Physiologically Based Pharmacokinetic Modeling of Clobazam and Stiripentol Co-Therapy in Dravet Syndrome
Abstract
1. Introduction
2. Materials and Methods
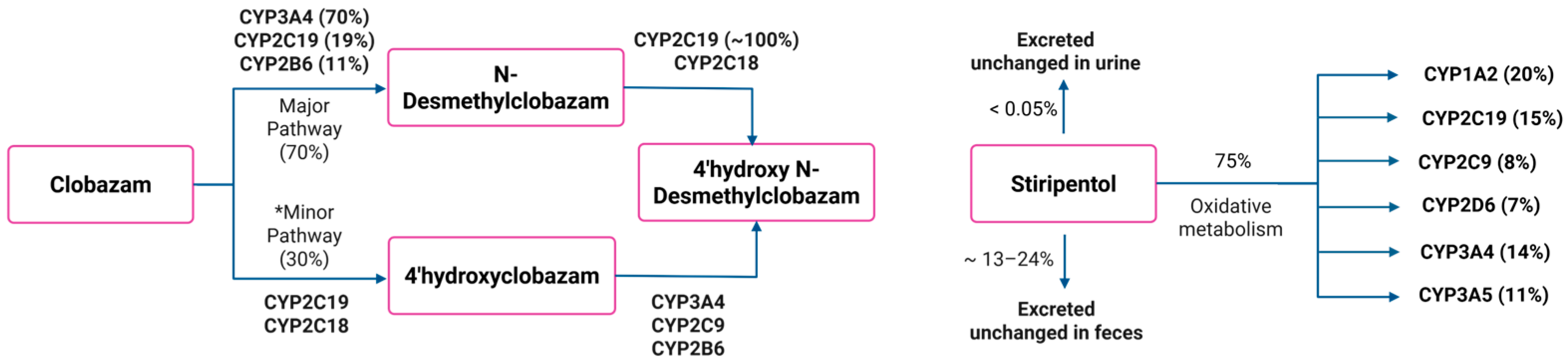
2.1. Development and Validation of the PBPK Model for CLB and Its Active Metabolite N-CLB in Healthy Adults
2.2. Development and Validation of the PBPK Model for STP in Healthy Adults
2.3. Simulation Trial Design for CLB and STP PBPK Models
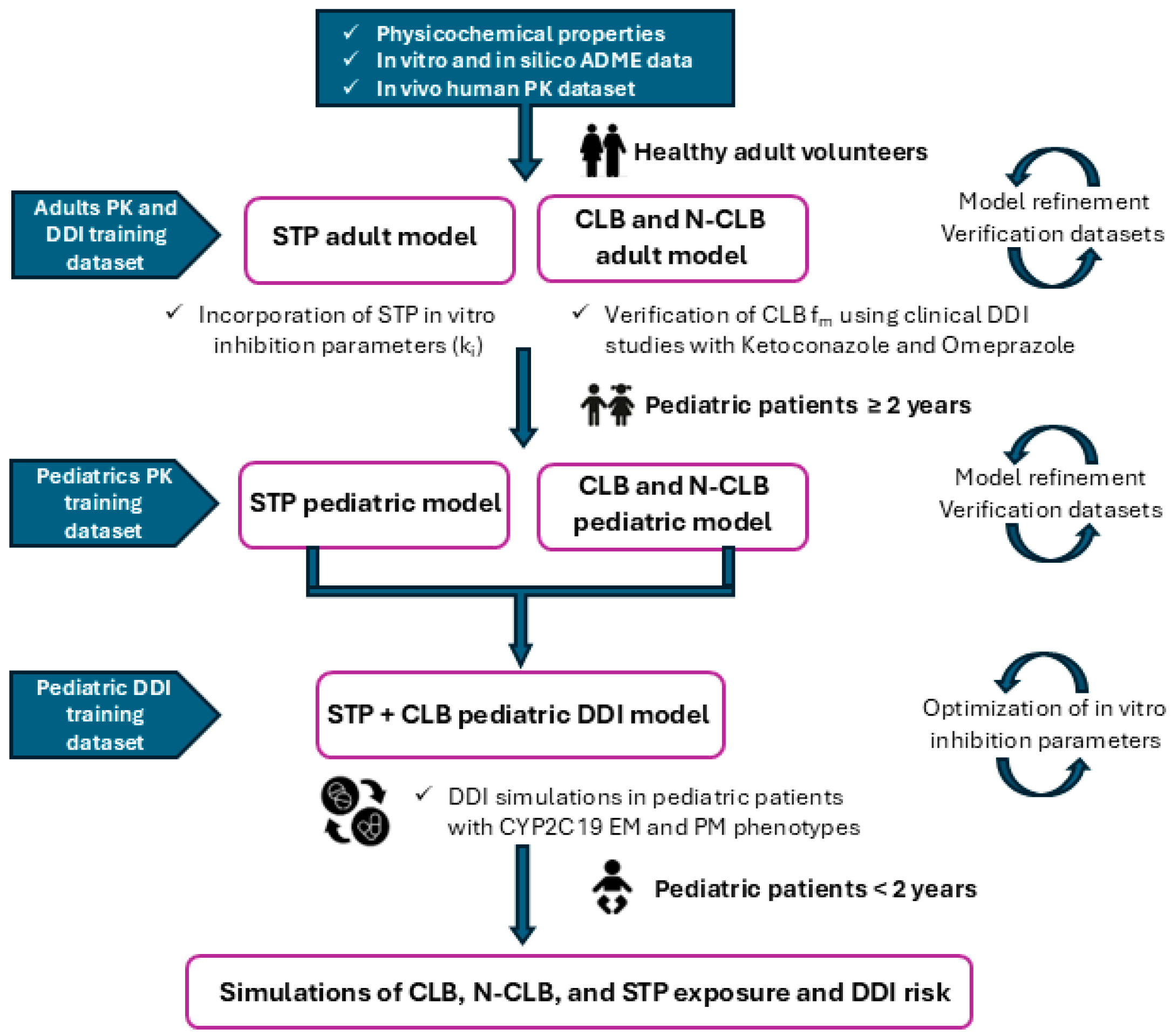
2.4. Development and Validation of CLB and STP PBPK Models in Pediatric Patients Above the Age of Two
2.5. Metabolism-Mediated DDI Risk Assessment of CLB and STP Co-Therapy in Pediatric Dravet Syndrome Patients
2.6. Extrapolation of the Validated PBPK Models of CLB and STP to Pediatric Patients Below the Age of Two
2.7. Global Sensitivity Analysis of CLB and STP PBPK Models
3. Results
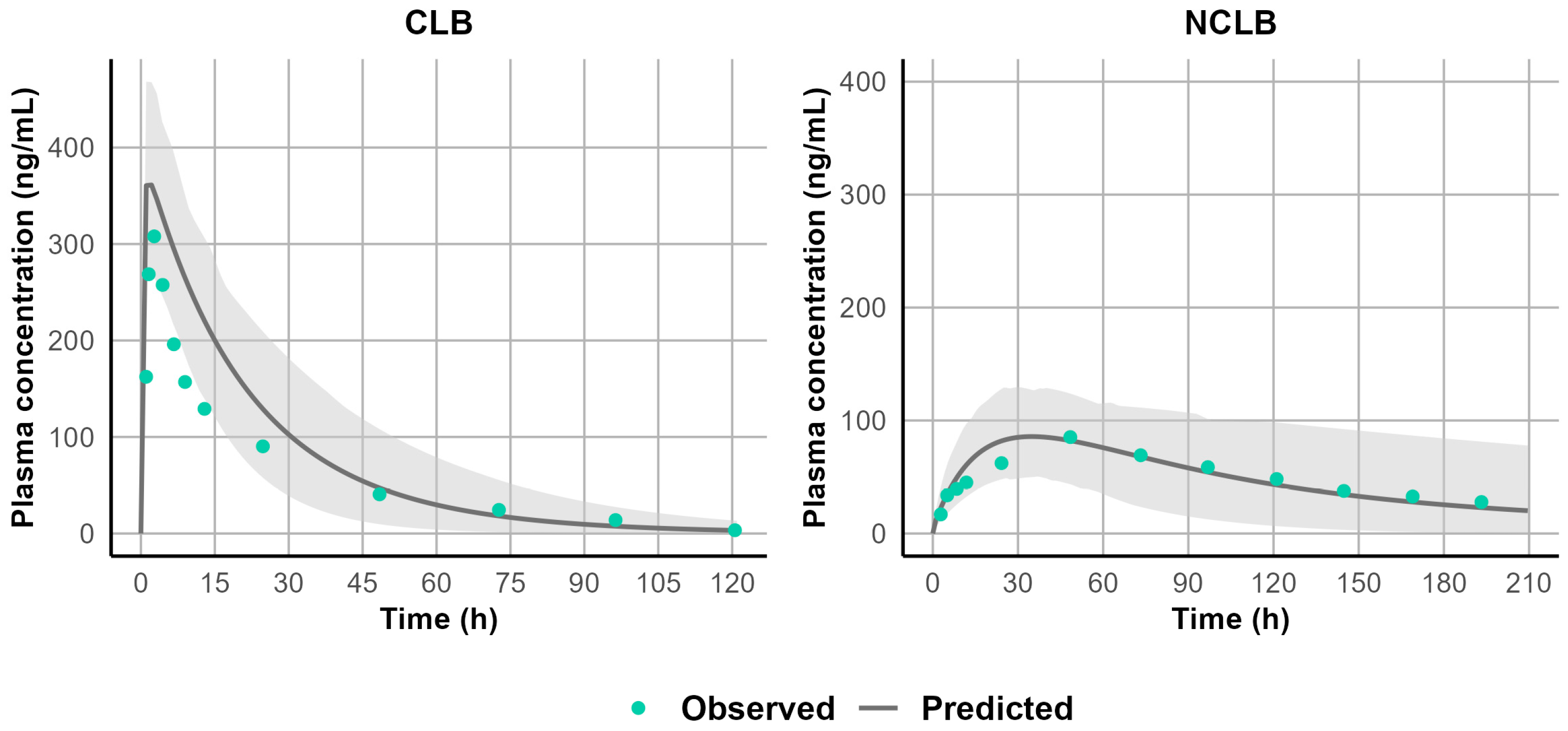
