Digital Twins in Personalized Medicine: Bridging Innovation and Clinical Reality
Abstract
1. Introduction
2. Literature Search Strategy
3. State of the Art: Research Advances in Digital Twins
3.1. From Concept to Computational Reality
3.2. Model Typologies: Mechanistic, Data-Driven, and Hybrid Approaches
3.3. Enabling Technologies: Infrastructure for Simulation and Personalization
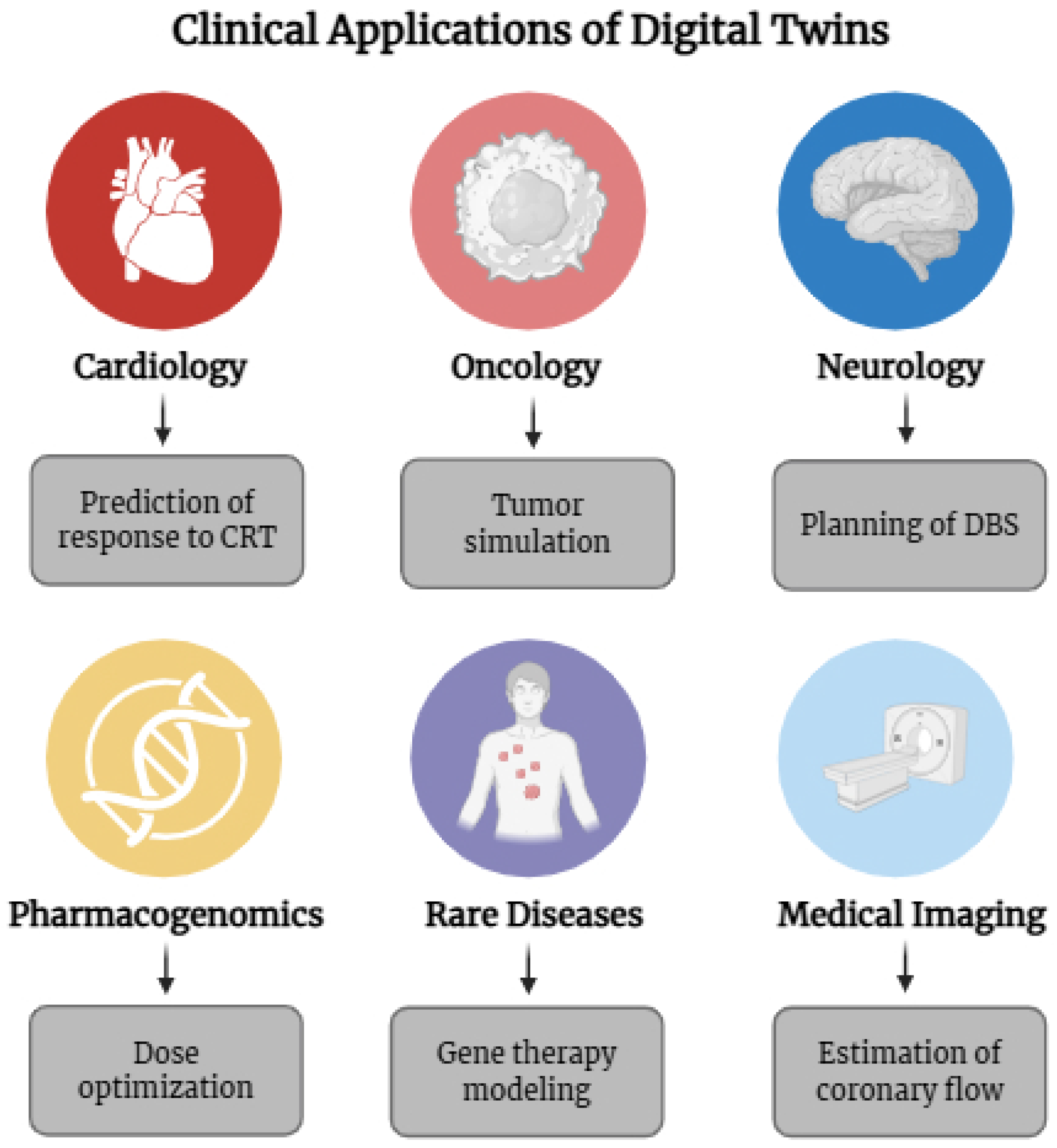
4. Clinical Applications of Digital Twins in Personalized Medicine
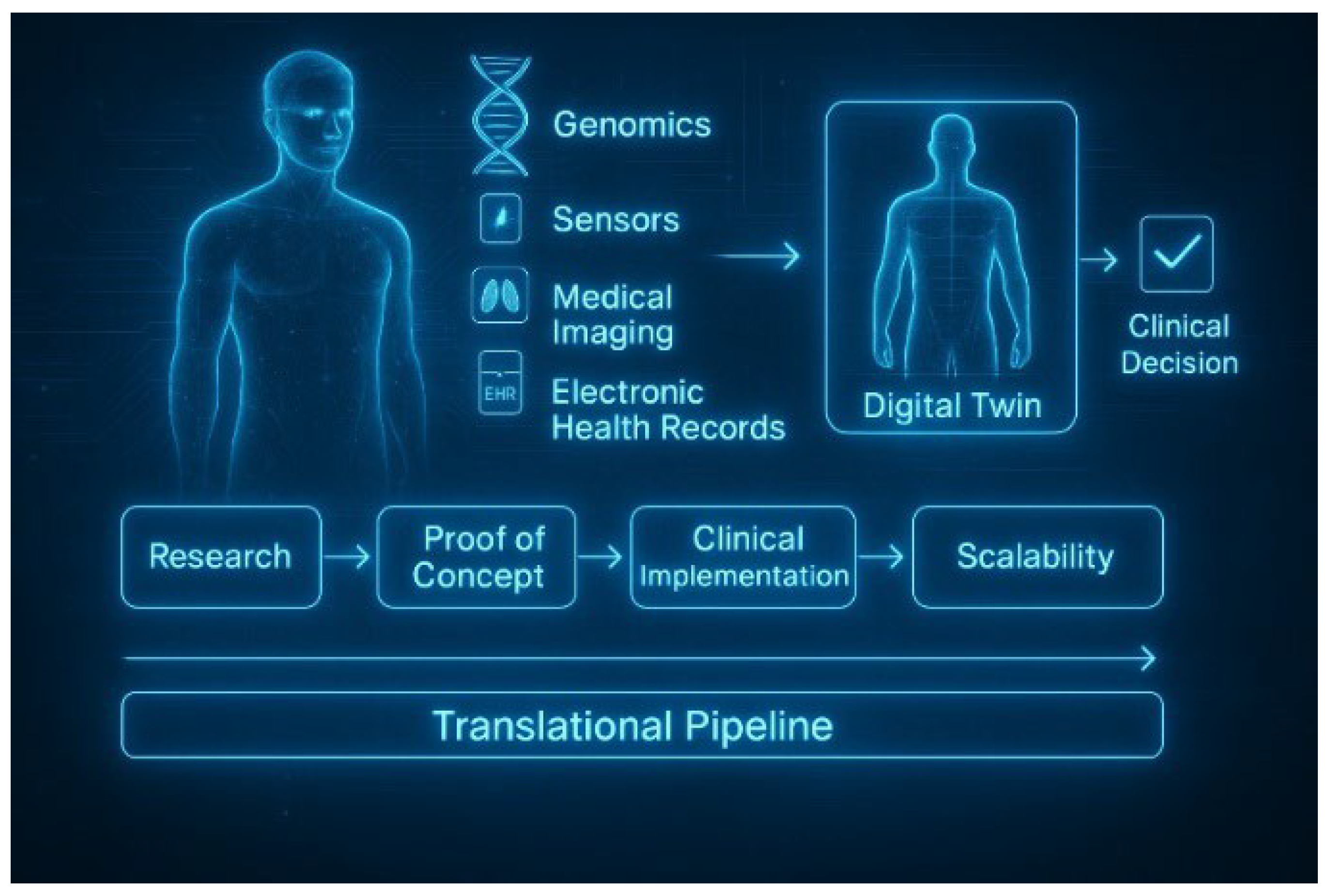
5. The Translational Gap: From Conceptual Promise to Clinical Practice
5.1. Scientific Maturity vs. Clinical Readiness
5.2. Lack of Standardized Validation Frameworks
5.3. Interoperability and Integration Barriers
5.4. Explainability, Clinical Trust, and Decision Accountability
5.5. Ethical and Regulatory Constraints
5.6. Health System Inequities and the Risk of Exclusion
5.7. Organizational Culture and Clinical Workflow Disruption
5.8. Summary: Beyond the Technology
6. Clinical Trials and Validation Strategies for Digital Twins
6.1. Why Are Conventional Trials Insufficient
6.2. Emerging Validation Approaches
6.3. Real-World Examples and Pilot Studies
6.4. Regulatory Perspectives and Frameworks in Development
6.5. Toward a Validation Ecosystem for Digital Twins
7. Future Perspectives: From Proof-of-Concept to Clinical Standard
8. Conclusion and Translational Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAV | Adeno-Associated Viruses |
| AI | Artificial Intelligence |
| CDSS | Clinical Decision Support System |
| CRT | Cardiac Resynchronization Therapy |
| DBS | Deep Brain Stimulation |
| DHT | Digital Human Twin |
| DT | Digital Twin |
| EHR | Electronic Health Record |
| FHIR | Fast Healthcare Interoperability Resources |
| NGS | Next-Generation Sequencing |
| OMOP | Observational Medical Outcomes Partnership |
| RCT | Randomized Controlled Trial |
| SaMD | Software as a Medical Device |
| SMA | Spinal Muscular Atrophy |
| XR | Extended Reality |
References
- Stefanicka-Wojtas, D.; Kurpas, D. Personalised Medicine-Implementation to the Healthcare System in Europe (Focus Group Discussions). J. Pers Med. 2023, 13, 380. [Google Scholar] [CrossRef]
- Pandey, A.; Gupta, S.P. Personalized Medicine: A Comprehensive Review. Orient. J. Chem. 2024, 40, 933–944. [Google Scholar] [CrossRef]
- Goetz, L.H.; Schork, N.J. Personalized medicine: Motivation, challenges, and progress. Fertil. Steril. 2018, 109, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; He, X.; Song, X.; Shu, L.; Li, Z. The Digital Twin in Medicine: A Key to the Future of Healthcare? Front. Med. 2022, 9, 907066. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhou, H.-Y.; Baptista-Hon, D.T.; Gao, Y.; Liu, X.; Oermann, E.; Xu, S.; Jin, S.; Zhang, J.; Sun, Z.; et al. Concepts and applications of digital twins in healthcare and medicine. Patterns 2024, 5, 101028. [Google Scholar] [CrossRef]
- Jain, K.K. Personalized medicine. Curr. Opin. Mol. Ther. 2002, 4, 548–558. [Google Scholar]
- Gates, J.; Yulianti, Y.; Pangilinan, G. Big Data Analytics for Predictive Insights in Healthcare. Int. Trans. Artif. Intell. 2024, 3, 54–63. [Google Scholar] [CrossRef]
- Papachristou, K.; Katsakiori, P.F.; Papadimitroulas, P.; Strigari, L.; Kagadis, G.C. Digital Twins’ Advancements and Applications in Healthcare, Towards Precision Medicine. J. Pers. Med. 2024, 14, 1101. [Google Scholar] [CrossRef]
- Katsoulakis, E.; Wang, Q.; Wu, H.; Shahriyari, L.; Fletcher, R.; Liu, J.; Achenie, L.; Liu, H.; Jackson, P.; Xiao, Y.; et al. Digital twins for health: A scoping review. NPJ Digit. Med. 2024, 7, 77. [Google Scholar] [CrossRef]
- Kamel Boulos, M.N.; Zhang, P. Digital Twins: From Personalised Medicine to Precision Public Health. J. Pers. Med. 2021, 11, 745. [Google Scholar] [CrossRef]
