Potential Chemopreventive Role of Proton Pump Inhibitors in Head and Neck Cancer: Insights from a Nested Case–Control Analysis of a National Health Screening Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Exposure (Proton Pump Inhibitor)
- (1)
- The participants were categorized into three groups: nonusers, current PPI users (prescribed at least once within the past 30 days), and PPI-exposed individuals (prescribed at least once within 31–365 days).
- (2)
- Participants were further divided into four groups based on the duration of PPI use: nonusers, 1–29 days of PPI use, 30–89 days of PPI use, and 90 or more days of PPI use.
2.3. Outcome (Head and Neck Cancer (HNC))
2.4. Participant Selection
2.5. Covariates
2.6. Statistical Analyses
3. Results
3.1. General Characteristics
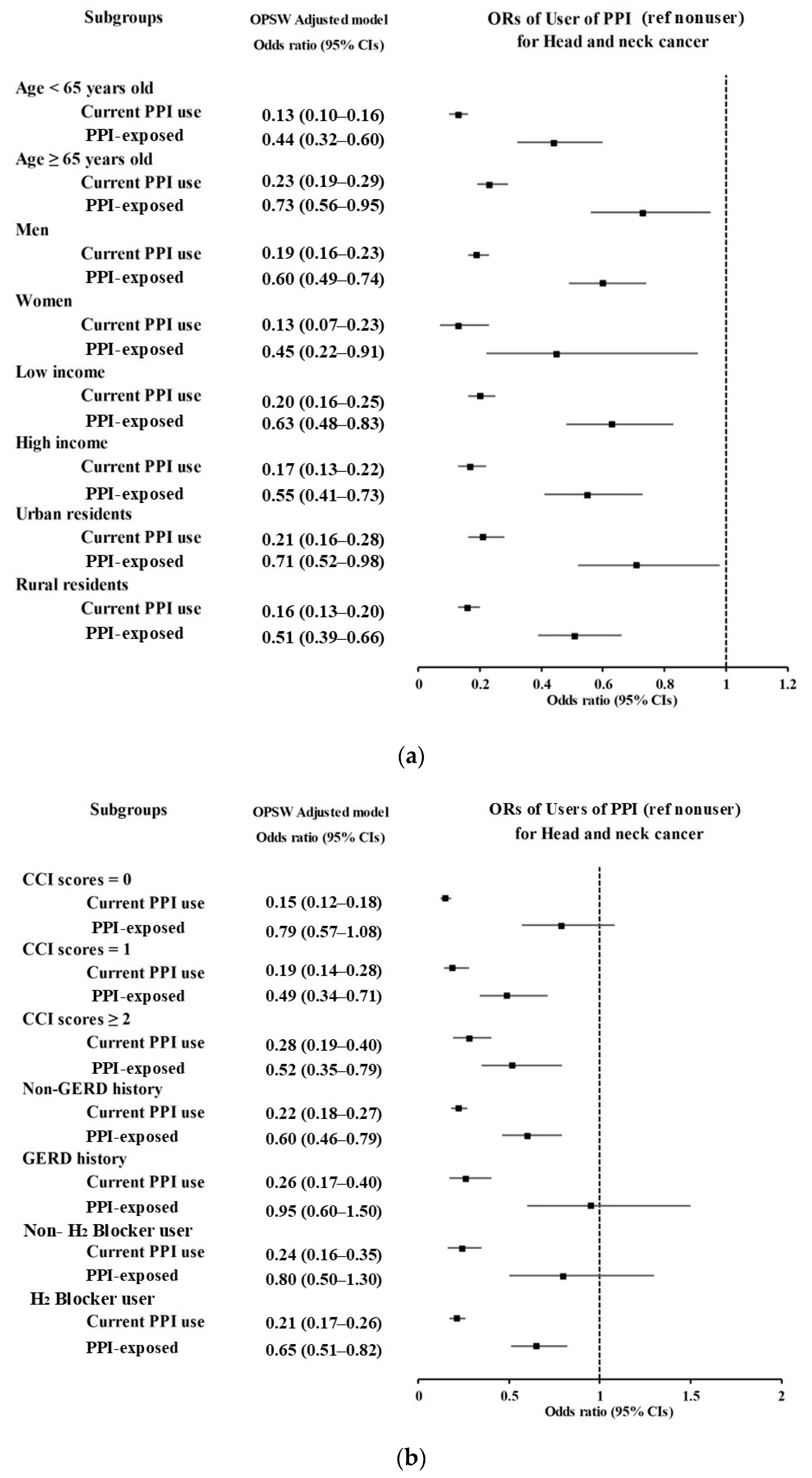
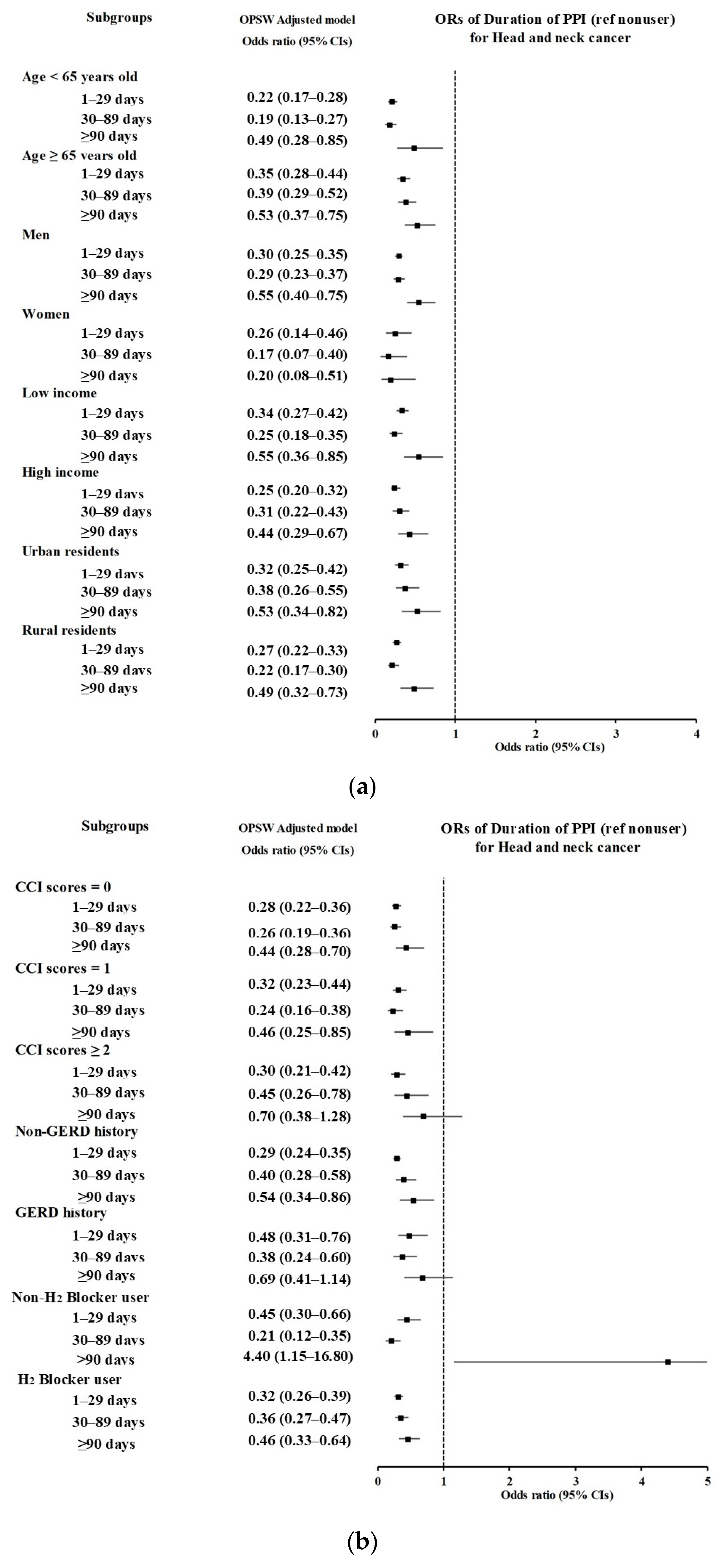
3.2. Associations of Prior Use of PPI and Its Duration with HNC
3.3. Associations Between the Use of PPI and HNC According to Subsites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, B.; Cai, H.D.; Xie, H.L.; Chen, D.Y.; Jiang, S.M.; Jia, L. Epidemiology of globus symptoms and associated psychological factors in