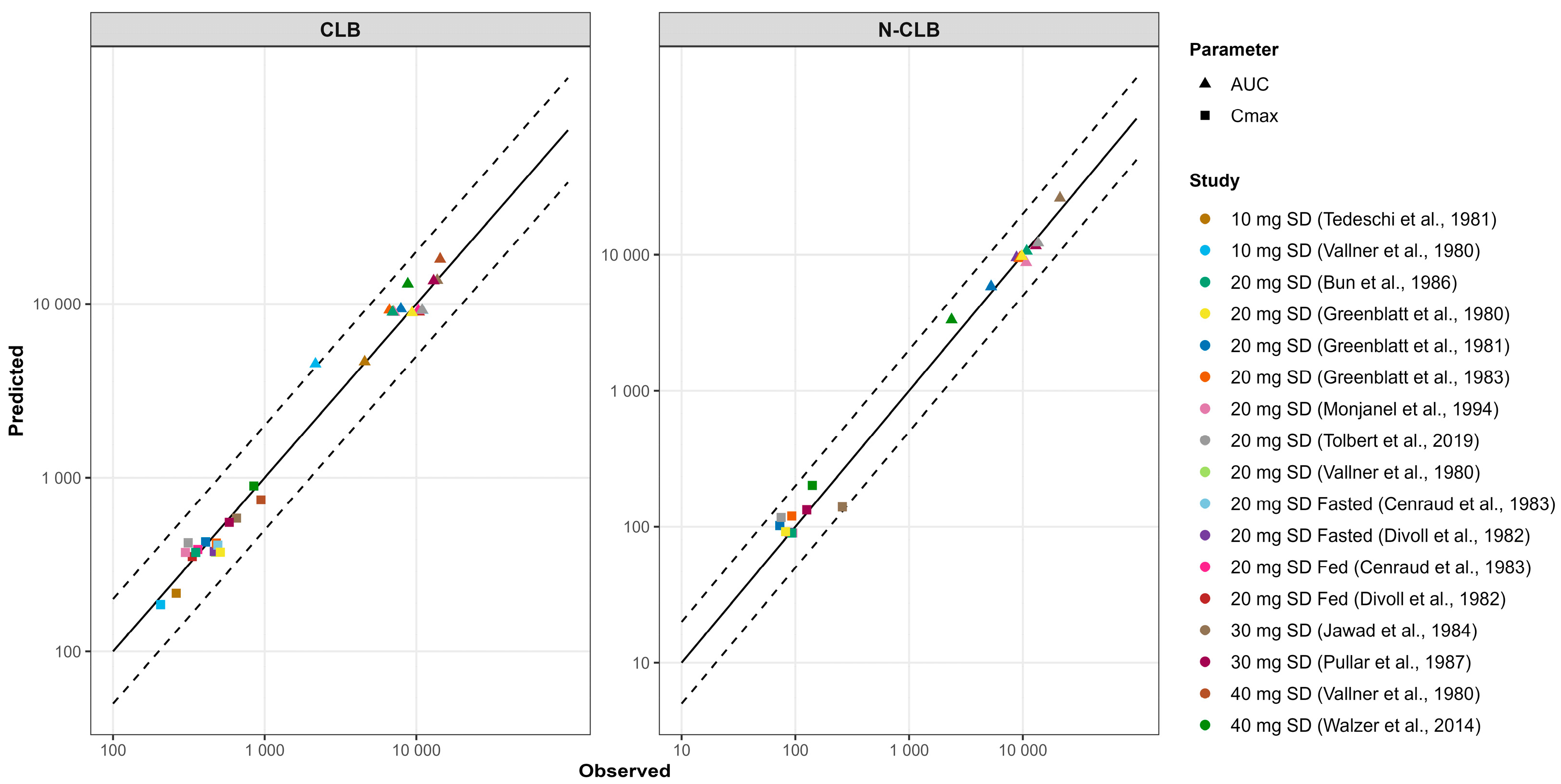
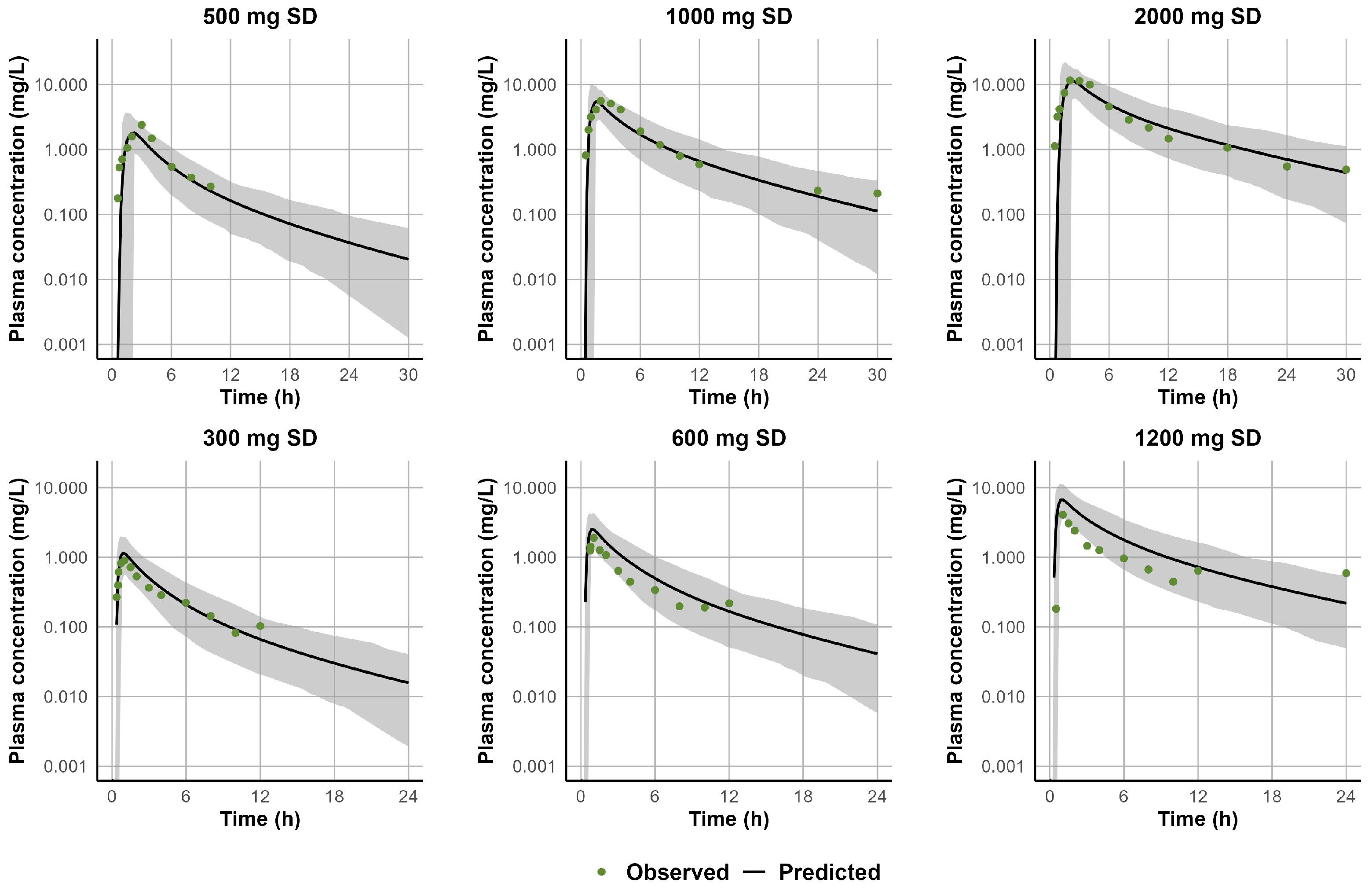
3.1. Development and Validation of the PBPK Model for CLB and Its Active Metabolite N-CLB in Healthy Adults
3.2. Development and Validation of the PBPK Model for STP in Healthy Adults
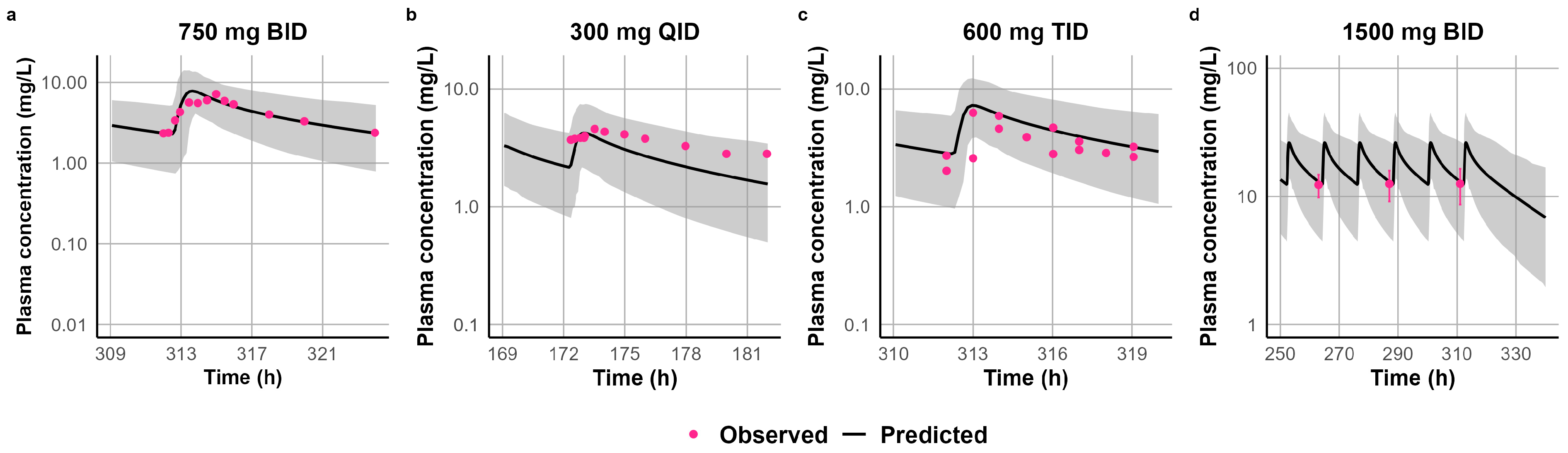
3.3. Development and Validation of CLB and STP PBPK Models in Pediatric Patients Above the Age of Two
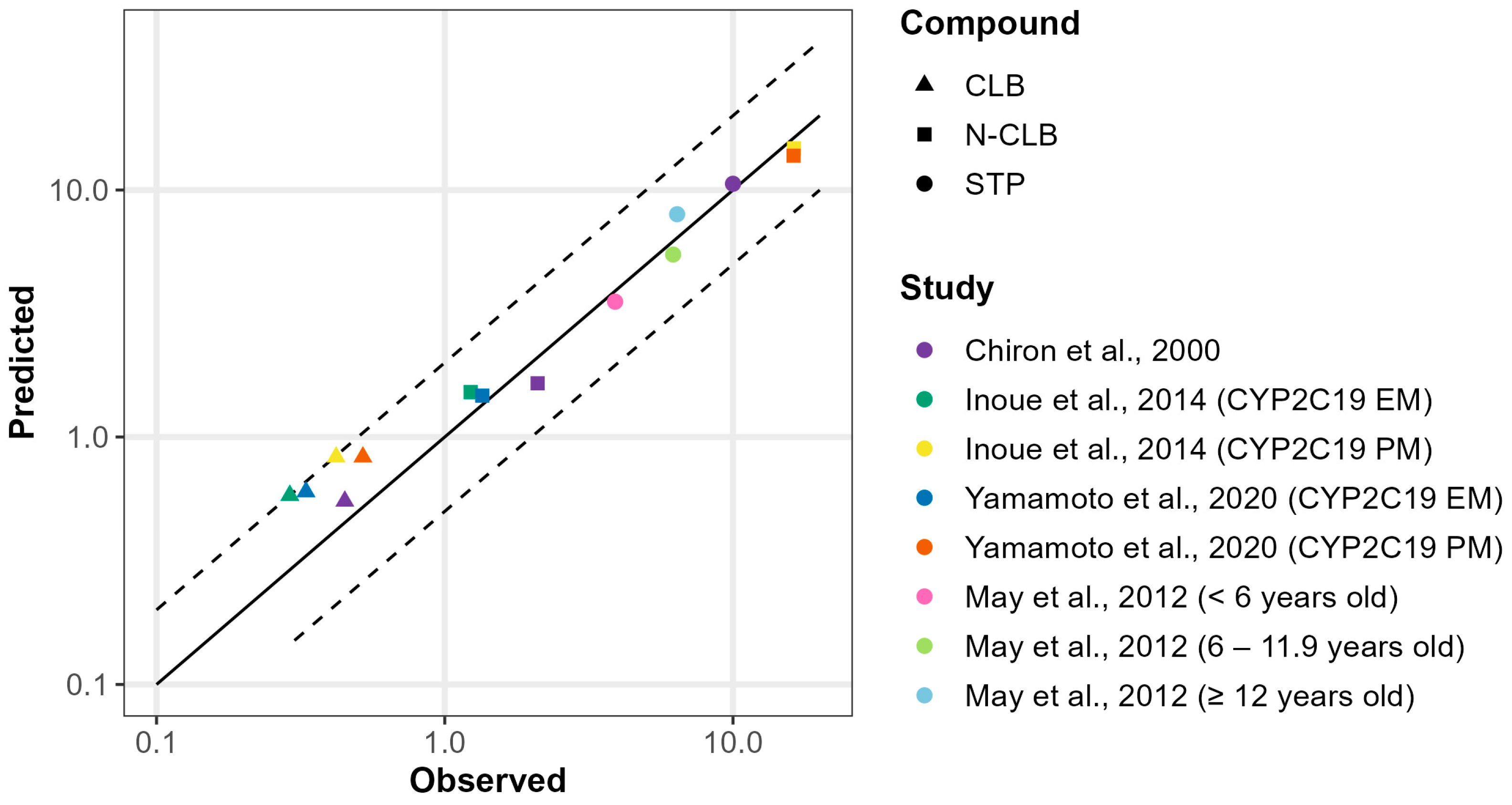
3.4. Metabolism-Mediated DDI Risk Assessment of CLB and STP Co-Therapy in Pediatric Dravet Syndrome Patient
3.5. Extrapolation of the Validated PBPK Models of CLB and STP to Pediatric Patients Below the Age of Two
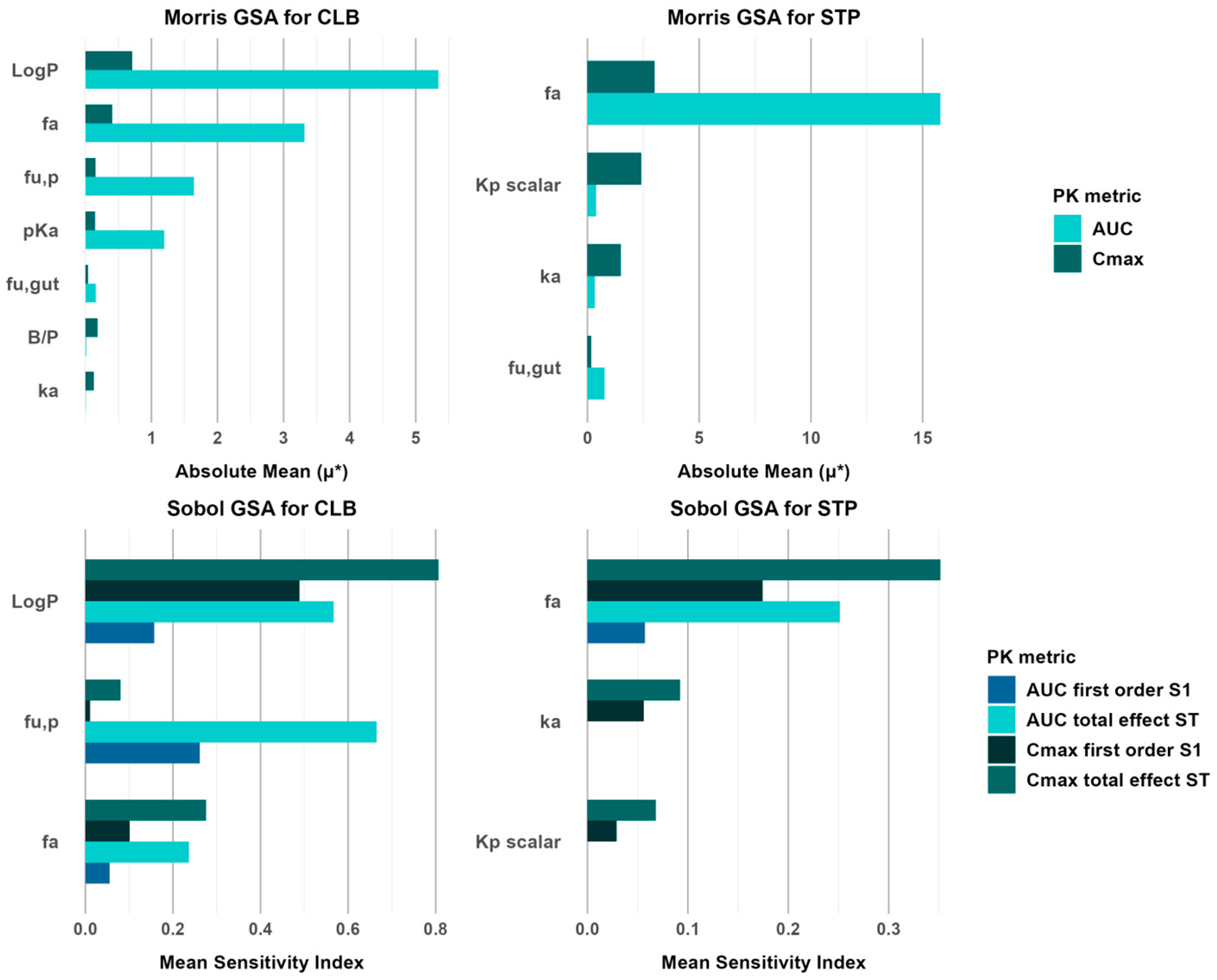
3.6. Global Sensitivity Analysis of CLB and STP PBPK Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sullivan, J.; Benítez, A.; Roth, J.; Andrews, J.S.; Shah, D.; Butcher, E.; Jones, A.; Cross, J.H. A