- Giansanti, D.; Morelli, S. Exploring the Potential of Digital Twins in Cancer Treatment: A Narrative Review of Reviews. J. Clin. Med. 2025, 14, 3574. [Google Scholar] [CrossRef] [PubMed]
- Coorey, G.; Figtree , G.A.; Fletcher, D.F.; Snelson, V.J.; Vernon, S.T.; Winlaw, D.; Grieve, S.M.; McEwan, A.; Yang, J.Y.H.; Qian, P.; et al. The health digital twin to tackle cardiovascular disease—A review of an emerging interdisciplinary field. NPJ Digit. Med. 2022, 5, 126. [Google Scholar] [CrossRef] [PubMed]
- Lorente, Q.; Villeneuve, E.; Merlo, C.; Boy, G.A.; Thermy, F. Sociotechnical System Digital Twin as an Organizational-enhancer Applied to Helicopter Engines Maintenance. In Proceedings of the 2022 IEEE International Conference on Industrial Engineering and Engineering Management (IEEM), Kuala Lumpur, Malaysia, 7–10 December 2022; 2022. [Google Scholar] [CrossRef]
- Kabalska, A.; Wagner, R. Rendering Frankenstein’s monsters? The emergence and impacts of human digital twins. Digit. Twins Appl. 2024, 1, 88–92. [Google Scholar] [CrossRef]
- Stahlberg, E.A.; Abdel-Rahman , M.; Aguilar, B.; Asadpoure, A.; Beckman, R.A.; Borkon, L.L.; Bryan, J.N.; Cebulla, C.M.; Chang, Y.H.; Chatterjee, A.; et al. Exploring approaches for predictive cancer patient digital twins: Opportunities for collaboration and innovation. Front. Digit. Health 2022, 4, 1007784. [Google Scholar] [CrossRef]
- Scientists Create Cancer Patients ‘Digital Twins’ to Predict How Well Treatments May Work. eCancer. 2024. Available online: https://ecancer.org/en/news/25568-scientists-create-cancer-patients-digital-twins-to-predict-how-well-treatments-may-work (accessed on 22 June 2025).
- SIEMENS. A Digital Twin of the Heart. Available online: https://www.siemens.com/global/en/company/about/history/specials/175-years/digital-twin-of-the-heart.html (accessed on 22 June 2025).
- Ringeval, M.; Etindele Sosso, F.A.; Cousineau, M.; Paré , G. Advancing Health Care With Digital Twins: Meta-Review of Applications and Implementation Challenges. J. Med. Internet Res. 2025, 27, e69544. [Google Scholar] [CrossRef]
- Attaran, S.; Attaran, M. Advancing Healthcare Through the Integration of Digital Twins Technology: Personalized Medicine’s Next Frontier. Future Internet 2024, 16, 477. [Google Scholar] [CrossRef]
- Manju, K.; Dimple, M.D. Revolutionizing Personalized Medicine with Digital Twin Technology. Int. J. Res. Publ. Rev. 2025, 6, 8300–8307. [Google Scholar]
- Hamburg, M.A.; Collins, F.S. The Path to Personalized Medicine. N. Engl. J. Med. 2010, 363, 301–304. [Google Scholar] [CrossRef]
- Oulefki, A.; Amira, A.; Foufou, S. Digital twins and AI transforming healthcare systems through innovation and data-driven decision making. Health Technol. 2025, 15, 299–321. [Google Scholar] [CrossRef]
- Ștefănigă, S.A.; Cordoș, A.A.; Ivascu, T.; Feier, C.V.I.; Muntean, C.; Stupinean, C.V.; Călinici, T.; Aluaș, M.; Bolboacă, S.D. Advancing Precision Oncology with Digital and Virtual Twins: A Scoping Review. Cancers 2024, 16, 3817. [Google Scholar] [CrossRef]
- Alsalloum, G.A.; Al Sawaftah, N.M.; Percival, K.M.; Husseini, G.A. Digital Twins of Biological Systems: A Narrative Review. IEEE Open J. Eng. Med. Biol. 2024, 5, 670–677. [Google Scholar] [CrossRef]
- Tortora, M.; Pacchiano, F.; Ferraciolli, S.F.; Criscuolo, S.; Gagliardo, C.; Jaber, K.; Angelicchio, M.; Briganti, F.; Caranci, F.; Tortora, F. Medical Digital Twin: A Review on Technical Principles and Clinical Applications. J. Clin. Med. 2025, 14, 324. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.D.; Krauthammer, M.; Witt, C.M.; Biller-Andorno, N.; Christen, M. A consensus statement on the use of digital twins in medicine. npj Digit. Med. 2025. [Google Scholar] [CrossRef]
- Wickramasinghe, N.; Jayaraman, P.P.; Forkan, A.R.M.; Ulapane, N.; Kaul, R.; Vaughan, S.; Zelcer, J. A vision for leveraging the concept of digital twins to support the provision of personalized cancer care. IEEE Internet Comput. 2021, 26, 17–24. [Google Scholar] [CrossRef]