China. J. Dig. Dis. 2016, 17, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Koufman, J.A.; Aviv, J.E.; Casiano, R.R.; Shaw, G.Y. Laryngopharyngeal reflux: Position statement of the committee on speech, voice, and swallowing disorders of the American Academy of Otolaryngology-Head and Neck Surgery. Otolaryngol. Head Neck Surg. 2002, 127, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Allen, J.E.; Barillari, M.R.; Karkos, P.D.; Jia, H.; Ceccon, F.P.; Imamura, R.; Metwaly, O.; Chiesa-Etomba, C.M.; Bock, J.M.; et al. Management of laryngopharyngeal reflux around the world: An international study. Laryngoscope 2021, 131, E1589–E1597. [Google Scholar] [CrossRef] [PubMed]
- Freedberg, D.E.; Kim, L.S.; Yang, Y.X. The risks and benefits of long-term use of proton pump inhibitors: Expert review and best practice advice from the American Gastroenterological Association. Gastroenterology 2017, 152, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Haastrup, P.F.; Thompson, W.; Søndergaard, J.; Jarbøl, D.E. Side effects of long-term proton pump inhibitor use: A review. Basic Clin. Pharmacol. Toxicol. 2018, 123, 114–121. [Google Scholar] [CrossRef]
- Choi, H.G.; Lee, H.K.; Kang, H.S.; Lim, H.; Kim, J.H.; Kim, J.H.; Kim, N.Y.; Cho, S.J.; Nam, E.S.; Min, K.W.; et al. Possible association between the use of proton pump inhibitors and H2 receptor antagonists, and esophageal cancer: A nested case–control study using a Korean National Health Screening Cohort. Pharmaceuticals 2022, 15, 517. [Google Scholar] [CrossRef]
- Liu, K.; Wang, Y.H.; Wang, J.; Chen, B.; Luo, N.; Gong, J. Meta-analysis of proton pump inhibitor use and the risk of developing gastric cancer or colorectal cancer. Anticancer. Drugs 2023, 34, 971–998. [Google Scholar] [CrossRef]
- Sarkar, M.; Hennessy, S.; Yang, Y.X. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann. Intern. Med. 2008, 149, 391–398. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.S.; Park, S.H.; Shin, S.A.; Kim, K. Cohort profile: The national health insurance service–national sample cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2017, 46, e15. [Google Scholar] [CrossRef]
- National Health Insurance Service. National Health Insurance Sharing Service [Internet]; National Health Insurance Service: Wonju, Republic of Korea, 2019; Available online: https://nhiss.nhis.or.kr/bd/ab/bdaba002cv.do (accessed on 24 March 2019).