systematic literature review on the global epidemiology of Dravet syndrome and Lennox–Gastaut syndrome: Prevalence, incidence, diagnosis, and mortality. Epilepsia 2024, 65, 1240–1263. [Google Scholar] [CrossRef]
- Strzelczyk, A.; Schubert-Bast, S. A Practical Guide to the Treatment of Dravet Syndrome with Anti-Seizure Medication. CNS Drugs 2022, 36, 217–237. [Google Scholar] [CrossRef]
- Tolbert, D.; Larsen, F. A comprehensive overview of the clinical pharmacokinetics of clobazam. J. Clin. Pharmacol. 2019, 59, 7–19. [Google Scholar] [CrossRef]
- Tanabe, T.; Awaya, Y.; Matsuishi, T.; Iyoda, K.; Nagai, T.; Kurihara, M.; Yamamoto, K.; Minagawa, K.; Maekawa, K. Management of and prophylaxis against status epilepticus in children with severe myoclonic epilepsy in infancy (SMEI.; Dravet syndrome)—A nationwide questionnaire survey in Japan. Brain Dev. 2008, 30, 629–635. [Google Scholar] [CrossRef]
- Giraud, C.; Tran, A.; Rey, E.; Vincent, J.; Tréluyer, J.-M.; Pons, G. In vitro characterization of clobazam metabolism by recombinant cytochrome P450 enzymes: Importance of CYP2C19. Drug Metab. Dispos. 2004, 32, 1279–1286. [Google Scholar] [CrossRef]
- de Leon, J.; Spina, E.; Diaz, F.J. Clobazam Therapeutic Drug Monitoring: A Comprehensive Review of the Literature With Proposals to Improve Future Studies. Ther. Drug Monit. 2013, 35, 30–47. [Google Scholar] [CrossRef]
- Huddart, R.; Leeder, J.S.; Altman, R.B.; Klein, T.E. PharmGKB summary: Clobazam pathway, pharmacokinetics. Pharmacogenet Genom. 2018, 28, 110–115. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Clinical Pharmacology and Biopharmaceutics Review for Stiripentol (STP). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/206709Orig1s000,207223Orig1s000ClinPharmR.pdf (accessed on 3 January 2025).