- Gharleghi, R.; Dessalles, C.A.; Lal, R.; McCraith, S.; Sarathy, K.; Jepson, N.; Otton, J.; Barakat, A.I.; Beier, S. 3D Printing for Cardiovascular Applications: From End-to-End Processes to Emerging Developments. Ann. Biomed. Eng. 2021, 49, 1598–1618. [Google Scholar] [CrossRef]
- Corral-Acero, J.; Margara, F.; Marciniak, M.; Rodero, C.; Loncaric, F.; Feng, Y.; Gilbert, A.; Fernandes, J.F.; Bukhari, H.A.; Wajdan, A.; et al. The ‘Digital Twin’to enable the vision of precision cardiology. Eur. Heart J. 2020, 41, 4556–4564. [Google Scholar] [CrossRef]
- Mikołajewska, E.; Masiak, J.; Mikołajewski, D. Applications of Artificial Intelligence-Based Patient Digital Twins in Decision Support in Rehabilitation and Physical Therapy. Electronics 2024, 13, 4994. [Google Scholar] [CrossRef]
- Sun, T.; He, X.; Li, Z. Digital twin in healthcare: Recent updates and challenges. Digit. Health 2023, 9, 20552076221149651. [Google Scholar] [CrossRef]
- Sel, K.; Osman, D.; Zare, F.; Masoumi Shahrbabak, S.; Brattain, L.; Hahn, J.-O.; Inan, O.T.; Mukkamala, R.; Palmer, J.; Paydarfar, D.; et al. Building Digital Twins for Cardiovascular Health: From Principles to Clinical Impact. J. Am. Heart Assoc. 2024, 13, e031981. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, L.; Ali, A.; Nugent, C.; Cleland, I.; Li, R.; Ding, J.; Ning, H. Human digital twin: A survey. J. Cloud Comput. 2024, 13, 131. [Google Scholar] [CrossRef]
- Al Kuwaiti, A.; Nazer, K.; Al-Reedy, A.; Al-Shehri, S.; Al-Muhanna, A.; Subbarayalu, A.V.; Al Muhanna, D.; Al-Muhanna, F.A. A Review of the Role of Artificial Intelligence in Healthcare. J. Pers. Med. 2023, 13, 951. [Google Scholar] [CrossRef] [PubMed]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Belle, A.; Thiagarajan, R.; Soroushmehr, S.R.; Navidi, F.; Beard, D.A.; Najarian, K. Big Data Analytics in Healthcare. Biomed. Res. Int. 2015, 2015, 370194. [Google Scholar] [CrossRef]
- Viceconti, M.; Hunter, P.; Hose, R. Big Data, Big Knowledge: Big Data for Personalized Healthcare. IEEE J. Biomed. Health Inform. 2015, 19, 1209–1215. [Google Scholar] [CrossRef]
- Pappalardo, F.; Russo, G.; Tshinanu, F.M.; Viceconti, M. In silico clinical trials: Concepts and early adoptions. Brief Bioinform 2019, 20, 1699–1708. [Google Scholar] [CrossRef]
- Niederer, S.A.; Lumens, J.; Trayanova, N.A. Computational models in cardiology. Nat. Rev. Cardiol. 2019, 16, 100–111. [Google Scholar] [CrossRef]
- Armoundas, A.A.; Narayan, S.M.; Arnett, D.K.; Spector-Bagdady, K.; Bennett, D.A.; Celi, L.A.; Friedman, P.A.; Gollob, M.H.; Hall, J.L.; Kwitek, A.E.; et al. Use of Artificial Intelligence in Improving Outcomes in Heart Disease: A Scientific Statement From the American Heart Association. Circulation 2024, 149, e1028–e1050. [Google Scholar] [CrossRef]
- Sendak, M.P.; D’Arcy, J.; Kashyap, S.; Gao, M.; Nichols, M.; Corey, K.; Ratliff, W.; Balu, S. A path for translation of machine learning products into healthcare delivery. EMJ Innov. 2020, 10, 19-00172. [Google Scholar] [CrossRef]
- Cabitza, F.; Rasoini, R.; Gensini, G.F. Unintended Consequences of Machine Learning in Medicine. Jama 2017, 318, 517–518. [Google Scholar] [CrossRef]
- Rieke, N.; Hancox, J.; Li, W.; Milletarì, F.; Roth, H.R.; Albarqouni, S.; Bakas, S.; Galtier, M.N.; Landman, B.A.; Maier-Hein, K.; et al. The future of digital health with federated learning. NPJ Digit. Med. 2020, 3, 119. [Google Scholar] [CrossRef]
- Ferrari, A.; Willcox, K. Digital twins in mechanical and aerospace engineering. Nat. Comput. Sci. 2024, 4, 178–183. [Google Scholar] [CrossRef]
- Jiang, F.; Ma, L.; Broyd, T.; Chen, K. Digital twin and its implementations in the civil engineering sector. Autom. Constr. 2021, 130, 103838. [Google Scholar] [CrossRef]