- Kim, S.Y.; Min, C.; Oh, D.J.; Choi, H.G. Bidirectional association between GERD and asthma: Two longitudinal follow-up studies using a national sample cohort. J. Allergy Clin. Immunol. Pract. 2020, 8, 1005–1013.e9. [Google Scholar] [CrossRef]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Thomas, L.E.; Li, F. Addressing extreme propensity scores via the overlap weights. Am. J. Epidemiol. 2019, 188, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.E.; Li, F.; Pencina, M.J. Overlap weighting: A propensity score method that mimics attributes of a randomized clinical trial. JAMA 2020, 323, 2417–2418. [Google Scholar] [CrossRef]
- Li, F.; Morgan, K.L.; Zaslavsky, A.M. Balancing covariates via propensity score weighting. J. Am. Stat. Assoc. 2018, 113, 390–400. [Google Scholar] [CrossRef]
- Eells, A.C.; Mackintosh, C.; Marks, L.; Marino, M.J. Gastroesophageal reflux disease and head and neck cancers: A systematic review and meta-analysis. Am. J. Otolaryngol. 2020, 41, 102653. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, B.; Lim, H.; Kim, M.; Kong, I.G.; Choi, H.G. Increased risk of larynx cancer in patients with gastroesophageal reflux disease from a national sample cohort. Clin. Otolaryngol. 2019, 44, 534–540. [Google Scholar] [CrossRef]
- McCormick, C.A.; Samuels, T.L.; Battle, M.A.; Frolkis, T.; Blumin, J.H.; Bock, J.M.; Wells, C.; Yan, K.; Altman, K.W.; Johnston, N. H+/K+ ATPase expression in the larynx of laryngopharyngeal reflux and laryngeal cancer patients. Laryngoscope 2021, 131, 130–135. [Google Scholar] [CrossRef]
- Papagerakis, S.; Bellile, E.; Peterson, L.A.; Pliakas, M.; Balaskas, K.; Selman, S.; Hanauer, D.; Taylor, J.M.G.; Duffy, S.; Wolf, G. Proton pump inhibitors and histamine 2 blockers are associated with improved overall survival in patients with head and neck squamous carcinoma. Cancer Prev. Res. 2014, 7, 1258–1269. [Google Scholar] [CrossRef]
- Lechien, J.R.; Saussez, S.; Harmegnies, B.; Finck, C.; Burns, J.A. Laryngopharyngeal reflux and voice disorders: A multifactorial model of etiology and pathophysiology. J. Voice 2017, 31, 733–752. [Google Scholar] [CrossRef]
- Nurgalieva, Z.Z.; Graham, D.Y.; Dahlstrom, K.R.; Wei, Q.; Sturgis, E.M. A pilot study of Helicobacter pylori infection and risk of laryngopharyngeal cancer. Head Neck 2005, 27, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, C.T.; Issaeva, N.; Vageli, D.P. In vitro model for gastroduodenal reflux–induced nuclear factor-kappaB activation and its role in hypopharyngeal carcinogenesis. Head Neck 2016, 38, E1381–E1391. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.A. What is the evidence that gastroesophageal reflux is involved in the etiology of laryngeal cancer? Curr. Opin. Otolaryngol. Head Neck Surg. 2005, 13, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.Y.; Zhang, S.S.; Zhong, J.T.; Zhou, S.H. Pepsin and laryngeal and hypopharyngeal carcinomas. Clin. Exp. Otorhinolaryngol. 2021, 14, 159–168. [Google Scholar] [CrossRef]