- Quilichini, P.P.; Chiron, C.; Ben-Ari, Y.; Gozlan, H. Stiripentol, a Putative Antiepileptic Drug, Enhances the Duration of Opening of GABAA-Receptor Channels. Epilepsia 2006, 47, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.; Rey, E.; Pons, G.; Rousseau, M.; d’Athis, P.; Olive, G.; Mather, G.G.; Bishop, F.E.; Wurden, C.J.; Labroo, R.; et al. Influence of stiripentol on cytochrome P450-mediated metabolic pathways in humans: In vitro and in vivo comparison and calculation of in vivo inhibition constants. Clin. Pharmacol. Ther. 1997, 62, 490–504. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, A.; Wirrell, E.C.; Youssef, P.E. Stiripentol for the treatment of seizures associated with Dravet syndrome in patients 6 months and older and taking clobazam. Expert. Rev. Neurother. 2023, 23, 297–309. [Google Scholar] [CrossRef]
- Sullivan, J.; Deighton, A.M.; Vila, M.C.; Szabo, S.M.; Maru, B.; Gofshteyn, J.S.; James, E.S.; Rico, S.; Zuberi, S.M. The clinical, economic, and humanistic burden of Dravet syndrome—A systematic literature review. Epilepsy Behav. 2022, 130, 108661. [Google Scholar] [CrossRef] [PubMed]
- Chiron, C.; Marchand, M.C.; Tran, A.; Rey, E.; d’Athis, P.; Vincent, J.; Dulac, O.; Pons, G.; STICLO Study Group. Stiripentol in severe myoclonic epilepsy in infancy: A randomised placebo-controlled syndrome-dedicated trial. Lancet 2000, 356, 1638–1642. [Google Scholar] [CrossRef] [PubMed]
- Wirrell, E.C.; Hood, V.; Knupp, K.G.; Meskis, M.A.; Nabbout, R.; Scheffer, I.E.; Wilmshurst, J.; Sullivan, J. International consensus on diagnosis and management of Dravet syndrome. Epilepsia 2022, 63, 1761–1777. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. DIACOMIT (Stiripentol) Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/206709s003,207223s003lbl.pdf (accessed on 18 March 2025).
- Chiron, C.; Chemaly, N.; Chancharme, L.; Nabbout, R. Initiating stiripentol before 2 years of age in patients with Dravet syndrome is safe and beneficial against status epilepticus. Dev. Med. Child. Neurol. 2023, 65, 1607–1616. [Google Scholar] [CrossRef]
- Denney, W.S.; Duvvuri, S.; Buckeridge, C. Abstracts Accepted for American Conference on Pharmacometrics 2015 (ACoP6). Simple, Automatic Noncompartmental Analysis: The PKNCA r Package. J. Pharmacokinet. Pharmacodyn. 2015, 42, 11–107. [Google Scholar] [CrossRef]
- Cenraud, B.; Guyot, M.; Levy, R.H.; Brachet-Liermain, A.; Morselli, P.L.; Moreland, T.A.; Loiseau, P. No effect of food intake on clobazam absorption. Br. J. Clin. Pharmacol. 1983, 16, 728–730. [Google Scholar] [CrossRef]
- Bun, H.; Coassolo, P.; Gouezo, F.; Serradimigni, A.; Cano, J.P. Time-dependence of clobazam and N-demethylclobazam kinetics in healthy volunteers. Int. J. Clin. Pharmacol. Ther. Toxicol. 1986, 24, 287–293. [Google Scholar]
- Greenblatt, D.J.; Divoll, M.; Puri, S.K.; Ho, I.; Zinny, M.A.; Shader, R.I. Reduced single-dose clearance of clobazam in elderly men predicts increased multiple-dose accumulation. Clin. Pharmacokinet. 1983, 8, 83–94. [Google Scholar] [CrossRef]
- Greenblatt, D.J.; Divoll, M.; Puri, S.K.; Ho, I.; Zinny, M.A.; Shader, R.I. Clobazam kinetics in the elderly. Br. J. Clin. Pharmacol. 1981, 12, 631–636. [Google Scholar] [CrossRef]
- Divoll, M.; Greenblatt, D.J.; Ciraulo, D.A.; Puri, S.K.; Ho, I.; Shader, R.I. Clobazam kinetics: Intrasubject variability and effect of food on adsorption. J. Clin. Pharmacol. 1982, 22, 69–73. [Google Scholar] [CrossRef]
- Rupp, W.; Badian, M.; Christ, O.; Hajdú, P.; Kulkarni, R.D.; Taeuber, K.; Uihlein, M.; Bender, R.; Vanderbeke, O. Pharmacokinetics of single and multiple doses of clobazam in humans. Br. J. Clin. Pharmacol. 1979, 7 (Suppl. 1), 51s–57s. [Google Scholar] [CrossRef] [PubMed]
- Patsalos, P.N.; Zugman, M.; Lake, C.; James, A.; Ratnaraj, N.; Sander, J.W. Serum protein binding of 25 antiepileptic drugs in a routine clinical setting: A comparison of free non-protein-bound concentrations. Epilepsia 2017, 58, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Sinha, V.K.; Snoeys, J.; Osselaer, N.V.; Peer, A.V.; Mackie, C.; Heald, D. From preclinical to human-prediction of oral absorption and drug–drug interaction potential using physiologically based pharmacokinetic (PBPK) modeling approach in an industrial setting: A workflow by using case example. Biopharm. Drug Dispos. 2012, 33, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Volz, M.; Christ, O.; Kellner, H.M.; Kuch, H.; Fehlhaber, H.W.; Gantz, D.; Hajdu, P.; Cavagna, F. Kinetics and metabolism of clobazam in animals and man. Br. J. Clin. Pharmacol. 1979, 7 (Suppl. 1), 41s–50s. [Google Scholar] [CrossRef]
- Levy, R.H.; Lane, E.A.; Guyot, M.; Brachet-Liermain, A.; Cenraud, B.; Loiseau, P. Analysis of parent drug-metabolite relationship in the presence of an inducer. Application to the carbamazepine-clobazam interaction in normal man. Drug Metab. Dispos. 1983, 11, 286–292. [Google Scholar] [CrossRef]
- Pullar, T.; Haigh, J.R.; Peaker, S.; Feely, M.P. Pharmacokinetics of N-desmethylclobazam in healthy volunteers and patients with epilepsy. Br. J. Clin. Pharmacol. 1987, 24, 793–797. [Google Scholar] [CrossRef]
- Contin, M.; Sangiorgi, S.; Riva, R.; Parmeggiani, A.; Albani, F.; Baruzzi, A. Evidence of polymorphic CYP2C19 involvement in the human metabolism of N-desmethylclobazam. Ther. Drug Monit. 2002, 24, 737–741. [Google Scholar] [CrossRef]
- Greenblatt, D.J. Electron-capture GLC determination of clobazam and desmethylclobazam in plasma. J. Pharm. Sci. 1980, 69, 1351–1352. [Google Scholar] [CrossRef]