- Schluse, M.; Priggemeyer, M.; Atorf, L.; Rossmann, J. Experimentable Digital Twins—Streamlining Simulation-Based Systems Engineering for Industry 4.0. IEEE Trans. Ind. Inform. 2018, 14, 1722–1731. [Google Scholar] [CrossRef]
- Guo, J.; Lv, Z. Application of Digital Twins in multiple fields. Multimed. Tools Appl. 2022, 81, 26941–26967. [Google Scholar] [PubMed]
- Tao, F.; Zhang, H.; Liu, A.; Nee, A.Y.C. Digital Twin in Industry: State-of-the-Art. IEEE Trans. Ind. Inform. 2019, 15, 2405–2415. [Google Scholar] [CrossRef]
- De Domenico, M.; Allegri, L.; Caldarelli, G.; d’Andrea, V.; Di Camillo, B.; Rocha, L.M.; Rozum, J.; Sbarbati, R.; Zambelli, F. Challenges and opportunities for digital twins in precision medicine from a complex systems perspective. NPJ Digit. Med. 2025, 8, 37. [Google Scholar]
- Qian, S.; Ugurlu, D.; Fairweather, E.; Toso, L.D.; Deng, Y.; Strocchi, M.; Cicci, L.; Jones, R.E.; Zaidi, H.; Prasad, S.; et al. Developing cardiac digital twin populations powered by machine learning provides electrophysiological insights in conduction and repolarization. Nat. Cardiovasc. Res. 2025, 4, 624–636. [Google Scholar] [CrossRef]
- Baillargeon, B.; Rebelo, N.; Fox, D.D.; Rebelo, R.L.; Kuhl, E. The Living Heart Project: A robust and integrative simulator for human heart function. Eur. J. Mech. A Solids 2014, 48, 38–47. [Google Scholar] [CrossRef]
- Viceconti, M.; De Vos, M.; Mellone, S.; Geris, L. Position Paper From the Digital Twins in Healthcare to the Virtual Human Twin: A Moon-Shot Project for Digital Health Research. IEEE J. Biomed. Health Inform. 2024, 28, 491–501. [Google Scholar] [CrossRef]
- El-Warrak, L.; Cde Farias, M. The State of the Art of Digital Twins in Health—A Quick Review of the Literature. Computers 2024, 13, 228. [Google Scholar] [CrossRef]
- Fairbairn, T.A.; Mullen, L.; Nicol, E.; Lip, G.Y.H.; Schmitt, M.; Shaw, M.; Tidbury, L.; Kemp, I.; Crooks, J.; Burnside, G.; et al. Implementation of a national AI technology program on cardiovascular outcomes and the health system. Nat. Med. 2025, 31, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Deisboeck, T.S.; Wang, Z.; Macklin , P.; Cristini, V. Multiscale Cancer Modeling. Annu. Rev. Biomed. Eng. 2011, 13, 127–155. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen , Z.; Wang, J.; Wang, X.; Qu, B.; Ma, L.; Zhao, W.; Zhang, G.; Xu, S. Dose Prediction Using a Three-Dimensional Convolutional Neural Network for Nasopharyngeal Carcinoma With Tomotherapy. Front. Oncol. 2021, 11, 752007. [Google Scholar] [CrossRef] [PubMed]
- Kapteyn, M.; Chaudhuri, A.; Lima, E.; Pash, G.; Bravo, R.; Willcox, K.; Yankeelov, T.E.; Hormuth, D.A., II. TumorTwin: A Python Framework for Patient-Specific Digital Twins in Oncology. arXiv 2025, arXiv:2505.00670. [Google Scholar]
- Aghamiri, S.S.; Amin, R. The Potential Use of Digital Twin Technology for Advancing CAR-T Cell Therapy. Curr. Issues Mol. Biol. 2025, 47, 321. [Google Scholar] [CrossRef]
- Chakshu, N.K.; Carson, J.; Sazonov, I.; Nithiarasu, P. A semi-active human digital twin model for detecting severity of carotid stenoses from head vibration—A coupled computational mechanics and computer vision method. Int. J. Numer. Methods Biomed. Eng. 2019, 35, e3180. [Google Scholar] [CrossRef] [PubMed]
- Jirsa, V.; Wang , H.; Triebkorn, P.; Hashemi, M.; Jha, J.; Gonzalez-Martinez, J.; Guye, M.; Makhalova, J.; Bartolomei, F. Personalised virtual brain models in epilepsy. Lancet Neurol. 2023, 22, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Gettman, D. Digital Twin Models for Simulating Patient Medication Outcomes. In Proceedings of the Pharmacy Practice: Spring and Fall Series on Transformative Change, Oakwood, VA, USA; 2008. [Google Scholar]