- Vageli, D.P.; Doukas, S.G.; Doukas, P.G.; Judson, B.L. Bile reflux and hypopharyngeal cancer. Oncol. Rep. 2021, 46, 244. [Google Scholar] [CrossRef]
- Sasaki, C.T.; Doukas, S.G.; Costa, J.; Vageli, D.P. Biliary reflux as a causal factor in hypopharyngeal carcinoma: New clinical evidence and implications. Cancer 2019, 125, 3554–3565. [Google Scholar] [CrossRef]
- Sarela, A.I.; Hick, D.G.; Verbeke, C.S.; Casey, J.F.; Guillou, P.J.; Clark, G.W. Persistent acid and bile reflux in asymptomatic patients with Barrett esophagus receiving proton pump inhibitor therapy. Arch. Surg. 2004, 139, 547–551. [Google Scholar] [CrossRef]
- Guilemany, J.M.; Langdon, C.; Ballesteros, F.; Blanch, J.L. Prognostic significance and association of Helicobacter pylori infection in pharyngolaryngeal cancer. Eur. Arch. Otorhinolaryngol. 2014, 271, 2539–2543. [Google Scholar] [CrossRef]
- Mohtasham, N.; Saghravanian, N.; Zare, R.; Saghafi, S.; Ghazi, N.; Mohajertehran, F.; Shahabinejad, M. Tumor tissue Helicobacter pylori and human papillomavirus infection in head and neck squamous cell carcinoma patients and association with clinicopathological indices: A cross-sectional medical survey. Dent. Res. J. 2022, 19, 8. [Google Scholar]
- Pabón-Carrasco, M.; Keco-Huerga, A.; Castro-Fernández, M.; Saracino, I.M.; Fiorini, G.; Vaira, D.; Pérez-Aísa, Á.; Tepes, B.; Jonaitis, L.; Voynovan, I.; et al. Role of proton pump inhibitors dosage and duration in Helicobacter pylori eradication treatment: Results from the European Registry on H. pylori management. United Eur. Gastroenterol. J. 2024, 12, 122–138. [Google Scholar] [CrossRef]
- Piovani, D.; Tsantes, A.G.; Schünemann, H.J.; Bonovas, S. Meta-analysis: Use of proton pump inhibitors and risk of gastric cancer in patients requiring gastric acid suppression. Aliment. Pharmacol. Ther. 2023, 57, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Arai, J.; Niikura, R.; Hayakawa, Y.; Kawahara, T.; Honda, T.; Hasatani, K.; Yoshida, N.; Nishida, T.; Sumiyoshi, T.; Kiyotoki, S.; et al. Chemoprevention of oesophageal squamous-cell carcinoma and adenocarcinoma: A multicentre retrospective cohort study. Digestion 2022, 103, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Snider, E.J.; Kaz, A.M.; Inadomi, J.M.; Grady, W.M. Chemoprevention of esophageal adenocarcinoma. Gastroenterol. Rep. 2020, 8, 253–260. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Before 2 PS Overlap Weighting Adjustment | After PS Overlap Weighting Adjustment | ||||
|---|---|---|---|---|---|---|
| Head and Neck Cancer | Control | Standardized Difference | Head and Neck Cancer | Control | Standardized Difference | |
| Total participants (n, %) | 1677 (100) | 6708 (100) | 1324 (100) | 1324 (100) | ||
| Age (%) | 0.00 | 0.00 | ||||
| Under 40 | 99 (5.90) | 396 (5.90) | 79 (5.92) | 79 (5.92) | ||
| 40–44 | 65 (3.88) | 260 (3.88) | 51 (3.88) | 51 (3.88) | ||
| 45–49 | 112 (6.68) | 448 (6.68) | 88 (6.67) | 88 (6.67) | ||
| 50–54 | 178 (10.61) | 712 (10.61) | 140 (10.56) | 140 (10.56) | ||
| 55–59 | 218 (13.00) | 872 (13.00) | 173 (13.06) | 173 (13.06) | ||
