid biopsy anticipates hyper progression and early death in advanced non-small cell lung cancer patients treated with immune checkpoint inhibitors. Br. J. Cancer 2022, 127, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Bragadin, A.B.; Del Bianco, P.; Zulato, E.; Attili, I.; Pavan, A.; Carlet, J.; Marra, L.; Guarneri, V.; Indraccolo, S.; Bonanno, L. Longitudinal liquid biopsy predicts clinical benefit from immunotherapy in advanced non-small cell lung cancer. npj Precis. Oncol. 2024, 8, 234. [Google Scholar] [CrossRef]
- Franciszkiewicz, K.; Boissonnas, A.; Boutet, M.; Combadiere, C.; Mami-Chouaib, F. Role of chemokines and chemokine receptors in shaping the effector phase of the antitumor immune response. Cancer Res. 2012, 72, 6325–6332. [Google Scholar] [CrossRef]
- Khan, S.; Khan, S.A.; Luo, X.; Fattah, F.J.; Saltarski, J.; Gloria-McCutchen, Y.; Lu, R.; Xie, Y.; Li, Q.; Wakeland, E.; et al. Immune dysregulation in cancer patients developing immune-related adverse events. Br. J. Cancer 2019, 120, 63–68. [Google Scholar] [CrossRef]
- Tyan, K.; Baginska, J.; Brainard, M.; Giobbie-Hurder, A.; Severgnini, M.; Manos, M.; Haq, R.; Buchbinder, E.I.; Ott, P.A.; Hodi, F.S.; et al. Cytokine changes during immune-related adverse events and corticosteroid treatment in melanoma patients receiving immune checkpoint inhibitors. Cancer Immunol. Immunother. 2021, 70, 2209–2221. [Google Scholar] [CrossRef]
- Li, J.; Hou, H.; Li, J.; Zhang, K. Angiopoietins/Tie2 signaling axis and its role in angiogenesis of psoriasis. Acta Histochem. 2025, 127, 152228. [Google Scholar] [CrossRef] [PubMed]
- Rouvier, E.; Luciani, M.F.; Mattéi, M.G.; Denizot, F.; Golstein, P. CTLA-8, cloned from an activated T cell, bearing AU- rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J. Immunol. 1993, 150, 5445–5456. [Google Scholar] [CrossRef]