- Hill, M.; White, C.; Wang, S.; Thomas, J.; DeVincentis, B.; Singh, N. Computational fluid dynamics based digital twins of fixed bed bioreactors validate scaling principles for recombinant adeno-associated virus gene therapy manufacturing. Biotechnol. Bioeng. 2024, 121, 2662–2677. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ghallab, A.; Hassan, R.; Dooley, S.; Hengstler, J.G.; Drasdo, D. A liver digital twin for in silico testing of cellular and inter-cellular mechanisms in regeneration after drug-induced damage. iScience 2024, 27, 108077. [Google Scholar] [CrossRef] [PubMed]
- Grieb, N.; Schmierer, L.; Kim, H.U.; Strobel, S.; Schulz, C.; Meschke, T.; Kubasch, A.S.; Brioli, A.; Platzbecker, U.; Neumuth, T.; et al. A digital twin model for evidence-based clinical decision support in multiple myeloma treatment. Front. Digit. Health 2023, 5, 1324453. [Google Scholar] [CrossRef] [PubMed]
- Faiella, E.; Pileri, M.; Ragone, R.; Grasso, R.F.; Zobel, B.B.; Santucci, D. Digital twins in radiology: A systematic review of applications, challenges, and future perspectives. Eur. J. Radiol. 2025, 189, 112166. [Google Scholar] [CrossRef] [PubMed]
- Pesapane, F.; Rotili, A.; Penco, S.; Nicosia, L. Digital Twins in Radiology. J. Clin. Med. 2022, 11, 6553. [Google Scholar] [CrossRef]
- Gabrielli, S.; Piras, E.; Ibarra, O. Digital Twins in the Future Design of Digital Therapeutics. In Proceedings of the UbiComp/ISWC’23: The 2023 ACM International Joint Conference on Pervasive and Ubiquitous Computing, Cancún, Mexico, 8–12 October 2023; pp. 602–605. [Google Scholar]
- Schwartz, S.M.; Wildenhaus, K.; Bucher, A.; Byrd, B. Digital Twins and the Emerging Science of Self: Implications for Digital Health Experience Design and “Small” Data. Front. Comput. Sci. 2020, 2, 31. [Google Scholar] [CrossRef]
- Rivera, L.F.; Jiménez, M.; Angara, P.; Villegas, N.M.; Tamura, G.; Müller, H.A. Towards Continuous Monitoring in Personalized Healthcare Through Digital TWINS. In Proceedings of the 29th Annual International Conference on Computer Science and Software Engineering, Toronto, ON, Canada, 4–6 November 2019; IBM Corp.: Riverton, NJ, USA, 2019; pp. 329–335. [Google Scholar]
- Björnsson, B.; Borrebaeck, C.; Elander, N.; Gasslander, T.; Gawel, D.R.; Gustafsson, M.; Jörnsten, R.; Lee, E.J.; Li, X.; Lilja, S.; et al. Digital twins to personalize medicine. Genome Med. 2019, 12, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Hose, D.R.; Lawford, P.V.; Huberts, W.; Hellevik, L.R.; Omholt, S.W.; van de Vosse, F.N. Cardiovascular models for personalised medicine: Where now and where next? Med. Eng. Phys. 2019, 72, 38–48. [Google Scholar] [CrossRef]
- Saberi, M.A.; Mcheick, H.; Adda, M. From Data Silos to Health Records Without Borders: A Systematic Survey on Patient-Centered Data Interoperability. Information 2025, 16, 106. [Google Scholar] [CrossRef]
- Smith, N.; Romanchikova, M.; Partarrieu, I.; Cooke, E.; Lemanska, A.; Thomas, S. NMS 2018-2021 Life-sciences and Healthcare Project "Digital Health: Curation of Healthcare Data" - Final Report. NPL Report. MS 31. 2021, p. 6. Available online: http://eprintspublications.npl.co.uk/id/eprint/9294 (accessed on 22 June 2025).
- Mitra, R.; McGough, S.F.; Chakraborti, T.; Holmes, C.; Copping, R.; Hagenbuch, N.; Biedermann, S.; Noonan, J.; Lehmann, B.; Shenvi, A.; et al. Learning from data with structured missingness. Nat. Mach. Intell. 2023, 5, 13–23. [Google Scholar] [CrossRef]
- Riahi, V.; Diouf, I.; Khanna , S.; Boyle, J.; Hassanzadeh, H. Digital Twins for Clinical and Operational Decision-Making: Scoping Review. J. Med. Internet Res. 2025, 27, e55015. [Google Scholar] [CrossRef]
- Lämmermann, L.; Hofmann, P.; Urbach, N. Managing artificial intelligence applications in healthcare: Promoting information processing among stakeholders. Int. J. Inf. Manag. 2024, 75, 102728. [Google Scholar] [CrossRef]
- Kuštelega, M.; Mekovec, R.; Shareef, A. Privacy and security challenges of the digital twin: Systematic literature review. JUCS J. Univers. Comput. Sci. 2024, 30, 1782–1806. [Google Scholar] [CrossRef]