| 60–64 | 225 (13.42) | 900 (13.42) | 176 (13.31) | 176 (13.31) | ||
| 65–69 | 220 (13.12) | 880 (13.12) | 174 (13.10) | 174 (13.10) | ||
| 70–74 | 233 (13.89) | 932 (13.89) | 183 (13.80) | 183 (13.80) | ||
| 75–79 | 173 (10.32) | 692 (10.32) | 137 (10.35) | 137 (10.35) | ||
| 80–84 | 102 (6.08) | 408 (6.08) | 82 (6.17) | 82 (6.17) | ||
| 85+ | 52 (3.10) | 208 (3.10) | 42 (3.16) | 42 (3.16) | ||
| Sex (%) | ||||||
| Male | 1255 (74.84) | 5020 (74.84) | 0.00 | 988 (74.60) | 988 (74.60) | 0.00 |
| Female | 422 (25.16) | 1688 (25.16) | 336 (25.40) | 336 (25.40) | ||
| Income (%) | ||||||
| 1 (lowest) | 370 (22.06) | 1480 (22.06) | 0.00 | 293 (22.09) | 293 (22.09) | 0.00 |
| 2 | 229 (13.66) | 916 (13.66) | 181 (13.65) | 181 (13.65) | ||
| 3 | 248 (14.79) | 992 (14.79) | 196 (14.78) | 196 (14.78) | ||
| 4 | 347 (20.69) | 1388 (20.69) | 274 (20.65) | 274 (20.65) | ||
| 5 (highest) | 483 (28.80) | 1932 (28.80) | 382 (28.83) | 382 (28.83) | ||
| Region of residence (%) | ||||||
| Urban | 716 (42.70) | 2864 (42.70) | 0.00 | 567 (42.82) | 567 (42.82) | 0.00 |
| Rural | 961 (57.30) | 3844 (57.30) | 757 (57.18) | 757 (57.18) | ||
| 1 CCI score (mean, SD) | 0.90 (1.23) | 0.68 (1.18) | 0.19 | 0.85 (1.05) | 0.85 (0.61) | 0.00 |
| Number of treatments with 3 GERD (mean, SD) | 0.79 (2.62) | 0.41 (1.77) | 0.17 | 0.65 (1.92) | 0.65 (1.13) | 0.00 |
| H2 blocker prescription dates (n, %) | 34.06 (69.49) | 25.29 (64.86) | 0.13 | 31.94 (58.82) | 31.94 (33.61) | 0.00 |
| User of 4 PPI (n, %) | 0.54 | 0.49 | ||||
| Nonuser | 1135 (67.68) | 5734 (85.48) | 909 (68.64) | 1104 (83.35) | ||
| Current PPI use | 323 (19.26) | 208 (3.10) | 248 (18.71) | 53 (4.03) | ||
| PPI-exposed | 219 (13.06) | 766 (11.42) | 168 (12.65) | 167 (12.61) | ||
| Duration of PPI use (n, %) | ||||||
| Nonuser | 1135 (67.68) | 5734 (85.48) | 0.43 | 909 (68.64) | 1104 (83.35) | 0.39 |
| 1–29 days | 323 (19.26) | 474 (7.07) | 253 (19.12) | 99 (7.45) | ||
| 30–89 days | 129 (7.69) | 260 (3.88) | 98 (7.39) | 60 (4.51) | ||
| ≥90 days | 90 (5.37) | 240 (3.58) | 64 (4.86) | 62 (4.69) | ||
| Characteristics | Number of Events (Exposure/Total, %) | Number of Controls (Exposure/Total, %) | Odds Ratios (95% Confidence Intervals) | |||
|---|---|---|---|---|---|---|
| Crude | p-Value | Adjusted Model with 1 OW † | p-Value | |||
| Odd ratios for head and neck cancer | ||||||
| Current PPI use | 323/1677 (19.26) | 208/6708 (3.1) | 7.85 (6.52–9.44) | <0.001 * | 0.14 (0.11–0.17) | <0.001 * |
| 2 PPI-exposed | 219/1677 (13.06) | 766/6708 (11.42) | 1.44 (1.23–1.70) | <0.001 * | 0.69 (0.60–0.79) | <0.001 * |
| Odd ratios for oral cavity cancer | ||||||
| Current PPI use | 41/409 (10.02) | 490/7976 (6.14) | 1.74 (1.24–2.44) | 0.001 * | 1.01 (0.80–1.27) | 0.964 |
| PPI-exposed | 53/409 (12.96) | 932/7976 (11.69) | 1.18 (0.88–1.60) | 0.27 | 0.89 (0.72–1.11) | 0.302 |
| Odd ratios for oropharynx cancer | ||||||
| Current PPI use | 27/195 (13.85) | 504/8190 (6.15) | 2.52 (1.65–3.84) | <0.001 * | 0.70 (0.53–0.94) | 0.016 * |