- Mills, K.H.G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2023, 23, 39. [Google Scholar] [CrossRef] [PubMed]
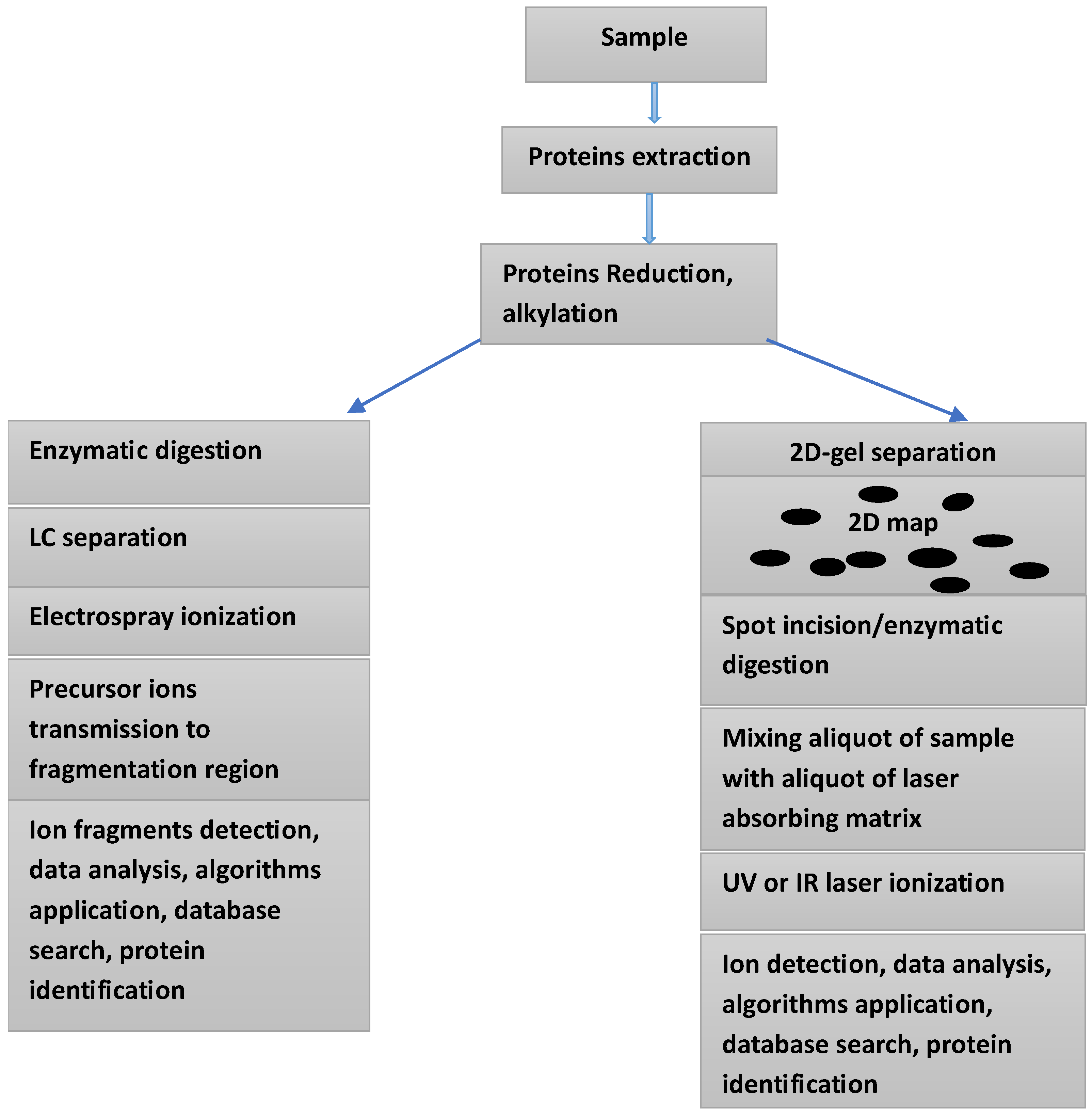
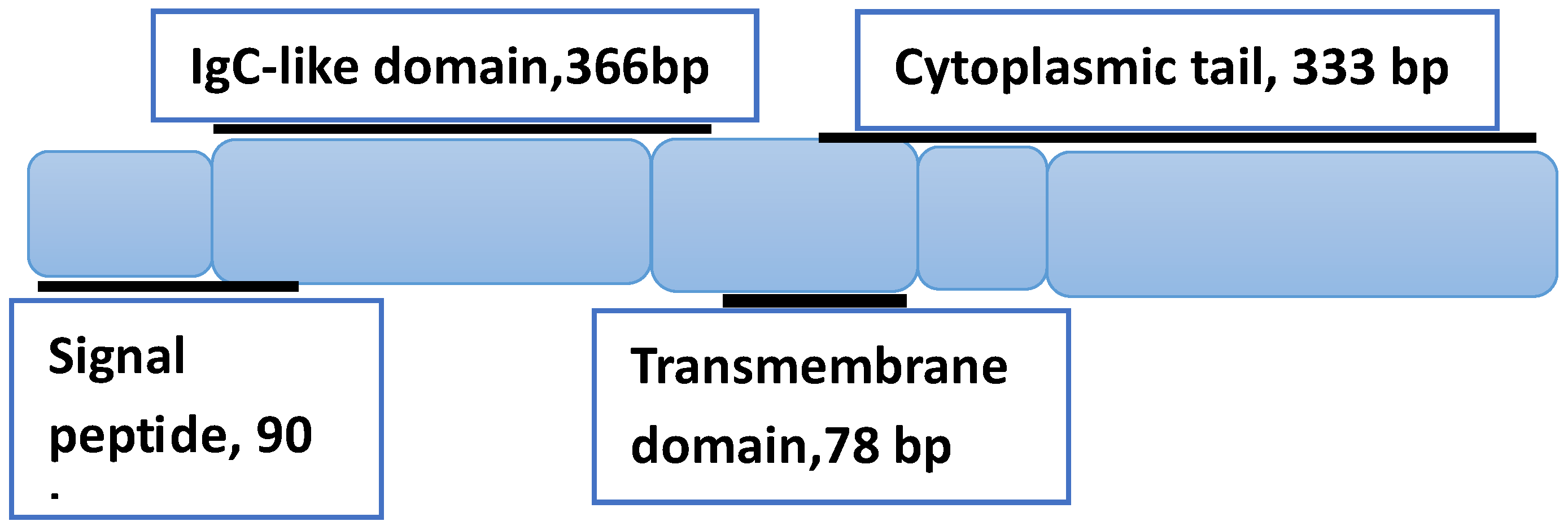
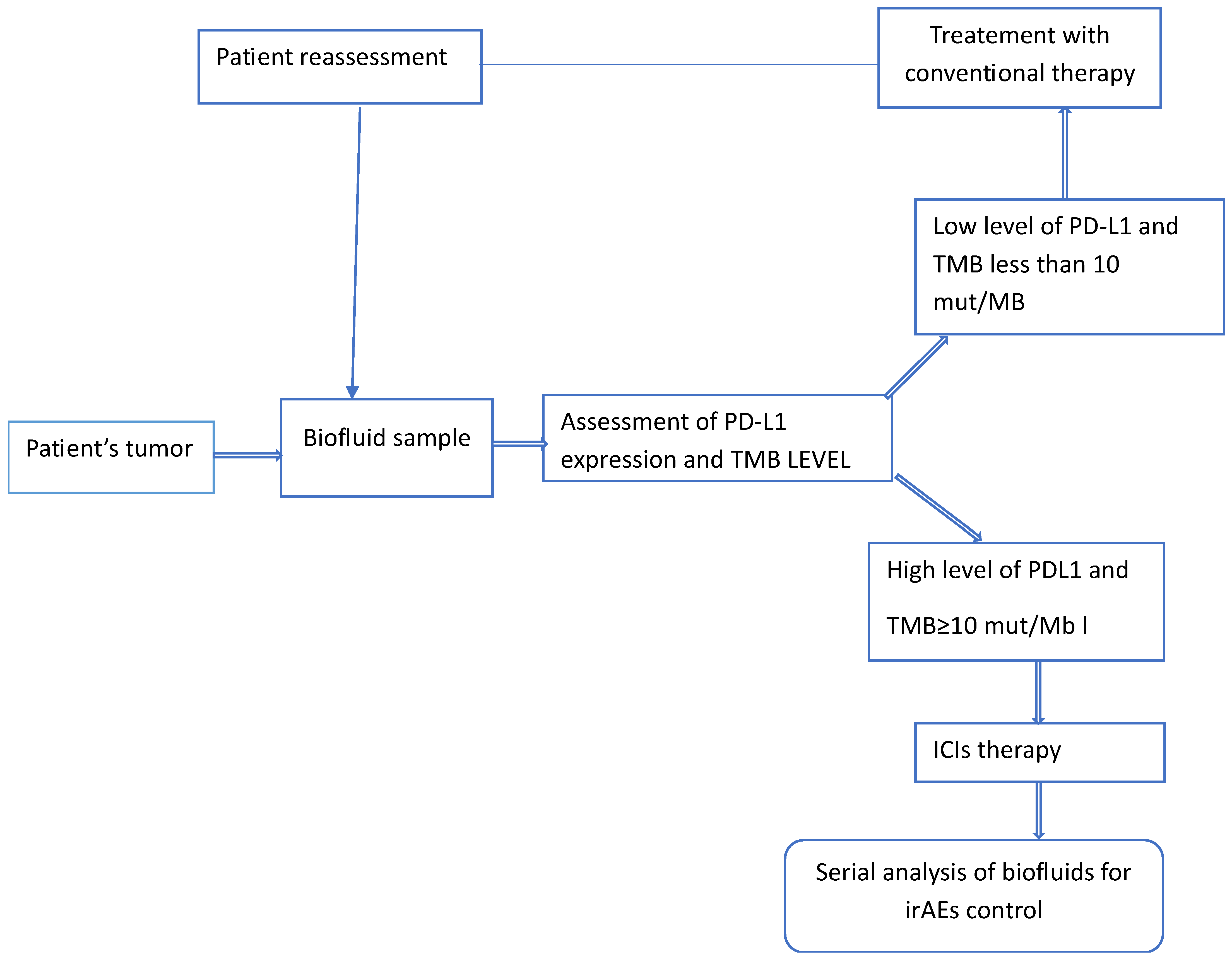
| Some irAEs Associated with ICIs Therapy | Observations | Ref. |
|---|---|---|
| Hepatotoxicity | This toxicity has a variable profile of both symptoms and severity, which renders it difficult to diagnose. It has a relatively low rate (1–2% in the case of anti-PD-1therapy) and a much higher rate (13–16% associated with combined CTLA-4/PD-1). | [1] |
| Endocrine toxicity | This adverse event primarily affects the thyroid gland; it is more common in anti-PD-1 therapy. In lung cancer treatment, the incidence of this event was about 18%. | [2] |
| Skin toxicity | Skin toxicity is very common in patients treated with ICIs. Incidence of 90% for patients treated with CTLA-4 and 70% for those treated with PD-1/PD-L1. | [3] |
| Neurotoxicity | Neurotoxicity associated with ICIs therapy is rather rare, yet it can result in a high rate of fatality. Due to their clinical diversity, diagnosing these forms of neurotoxicity remains a challenging task. | [4] |
| Cardiac toxicity | Cardiac toxicity due to ICIs treatment is rather rare (1–2%), yet because of its high potential lethality it has to be under close and continuous monitoring. | [5] |
| Lung toxicity | Pulmonary toxicity occurs in less than 3% of the treated patients. Patients treated with PD-1 inhibitor were more exposed to this toxicity compared to those treated with PD-L1. | [6] |
| Dermatologic toxicities | Cutaneous toxicities are the most common irAEs, which can affect 60–70% of patients treated with a combination of anti-PD-1/PD-L1 and anti-CTLA-4 inhibitors. | [7] |