- Hummel, P.; Braun , M.; Tretter, M.; Dabrock, P. Data sovereignty: A review. Big Data Soc. 2021, 8, 2053951720982012. [Google Scholar] [CrossRef]
- Mittelstadt, B. Principles alone cannot guarantee ethical AI. Nat. Mach. Intell. 2019, 1, 501–507. [Google Scholar] [CrossRef]
- Li, G. A New Regulatory Road in Clinical Trials: Digital Twins. Appl. Clin. Trials 2024, 33, 9. [Google Scholar]
- Wang, Y.; Su, Z.; Guo, S.; Dai, M.; Luan, T.H.; Liu, Y. A survey on digital twins: Architecture, enabling technologies, security and privacy, and future prospects. IEEE Internet Things J. 2023, 10, 14965–14987. [Google Scholar] [CrossRef]
- Rawson, T.M.; Zhu, N.; Galiwango, R.; Cocker, D.; Islam, M.S.; Myall, A.; Vasikasin, V.; Wilson, R.; Shafiq, N.; Das , S.; et al. Using digital health technologies to optimise antimicrobial use globally. Lancet Digit. Health 2024, 6, e914–e925. [Google Scholar] [CrossRef] [PubMed]
- Karsh, B.-T. Clinical Practice Improvement and Redesign: How Change in Workflow Can Be Supported by Clinical Decision Support; AHRQ Publication No. 09-0054-EF; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2009. [Google Scholar]
- An, G. Specialty grand challenge: What it will take to cross the valley of death: Translational systems biology, ”true” precision medicine, medical digital twins, artificial intelligence and in silico clinical trials. Front. Syst. Biol. 2022, 2, 901159. [Google Scholar] [CrossRef]
- Helbing, D.; Sánchez-Vaquerizo, J.A. Digital twins: Potentials, ethical issues and limitations. In Handbook on the Politics and Governance of Big Data and Artificial Intelligence; Edward Elgar Publishing: Cheltenham, UK, 2023; pp. 64–104. [Google Scholar]
- Mazzetto, S. A Review of Urban Digital Twins Integration, Challenges, and Future Directions in Smart City Development. Sustainability 2024, 16, 8337. [Google Scholar] [CrossRef]
- Quek, H.Y.; Sielker, F.; Akroyd, J.; Bhave, A.N.; von Richthofen, A.; Herthogs, P.; van der Laag Yamu, C.; Wan, L.; Nochta, T.; Burgess, G.; et al. The conundrum in smart city governance: Interoperability and compatibility in an ever-growing ecosystem of digital twins. Data Policy 2023, 5, e6. [Google Scholar] [CrossRef]
- Zhao, F.; Wu Y, Y.; Hu, M.; Chang, C.W.; Liu, R.; Qiu, R.; Yang , X. Current Progress of Digital Twin Construction Using Medical Imaging. arXiv 2024, arXiv:2411.08173. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, K.P.; Brito, G.; Boulos, M.N.K. Health digital twins in life science and health care innovation. Annu. Rev. Pharmacol. Toxicol. 2024, 64, 159–170. [Google Scholar] [CrossRef]
- Venkatesh, K.P.; MRaza, M.; Kvedar, J.C. Health digital twins as tools for precision medicine: Considerations for computation, implementation, and regulation. NPJ Digit. Med. 2022, 5, 150. [Google Scholar] [CrossRef]
- Allen, A.; Siefkas, A.; Pellegrini, E.; Burdick, H.; Barnes, G.; Calvert, J.; Mao, Q.; Das, R. A digital twins machine learning model for forecasting disease progression in stroke patients. Appl. Sci. 2021, 11, 5576. [Google Scholar] [CrossRef]
- Ricci, A.; Croatti, A.; Montagna, S. Pervasive and Connected Digital Twins—A Vision for Digital Health. IEEE Internet Comput. 2022, 26, 26–32. [Google Scholar] [CrossRef]
- Zobel-Roos, S.; Schmidt, A.; Mestmäcker, F.; Mouellef, M.; Huter, M.; Uhlenbrock, L.; Kornecki, M.; Lohmann, L.; Ditz, R.; Strube, J. Accelerating biologics manufacturing by modeling or: Is approval under the QbD and PAT approaches demanded by authorities acceptable without a digital-twin? Processes 2019, 7, 94. [Google Scholar] [CrossRef]