| PPI-exposed | 25/195 (12.82) | 960/8190 (11.72) | 1.22 (0.80–1.88) | 0.355 | 0.79 (0.59–1.07) | 0.124 |
| Odd ratios for nasopharynx cancer | 183 (13.80) | 183 (13.80) | ||||
| Current PPI use | 21/167 (12.57) | 510/8218 (6.21) | 2.14 (1.34–3.42) | 0.002 * | 0.68 (0.49–0.94) | 0.018 * |
| PPI-exposed | 16/167 (9.58) | 969/8218 (11.79) | 0.86 (0.51–1.44) | 0.56 | 1.06 (0.74–1.52) | 0.743 |
| Odd ratios for hypopharynx cancer | ||||||
| Current PPI use | 41/143 (28.67) | 490/8242 (5.95) | 7.01 (4.76–10.3) | <0.001 * | 0.33 (0.25–0.43) | <0.001 * |
| PPI-exposed | 21/143 (14.69) | 964/8242 (11.7) | 1.83 (1.12–2.96) | 0.015 * | 0.71 (0.51–0.99) | 0.042 * |
| Odd ratios for salivary gland cancer | ||||||
| Current PPI use | 30/177 (16.95) | 501/8208 (6.1) | 2.99 (1.99–4.48) | <0.001 * | 0.42 (0.32–0.56) | <0.001 * |
| PPI-exposed | 12/177 (6.78) | 973/8208 (11.85) | 0.62 (0.34–1.11) | 0.109 | 1.57 (1.05–2.36) | 0.027 * |
| Odd ratios for nasal cavity/sinus cancer | ||||||
| Current PPI use | 9/131 (6.87) | 522/8254 (6.32) | 1.17 (0.59–2.32) | 0.66 | 1.48 (0.93–2.36) | 0.096 |
| PPI-exposed | 22/131 (16.79) | 963/8254 (11.67) | 1.55 (0.97–2.47) | 0.067 | 0.72 (0.52–1.00) | 0.049 * |
| Odd ratios for larynx cancer | ||||||
| Current PPI use | 150/444 (33.78) | 381/7941 (4.8) | 11.5 (9.11–14.4) | <0.001 * | 0.19 (0.16–0.22) | <0.001 * |
| PPI-exposed | 66/444 (14.86) | 919/7941 (11.57) | 2.09 (1.58–2.77) | <0.001 * | 0.59 (0.48–0.72) | <0.001 * |
| Characteristics | Number of Events (Exposure/Total, %) | Number of Controls (Exposure/Total, %) | Odds Ratios (95% Confidence Intervals) | |||
|---|---|---|---|---|---|---|
| Crude | p-Value | Adjusted Model with 1 OW † | p-Value | |||
| Odd ratios for head and neck cancer | ||||||
| 1–29 days | 323/1677 (19.26) | 474/6708 (7.07) | 3.44 (2.95–4.02) | <0.001 * | 0.30 (0.26–0.35) | <0.001 * |
| 30–89 days | 129/1677 (7.69) | 260/6708 (3.88) | 2.51 (2.01–3.12) | <0.001 * | 0.44 (0.36–0.53) | <0.001 * |
| ≥90 days | 90/1677 (5.37) | 240/6708 (3.58) | 1.89 (1.47–2.43) | <0.001 * | 0.61 (0.49–0.77) | <0.001 * |
| Odd ratios for oral cavity cancer | ||||||
| 1–29 days | 63/409 (15.4) | 734/7976 (9.2) | 1.79 (1.35–2.37) | <0.001 * | 0.81 (0.67–0.99) | 0.035 * |
| 30–89 days | 16/409 (3.91) | 373/7976 (4.68) | 0.89 (0.53–1.49) | 0.664 | 1.44 (1.01–2.05) | 0.045 * |
| ≥90 days | 15/409 (3.67) | 315/7976 (3.95) | 0.99 (0.58–1.68) | 0.973 | 1.12 (0.76–1.65) | 0.573 |
| Odd ratios for oropharynx cancer | ||||||
| 1–29 days | 32/195 (16.41) | 765/8190 (9.34) | 1.97 (1.33–2.91) | <0.001 * | 0.71 (0.55–0.92) | 0.009 * |
| 30–89 days | 8/195 (4.1) | 381/8190 (4.65) | 0.99 (0.48–2.03) | 0.973 | 1.18 (0.73–1.92) | 0.491 |
| ≥90 days | 12/195 (6.15) | 318/8190 (3.88) | 1.77 (0.97–3.23) | 0.061 | 0.50 (0.31–0.78) | 0.003 * |
| Odd ratios for nasopharynx cancer | 183 (13.80) | 183 (13.80) | ||||
| 1–29 days | 23/167 (13.77) | 774/8218 (9.42) | 1.54 (0.98–2.41) | 0.06 | 0.82 (0.61–1.11) | 0.196 |
| 30–89 days | 10/167 (5.99) | 379/8218 (4.61) | 1.37 (0.71–2.62) | 0.346 | 0.77 (0.49–1.22) | 0.263 |