| Renal toxicities | Acute kidney injury is closely associated with ICIs therapy, occurs in about 5% of patients receiving a combination of ICI therapy, and 2% of those treated with ICI monotherapy. | [8] |
| Predictive Biomarkers for ICIs Response | Observations |
|---|---|
| Programmed Death Ligand 1 (PD-L1) | PD-L1 was approved by the FDA in2015 as a predictive biomarker in response to ICIs treatment of non-small cell lung cancer (NSCLC). Different assays-approved by the FDA use immunohistochemistry (IHC). The use of different assays and different modes of implementation are considered responsible for inconsistency in reported results. |
| Tumor Mutational Burden (TMB). | TMB was approved by the FDA in 2020 as a predictive biomarker in response to ICIs treatment of unresectable or metastatic solid tumors. This biomarker measures the total number of somatic non-synonymous mutations present within a cancer genome. Reported inconsistencies in clinical trials are commonly attributed to infrequent use of whole-genome sequencing (WGS) in clinical practice. |
| Microsatellite Instability/Defective Mismatch Repair (MSI/dMMR). | MSI/dMMR was the second predictive biomarker to be approved by the FDA in 2017. This marker is designated for measuring response to ICIs treatment against unresectable or metastatic solid tumors. There are three different assays available in clinical tests: IHC for detecting dMMR, PCR, and NGS for detecting MSI. As is the case with PD-L1, inconsistency in the reported results is due to the use of different assays and different approaches in their implementation. |
| Homolog 3 protein (B7-H3), also known as CD276. | High expression level of this protein compared to healthy tissues is the main parameter under investigation to establish whether such differences can be considered a predictive biomarker for advanced solid tumors. |