- Green, J.I.J. Medical Device Regulations and custom-made device documentation: Ten frequently asked questions and their answers. Prim. Dent. J. 2022, 11, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Chen, J.; Xie, S.; Li, X.; Liu, H.; Wang, L.; Hong, C.; Li, G.; Li, H.; Chen, K. Establishment of CFD-ANN-NSGA-II model for stirred reactor design. Chem. Eng. Sci 2025, 311. [Google Scholar] [CrossRef]
- Leckenby, E.; Dawoud, D.; Bouvy, J.; Jónsson , P. The sandbox approach and its potential for use in health technology assessment: A literature review. Appl. Health Econ. Health Policy 2021, 19, 857–869. [Google Scholar] [CrossRef]
- Elvidge, J.; Crabb, N.; Delnoij, D.; Knies, S.; Lundin, D.; Houÿez, F.; Röning, J.; Wang, J.; Jiu, L.; Bennett, A.; et al. Implementing a sandbox approach in health technology assessment: Benefits and recommendations. Int. J. Technol. Assess. Health Care 2024, 40, e44. [Google Scholar] [CrossRef]
- Khalyasmaa, A.I.; Stepanova, A.I.; Eroshenko, S.A.; Matrenin , P.V. Review of the digital twin technology applications for electrical equipment lifecycle management. Mathematics 2023, 11, 1315. [Google Scholar] [CrossRef]
- Knapp, A.C.; Cruz, D.A.; Mehrad, B.; Laubenbacher, R.C. Personalizing computational models to construct medical digital twins. J. R. Soc. Interface 2025, 22, 20250055. [Google Scholar] [CrossRef] [PubMed]
- Batty, M. Digital twins; Sage Publications Sage: London, UK, 2018; pp. 817–820. [Google Scholar]
- Samson, O. The Role of Digital Twins in Optimizing Healthcare Coverage Strategies. 2024. [Google Scholar]
- Armeni, P.; Polat, I.; De Rossi, L.M.; Diaferia, L.; Meregalli, S.; Gatti, A. Digital twins in healthcare: Is it the beginning of a new era of evidence-based medicine? A critical review. J. Pers. Med. 2022, 12, 1255. [Google Scholar] [CrossRef] [PubMed]
- Gomis, M.; Berdún, J.; Borrás-Santos, A.; De Dios López, A.; Fernández-Montells Rama, B.; García-Esquirol, Ó.; Gratacòs, M.; Ontiveros Rodríguez, G.D.; Pelegrín Cruz, R.; Real, J.; et al. Clinical Validation of Digital Healthcare Solutions: State of the Art, Challenges and Opportunities. Healthcare 2024, 12, 1057. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Nayyar, A. Exploring diverse use cases of digital twins projecting digital transformation: Unlocking potential, addressing challenges and viable solutions. In Digital Twins for Smart Cities and Villages; Elsevier: Amsterdam, The Netherlands, 2025; pp. 631–655. [Google Scholar]
- Karanasiou, G.; Edelman, E.; Boissel, F.H.; Byrne, R.; Emili, L.; Fawdry, M.; Filipovic, N.; Flynn, D.; Geris, L.; Hoekstra, A.; et al. Advancing In Silico Clinical Trials for Regulatory Adoption and Innovation. IEEE J. Biomed. Health Inform. 2024. [CrossRef] [PubMed]
- Monlezun, D.J. The Thinking Healthcare System: Artificial Intelligence and Human Equity; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Rodriguez-Chavez, I.R.; Licholai, G. Decentralized Clinical Trials: A New Paradigm for New Medical Product Development and Digital Therapeutics. In Digital Therapeutics; Chapman and Hall/CRC: Boca Raton, FL, USA, 2022; pp. 373–404. [Google Scholar]
- Subasi, A.; Subasi, M.E. Digital twins in healthcare and biomedicine. In Artificial Intelligence, Big Data, Blockchain and 5G for the Digital Transformation of the Healthcare Industry; Elsevier: Amsterdam, The Netherlands, 2024; pp. 365–401. [Google Scholar]
- Lujan, G.; Quigley, J.C.; Hartman, D.; Parwani, A.; Roehmholdt, B.; Van Meter, B. Dissecting the business case for adoption and implementation of digital pathology: A white paper from the Digital Pathology Association. J. Pathol. Inform. 2021, 12, 17. [Google Scholar] [CrossRef]
- Montezuma, D.; Monteiro, A.; Fraga, J.; Ribeiro, L.; Gonçalves, S.; Tavares, A.; Monteiro, J.; Macedo-Pinto, I. Digital Pathology Implementation in Private Practice: Specific Challenges and Opportunities. Diagnostics 2022, 12, 529. [Google Scholar] [CrossRef]