| ≥90 days | 4/167 (2.4) | 326/8218 (3.97) | 0.64 (0.23–1.73) | 0.376 | 1.30 (0.64–2.66) | 0.472 |
| Odd ratios for hypopharynx cancer | ||||||
| 1–29 days | 35/143 (24.48) | 762/8242 (9.25) | 3.85 (2.57–5.76) | <0.001 * | 0.44 (0.34–0.57) | <0.001 * |
| 30–89 days | 13/143 (9.09) | 376/8242 (4.56) | 2.90 (1.60–5.25) | <0.001 * | 0.54 (0.36–0.81) | 0.003 * |
| ≥90 days | 14/143 (9.79) | 316/8242 (3.83) | 3.71 (2.08–6.62) | <0.001 * | 0.43 (0.27–0.67) | <0.001 * |
| Odd ratios for salivary gland cancer | ||||||
| 1–29 days | 31/177 (17.51) | 766/8208 (9.33) | 2.02 (1.36–3.00) | <0.001 * | 0.60 (0.46–0.79) | <0.001 * |
| 30–89 days | 8/177 (4.52) | 381/8208 (4.64) | 1.05 (0.51–2.15) | 0.9 | 1.07 (0.64–1.77) | 0.799 |
| ≥90 days | 3/177 (1.69) | 327/8208 (3.98) | 0.46 (0.15–1.44) | 0.183 | 2.19 (0.95–5.04) | 0.064 |
| Odd ratios for nasal cavity/sinus cancer | ||||||
| 1–29 days | 14/131 (10.69) | 783/8254 (9.49) | 1.21 (0.69–2.13) | 0.507 | 1.14 (0.79–1.65) | 0.477 |
| 30–89 days | 12/131 (9.16) | 377/8254 (4.57) | 2.16 (1.17–3.96) | 0.013 * | 0.57 (0.37–0.87) | 0.01 * |
| ≥90 days | 5/131 (3.82) | 325/8254 (3.94) | 1.04 (0.42–2.57) | 0.93 | 1.16 (0.60–2.24) | 0.66 |
| Odd ratios for larynx cancer | ||||||
| 1–29 days | 120/444 (27.03) | 677/7941 (8.53) | 5.16 (4.08–6.53) | <0.001 * | 0.30 (0.25–0.35) | <0.001 * |
| 30–89 days | 60/444 (13.51) | 329/7941 (4.14) | 5.31 (3.91–7.21) | <0.001 * | 0.28 (0.23–0.36) | <0.001 * |
| ≥90 days | 36/444 (8.11) | 294/7941 (3.7) | 3.57 (2.46–5.17) | <0.001 * | 0.50 (0.37–0.68) | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.S.; Jo, S.; Kang, H.S.; Kwon, M.J.; Wee, J.H.; Kang, J.W.; Choi, H.G.; Kim, H. Potential Chemopreventive Role of Proton Pump Inhibitors in Head and Neck Cancer: Insights from a Nested Case–Control Analysis of a National Health Screening Cohort. J. Pers. Med. 2025, 15, 8. https://doi.org/10.3390/jpm15010008
Lee JS, Jo S, Kang HS, Kwon MJ, Wee JH, Kang JW, Choi HG, Kim H. Potential Chemopreventive Role of Proton Pump Inhibitors in Head and Neck Cancer: Insights from a Nested Case–Control Analysis of a National Health Screening Cohort. Journal of Personalized Medicine. 2025; 15(1):8. https://doi.org/10.3390/jpm15010008
Chicago/Turabian StyleLee, Joong Seob, Soomin Jo, Ho Suk Kang, Mi Jung Kwon, Jee Hye Wee, Jeong Wook Kang, Hyo Geun Choi, and Heejin Kim. 2025. "Potential Chemopreventive Role of Proton Pump Inhibitors in Head and Neck Cancer: Insights from a Nested Case–Control Analysis of a National Health Screening Cohort" Journal of Personalized Medicine 15, no. 1: 8. https://doi.org/10.3390/jpm15010008
APA StyleLee, J. S., Jo, S., Kang, H. S., Kwon, M. J., Wee, J. H., Kang, J. W., Choi, H. G., & Kim, H. (2025). Potential Chemopreventive Role of Proton Pump Inhibitors in Head and Neck Cancer: Insights from a Nested Case–Control Analysis of a National Health Screening Cohort. Journal of Personalized Medicine, 15(1), 8. https://doi.org/10.3390/jpm15010008