| B and T lymphocyte attenuator (BTLA). | This co-inhibitory receptor shares structural similarity with two extensively studied immune checkpoints, 4 CTLA-4 and PD-1. The correlation between BTLA expression and the prognosis of different forms of cancer is under investigation. |
| Immune Checkpoints |
|---|
| Cell death ligand-L1(PD-L1) Programmed cell death-1 (PD-1) Cytotoxic T lymphocyte antigen-4 (CTLA-4) Lymphocyte activation gene-3 (LAG-3) T cell immunoglobulin and ITIM domain (TIGIT) T cell immunoglobulin and mucin-domain containing-3 (TIM-3) V-domain immunoglobulin suppressor of T cell activation (VISTA) B7 homolog 3 protein (B7-H3) Inducible T cell costimulatory (ICOS) B and T lymphocyte attenuator (BTLA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agostini, M.; Traldi, P.; Hamdan, M. The Search for Predictive Biomarkers in Response to Immune Checkpoint Inhibitors and Associated Adverse Events. J. Pers. Med. 2025, 15, 596. https://doi.org/10.3390/jpm15120596
Agostini M, Traldi P, Hamdan M. The Search for Predictive Biomarkers in Response to Immune Checkpoint Inhibitors and Associated Adverse Events. Journal of Personalized Medicine. 2025; 15(12):596. https://doi.org/10.3390/jpm15120596
Chicago/Turabian StyleAgostini, Marco, Pietro Traldi, and Mahmoud Hamdan. 2025. "The Search for Predictive Biomarkers in Response to Immune Checkpoint Inhibitors and Associated Adverse Events" Journal of Personalized Medicine 15, no. 12: 596. https://doi.org/10.3390/jpm15120596
APA StyleAgostini, M., Traldi, P., & Hamdan, M. (2025). The Search for Predictive Biomarkers in Response to Immune Checkpoint Inhibitors and Associated Adverse Events. Journal of Personalized Medicine, 15(12), 596. https://doi.org/10.3390/jpm15120596