- Eminaga, O.; Abbas, M.; Kunder, C.; Tolkach, Y.; Han, R.; Brooks, J.D. Critical evaluation of artificial intelligence as a digital twin of pathologists for prostate cancer pathology. Sci. Rep. 2024, 14, 5284. [Google Scholar] [CrossRef]
- Munari, E.; Scarpa, A.; Cima, L.; Pozzi, M.; Pagni, F.; Vasuri, F. Cutting-edge technology and automation in the pathology laboratory. Virchows Arch. 2024, 484, 555–566. [Google Scholar] [CrossRef]
- Shen, M.-D.; Chen, S.-B.; Ding, X.-D. The effectiveness of digital twins in promoting precision health across the entire population: A systematic review. NPJ Digit. Med. 2024, 7, 145. [Google Scholar] [CrossRef]
- Jameil, A.K.; Al-Raweshidy, H. A digital twin framework for real-time healthcare monitoring: Leveraging AI and secure systems for enhanced patient outcomes. Discov. Internet Things 2025, 5, 1–27. [Google Scholar] [CrossRef]
| Medical Specialty | Clinical Application | Implementation Status |
|---|---|---|
| Cardiology | Optimization of cardiac resynchronization therapy | Validated in studies [27,52,53,54] |
| Oncology | Tumor response simulation and personalized immunotherapy planning | Pilot/Experimental [27,53,55,56,57,58] |
| Neurology | Planning of deep brain stimulation in Parkinson’s disease | Experimental [59,60] |
| Pharmacogenomics | Dose adjustment based on genetic polymorphisms (CYP450) | Experimental [61,62,63] |
| Rare Diseases | Modeling of vector distribution in gene therapy | Pilot/Validated [63,64] |
| Medical Imaging | Coronary flow estimation via CT angiography (e.g., HeartFlow) | Clinical/Commercial [52,65,66,67] |
| Barrier Type | Description | Clinical Impact | Real-World Example |
|---|---|---|---|
| Technical | Lack of interoperability between hospital systems | Fragmented data, outdated models | EHRs are incompatible with sensors [72] |
| Regulatory | Absence of specific guidelines for DT approval | Delayed certification and institutional trust | No legal framework for hybrid models [37,38] |
| Validation | Lack of standardized validation frameworks | Lack of clinical trust, delays in adoption of the method | No agreed endpoints for in silico validation [10,34] |
| Ethical | Dynamic consent and continuous use of patient data | Risk of unauthorized or opaque use | Updates without renewed patient consent [43,77,78,79,80] |
| Trust/Explainability | Limited explainability and unclear decision accountability | Low clinician confidence, reluctant to use in practice | Opacity of AI-driven DT predictions in oncology/neurosurgery [42,75,76] |
| Organizational | Staff resistance and lack of training | Low adoption despite the availability of tools | Clinicians ignoring DT-generated alerts [84] |
| Computational | Insufficient infrastructure for real-time data processing | Inability to update the model dynamically | Hospitals lacking adequate servers or cloud resources [73] |
| Equity/Inclusion | Models trained on non-representative populations | Risk of algorithmic bias and errors in vulnerable populations | Underrepresentation of genetic and socioeconomic minorities [8,10,82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.; Vale, N. Digital Twins in Personalized Medicine: Bridging Innovation and Clinical Reality. J. Pers. Med. 2025, 15, 503. https://doi.org/10.3390/jpm15110503
Silva A, Vale N. Digital Twins in Personalized Medicine: Bridging Innovation and Clinical Reality. Journal of Personalized Medicine. 2025; 15(11):503. https://doi.org/10.3390/jpm15110503
Chicago/Turabian StyleSilva, Abigail, and Nuno Vale. 2025. "Digital Twins in Personalized Medicine: Bridging Innovation and Clinical Reality" Journal of Personalized Medicine 15, no. 11: 503. https://doi.org/10.3390/jpm15110503
APA StyleSilva, A., & Vale, N. (2025). Digital Twins in Personalized Medicine: Bridging Innovation and Clinical Reality. Journal of Personalized Medicine, 15(11), 503. https://doi.org/10.3390/jpm15110503