- Jawad, S.; Richens, A.; Oxley, J. Single dose pharmacokinetic study of clobazam in normal volunteers and epileptic patients. Br. J. Clin. Pharmacol. 1984, 18, 873–877. [Google Scholar] [CrossRef]
- Monjanel-Mouterde, S.; Antoni, M.; Bun, H.; Botta-Frindlund, D.; Gauthier, A.; Durand, A.; Cano, J.P. Pharmacokinetics of a single oral dose of clobazam in patients with liver disease. Pharmacol. Toxicol. 1994, 74, 345–350. [Google Scholar] [CrossRef]
- Morrison, G.; Crockett, J.; Blakey, G.; Sommerville, K. A Phase 1, Open-Label, Pharmacokinetic Trial to Investigate Possible Drug-Drug Interactions Between Clobazam, Stiripentol, or Valproate and Cannabidiol in Healthy Subjects. Clin. Pharmacol. Drug Dev. 2019, 8, 1009–1031. [Google Scholar] [CrossRef] [PubMed]
- Ochs, H.R.; Greenblatt, D.J.; Lüttkenhorst, M.; Verburg-Ochs, B. Single and multiple dose kinetics of clobazam, and clinical effects during multiple dosage. Eur. J. Clin. Pharmacol. 1984, 26, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, G.; Riva, R.; Baruzzi, A. Clobazam plasma concentrations: Pharmacokinetic study in healthy volunteers and data in epileptic patients. Br. J. Clin. Pharmacol. 1981, 11, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Vallner, J.J.; Kotzan, J.A.; Stewart, J.T.; Honigberg, I.L.; Needham, T.E.; Brown, W.J. Plasma levels of clobazam after 10-, 20-, and 40-mg tablet doses in healthy subjects. J. Clin. Pharmacol. 1980, 20, 444–451. [Google Scholar] [CrossRef]
- Walzer, M.; Bekersky, I.; Blum, R.A.; Tolbert, D. Pharmacokinetic drug interactions between clobazam and drugs metabolized by cytochrome P450 isoenzymes. Pharmacotherapy 2012, 32, 340–353. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Clinical Pharmacology and Biopharmaceutics Review for Clobazam. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/202067Orig1s000ClinPharmR.pdf (accessed on 3 January 2025).
- Peigné, S.; Rey, E.; Le Guern, M.E.; Dulac, O.; Chiron, C.; Pons, G.; Jullien, V. Reassessment of stiripentol pharmacokinetics in healthy adult volunteers. Epilepsy Res. 2014, 108, 909–916. [Google Scholar] [CrossRef]
- Moreland, T.A.; Astoin, J.; Lepage, F.; Tombret, F.; Levy, R.H.; Baillie, T.A. The metabolic fate of stiripentol in man. Drug Metab. Dispos. 1986, 14, 654–662. [Google Scholar] [CrossRef]
- Levy, R.H.; Lin, H.S.; Blehaut, H.M.; Tor, J.A. Pharmacokinetics of stiripentol in normal man: Evidence of nonlinearity. J. Clin. Pharmacol. 1983, 23, 523–533. [Google Scholar] [CrossRef]
- Yun, Y.E.; Edginton, A.N. Correlation-based prediction of tissue-to-plasma partition coefficients using readily available input parameters. Xenobiotica 2013, 43, 839–852. [Google Scholar] [CrossRef]
- Lin, H.-s.; Levy, R.H. Pharmacokinetic Profile of a New Anticonvulsant, Stiripentol, in the Rhesus Monkey. Epilepsia 1983, 24, 692–702. [Google Scholar] [CrossRef]
- Levy, R.H.; Loiseau, P.; Guyot, M.; Blehaut, H.M.; Tor, J.; Moreland, T.A. Michaelis-Menten kinetics of stiripentol in normal humans. Epilepsia 1984, 25, 486–491. [Google Scholar] [CrossRef]
- Inoue, Y.; Ohtsuka, Y. Effectiveness of add-on stiripentol to clobazam and valproate in Japanese patients with Dravet syndrome: Additional supportive evidence. Epilepsy Res. 2014, 108, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Takahashi, Y.; Ikeda, H.; Imai, K.; Kagawa, Y.; Inoue, Y. Impact of CYP2C19 Phenotypes on Clinical Efficacy of Stiripentol in Japanese Patients With Dravet Syndrome. Ther. Drug Monit. 2020, 42, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Kunze, K.L.; Trager, W.F.; Kharasch, E.D.; Levy, R.H. Comparison of in vitro and in vivo inhibition potencies of fluvoxamine toward CYP2C19. Drug Metab. Dispos. 2003, 31, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Cazali, N.; Tran, A.; Treluyer, J.M.; Rey, E.; d’Athis, P.; Vincent, J.; Pons, G. Inhibitory effect of stiripentol on carbamazepine and saquinavir metabolism in human. Br. J. Clin. Pharmacol. 2003, 56, 526–536. [Google Scholar] [CrossRef]
- Giraud, C.; Treluyer, J.M.; Rey, E.; Chiron, C.; Vincent, J.; Pons, G.; Tran, A. In vitro and in vivo inhibitory effect of stiripentol on clobazam metabolism. Drug Metab. Dispos. 2006, 34, 608–611. [Google Scholar] [CrossRef]
- Abduljalil, K.; Cain, T.; Humphries, H.; Rostami-Hodjegan, A. Deciding on Success Criteria for Predictability of Pharmacokinetic Parameters from In Vitro Studies: An Analysis Based on In Vivo Observations. Drug Metab. Dispos. 2014, 42, 1478–1484. [Google Scholar] [CrossRef]
- May, T.W.; Boor, R.; Mayer, T.; Jürgens, U.; Rambeck, B.; Holert, N.; Korn-Merker, E.; Brandt, C. Concentrations of Stiripentol in Children and Adults With Epilepsy: The Influence of Dose, Age, and Comedication. Ther. Drug Monit. 2012, 34, 390–397. [Google Scholar] [CrossRef]
- Salem, F.; Johnson, T.N.; Abduljalil, K.; Tucker, G.T.; Rostami-Hodjegan, A. A re-evaluation and validation of ontogeny functions for cytochrome P450 1A2 and 3A4 based on in vivo data. Clin. Pharmacokinet. 2014, 53, 625–636. [Google Scholar] [CrossRef]
- Tateishi, T.; Nakura, H.; Asoh, M.; Watanabe, M.; Tanaka, M.; Kumai, T.; Takashima, S.; Imaoka, S.; Funae, Y.; Yabusaki, Y.; et al. A comparison of hepatic cytochrome P450 protein expression between infancy and postinfancy. Life Sci. 1997, 61, 2567–2574. [Google Scholar] [CrossRef]
- Croom, E.L.; Stevens, J.C.; Hines, R.N.; Wallace, A.D.; Hodgson, E. Human hepatic CYP2B6 developmental expression: The impact of age and genotype. Biochem. Pharmacol. 2009, 78, 184–190. [Google Scholar] [CrossRef]
- Pearce, R.E.; Gaedigk, R.; Twist, G.P.; Dai, H.; Riffel, A.K.; Leeder, J.S.; Gaedigk, A. Developmental Expression of CYP2B6: A Comprehensive Analysis of mRNA Expression, Protein Content and Bupropion Hydroxylase Activity and the Impact of Genetic Variation. Drug Metab. Dispos. 2016, 44, 948–958. [Google Scholar] [CrossRef]
- Koukouritaki, S.B.; Manro, J.R.; Marsh, S.A.; Stevens, J.C.; Rettie, A.E.; McCarver, D.G.; Hines, R.N. Developmental expression of human hepatic CYP2C9 and CYP2C19. J. Pharmacol. Exp. Ther. 2004, 308, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Hines, R.N. Ontogeny of human hepatic cytochromes P450. J. Biochem. Mol. Toxicol. 2007, 21, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Treluyer, J.M.; Gueret, G.; Cheron, G.; Sonnier, M.; Cresteil, T. Developmental expression of CYP2C and CYP2C-dependent activities in the human liver: In-vivo/in-vitro correlation and inducibility. Pharmacogenetics 1997, 7, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.C.; Marsh, S.A.; Zaya, M.J.; Regina, K.J.; Divakaran, K.; Le, M.; Hines, R.N. Developmental changes in human liver CYP2D6 expression. Drug Metab. Dispos. 2008, 36, 1587–1593. [Google Scholar] [CrossRef]
- Treluyer, J.M.; Jacqz-Aigrain, E.; Alvarez, F.; Cresteil, T. Expression of CYP2D6 in developing human liver. Eur. J. Biochem. 1991, 202, 583–588. [Google Scholar] [CrossRef]
- McNally, K.; Cotton, R.; Loizou, G.D. A Workflow for Global Sensitivity Analysis of PBPK Models. Front. Pharmacol. 2011, 2, 31. [Google Scholar] [CrossRef]
- Liu, D.; Li, L.; Rostami-Hodjegan, A.; Bois, F.Y.; Jamei, M. Considerations and Caveats when Applying Global Sensitivity Analysis Methods to Physiologically Based Pharmacokinetic Models. AAPS J. 2020, 22, 93. [Google Scholar] [CrossRef]
- Patsalos, P.N.; Berry, D.J.; Bourgeois, B.F.; Cloyd, J.C.; Glauser, T.A.; Johannessen, S.I.; Leppik, I.E.; Tomson, T.; Perucca, E. Antiepileptic drugs--best practice guidelines for therapeutic drug monitoring: A position paper by the subcommission on therapeutic drug monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia 2008, 49, 1239–1276. [Google Scholar] [CrossRef]
- Farwell, J.R.; Anderson, G.D.; Kerr, B.M.; Tor, J.A.; Levy, R.H. Stiripentol in Atypical Absence Seizures in Children: An Open Trial. Epilepsia 1993, 34, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.H.; Loiseau, P.; Guyot, M.; Blehaut, H.M.; Tor, J.; Moreland, T.A. Stiripentol kinetics in epilepsy: Nonlinearity and interactions. Clin. Pharmacol. Ther. 1984, 36, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Kosaki, K.; Tamura, K.; Sato, R.; Samejima, H.; Tanigawara, Y.; Takahashi, T. A major influence of CYP2C19 genotype on the steady-state concentration of N-desmethylclobazam. Brain Dev. 2004, 26, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.M.; Rodriguez-Lopez, J.M.; Webb, A.J. Elevations in Norclobazam Concentrations and Altered Mental Status in CYP2C19 Poor Metabolizer Phenotype: A Case Report. Neurohospitalist 2023, 13, 434–437. [Google Scholar] [CrossRef]
- Ogungbenro, K.; Aarons, L. A physiologically based pharmacokinetic model for clobazam and stiripentol in adults and children. Pharm. Res. 2015, 32, 144–157. [Google Scholar] [CrossRef]
- Peigné, S.; Chhun, S.; Tod, M.; Rey, E.; Rodrigues, C.; Chiron, C.; Pons, G.; Jullien, V. Population Pharmacokinetics of Stiripentol in Paediatric Patients with Dravet Syndrome Treated with Stiripentol, Valproate and Clobazam Combination Therapy. Clin. Pharmacokinet. 2018, 57, 739–748. [Google Scholar] [CrossRef]
- Murray, M. Mechanisms of inhibitory and regulatory effects of methylenedioxyphenyl compounds on cytochrome P450-dependent drug oxidation. Curr. Drug Metab. 2000, 1, 67–84. [Google Scholar] [CrossRef]
- Masubuchi, Y.; Takahashi, C.; Gendo, R. Time-Dependent Inhibition of CYP1A2 by Stiripentol and Structurally Related Methylenedioxyphenyl Compounds via Metabolic Intermediate Complex Formation. Drug Metab. Dispos. 2023, 52, 63–68. [Google Scholar] [CrossRef]
- Theis, J.G.; Koren, G.; Daneman, R.; Sherwin, A.L.; Menzano, E.; Cortez, M.; Hwang, P. Interactions of clobazam with conventional antiepileptics in children. J. Child. Neurol. 1997, 12, 208–213. [Google Scholar] [CrossRef]
- Björkman, S. Prediction of cytochrome p450-mediated hepatic drug clearance in neonates, infants and children: How accurate are available scaling methods? Clin. Pharmacokinet. 2006, 45, 1–11. [Google Scholar] [CrossRef]
- Tran, A.; Rey, E.; Pons, G.; Pariente-Khayat, A.; d’Athis, P.; Sallerin, V.; Dupont, C. Pharmacokinetic-pharmacodynamic study of oral lansoprazole in children. Clin. Pharmacol. Ther. 2002, 71, 359–367. [Google Scholar] [CrossRef]
- Marier, J.F.; Dubuc, M.C.; Drouin, E.; Alvarez, F.; Ducharme, M.P.; Brazier, J.L. Pharmacokinetics of omeprazole in healthy adults and in children with gastroesophageal reflux disease. Ther. Drug Monit. 2004, 26, 3–8. [Google Scholar] [CrossRef]
- Johnson, T.N.; Rostami-Hodjegan, A.; Tucker, G.T. Prediction of the clearance of eleven drugs and associated variability in neonates, infants and children. Clin. Pharmacokinet. 2006, 45, 931–956. [Google Scholar] [CrossRef]
- Zhou, W.; Johnson, T.N.; Bui, K.H.; Cheung, S.Y.A.; Li, J.; Xu, H.; Al-Huniti, N.; Zhou, D. Predictive Performance of Physiologically Based Pharmacokinetic (PBPK) Modeling of Drugs Extensively Metabolized by Major Cytochrome P450s in Children. Clin. Pharmacol. Ther. 2018, 104, 188–200. [Google Scholar] [CrossRef]
- Small, B.G.; Johnson, T.N.; Rowland Yeo, K. Another Step Toward Qualification of Pediatric Physiologically Based Pharmacokinetic Models to Facilitate Inclusivity and Diversity in Pediatric Clinical Studies. Clin. Pharmacol. Ther. 2023, 113, 735–745. [Google Scholar] [CrossRef]
- Bansal, S.; Paine, M.F.; Unadkat, J.D. Comprehensive Predictions of Cytochrome P450 (P450)-Mediated In Vivo Cannabinoid-Drug Interactions Based on Reversible and Time-Dependent P450 Inhibition in Human Liver Microsomes. Drug Metab. Dispos. 2022, 50, 351–360. [Google Scholar] [CrossRef] [PubMed]


| Compound | CLB | N-CLB | |||
|---|---|---|---|---|---|
| Parameter (Units) | Value | Reference | Value | Reference | |
| Physiochemical Properties | Molecular weight (g/mol) | 300.74 | ChEMBL | 286.71 | ADMET Predictor |
| LogP | 2.14 | ALOGPS | 2.45 | ||
| Compound Type | Monoprotic base | Monoprotic base | |||
| pKa | 6.65 | ChEMBL | 3.93 | ||
| B/P ratio | 0.69 | ADMET Predictor | 0.69 | ADMET Predictor | |
| fu,p | 0.1 | [24] | 0.11 | [24] | |
| Absorption | Absorption Model | First-Order Absorption | Simcyp | First Order Absorption | Simcyp |
| fa | 0.93 | [38] | |||
| ka (1/h) | 2.11 (Fasted) 1.25 (Fed) | [19] Estimated to capture clinical data | |||
| fu,gut | 0.1 | Assumed as fu,p | 0.11 | Assumed as fu,p | |
| Distribution | Distribution Model | Full PBPK | The Rodgers and Rowland method | Full PBPK | The Rodgers and Rowland method |
| Vss (L/kg) | 0.56 | Predicted | 1.04 | Predicted | |
| Kp scalar | 1 | No adjustment needed to capture observed Vss | 0.8 | Optimized to fit clinical data | |
| Elimination | CLh (L/h) | 2 | [3] | 1.09 | [28] |
| CLR (L/h) | 0.05 | [26] | 0.08 | [28] | |
| Elimination Model | Enzyme Kinetics | Enzyme Kinetics | |||
| CLint,CYP2C19 (µL/min/nmol P450) | 0.173 | Back-calculated using the well-stirred liver model | 0.636 | Back-calculated using the well-stirred liver model | |
| CLint,CYP3A4 (µL/min/nmol P450) | 0.019 | Back-calculated using the well-stirred liver model | |||
| CLint,CYP2B6 (µL/min/nmol P450) | 0.022 | Back-calculated using the well-stirred liver model | |||
| Compound | STP | ||
|---|---|---|---|
| Parameter (Units) | Value | Reference | |
| Physiochemical properties | Molecular weight (g/mol) | 234.29 | PubChem |
| LogP | 2.94 | [15] | |
| Compound type | Neutral | ADMET predictor | |
| B/P ratio | 0.58 | [41] | |
| fu,p | 0.01 | ||
| Absorption | Absorption model | First Order Absorption | Simcyp |
| fa | 0.82 | [40] | |
| ka (1/h) | 1.4 | Fitted to capture Cmax | |
| fu,gut | 0.01 | Assumed as fu,p | |
| Lag time (h) | 0.5–1 | Fitted to capture tmax | |
| Distribution | Distribution model | Full PBPK | The Rodgers and Rowland method |
| Vss (L/kg) | 1.74 | Predicted | |
| Kp scalar | 4.2 | Predicted | |
| Elimination | CLpo (L/h) | 8–70 | Fitted a |
| fm, CYP1A2 | 0.2 | [8] | |
| fm, CYP2C19 | 0.15 | ||
| fm, CYP2C9 | 0.08 | ||
| fm, CYP2D6 | 0.075 | ||
| fm, CYP3A4 | 0.14 | ||
| fm, CYP3A5 | 0.11 | ||
| Interaction b | CYP1A2 ki (μM) | 3.3 | [8] |
| CYP2B6 ki (μM) | 7 | [8] | |
| CYP2C19 ki (μM) | 0.0139 | Optimized c | |
| CYP2C8 ki (μM) | 3.4 | [8] | |
| CYP2C9 ki (μM) | 65 | [8] | |
| CYP2D6 ki (μM) | 9.3 | [10] | |
| CYP3A4 ki (μM) | 2.5 | [48] | |
| P-gp ki (μM) | 46 | [8] | |
| BCRP ki (μM) | 1.17 | [8] | |
| Study | Role in PBPK Model | CYP2C19 Phenotype | Dosing Regimen | Compound | Parameter | Simulated | Observed | Simulated/Observed |
|---|---|---|---|---|---|---|---|---|
| Chiron et al., 2000 [13] | DDI simulation between STP + CLB in pediatrics | Not reported | STP 25 mg/kg BID, CLB 1 mg/kg QD | CLB | Cmin,inh (mg/L) | 0.88 | 0.84 (0.66–1.02) | 1.05 |
| Cmin ratio | 1.77 | 1.9 | 0.93 | |||||
| N-CLB | Cmin,inh (mg/L) | 9.00 | 11.6 (10.3–12.9) | 0.78 | ||||
| Cmin ratio | 7.75 | 5.5 | 1.4 | |||||
| Inoue et al., 2014 [45] | DDI simulation between STP + CLB in Japanese population | EMs | STP 25 mg/kg BID, CLB 1 mg/kg QD | CLB | Cmin,inh (mg/L) | 0.94 | 0.52 ± 0.28 | 1.81 |
| Cmin ratio | 1.64 | 1.86 | 0.88 | |||||
| N-CLB | Cmin,inh (mg/L) | 10.03 | 7.50 ± 3.58 | 1.34 | ||||
| Cmin ratio | 7.9 | 6.1 | 1.30 | |||||
| PMs | CLB | Cmin,inh (mg/L) | 1.08 | 0.72 ± 0.57 | 1.5 | |||
| Cmin ratio | 1.31 | 1.71 | 0.77 | |||||
| N-CLB | Cmin,inh (mg/L) | 13.29 | 10.07 ± 3.53 | 1.32 | ||||
| Cmin ratio | 0.90 | 0.62 | 1.45 | |||||
| Yamamoto et al., 2020 [46] | EMs | STP 17.5 mg/kg BID, CLB 1 mg/kg QD | CLB | Cmin,inh (mg/L) | 0.91 | 0.59 ± 0.07 | 1.54 | |
| Cmin ratio | 1.54 | 1.79 | 0.86 | |||||
| N-CLB | Cmin,inh (mg/L) | 8.91 | 6.38 ± 0.49 | 1.40 | ||||
| Cmin ratio | 7.14 | 4.73 | 1.51 | |||||
| PMs | CLB | Cmin,inh (mg/L) | 1.01 | 0.66 ± 0.08 | 1.53 | |||
| Cmin ratio | 1.22 | 1.27 | 0.96 | |||||
| N-CLB | Cmin,inh (mg/L) | 12.75 | 10.26 ± 0.98 | 1.24 | ||||
| Cmin ratio | 0.93 | 0.63 | 1.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eltanameli, B.; Al Sahlawi, S.; Cristofoletti, R. Physiologically Based Pharmacokinetic Modeling of Clobazam and Stiripentol Co-Therapy in Dravet Syndrome. J. Pers. Med. 2025, 15, 549. https://doi.org/10.3390/jpm15110549
Eltanameli B, Al Sahlawi S, Cristofoletti R. Physiologically Based Pharmacokinetic Modeling of Clobazam and Stiripentol Co-Therapy in Dravet Syndrome. Journal of Personalized Medicine. 2025; 15(11):549. https://doi.org/10.3390/jpm15110549
Chicago/Turabian StyleEltanameli, Bassma, Sulafa Al Sahlawi, and Rodrigo Cristofoletti. 2025. "Physiologically Based Pharmacokinetic Modeling of Clobazam and Stiripentol Co-Therapy in Dravet Syndrome" Journal of Personalized Medicine 15, no. 11: 549. https://doi.org/10.3390/jpm15110549
APA StyleEltanameli, B., Al Sahlawi, S., & Cristofoletti, R. (2025). Physiologically Based Pharmacokinetic Modeling of Clobazam and Stiripentol Co-Therapy in Dravet Syndrome. Journal of Personalized Medicine, 15(11), 549. https://doi.org/10.3390/jpm15110549








